Abstract
Ameloblastin is mainly known as a dental enamel protein, synthesized and secreted into developing enamel matrix by the enamel-forming ameloblasts. The function of ameloblastin in tooth development remains unclear, but it has been suggested to be involved in processes varying from regulating crystal growth to activity as a growth factor or partaking in cell signaling. Recent studies suggest that some enamel matrix proteins also might have important functions outside enamel formation. In this context ameloblastin has recently been reported to induce dentin and bone repair, as well as being present in the early bone and cartilage extracellular matrices during embryogenesis. However, what cells express ameloblastin in these tissues still remain unclear. Thus, the expression of ameloblastin was examined in cultured primary mesenchymal cells and in vivo during healing of bone defects in a “proof of concept” animal study. The real time RT-PCR analysis revealed human ameloblastin (AMBN) mRNA expression in human mesenchymal stem cells and primary osteoblasts and chondrocytes. Expression of AMBN mRNA was also confirmed in human CD34 positive cells and osteoclasts. Western and dot blot analysis of cell lysates and medium confirmed the expression and secretion of ameloblastin from mesenchymal stem cells, primary human osteoblasts and chondrocytes. Expression of ameloblastin was also detected in newly formed bone in experimental bone defects in adult rats. Together these findings suggest a role of this protein in early bone formation and repair.
Keywords: Ameloblastin expression, Bone formation, Bone repair, Mesenchymal stem cells
INTRODUCTION
Formation of hard tissues generally follows a common scheme including mesenchymal cell recruitment, proliferation, differentiation and finally extracellular matrix secretion and biomineralization. Several of the involved matrix molecules, i.e. collagens and proteoglycans, are common to most hard tissues, regardless of their location or function. Enamel, on the other hand, is the only mineralized tissue formed by ectodermal cells in a highly specific process employing molecules such as amelogenin, enamelin and ameloblastin proteins to control the biomineral. The major role for these molecules is believed to be nucleating, templating and controlling hydroxyapatite crystal growth during enamel biomineralization. However, ameloblastin [1–3] and amelogenin [4–6] expression have also been detected in mesenchymal cells during embryogenesis or tissue repair, suggesting that at least some of the enamel matrix proteins also have roles outside enamel tissue and that these molecules could play a more general role during the formation of skeletal tissues. Ameloblastin, also known as amelin or sheathlin [7–9], is a well-conserved gene among species and present throughout evolution and ontogenesis of teeth [10]. It was originally identified as an enamel-specific protein [8,9,11 12] and its presence appeared to be critical for proper enamel prism formation [13,14]. Ameloblastin is suggested to be a two domain, intrinsically unstructured protein [15] that binds calcium [16] and is subject to intensive proteolysis by the matrix proteases enamelysin and kallikrein 4 [17,18].
Based to a large extent on localization by histochemical methods and transgenic experiments, ameloblastin has been proposed to be both a structural component of the enamel matrix, as well as functioning as a growth factor or signaling molecule during tooth growth [1–3,8,19–24]. The ameloblastin protein was first localized in ameloblast cells [7,11] that were shown to secrete it extracellularly when it accumulates into the sheath space between the enamel prisms of maturing enamel [25], as well as being expressed in Hertwig’s root sheath cells during root formation [8]. Later, ameloblastin expression has been detected in early odontoblasts [1], in trauma-induced reparative dentin [2], and in osteoblast like cells during early stages of bone formation [3]. Genetic manipulations of ameloblastin show severe enamel hypoplasia [26] and reduced alveolar bone formation [27] in the AmbnΔ5–6 mutant mouse model [27], and overproduction of ameloblastin in transgenic mice leads to formation of thinner and more porous enamel, with disrupted rod patterns and abnormal crystallites [28]. In the AmbnΔ5–6 mutant mouse model, ameloblasts (and associated cell layers of the enamel organ) detach from the tooth surface as they enter the secretory stage. This directly or indirectly stops the typical differentiation sequence and abort enamel formation [26]. The true function(s) of ameloblastin remains obscure, but the fact that ameloblastin expression is not restricted to enamel formation seems now to be well established. Recently, we reported that murine ameloblastin (Ambn) mRNA and protein are expressed during craniofacial bone development in rats at embryonic and early post-natal stages [3]. During intramembranous ossification, ameloblastin expression was detected in the superficial layer of the condensed vascularized connective tissue and in the cellular layer covering the surface of newly formed woven bone. In endochondral ossification, the protein was found in the extracellular matrix of the cartilage templates and in the perichondrium. Ameloblastin is expressed during ongoing growth in developmental and remodeling processes, however, the expression decreases at the end of the process, and is not detectable in mature bone [3]. The study presented here addresses the ubiquity of ameloblastin expression in precursor cells from blood (CD34+), mesenchymal stem cells, as well as primary human osteoblasts, chondrocytes and osteoclasts.
MATERIALS AND METHODS
Cell Cultures
Human mesenchymal stem cells (MSC) were purchased from Cambrex Bio Science, Walkersville, MD, USA (BMC-1D) and cultured in Mesenchymal Stem Cell Growth Medium (MSCGM, Cambrex Bio Science). Bone marrow stem cells (BMSC) were isolated from bone marrow from the iliac crest from two different donors (BMSC). The mononuclear mesenchymal stem cells were isolated using Lymphoprep (AXIS-SHIELD, Oslo, Norway). The mononuclear cells isolated from the lymphoprep were subsequently cultured in MEM-α medium (Invitrogen Life Technologies, Grand Island, NY, USA) with 20 % foetal calf serum (FCS; Biowest, Loire Valley, France), with 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen Life Technologies).
Mesenchymal stem cells from human liposuction waste material from two different donors; Adipose derived adult stem cells (ADAS) were cultured in Dulbecco’s Modified Eagle’s Medium/ Nutrient Mixture F-12 Ham (1:1 v/v; Sigma-Aldrich, St.Louis, MO, USA) supplemented with 20 % FCS, 100 U/ml penicillin and 100 μg/ml streptomycin, and MSC were sorted by flow cytometry. The BMSC and ADAS cells were subcultured in MEM-α medium (PAA Laboratories, Pasching Austria) with 10 % FCS (PAA) and 100 U/ml penicillin, 0,1 mg/ml streptomycin (Sigma) and Dulbecco’s Modified Eagle’s Medium (DMEM; PAA Laboratories with 10 % FCS (PAA Laboratories), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma), respectively. The immunophenotype of the undifferentiated MSCs from bone marrow and adipose tissue were characterized by flow cytometry to be CD45 low or negative, and CD10, CD13 and CD90 positive. Further their ability to differentiate in osteogenic and adipose directions, as well as clonogenic growth in a low density CFU-f (colony forming unit-fibroblast) assay was tested [29,30].
