Abstract
Objective
It is unknown whether muscle wasting accounts for impaired physical function in adults on maintenance hemodialysis (MHD).
Design
Observational study
Setting
Outpatient dialysis units and a fall clinic
Subjects
108 MHD and 122 elderly non-hemodialysis (non-HD) participants
Exposure variable
Mid-thigh muscle area was measured by magnetic resonance imaging.
Main outcome measure
Physical function was measured by distance walked in six minutes (6MW).
Results
Compared to non-HD elderly participants, MHD participants were younger (49.2 ± 15.8 yrs vs. 75.3 ± 7.1 yrs, p<0.001) and had higher mid-thigh muscle area (106.2 ± 26.8 cm2 vs. 96.1 ± 21.1 cm2, p=0.002). However, the 6MW distance was lower in MHD participants (322.9 ± 110.4 m vs. 409.0 ± 128.3 m, p<0.001). In multiple regression analysis adjusted for demographics, comorbid conditions and mid-thigh muscle area, MHD patients walked significantly less distance (−117 m, 95% −177 to −56 m, p<0.001) than the non-HD elderly.
Conclusions
Even when compared to elderly non-HD participants, younger MHD participants have poorer physical function that was not explained by muscle mass or comorbid conditions. We speculate that the uremic milieu may impair muscle function independent of muscle mass. The mechanism of impaired muscle function in uremia needs to be established in future studies.
Introduction
Frailty is classically defined by decreased grip strength, slower walking time, exhaustion, low physical activity level, and unintentional weight loss1. Frailty is highly prevalent in those undergoing maintenance hemodialysis (MHD)2 and is strongly associated with mortality and adverse health outcomes in this population3. Frailty is also common in older individuals experiencing age-related decrements in physical and muscle function1. Muscle wasting is also common in the MHD4 and elderly populations5.
It is unclear whether decreased physical function in MHD patients is because of decreased muscle mass or whether uremia per se impairs muscle function. Furthermore, increased prevalence of comorbid conditions such as heart failure, peripheral vascular disease and diabetes mellitus in the MHD population might also explain the decreased physical function in this population. Therefore, we examined the hypothesis that decreased physical function in MHD patients is largely explained by decreased muscle mass and increased comorbid conditions in the MHD population by pooling data from a dialysis cohort and a non-MHD elderly cohort.
Methods
Study population
We combined data from a dialysis cohort and a non-dialysis cohort. In both of these cohorts, magnetic resonance imaging (MRI) was performed to measure mid-thigh muscle area and 6MW was performed to measure physical function.
Protein Intake, Cardiovascular disease and Nutrition In stage V CKD (PICNIC) is a prospective observational study examining the impact of nutrient intake on vascular health, body composition and physical functioning in adult (≥18 years) patients on MHD for at least 3 months at the University of Utah and Vanderbilt University Medical Center (VUMC) outpatient dialysis units (NCT00566670). Exclusion criteria for the MHD cohort included patients with medical conditions with increased short-term mortality such as symptomatic heart failure, active malignancy (excluding squamous and basal cell skin cancers) and acquired immune deficiency syndrome; patients with inability to walk or those using a wheel-chair; patients with contraindications to MRI such as pacemakers; and patients with atrial fibrillation which may interfere with measurement of pulse wave velocity.
The non-HD population was comprised of participants in an ongoing longitudinal study of older adults (≥65 years) at high risk of falls (NCT01080196). This ongoing study is examining the effect of a multi-component exercise-training program on fall prevention at the University of Utah Department of Physical Therapy. Fall prevention participants were included if they were community ambulators with at least two co-morbid health conditions and a history of at least one fall in the previous year. Exclusion criteria for the fall prevention cohort included dementia, progressive central nervous system disorder, myopathic or rheumatologic disease that adversely impacted muscle, and any absolute contraindications for MRI.
Data collection
Baseline study data from both studies were used in this cross-sectional investigation. In both studies, standardized questionnaires were used to obtain demographic, past medical history, medications and socioeconomic data. Height and weight were measured following standardized protocols. BMI was calculated as weight in kilograms divided by height in meters squared.