Human peripheral mononuclear cells (PBMC) were isolated from blood by Ficoll-Hypaque gradient centrifugation (Lymphoprep; Nyegaard, Norway), and CD34+ cells were positively selected using anti-CD34 coated magnetic beads (Dynal, Oslo, Norway) as described by Smeland et al. (31). Osteoclasts (Ocl) were differentiated from human PBMC by adding macrophage-colony-stimulating factor (M-CSF) and receptor for activation of NF-κB (RANK) ligand to the medium (50 ng/ml each). The cells were stained for tartrate resistant acid phosphatase (TRAP) activity using naphtol AS-BI phosphate and Fast Garnet in the presence of sodium tartrate, as described by the manufacturer (Sigma). Cells that were TRAP positive and contained three or more nuclei were counted as osteoclasts. All donor recruitment and cell sampling were performed in accordance with a protocol approved by the local ethical committee, and made anonymous according to the rules of the Norwegian bio-bank laws and health register authorities.
Commercially available human primary osteoblasts (NHO) from tibia (NHOst cell system; Cambrex Bio Science) were grown in Osteoblast Growth Media (OGM) (Cambrex Bio Science). Osteoblasts cultured to facilitate differentiation were exposed to hydrocortisone hemisuccinate (200 nM) and β-glycerophosphate (10 mM) (Cambrex Bio Science) in the OGM medium. Commercially available human primary chondrocytes (NHAC) from knee joints were purchased from two different companies (hCa, Cell Applications, CA, San Diego, USA and NHAC-kn, Cambrex Bio Science, respectively). These cells were grown in Chondrocyte Growth Medium (Cell Applications and Cambrex Bio Science, respectively). Chondrocyte Differentiation Medium (CDM, containing FBS 5%, gentamicin sulfate-amphotericin B 0.1%, TGFβ-1 0.5%, R3-IGF-1 0.2%, insulin 0.2%, transferrin 0.2% and ascorbic acid 2.5%) was added to chondrocyte cell cultures to facilitate differentiation.
Human dental pulp cells (Dominon Pharmakine, Derio, Spain) and Human periodontal ligament (PDL) cells (ATCC, Manassas, VA, USA) were cultured in DMEM (PAA Laboratories) supplemented with 10 % FCS (PAA Laboratories), 100 U/ml penicillin and 0,1 mg/ ml streptomycin (Sigma). Dental pulp cells have previously been found to express AMBN [32] and was used as positive control.
The mouse enamel organ epithelial cell line, LS8, is an immortalized ameloblast-like cell line that expresses enamel-specific genes such as Ambn [33–35], was grown in DMEM (PAA Laboratories), 10% FCS (PAA Laboratories), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma).
The mouse osteoblastic cell line MC3T3-E1 (No ACC 210) was obtained from Deutsche Sammlung von Microorganismen und Zellkulturen (DSMZ, Braunschweig, Germany) and maintained in α-MEM (PAA Laboratories) containing 20 mM HEPES, 10% FCS (PAA Laboratories), and 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma).
To maintain cell viability half of the medium was changed twice weekly in the stem cell cultures, whereas the entire medium was changed every second day in the other cell cultures. The cells were grown in a humidified 95% air, 5% CO2 atmosphere at 37°C throughout the experiments.
mRNA Isolation
The cells were harvested 3, 12, 24, 48, 72 or 168 hours after confluence, and lysed in lysis/binding buffer (100 mM Tris-HCl, pH 8.0, 500 mM LiCl, 10 mM EDTA, pH 8.0, 0.5 mM dithiothreitol (DTT), and 1% sodium dodecyl sulfate (SDS). mRNA was isolated using magnetic beads (oligo (dT)25 as described by the manufacturer (Dynal AS, Oslo, Norway). Beads containing mRNA were re-suspended in 10 mM Tris-HCl, pH 8.0, and stored at −70°C until use. One microliter of the mRNA-containing solution was applied directly to obtain a first-strand complementary DNA (cDNA) using the iScript cDNA Synthesis Kit which contains both oligo (dT) and random hexamer primers (BioRad, Hercules, CA, USA).
Real-Time Reverse Transcriptase–PCR
Real time RT-PCR reactions were performed and monitored using iCycler iQ (BioRad). The 2X iQ SYBR Green Supermix was based on iTaq DNA polymerase (BioRad). cDNA samples were analyzed for the genes of interest using specific primers (Table I). The amplification program consisted of a pre-incubation step for denaturation of the template cDNA (3 min, 95°C), followed by 50 cycles consisting of a denaturation step (15 s, 95°C), an annealing step (30 s 60°C) and an extension step (30 s 72°C). After each cycle, fluorescence was measured at 72°C. A negative control without cDNA template was run in each assay. To allow relative quantification after PCR, standard curves were constructed from the standard reactions for each target and two housekeeping genes; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin, by plotting Ct values, that is, the cycle number at which the fluorescence signal exceeds background versus log cDNA dilution. The Ct readings for each of the unknown samples were then used to calculate the amount of either the target or housekeeping gene relative to the standard. Levels of mRNA for target genes were normalised relative to the housekeeping genes. Oligonucleotide primer sequences used for the real-time RT-PCR and the specific parameters are shown in Table 1. PCR products were subjected to a melting curve analysis on the iCycler and subsequently to 2% agarose/TAE gel electrophoresis to confirm amplification specificity, Tm, and amplicon size (Table 1). The PCR products were excised from the agarose gel and the DNA was released from the gel by heating in TAE buffer (Omega GmbH, Mannheim, Germany). Subsequently, the DNA product was re-amplified by PCR using the primers and conditions described earlier. The resulting cDNAs were submitted to sequence analysis (Cogenics, Essex, UK). The PCR products were identified as either Homo sapiens ameloblastin (NM_016519) or Mus musculus ameloblastin (NM_009664) depending on the origin of the investigated cell cultures.
Table 1.
Primers used in real-time RT-PCR analysis of ameloblastin mRNA expression.
| Gene | Primer sequence | Species | Amplicon size (bp) |
|---|---|---|---|
| AMBN | S 5’ - AGCCATGTTTCCAGGATTTG - 3’ A 5’ - TGCACCTCCTTCTTCGTTCT - 3’ |
Human | 142 |
| Ambn | S 5’ - GCGTTTCCAAGAGCCCTGATAAC - 3’ A 5’ - AAGAAGCAGTGTCACATTTCCTGG - 3’ |
Mouse | 366 |
| GAPDH | S 5’-TGCACCACCAACTGCTTAGC - 3’ A 5’-GGCATGGACTGTGGTCATGAG - 3′ |
Human | 87 |
| GAPDH | S 5’-ACCCAGAAGACTGTGGATGG- 3’ A 5’-CACATTGGGGGTAGGAACAC - 3` |
Mouse | 171 |
| Beta-Actin | S 5’ - CTGGAACGGTGAAGGTGACA- 3’ A 5’ - AAGGGACTTCCTGTAACAATGCA - 3’ |
Human | 140 |
| Beta-Actin | S 5’ - GCTTCTTTGCAGCTCCTTCGT- 3’ A 5’ - ATATCGTCATCCATGGCGAAC - 3’ |
Mouse | 64 |
Dot Blot and Western Blot Analysis
Human pulp cells, MSC, ADAS, BMSC, NHO, NHAC and PDL cells were cultured in their respective cell culture media in T75 flasks, at 90 % confluence media were changed and cells and cell culture media harvested after 24 h incubation. The cells were washed twice with PBS, and lysed in 1 ml of Nonidet P-40 (NP-40) buffer (20 mM Tris HCl pH 8, 137 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 ng/ml aprotinin and 10 ng/ml leupeptin).
In the dot blot analysis samples of cell lysate and cell culture medium (20 μl) were added to pre-soaked nitrocellulose membranes (BioRad) in a dot blot manifold (Bio-Dot Microfiltration Apparatus; Bio-Rad). Purified recombinant rat ameloblastin-thioredoxin fusion protein (rrAmbn) ([1,23] ranging from 200 μg/ml to 2.5 μg/ml was used as reference samples, and Phosphate buffered saline (PBS) was used as blank negative control. Excess binding capacity of the nitrocellulose membrane was blocked by incubation in 0.5% non-fat, dried milk (Sigma) and 0.1% Tween-20 (Bio-Rad) for two hour at room temperature before the antiserum solution was added. Expression of the ameloblastin protein was identified by anti-ameloblastin (mix of H-300, sc-50533 and N-18, sc-33100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and verified against irrelevant controls of non-immune serum (mix of pre-immune Control Rabbit serum, NRBS-1, and pre-immune Control Goat Serum, NGTS-500; Alpha Diagnostic International Inc., San Antonio, USA), all used in 1:100 dilution in TTBS. After incubation overnight at 4°C, the membranes were washed 3 times in TTBS before further incubation in a 1:1000 dilution of horseradish peroxidase-label secondary antibody (Goat-anti-rabbit IgG-HRP, Southern Biotechnology, Birmingham, AL, USA). The 3,3- diaminobenzidine (tetrahydrochloride)-based horseradish peroxidase reaction product was visualized using 3% H2O2, 0.3 g/liter diaminobenzidine, and 0.5 g/liter NiCl2 in 50 mM (NH4)HCO3 [36]. Cross reactivity or false positive signals were tested using the non-immune serum control or only secondary antibody on aliquots of the samples. Images were taken and the membranes incubated in Swine-anti-goat IgG-HRP (Southern Biotechnology) diluted 1:1000 for 1 h at room temperature. The washing and staining procedure was repeated and new images taken.
In the western blot analysis, samples of cell lysate and cell culture media were diluted 1:2 in Laemmli samples buffer (BioRad), and separated on10 % Tris-HCl gels (Bio-Rad) (running buffer: 25 mM Tris-HCl, pH 8.3; 192 mM glycine, 0.1 % w/v SDS) at 100V prior to electro-blotting onto nitrocellulose membrane (0.2 m) (BioRad). Broad range prestained SDS-PAGE standards (162-0318; Bio-Rad) was used as reference. The transfer was performed in 25 mM Tris-base, 192 mM glycine and 20 % methanol, pH 8.3, for 45 min at 100 V. The immunodetection and staining procedure was followed as described above.
Effect of rrAmbn on AMBN expression
rrAmbn was dissolved to 1 mg/ml in sterile water and was added to the cultures to a final concentration of 10 ug/ml medium. Primary human mesenchymal stem cells (BMSC and ADAS), isolated from the same donors, were cultured according to the methods described in the above section. The cells were cultured in 6 well plates and grown to about 90 % confluence. At this stage rrAmbn was added once to the cultures. An equal number of identical parallel cultures received only sterile medium to serve as controls. After 3, 12 and 24 hours following the onset of rrAmbn stimulation, three test cultures and three control cultures were harvested for each time point, and submitted to mRNA extraction. Extraction of mRNA and real time RT-PCR was carried out as described in the above sections. The mRNA for each individual treatment and given time points was pooled into and analyzed by real time Reverse Transcriptase PCR analysis. Specific primers against human AMBN and GAPDH and β-actin used as reference housekeeping genes (Table I) were used to investigate relative changes in expression of AMBN during the experiment.
Proof of Concept Animal Experiment
All animal procedures were in accordance with and approved by the local ethical committee of the University of Ulm, Germany, and performed in accordance with German and EU regulations.
Adult female Sprague Dawley rats (n=4) with a weight of approximately 200 grams were given a subcutaneous injection (Atropin 0.05 mg/kg,) 15 min before the commencement of the surgical procedure. All rats were anaesthetized by means of an intraperitoneal injection 5 min before the beginning of the surgery (Xylazin 12 mg/kg and Ketamin 75 mg/kg). The cleanshaven heads and necks as well as the oral cavities of the animals were washed presurgically with ethanol chlorhexidin (Ethanol-Chlorhexidin; Pharmacia, Uppsala, Sweden; 5.0 g Chlorhexidindigluconate per 1000 ml), and all experiments were performed under aseptic conditions. An infrared lamp was used to maintain body temperature.
A horizontal incision of about 10 mm was made along the buccodistal aspect of the mandible behind and below the location of the molar teeth. After a blunt subcutaneous dissection to mobilise the skin, the distal part of the jaw bone was uncovered. Damage to the major blood vessels and nerves as well as perforation to the oral cavity were avoided. After exposing the buccal bone a penetrating cylindrical bilateral defect with a diameter of 2.5 mm through the ramus was drilled using a low-speed trepan burr and ample irrigation with sterile saline. The periosteum covering the area of both ends of the holes was completely removed to ensure that defects healed with fibrous tissue filling and not by direct bone formation. After this procedure the soft tissue, muscles and blood vessels were carefully repositioned and the wound closed by sutures (Perma hand Seide 4-0, Ethicon, Norderstedt, Germany). Bilateral defects were created in each animal (n=2 per animal), and pre-cut soft lyophilized collagen sheets (Lyoplant; Braun Aesculap,Tutsingen, Germany) with PBS were implanted in the defects (n=8). After surgery, the animals were placed in separate cages under an infrared lamp until the end of the anaesthesia.