Magnetic Resonance Imaging
MRI scans of the legs were performed at both University of Utah and VUMC sites at the baseline visit. For the MHD cohort, this was on a non-dialysis day. Mid-thigh muscle area (MTMA) was quantified by imaging both legs in the axial plane at the midpoint of the femur. A 3-point Dixon method6 was used to create separated fat and non-fat images, with phase unwrapping by iterative solution of the Poisson equation7. Percent fat volume fraction and percent non-fat volume fraction were calculated from the signal intensity of the fat and non-fat MRI images using the gradient recalled echo signal equation and a tissue signal model8. Cross-sectional lean muscle area was calculated in a single axial image at the midpoint of the femur by adding the percent non-fat fraction value of each pixel over the entire leg cross-section and multiplying by pixel area.
Imaging at the University of Utah was performed on a 3 Tesla Siemens Trio scanner. Imaging at VUMC was performed with a 3T Philips Achieva scanner. Image processing, calculation of fat- and non-fat volume fraction images, and measurement of fat and muscle cross-sectional areas were performed at the University of Utah by the same observers for the dialysis and non-dialysis cohorts following a standardized protocol.
Six-minute walk distance (6MW)
Physical function assessing locomotion was measured objectively using the 6MW test. This test was performed per American Thoracic Society standards using a flat surface on an indoor walking course9. For the MHD cohort, testing was performed on non-dialysis days. In all participants testing was proctored by the study coordinator. Each participant walked along the indoor course at a self-determined pace for a total of six minutes with a distance measuring wheel. Participants were allowed to rest briefly by leaning against the wall or sitting during the test, or stop prematurely if they were unable to complete the test. There was no practice test, warm up period, or incentive provided for performance.
Statistical Methods
Baseline characteristics of MHD and non-HD groups are presented by mean and standard deviation for continuous variables and proportions for categorical variables. Continuous variables between the subgroups were compared by t-test and categorical variables by Chi-square test or Fisher’s exact test. To examine the relationships between MHD status with 6MW, multivariable linear regression analyses were performed with or without adjustment for age, gender, race, smoking, alcohol, coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes, lung disease and mid-thigh muscle area. Age ≥ 65 years was included as a dichotomous variable in the model since all participants are 65 years of age or older in the non-HD subgroup. Additionally, we related 6MW distance with MTMA separately in MHD and non-HD subgroups in multivariate linear regression models adjusted for the above covaraiates.. To satisfy the assumption of linear regression analyses, the independent, homoscedasticity and normality of residuals were tested and no violations of the assumptions were found. All analyses were conducted with Stata 12 (Stata Inc, College station, TX).
Results
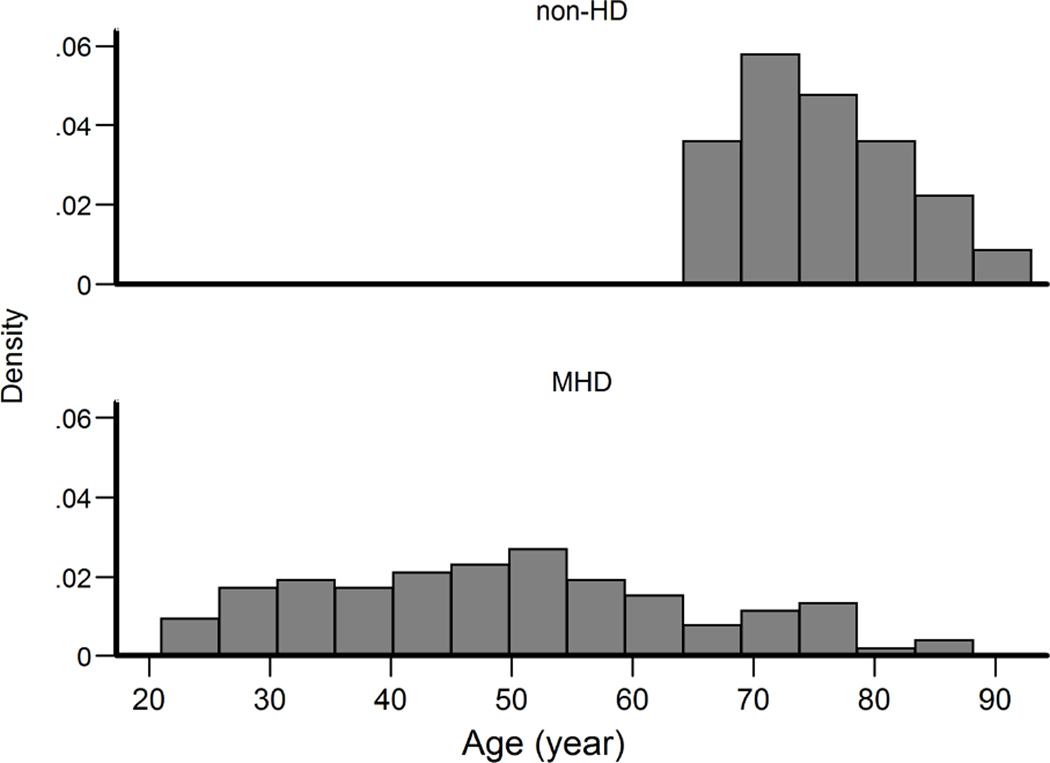
The study population consisted of 108 MHD participants and 122 older (≥65 years) high fall risk participants (non-HD group). Baseline characteristics of all participants are reported in Table 1 by MHD or non-HD status. Percentages or mean (± standard deviation) are presented. Age distribution of the MHD and non-HD participants are depicted in Figure 1. MHD participants were younger (49.2 ± 15.8 years) than non-HD participants (75.3 ± 7.1 years) (p<0.001). 57% of the MHD participants, and 33% of the non-HD participants were male (p<0.001), and 62% of the MHD and 98% of the non-HD participants were Caucasian (p<0.001).