After 14 days, the animals were sacrificed and the segments of the lower jaws containing the bone defects were removed en block together with adjacent teeth and alveolar bone and fixed in freshly prepared 4 % buffered paraformaldehyde for about 16 h, decalcified in 12.5 % EDTA for 14 days, rinsed in 1 % PBS for 1 d and, finally, embedded in paraffin. RNase-free water was used for all solutions. Semi-serial sections (7 μm) were mounted on aminopropyltriethoxysilane (Sigma ) coated glass slides. The sections were stained according to van Gieson or with hematoxylin-eosin and used for immunohistochemistry.
Immunohistochemistry
Tyramide signal amplification (TSA) Biotin system (Perkin Elmer, Boston, MA, USA) was used to perform immunohistochemical staining. Sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol. To quench the endogenous peroxidase activity, slides were incubated for 15 min with 1% H2O2 in Tris-buffered saline (TBS; 20 mM Tris-HCl, 150 mM NaCl, pH 7.6). Sections were then treated with Proteinase K (0.1 mg/ml; Sigma) followed by incubation for 30 min with the blocking reagent provided in the kit. Sections were incubated overnight at 4°C with affinity purified antibody against recombinant fusion ameloblastin, diluted 1:1000 [1]. Control sections were incubated with blocking buffer. The immune reaction was detected, according to the manufacturer’s instructions, by subsequent incubation with horseradish peroxidase (HRP)-labelled goat anti-rabbit antibody (DakoCytomation Denmark, Glostrup, Denmark), biotinyl tyramide and HRP-labelled streptavidine (provided in the kit). The immune reaction was finally visualized with diaminobenzidine (DAB; 0.7 mg/ml; Sigma) and light microscopy-photographed using a light microscope.
RESULTS
AMBN mRNA expression in cultured mesenchymal cells
The real time RT-PCR analysis produced a positive AMBN signal for all human mesenchymal cells tested (Figure 1). The size of the products corresponded perfectly with the predicted PCR products (Table 1), and sequencing of the PCR products from two random donors; bone marrow derived cells (BMSC) and normal human osteoblasts (NHO) confirmed that the PCR produced DNA indeed was identical to the targeted AMBN mRNA sequence (NCBI reference sequence NM_016519.4). As a general trend, the AMBN PCR signal, when normalized to expression of the housekeeping genes, decreased the more differentiated the primary cells were, but this trend could not be statistically confirmed. AMBN mRNA expression was also confirmed in primary human precursor cells of haematopoietic origin; (CD34+ cells and osteoclast cells (hOcl)) as well as in human precursor cells of mesenchymal origin; bone marrow derived cells (BMC-1D, BMSC) and adipose derived stem cells (ADAS). In more highly differentiated primary human cells from calcified tissues both the primary human osteoblasts from tibia (NHO), and the primary human chondrocytes (hCa) from articular cartilage, we found the expression of AMBN mRNA to be low but still clearly detectable (Figure 1).
Figure 1.
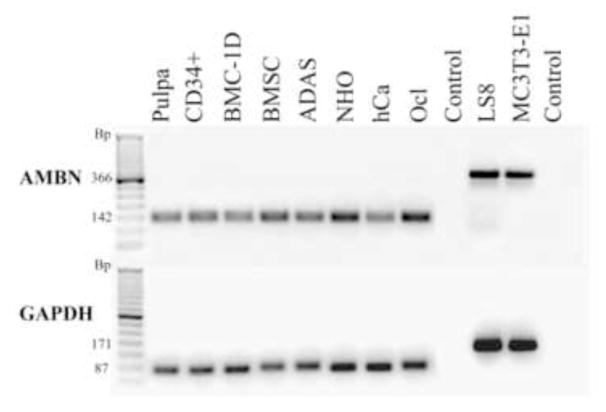
Real time RT-PCR products from various mesenchymal cells were separated on a 2% agarose gel and visualized by ethidium bromide staining. All cells in this panel produced a positive signal for AMBN (top panel). The PCR reaction produced one discreet band for each cell type each conforming to the designated size (see Table 1) validating that the PCR reaction was specific. This was also confirmed by sequencing of the DNA isolated from the PCR signals. Human dental pulp cells and LS8 cells were included for positive controls. The “control” lane was from a negative PCR reaction run without template to test for non-specific amplification, primer oligomerization and contamination from genomic DNA. For comparison and quantification all cell types were also analyzed for the housekeeping gene GAPDH (lower panel). (Pulpa represent human dental pulp cells, CD34+ is primary human CD34+ cells, BMC-1D and BMSC are primary bone marrow derived adult stem cells, ADAS is primary adipose tissue derived adult stem cells, NHO is primary adult human osteoblasts, hCa is primary adult human chondrocytes, Ocl is primary human osteoclasts, LS8 is ameloblast-like murine cell line and MC3T3- E1 is murine osteoblast-like cell line).
Ambn mRNA expression was also detected in the mouse pre-osteoblast like cell line MC3T3- E1. The presence of Ambn mRNA in these cells was also confirmed by sequencing of the product from the real time RT-PCR assay, revealing a nucleotide sequence corresponding to the predicted NCBI reference Ambn mRNA NM_009664.1.
Ameloblastin protein expression in cultured human mesenchymal cells
The translation of the detected AMBN mRNA signals in selected mesenchymal cells was confirmed by detection of the ameloblastin protein in the cell lysates as well as cell culture medium from cells harvested at confluence, by both dot blot (Fig. 2) and Western blotting (Fig. 3) using an ameloblastin specific antibody. Compared to the positive controls, the rAmeloblastin fusion protein, the cell lysate and medium of all cell types produced a positive signal in dot blot (Fig. 2). Pulp cells and PDL cells produced the strongest signal in cell lysate, whereas the signal in cell culture media was almost similar for all cell types tested (Fig. 2) The molecular weight of the ameloblastin protein in both cell lysate and cell culture media (medium) were identified to be approx. 62 kDa (Fig. 3). We could not identify any ameloblastin in the cell culture media using the antibody towards the C-terminal part of ameloblastin (H-300) (data not shown), but the antibody towards the N-terminal part of the protein (N-18) identified AMBN signals of approximately the same size as in cell lysates (Fig. 3 - medium). The AMBN observed in cell medium also showed signs of degradation or processing, causing less distinct signals. An unspecific low molecular band was observed in the cell culture medium and in the irrelevant antiserum control, but not in the cell lysate (Fig. 3).
Figure 2.

Dot blot of cell culture medium (1) and cell lysates (2) from various mesenchymal cells, harvested at confluence, show that all the cells tested express and secrete the ameloblastin protein in amounts detectable by specific anti-ameloblastin antibody (ant-Ambn). Control is irrelevant antisera. Recombinant ameloblastin (rAMBN) in the form of a rat recombinant ameloblastin protein was used as reference in concentrations ranging from 200 μg/ml to 2.5 μg/ml. Cell lysate and cell culture medium were harvested after 24 h incubation from 90 % confluent primary human primary human dental pulp cells (pulp), MSC (mesenchymal stem cells), ADAS (adipocyte derived adult stem cells), BMSC (primary bone marrow stem cells), NHO (primary normal human osteoblasts), NHAC (primary adult chondrocytes) and PDL (primary human periodontal ligament fibroblasts).