Table1.
Baseline characteristics of MHD and Non-HD groups
| Characteristic | MHD n=108 |
Non-HD n=122 |
P value |
|---|---|---|---|
| Age | 49.2 ± 15.8 | 75.3 ± 7.1 | <0.001 |
| Male (%) | 56.5 | 32.8 | <0.001 |
| White (%) | 62.0 | 98.4 | <0.001 |
| Coronary artery disease (%) | 20.4 | 9.8 | 0.02 |
| Congestive heart failure (%) | 17.6 | 4.1 | <0.001 |
| Peripheral vascular disease (%) | 14.8 | 1.6 | 0.88 |
| Cerebrovascular disease (%) | 15.7 | 19.7 | 0.44 |
| Lung disease (%) | 13.9 | 11.5 | 0.58 |
| Diabetes (%) | 40.7 | 18.9 | <0.001 |
| Alcohol use (%) | 53.7 | 37.7 | 0.01 |
| Smoker (%) | 50.0 | 0.8 | <0.001 |
| Body mass index (kg/m2) | 27.9 ± 6.6 | 27.8 ± 5.5 | 0.94 |
| Mid-thigh muscle area (cm2) | 106.2 ± 26.8 | 96.1 ± 21.1 | 0.002 |
| Six-minute walk distance (m) | 322.9 ± 110.4 | 409.0 ± 128.3 | <0.001 |
Figure 1.
Age distribution of non-HD and HD cohorts
The mean BMI of the MHD participants was 27.9 ± 6.6 kg/m2 and of the non-HD participants was 27.8 ± 5.5 kg/m2 (p=0.55). Mean MTMA was 106.2 ± 26.8 cm2 in the MHD participants and 96.1 ± 21.1 cm2 in the non-HD participants (p=0.002). Median duration of ESRD of the MHD participants was 2.4 (interquartile range of 0.8 to4.8) years.
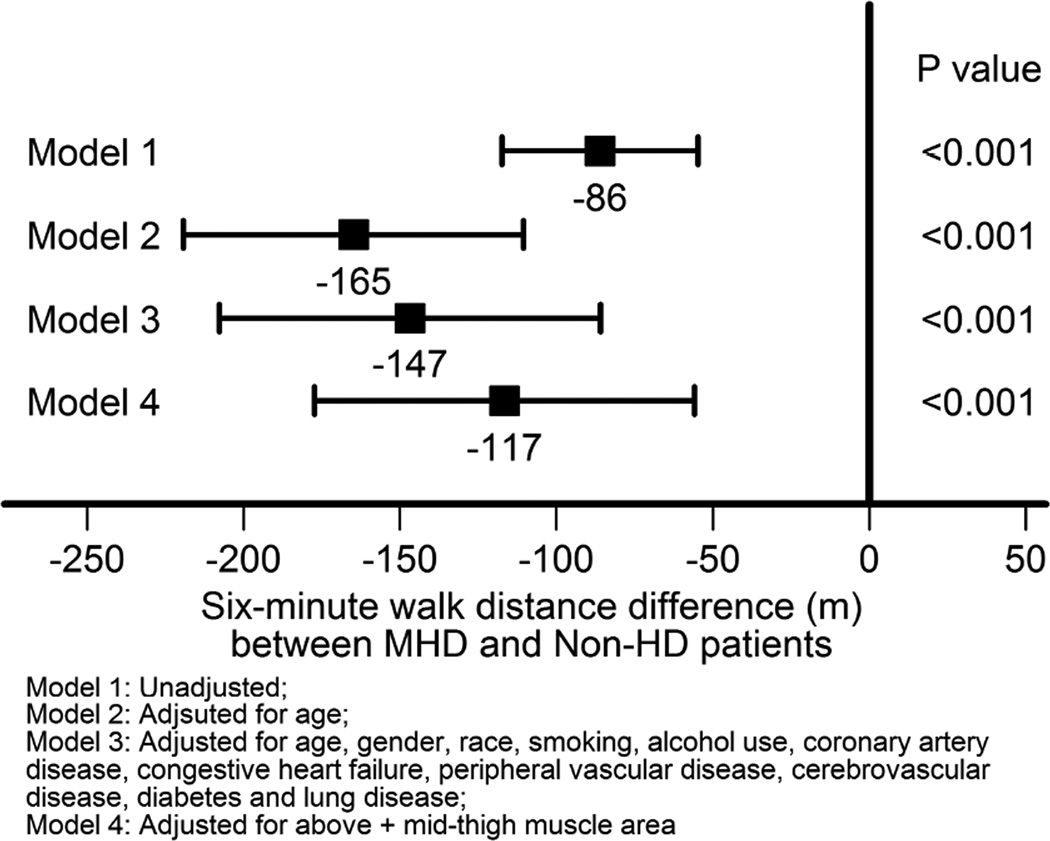
Figure 2 summarizes the relationship of MHD status with 6MW distances. Unadjusted, compared to the non-HD group, the MHD group walked 86 m (95% CI, 55 to 117 m) shorter distances in 6 minutes. When adjusted for demographics, this relationship was stronger (−165 m, 95% CI −219 to −110 m). With further adjustments for demographics, comorbid conditions and MTMA, MHD participants still had substantially lower 6MW distances (−117 m, 95% CI −177 to −55 m).
Figure 2.
Associations of MHD and non-HD status with 6MW distance (meters)
Table 2 summarizes the associations of demographics, comorbid conditions, dialysis status and mid-thigh muscle area with 6MW distances in the entire cohort. Older age, higher BMI and dialysis status had significant negative association with 6MW distance. On the other hand, higher mid-thigh muscle area had a strong positive association with 6MW distance.
Table 2.
Multivariable linear regression model* relating 6MW distance (meter) to demographics, comorbid conditions, dialysis status and mid-thigh muscle area
| β & 95% CI | P value | |
|---|---|---|
| Each SD$ increase in age | −41.7 (−70.5, −12.9) | 0.005 |
| Male | −29.1 (−69.9, 11.6) | 0.16 |
| White race | −28.4 (−71.1, 14.2) | 0.19 |
| Each SD# increase in body mass index | −36.7 (−53.9, −19.6) | <0.001 |
| Smoking | −1.8 (−45.2, 41.6) | 0.94 |
| Alcohol use | 4.1 (−26.9, 35.1) | 0.79 |
| Coronary artery disease | −24.0 (−68.5, 20.5) | 0.29 |
| Congestive heart failure | −39.1 (−90.5, 12.3) | 0.14 |
| Peripheral vascular disease | −29.0 (−87.5, 29.6) | 0.33 |
| Cerebrovascular disease | −20.0 (−57.5, 17.5) | 0.30 |
| Diabetes | −20.9 (−55.9, 14.1) | 0.24 |
| Lung disease | 12.6 (−30.5, 55.7) | 0.57 |
| MHD vs non-HD status | −117 (−177, −56) | <0.001 |
| Each SD^ increase in mid-thigh muscle area | 44.6 (22.7, 66.5) | <0.001 |
All variables are in the same model. Also adjusted for age ≥ 65 years as a dichotomous variable as the entire non-HD population was 65 years of age or older.
Each SD of age was 17.7 years
Each SD of BMI was 6.0 kg/m2
Each of mid-thigh muscle area was 24.4 cm2
In additional analyses, each SD increase in mid-thigh muscle area had a trend towards positive association with 6MW distance in the non-HD subgroup (24.5, 95% CI −8.5 to 57.5 meters, p =0.14) whereas this association was stronger in the MHD subgroup (50.9, 95% CI 24.1 to 77.7 meters, p <0.001).