Figure 3.
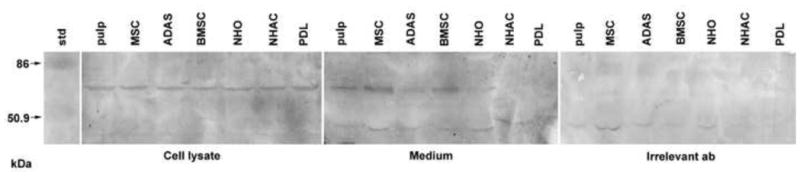
Western blot of ameloblastin in cell lysate and secreted to the cell culture medium. Irrelevant antisera were used as control. Cell lysate and cell culture medium were harvested after 24 h incubation from confluent primary human primary human dental pulp cells (pulp), MSC (mesenchymal stem cells), ADAS (adipocyte derived adult stem cells), BMSC (primary bone marrow stem cells), NHO (primary normal human osteoblasts), NHAC (primary adult chondrocytes) and PDL (primary human periodontal ligament fibroblasts). Std is pre-stained molecular weight standard.
Ameloblastin regulates its own expression in human mesenchymal stem cells
The effect of rrAmbn on AMBN mRNA expression was tested in primary human mesenchymal stem cells (BMSC and ADAS) obtained from the same donor. The cells were stimulated with rrAmbn added as a single dose to the medium, and its effect on AMBN mRNA expression normalized to expression of two housekeeping genes, was monitored for 24 hours and compared to AMBN expression in unstimulated cells (negative controls) (Figure 4). In unstimulated cells the AMBN mRNA expression was steady at a level of less than 2% of the housekeeping genes throughout the observation period. In the stimulated cells however, after the rrAmbn pulse, the expression of AMBN mRNA rapidly increased almost tenfold (p=0.029), peaking at 3 hours, before steadily declining back towards the background levels. After 12 hours the AMBN expression in the stimulated cells was only about 4% of that of the housekeeping genes, twice that of the controls. After 24 hours the AMBN mRNA expression levels had returned to control levels.
Figure 4.

The primary human mesenchymal stem cells (BMSC and ADAS) stimulated with rrAmbn added as a single pulse to the medium showed a rapid, ten-fold increase in AMBN expression peaking after three hours before steadily declining towards background levels. After 12 hours the AMBN expression in the stimulated cells was only about two times that of the unstimulated cells and after 24 hours the stimulated AMBN mRNA expression levels had completely returned to the level of the controls. In unstimulated cells the AMBN mRNA expression remained stable at a level of about 2% of the housekeeping genes throughout the observation period. The values are mean of two real time RT-PCR reactions run on pooled triplets of cell culture samples (n=6). Error bars are not included in this panel for better visualization, but the values at 3 and 12 hours were statistically significant (p=0.029). At 24 hours the difference between stimulated and unstimulated samples was not statistically significant (p>0.05) when using paired samples and Mann-Whitney Rank Sum Test.
In vivo Ameloblastin expression during early bone healing
Ameloblastin protein expression was identified by immunohistochemistry in sections of newly formed bone from experimental bilateral penetrating defects in the mandibular ramus of adult rats (Figure 5). Two weeks after surgery, new bone was observed lining the borders of the circular bony defect. The bone had the character of normal woven or trabecular bone growing by appositional growth from the original bone margins with growth towards the defect centre. This newly formed bone showed an intense immuno-staining for ameloblastin expression whereas the original, mineralized bone did not stain for ameloblastin expression. The anti-ameloblastin staining was mainly associated with the immature bone extracellular matrix adjacent to lining cells, osteoblasts and perivascular cells, while cells and matrix in the more mature parts of the bone and the osteocytes appeared to be ameloblastin negative. In the mature, original bone no anti-ameloblastin staining was observed.
Figure 5.
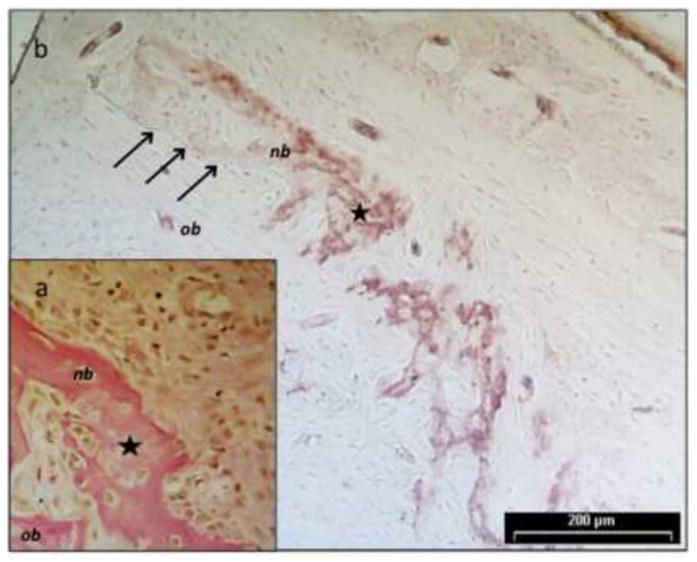
Ameloblastin protein expression was identified by immunohistochemistry in sections of newly formed bone from experimental bilateral defects in the mandibular ramus of adult rats. Two weeks after surgery, new bone was observed lining the borders of the circular bony defect. The new bone (nb) had the character of normal trabecular bone growing by appositional growth from the original bone (ob) margins (arrows) and in towards the bone marrow like centre of the defects. This newly formed bone matrix showed a strong immunostaining for ameloblastin expression whereas the original, mineralized bone did not. The localization of ameloblastin was mainly associated with the osteoblasts and the immature bone matrix and in some perivascular regions. In the mature, original bone, no ameloblastin expressing cells or matrix components were identified with the anti-ameloblastin antibody. Panel a) is a micrograph of a Van Gieson stained tissue section representing the peripheral part of the bilateral defect showing new bone growing appositionally onto the old bone delineating the defect. Panel b) is a parallel tissue section from the same tissue block as a) incubated with the rabbit anti-ameloblastin antibody. The specific anti-ameloblastin reaction was subsequently detected by the use of a horseradish peroxidase (HRP)-labelled goat anti-rabbit antibody, and finally visualized with diaminobenzidine (DAB). Slides were photographed using a light microscope at 400x.The star represent a reference point for alignment of the panels. Scale is the same in both panels; the bar in panel b) is 200 micrometers.