Discussion
The results of this study suggest that when compared to older adults at a high fall risk, MHD patients had lower physical function despite a younger age and higher locomotor muscle area. These findings persisted even when adjusted for the higher comorbidity burden in the MHD patients. Our findings, however, are in contrast to our hypothesis and show that beyond muscle wasting, the function of the locomotor muscle is poorer in the uremic milieu.
Muscle wasting is prevalent in MHD patients, even more so than in those with non-dialysis-dependent chronic kidney disease patients10. Advanced kidney disease is associated with protein wasting, especially in skeletal locomotor muscle, resulting from both impaired protein synthesis11 as well as increased protein degradation12. The clinical significance of this is two-fold: muscle wasting is a predictor of increased mortality in end stage renal disease (ESRD)13–14, and is linked with poor physical function15. Our finding of poorer locomotor performance in patients undergoing MHD, even in the presence of higher thigh muscle area relative to older adults at high fall risk, also highlights the likelihood that the dysfunction observed is not due solely to a lack of muscle, but a decrement of function in uremic muscle.
Whether the impaired functional ability of muscle in MHD patients is related to other metabolic disturbances such as mitochondrial defects, oxidative stress, inflammation, or other muscle structural abnormalities associated with uremia has not been fully elucidated. Patients on MHD have been shown to have an intrinsic functional defect in mitochondrial energy metabolism in their skeletal muscles that results in an impaired rate of the production of high energy organophosphorus compounds following exercise16. This, in turn results in slower recovery from muscle contraction16 and early muscle fatigue during exercise17.
Muscle fatigue is often reported by patients on dialysis and therefore may have played a role in the decreased physical function in this group. Whether the fatigability of the muscle of MHD patients however, is greater than normal subjects is not clear. Alternatively, changes in skeletal muscle properties might also explain the decreased functional ability of uremic muscle. Morphologic changes characteristic of aging including Type II fiber atrophy have been reported in MHD patients18. While not definitive in MHD, morphologic changes that include central nuclei, ring fibers, fiber splitting, moth eaten fibers and vacuoles that have been identified in aging muscle all indicate structural deterioration that may decrease the force generating capacity of skeletal muscle19. Finally, impaired activation of the motor neurons by the central nervous system has also been suggested as a contributor to impaired muscle function in uremic muscle19. We did not measure strength in the current study and cannot say that strength was impaired in our MHD cohort to a greater extent than it was in our older non-MHD group; however, significant impairment in quadriceps muscle force production has been reported in dialysis patients20. It may be that abnormalities in skeletal muscle properties or neural function were greater in our MHD cohort, resulting in a decrement in muscle quality or force produced per unit area of muscle. This potential decrease in muscle quality may have been reflected in worse physical function in the MHD patients relative to the non-HD subjects. Future studies should seek to identify whether muscle quality is affected in MHD.
A strength of our study, in addition to the use of MRI, is the use of the 6MW test for objective measurement of physical function. Physical function deficit in adults with chronic health conditions is classically defined as an impaired walking distance. The 6MW test is highlighted here because it is a performance-based measure of physical function21, distinct from thigh muscle size or co-morbid health conditions. This walking test is also self-paced and may better reflect the functional exercise level for daily physical activities22. Another strength is the comparison of the MHD cohort to a group of older adults who are at a high fall risk and have significantly less thigh muscle size.
A limitation of this study is the merging of two different datasets of MHD and non-MHD participants. In the control group no stored samples were available to test whether markers of inflammation, insulin resistance and oxidative stress explain the functional ability differences between the two groups. Renal function in the non-HD group was not assessed so investigating the independence of physical function and muscle size across different levels of renal function in our data was not possible. Nonetheless, in both of these datasets, mid-thigh muscle area was measured by the same technique by the same observers and 6MW distances were obtained in both the datasets with the same protocol. Finally, while the finding that the non-HD participants had a lower prevalence of smoking and some co-morbid health conditions may suggest that they were more physically active; however, all self reported low physical activity and had an average gait speed of 1.10 meters per second. Both of these suggest that the non-HD group was, in fact, a physically inactive group of older adults.