DISCUSSION
Ameloblastin was originally described as a tooth-specific enamel matrix protein expressed only by ameloblast cells [7,8,11]. In later studies, however, it was reported that ameloblastin was also expressed during the development of mesenchymal dental hard tissues [1], during trauma-induced reparative dentin formation [2] and during embryonic and post-natal stages of bone formation [3]. Accordingly, its function has been implicated in enamel biomineralization [13,37,38], and in interactions between the ameloblasts and the enamel extracellular matrix [7,26]. Furthermore, it has been suggested that ameloblastin could act as a signal molecule in epithelial-mesenchymal interactions leading to cell type specific differentiation [1,21,32]. The Ambn mutant mouse model shows a severe enamel hypoplasia [26] and uncontrolled differentiation of ameloblast cells [39]. Both in vitro and in vivo experiments have revealed that ameloblastin induces hard tissue regeneration, by influencing differentiation and growth of mesenchymal cells at the healing site [40,41]. Fukumoto et al. initially reported that the supposed ameloblastin null mouse has normal craniofacial bone development [26]. However, more detailed studies of these mice have shown the described ameloblastin null mutation is actually producing a shorter form of the ameloblastin protein that is translated from truncated RNA missing exons 5 and 6 [27]. These mice are reported to exhibit a more porous interdental bone and have generally reduced thickness of the alveolar bone process [27]. No specific analysis of skeletal bone quality, morphology or physiology, like bone density, strength tests and fracture healing, have so far been reported in these ameloblastin mutant animals. However, the mineral content in jaw-bone of the AmbnΔ5–6 mutant mouse model was analyzed, and no differences between wild type and mutant mice was found [42]. Creation of a complete knockout model, or use of other knock down techniques, is probably called for to reveal the possible function(s) for ameloblastin in embryonic and adult bone.
In the present study it is demonstrated that the AMBN gene is transcribed and translated in human stem cells and primary human bone cells like osteoblasts and chondrocytes as well as cells of human haematopoietic origin, such as CD34+ cells and osteoclasts. The observation that AMBN mRNA expression tend to decrease with increasing differentiation of stem cells and bone precursor cells suggest that ameloblastin is mainly involved in the early stages of cell differentiation. Ameloblastin is assumed to be a signaling molecule [1,21,32], and the observed putative autoregulation in mesenchymal stem cells indicate a regulation profile often mediated by signal molecules [43]. This is also supported by the bioinformatic analysis of ameloblastin showing that the protein has a structure and internal organization similar to other known signaling molecules [15,44]. This notion also fits well with the observations that ameloblastin is involved in embryonic endochondral and intramembranous bone formation [3], and in the early stages of healing after trauma to the dental pulp and dentin formation [2,23] possibly by recruiting precursor cells e.g. pre-odontoblasts and pre-osteoblasts for healing. That AMBN gene expression is also present in CD34+ cells do not come as a surprise since CD34+ cells have been shown to have the potential to differentiate into both osteoblasts [45–48] as well as monocytes and osteoclast precursors [49,50].
The role of a putative autoregulation of ameloblastin expression is not totally clear since no receptor pathway is known to involve ameloblastin as a ligand. Feed-back regulation is however a well recognized mechanism for cells to regulate cell signaling and cell homing. The fact that ameloblastin seems to be regulated by administration of rrAmbn, at least in some mesenchymal stem cells, suggests a role for this protein, or its derivatives, in one or more signaling pathways. To understand the true nature of ameloblastin it will thus be important to pinpoint the pathway(s) involved.
The in vivo demonstration of ameloblastin expression in adult healing bone indicate the role of this molecule also in adult bone formation after. The expression pattern in vivo and in vitro suggests that the expression of ameloblastin is associated with the newly secreted, non-mineralized bone matrix and the bone forming cells. It has been suggested that bone remodeling in cortical and in trabecular bone largely occurs in highly vascular bone remodeling compartments (BRCs) that are covered by a layer of bone lining cells recruited from CD34+ cells [51] and that it is possible that the BRCs also contribute directly to this population of circulating cells [52]. A possible role of ameloblastin in this case could be related to homing and recruiting of CD34+ cells, and in committing said cells to an osteoblastic or osteoclastic pathway. However, this hypothetical effect needs to be addressed in experimental studies and possibly examined in transgenic animals for confirmation.
The role of ameloblastin in bone formation and repair remains obscure even though it is tempting to suggest that the molecule is mainly related to the early steps of bone formation and healing. Further studies on the function and kinetics of ameloblastin expression are needed before the importance or redundancy of this molecule is confirmed. We already know that mutations in the AMBN gene is linked to one inheritable amelogenesis imperfecta [53], but it is not known whether mutations in the AMBN gene can also be linked to inheritable bone conditions. Given the general distribution of ameloblastin expression one cannot at this stage rule out the involvement of ameloblastin in bone and cartilage diseases. Subsequently, the role of ameloblastin expression should be further investigated, especially in relation to bone metabolic diseases, in fracture healing complications, in osseointegration of implants and of course in congenital bone diseases of unknown origin.
Acknowledgments
The authors would like to thank Dr. Heidi S. Berner, Faculty of Dentistry, University of Oslo (UiO), Oslo, Norway for assistance with osteoblast culturing, Kari W. Slørdahl, Norwegian University of Science and Technology (NTNU), Trondheim, Norway for helping out with the osteoclast culturing and Aina M. Lian and M. Britt Kvam, Faculty of Dentistry, University of Oslo (UiO), Oslo, Norway for skilful assistance. This work was supported by grant no.171038 to the authors from the Norwegian Research Council (NFR), grants from the Norwegian Cancer Society and the Faculty of Dentistry, University of Oslo, Norway.
Contributor Information
Margareth V. Tamburstuen, Email: margarvi@odont.uio.no.
Janne E. Reseland, Email: j.e.reseland@odont.uio.no.
Axel Spahr, Email: axel.spahr@uniklinik-ulm.de.
Steven J. Brookes, Email: s.j.brookes@leeds.ac.uk.
Gunnar Kvalheim, Email: gunnar.kvalheim@medisin.uio.no.
Ivan Slaby, Email: islaby@volny.cz.
Malcolm L. Snead, Email: mlsnead@usc.edu.
S. Petter Lyngstadaas, Email: spl@odont.uio.no.
References
- 1.Fong CD, Cerny R, Hammarstrom L, Slaby I. Sequential expression of an amelin gene in mesenchymal and epithelial cells during odontogenesis in rats. Eur J Oral Sci. 1998;106(Suppl 1):324–30. doi: 10.1111/j.1600-0722.1998.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 2.Spahr A, Lyngstadaas SP, Slaby I, Haller B, Boeckh C, Tsoulfidou F, et al. Expression of amelin and trauma-induced dentin formation. Clin Oral Investig. 2002;6:51–7. doi: 10.1007/s00784-001-0139-y. [DOI] [PubMed] [Google Scholar]
- 3.Spahr A, Lyngstadaas SP, Slaby I, Pezeshki G. Ameloblastin expression during craniofacial bone formation in rats. Eur J Oral Sci. 2006;114:504–11. doi: 10.1111/j.1600-0722.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 4.Papagerakis P, MacDougall M, Hotton D, Bailleul-Forestier I, Oboeuf M, Berdal A. Expression of amelogenin in odontoblasts. Bone. 2003;32:228–40. doi: 10.1016/s8756-3282(02)00978-x. [DOI] [PubMed] [Google Scholar]
- 5.Janones DS, Massa LF, Arana-Chavez VE. Immunocytochemical examination of the presence of amelogenin during the root development of rat molars. Arch Oral Biol. 2005;50:527–32. doi: 10.1016/j.archoralbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Haze A, Taylor AL, Blumenfeld A, Rosenfeld E, Leiser Y, Dafni L, et al. Amelogenin expression in long bone and cartilage cells and in bone marrow progenitor cells. Anat Rec [Hoboken ] 2007;290:455–60. doi: 10.1002/ar.20520. [DOI] [PubMed] [Google Scholar]
- 7.Cerny R, Slaby I, Hammarstrom L, Wurtz T. A novel gene expressed in rat ameloblasts codes for proteins with cell binding domains. J Bone Miner Res. 1996;11:883–91. doi: 10.1002/jbmr.5650110703. [DOI] [PubMed] [Google Scholar]
- 8.Fong CD, Slaby I, Hammarstrom L. Amelin: an enamel-related protein, transcribed in the cells of epithelial root sheath. J Bone Miner Res. 1996;11:892–8. doi: 10.1002/jbmr.5650110704. [DOI] [PubMed] [Google Scholar]
- 9.Hu CC, Fukae M, Uchida T, Qian Q, Zhang CH, Ryu OH, et al. Sheathlin: cloning, cDNA/polypeptide sequences, and immunolocalization of porcine enamel sheath proteins. J Dent Res. 1997;76:648–57. doi: 10.1177/00220345970760020501. [DOI] [PubMed] [Google Scholar]
- 10.Sire JY, Delgado S, Fromentin D, Girondot M. Amelogenin: lessons from evolution. Arch Oral Biol. 2005;50:205–12. doi: 10.1016/j.archoralbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Krebsbach PH, Lee SK, Matsuki Y, Kozak CA, Yamada KM, Yamada Y. Full-length sequence, localization, and chromosomal mapping of ameloblastin. A novel tooth-specific gene. J Biol Chem. 1996;271:4431–5. doi: 10.1074/jbc.271.8.4431. [DOI] [PubMed] [Google Scholar]
- 12.MacDougall M, Simmons D, Gu TT, Forsman-Semb K, Mardh CK, Mesbah M, et al. Cloning, characterization and immunolocalization of human ameloblastin. Eur J Oral Sci. 2000;108:303–10. doi: 10.1034/j.1600-0722.2000.108004303.x. [DOI] [PubMed] [Google Scholar]
- 13.Uchida T, Murakami C, Dohi N, Wakida K, Satoda T, Takahashi O. Synthesis, secretion, degradation, and fate of ameloblastin during the matrix formation stage of the rat incisor as shown by immunocytochemistry and immunochemistry using region-specific antibodies. J Histochem Cytochem. 1997;45:1329–40. doi: 10.1177/002215549704501002. [DOI] [PubMed] [Google Scholar]
- 14.Lyngstadaas SP. Synthetic hammerhead ribozymes as tools in gene expression. Crit Rev Oral Biol Med. 2001;12:469–78. doi: 10.1177/10454411010120060201. [DOI] [PubMed] [Google Scholar]
- 15.Vymetal J, Slaby I, Spahr A, Vondrasek J, Lyngstadaas SP. Bioinformatic analysis and molecular modelling of human ameloblastin suggest a two-domain intrinsically unstructured calcium-binding protein. Eur J Oral Sci. 2008;116:124–34. doi: 10.1111/j.1600-0722.2008.00526.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamakoshi Y, Tanabe T, Oida S, Hu CC, Simmer JP, Fukae M. Calcium binding of enamel proteins and their derivatives with emphasis on the calcium-binding domain of porcine sheathlin. Arch Oral Biol. 2001;46:1005–14. doi: 10.1016/s0003-9969(01)00070-x. [DOI] [PubMed] [Google Scholar]
- 17.Iwata T, Yamakoshi Y, Hu JC, Ishikawa I, Bartlett JD, Krebsbach PH, et al. Processing of ameloblastin by MMP-20. J Dent Res. 2007;86:153–7. doi: 10.1177/154405910708600209. [DOI] [PubMed] [Google Scholar]
- 18.Simmer JP, Hu JCC. Expression, structure, and function of enamel proteinases. Connective Tissue Research. 2002;43:441–9. doi: 10.1080/03008200290001159. [DOI] [PubMed] [Google Scholar]
- 19.Veis A, Tompkins K, Alvares K, Wei K, Wang L, Wang XS, et al. Specific amelogenin gene splice products have signaling effects on cells in culture and in implants in vivo. J Biol Chem. 2000;275:41263–72. doi: 10.1074/jbc.M002308200. [DOI] [PubMed] [Google Scholar]
- 20.Veis A. Amelogenin gene splice products: potential signaling molecules. Cell Mol Life Sci. 2003 Jan;60(1):38–55. doi: 10.1007/s000180300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeichner-David M, Chen LS, Hsu Z, Reyna J, Caton J, Bringas P. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur J Oral Sci. 2006;114(Suppl 1):244–53. doi: 10.1111/j.1600-0722.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett JD, Ganss B, Goldberg M, Moradian-Oldak J, Paine ML, Snead ML, et al. 3. Protein-protein interactions of the developing enamel matrix. Curr Top Dev Biol. 2006;74:57–115. doi: 10.1016/S0070-2153(06)74003-0. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Slaby I, Spahr A, Pezeshki G, Matsumoto K, Lyngstadaas SP. Ameloblastin Fusion Protein Enhances Pulpal Healing and Dentin Formation in Porcine Teeth. Calcif Tissue Int. 2006;78:278–84. doi: 10.1007/s00223-005-0144-2. [DOI] [PubMed] [Google Scholar]
- 24.Sonoda A, Iwamoto T, Nakamura T, Fukumoto E, Yoshizaki K, Yamada A, et al. Critical role of heparin binding domains of ameloblastin for dental epithelium cell adhesion and ameloblastoma proliferation. J Biol Chem. 2009;284:27176–84. doi: 10.1074/jbc.M109.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida T, Fukae M, Tanabe T, Yamakoshi Y, Satoda T, Murakami C, et al. Immunochemical and Immunocytochemical Study of A 15-Kda Non-Amelogenin and Related Proteins in the Porcine Immature Enamel - Proposal of A New Group of Enamel Proteins Sheath Proteins. Biomedical Research-Tokyo. 1995;16:131–40. [Google Scholar]