In summary, we conclude that even when compared to a control group of an elderly cohort at high risk of falls, MHD patients had poorer physical function. These differences in physical function between MHD and non-HD participants are not explained by thigh muscle area or measured co-morbid conditions. Therefore, the impaired locomotor muscle function thought to be common in patients on dialysis is likely the result of intrinsic changes in the muscle’s quality rather than quantity alone. Because muscle quantity does not explain the differences in physical function, future studies should investigate interventions aimed at improving muscle quality (force produced per unit of muscle area) in this population.
Practical Application
Higher muscle mass is associated with better physical function in dialysis patients. However, differences in muscle mass do not account for the lower functional status in dialysis patients compared to those not on dialysis.
Acknowledgements
This study was supported by the following: RO1-DK077298 and RO1-DK078112 awarded to SB, K24 DK62849 to TAI, 5R01-AG031255-05 to PCL, University of Utah Study Design and Biostatistics Center, with funding in part from research grant numbers UL1-RR025764, 1UL-1RR024975, UL1 TR000445 and C06-RR11234 from the National Center for Advancing Translational Sciences of the National Institute of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report.
References
- 1.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012 Jul 23;172(14):1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007 Nov;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 4.Isoyama N, Qureshi AR, Avesani CM, et al. Comparative Associations of Muscle Mass and Muscle Strength with Mortality in Dialysis Patients. [July 29, 2014];Clinical Journal of the American Society of Nephrology. 2014 doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002 May;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 6.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1991 Apr;18(2):371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 7.Moon-Ho Song S, Napel S, Pelc NJ, Glover GH. Phase unwrapping of MR phase images using Poisson equation. IEEE transactions on image processing : a publication of the IEEE Signal Processing Society. 1995;4(5):667–676. doi: 10.1109/83.382500. [DOI] [PubMed] [Google Scholar]
- 8.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. Journal of magnetic resonance imaging : JMRI. 1995 May-Jun;5(3):281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 9.ATS statement: guidelines for the six-minute walk test. American journal of respiratory and critical care medicine. 2002 Jul 1;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006 Aug;21(8):2210–2216. doi: 10.1093/ndt/gfl064. [DOI] [PubMed] [Google Scholar]
- 11.Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000 Feb;278(2):E219–E225. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- 12.Graham KA, Reaich D, Channon SM, Downie S, Goodship TH. Correction of acidosis in hemodialysis decreases whole-body protein degradation. J Am Soc Nephrol. 1997 Apr;8(4):632–637. doi: 10.1681/ASN.V84632. [DOI] [PubMed] [Google Scholar]
- 13.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. [09/ 2003];J Am Soc Nephrol. 14(9):2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 14.Noori N. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. // [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinson M, Ikizler TA, Morrell G, et al. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin J Am Soc Nephrol. 2014 Jun 6;9(6):1082–1090. doi: 10.2215/CJN.09200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp GJ, Crowe AV, Anijeet HKI, et al. Abnormal mitochondrial function and muscle wasting, but normal contractile efficiency, in haemodialysed patients studied non-invasively in vivo. [June 1, 2004];Nephrology Dialysis Transplantation. 2004 19(6):1520–1527. doi: 10.1093/ndt/gfh189. [DOI] [PubMed] [Google Scholar]
- 17.Sangkabutra T, Crankshaw DP, Schneider C, et al. Impaired K+ regulation contributes to exercise limitation in end-stage renal failure. Kidney Int. 2003 Jan;63(1):283–290. doi: 10.1046/j.1523-1755.2003.00739.x. [DOI] [PubMed] [Google Scholar]
- 18.Sakkas GK, Sargeant AJ, Mercer TH, et al. Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol Dial Transplant. 2003 Sep;18(9):1854–1861. doi: 10.1093/ndt/gfg237. [DOI] [PubMed] [Google Scholar]
- 19.Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in "muscle quality". J Cachexia Sarcopenia Muscle. 2012 Jun;3(2):95–109. doi: 10.1007/s13539-011-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahal IH, Bell GM, Bone JM, Edwards RH. Physiological abnormalities of skeletal muscle in dialysis patients. Nephrology, Dialysis, Transplantation: Official Publication Of The European Dialysis And Transplant Association - European Renal Association. 1997;12(1):119–127. doi: 10.1093/ndt/12.1.119. [DOI] [PubMed] [Google Scholar]
- 21.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003 Feb;123(2):387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 22.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. American journal of respiratory and critical care medicine. 2003 May 1;167(9):1287. doi: 10.1164/ajrccm.167.9.950. [DOI] [PubMed] [Google Scholar]