- 26.Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167:973–83. doi: 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wazen RM, Moffatt P, Zalzal SF, Yamada Y, Nanci A. A mouse model expressing a truncated form of ameloblastin exhibits dental and junctional epithelium defects. Matrix Biol. 2009;28:292–303. doi: 10.1016/j.matbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paine ML, Wang HJ, Luo W, Krebsbach PH, Snead ML. A transgenic animal model resembling amelogenesis imperfecta related to ameloblastin overexpression. J Biol Chem. 2003;278:19447–52. doi: 10.1074/jbc.M300445200. [DOI] [PubMed] [Google Scholar]
- 29.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, et al. Characterization of human bone marrow fibroblast colony-forming cells [CFU-F] and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 31.Smeland EB, Rusten L, Jacobsen SE, Skrede B, Blomhoff R, Wang MY, et al. All-trans retinoic acid directly inhibits granulocyte colony-stimulating factor-induced proliferation of CD34+ human hematopoietic progenitor cells. Blood. 1994;84:2940–5. [PubMed] [Google Scholar]
- 32.Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–70. doi: 10.1046/j.0909-8836.1998.eos106510.x. [DOI] [PubMed] [Google Scholar]
- 33.Chen LS, Couwenhoven RI, Hsu D, Luo W, Snead ML. Maintenance of amelogenin gene expression by transformed epithelial cells of mouse enamel organ. Arch Oral Biol. 1992;37:771–8. doi: 10.1016/0003-9969(92)90110-t. [DOI] [PubMed] [Google Scholar]
- 34.Dhamija S, Liu Y, Yamada Y, Snead ML, Krebsbach PH. Cloning and characterization of the murine ameloblastin promoter. J Biol Chem. 1999;274:20738–43. doi: 10.1074/jbc.274.29.20738. [DOI] [PubMed] [Google Scholar]
- 35.Dhamija S, Krebsbach PH. Role of Cbfa1 in ameloblastin gene transcription. J Biol Chem. 2001;276:35159–64. doi: 10.1074/jbc.M010719200. [DOI] [PubMed] [Google Scholar]
- 36.Mesulam MM. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978;26:106–17. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 37.Nanci A, Zalzal S, Lavoie P, Kunikata M, Chen W, Krebsbach PH, et al. Comparative immunochemical analyses of the developmental expression and distribution of ameloblastin and amelogenin in rat incisors. J Histochem Cytochem. 1998;46:911–34. doi: 10.1177/002215549804600806. [DOI] [PubMed] [Google Scholar]
- 38.Brookes SJ, Kirkham J, Shore RC, Wood SR, Slaby I, Robinson C. Amelin extracellular processing and aggregation during rat incisor amelogenesis. Arch Oral Biol. 2001;46:201–8. doi: 10.1016/s0003-9969(00)00121-7. [DOI] [PubMed] [Google Scholar]
- 39.Fukumoto S, Yamada A, Nonaka K, Yamada Y. Essential roles of ameloblastin in maintaining ameloblast differentiation and enamel formation. Cells Tissues Organs. 2005;181:189–95. doi: 10.1159/000091380. [DOI] [PubMed] [Google Scholar]
- 40.Fukae M, Kanazashi M, Nagano T, Tanabe T, Oida S, Gomi K. Porcine sheath proteins show periodontal ligament regeneration activity. Eur J Oral Sci. 2006;114(Suppl 1):212–8. doi: 10.1111/j.1600-0722.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- 41.Kanazashi M, Gomi K, Nagano T, Tanabe T, Arai T, Fukae M. The 17-kDa sheath protein in enamel proteins induces cementum regeneration in experimental cavities created in a buccal dehiscence model of dogs. J Periodontal Res. 2006;41:193–9. doi: 10.1111/j.1600-0765.2005.00859.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith CE, Wazen R, Hu Y, Zalzal SF, Nanci A, Simmer JP, et al. Consequences for enamel development and mineralization resulting from loss of function of ameloblastin or enamelin. Eur J Oral Sci. 2009;117:485–97. doi: 10.1111/j.1600-0722.2009.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman M. Feedback control of intercellular signalling in development. Nature. 2000;408:313–9. doi: 10.1038/35042500. [DOI] [PubMed] [Google Scholar]
- 44.Dosztanyi Z, Meszaros B, Simon I. Bioinformatical approaches to characterize intrinsically disordered/unstructured proteins. Brief Bioinform. 2010;11(2):225–43. doi: 10.1093/bib/bbp061. [DOI] [PubMed] [Google Scholar]
- 45.Chen JL, Hunt P, McElvain M, Black T, Kaufman S, Choi ES. Osteoblast precursor cells are found in CD34+ cells from human bone marrow. Stem Cells. 1997;15:368–77. doi: 10.1002/stem.150368. [DOI] [PubMed] [Google Scholar]
- 46.Olmsted-Davis EA, Gugala Z, Camargo F, Gannon FH, Jackson K, Kienstra KA, et al. Primitive adult hematopoietic stem cells can function as osteoblast precursors. Proc Natl Acad Sci U S A. 2003;100:15877–82. doi: 10.1073/pnas.2632959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. 2006;169:1440–57. doi: 10.2353/ajpath.2006.060064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsumoto T, Kuroda R, Mifune Y, Kawamoto A, Shoji T, Miwa M, et al. Circulating endothelial/skeletal progenitor cells for bone regeneration and healing. Bone. 2008;43:434–9. doi: 10.1016/j.bone.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Huang S, Terstappen LW. Formation of haematopoietic microenvironment and haematopoietic stem cells from single human bone marrow stem cells. Nature. 1992;360:745–9. doi: 10.1038/360745a0. [DOI] [PubMed] [Google Scholar]
- 50.Ciraci E, Barisani D, Parafioriti A, Formisano G, Arancia G, Bottazzo G, et al. CD34 human hematopoietic progenitor cell line, MUTZ-3, differentiates into functional osteoclasts. Exp Hematol. 2007;35:967–77. doi: 10.1016/j.exphem.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16:1575–82. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 52.Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, et al. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–7. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mardh CK, Backman B, Simmons D, Golovleva I, Gu TT, Holmgren G, et al. Human ameloblastin gene: genomic organization and mutation analysis in amelogenesis imperfecta patients. Eur J Oral Sci. 2001;109:8–13. doi: 10.1034/j.1600-0722.2001.00979.x. [DOI] [PubMed] [Google Scholar]


