Abstract
Objective
To identify characteristics associated with inability to progress to open-set speech recognition in children who are 5 years post cochlear implantation.
Study Design
Prospective, longitudinal and multidimensional assessment of auditory development over 5 years.
Setting
Six tertiary cochlear implant (CI) referral centers in the US.
Patients
Children with severe-to-profound hearing loss who underwent implantation before age 5 years enrolled in the Childhood Development after Cochlear Implant (CDaCI) study, categorized by level of speech recognition ability.
Intervention(s)
Cochlear implantation prior to 5 years of age and annual assessment of emergent speech recognition skills.
Main outcome measure(s)
Progression to open-set speech recognition by 5 years after implantation.
Results
Less functional hearing prior to implantation, older age at onset of amplification, lower maternal sensitivity to communication needs, minority status, and complicated perinatal history were associated with inability to obtain open set speech recognition by 5 years.
Conclusions
Characteristics of a subpopulation of children with CIs that were associated with an inability to achieve open-set speech recognition after 5 years of CI experience were investigated. These data distinguish pediatric CI recipients at risk for poor auditory development and highlight areas for future interventions to enhance support of early implantation.
Introduction
Cochlear implantation has proven to be widely successful for the vast of majority of children with severe to profound hearing loss. Numerous investigators have identified individual, familial, and environmental variables that influence auditory and language outcomes in children with cochlear implants (CIs) [1–4]. Characteristics of the “star” performers have been reviewed to identify underlying processes explaining individual differences in performance outcomes, such as early implantation, and cognitive factors (e.g., working memory) [5–7]. In the present study, the lower range of the performance continuum was investigated with an emphasis on speech recognition.
Since the inception of pediatric cochlear implantation, speech recognition has been investigated as means to assess success. Speech recognition test batteries typically involve a continuum of tasks, spanning from closed-set discrimination and identification to open-set recognition, to evaluate a wide range of performance in children of different ages and skill sets. Chief among the early eligibility criteria for pediatric implantation was the inability of the deaf child to advance beyond limited detection and identification of the segmental aspects of speech using appropriately fit hearing aids [8]. Even as CI eligibility criteria were expanded to include children who demonstrated minimum aided open-set recognition skills, the clinical goal has been to maximize a child’s ability to understand spoken communication beyond that which could be expected using appropriately fit amplification [8, 9]
During the early single-channel pediatric trials in the 1980s, only a small subset of children were shown to progress from the easier closed-set tasks to the more difficult open-set tasks [10, 11]. With modern, multichannel CIs and expanding demographics (i.e., greater levels of residual hearing and earlier ages at implantation), open-set speech recognition has become the expectation. The ability to perform open-set speech recognition tasks has become a marker of the auditory skills necessary for spoken language development [12, 13].
Several factors have emerged as important for predicting open-set speech recognition, including earlier age at implantation [14, 15], oral communication mode [14, 16, 17], and use of updated speech processors [16]. Today, children who do not achieve this milestone, even after years of CI use, are in the minority. The inability to achieve open-set speech recognition has most often been attributed to motor delays[18], cognitive deficits [18], and/or cochlear/neural abnormalities[19]. However, the process by which children transition from closed- to open-set speech recognition has never been fully explored and may determine key factors that underlie the development of speech recognition in children with CIs.
Few studies have addressed the characteristics of pediatric CI users who do not reach open-set speech recognition, even after extensive CI use. Retrospective analyses have been conducted to examine the lack of speech recognition development in children with CIs. To the best of our knowledge, there have been no prospective studies focusing on this subpopulation within a longitudinal cohort. A retrospective study [20] identified 5 young CI users who failed to improve on an open-set speech recognition measure (PB-K) after 2 years of implant use. The authors compared pre- and post-implant characteristics of the poor performers with 2 groups of cochlear implant users (randomly selected, age-matched). Older age at implantation and a longer time without access to sound was associated with lack of improvement on the PB-K. The authors also identified difficult mapping process and poor habilitation as contributing factors post-implant.
The Childhood Development after Cochlear Implantation (CDaCI) study uses multidimensional prospective data collection to assess children receiving a cochlear implant, both before and after implantation. A better understanding of the pre- and post-implant variables associated with children’s inability to achieve open-set speech recognition competency with the CI is important for guiding parents and interventionists in detecting potential difficulties and it enables researchers and clinicians to develop interventions for these children during critical moments in auditory development.
In this study, we investigated subject characteristics that could be associated with inability to develop open-set speech recognition with a cochlear implant. We compared baseline characteristics in children from the CDaCI cohort who demonstrated open-set speech recognition skills to those with lower-level performance at the 5-year post-activation interval, a greater length of time than the more usual 2 years of implant use [21, 22].
We hypothesized that two sets of baseline variables will have an effect on speech recognition skill development at 5 years post-CI activation [2, 23–27]. The first set of characteristics are those associated with the CI recipient (family variables, subject gender, race, maternal education, income, combined index of mother’s sensitivity, combined IQ score, and whether the birth was normal or involved perinatal complications). The second set of variables are specific to baseline measures of auditory input prior to implantation (age at detection of hearing loss, age at amplification, unaided and aided 4-frequency average (better ear), radiological findings, age at implantation).
Methods
Data were collected from 6 CI centers and 2 affiliated preschools as part of the national CDaCI longitudinal study. Children with severe to profound SNHL (≥70 dB loss, n=188) and children with normal hearing (n=97) were enrolled into the study between 2002 and 2004. The demographic data for this cohort have been published previously [28]. The present study includes only the children with hearing loss who met current CI candidacy criteria, and were:
≤ 5 years old;
Score better than 70 on the Bayley Scales of Infant Development Mental Scale or Motor Scale (BSID) (28, 29) or Leiter International Performance Scale-Revised (Leiter-R) greater than 66 (30);
English as the primary language spoken in the home;
Commitment of bilingual parent(s) to educate their child in English-speaking programs; and
No identified developmental condition that might preclude child participation in language assessment (Reynell Developmental Language Scales, RDLS)[31].
Three cases with hearing loss did not undergo post-operative evaluations and, therefore, were not considered further here. The current study included only those CI recipients with speech recognition scores obtained beyond study enrollment date or baseline (n=185). The demographic characteristics of the children appear in Table 1.
Table 1.
Sample Characteristics
| Performance Group at Five Years | ||||
|---|---|---|---|---|
| Variable |
All Subjects (n=185) |
Open-set by 5 Y (n=155) |
No Open-Set at 5 Y (n=18) |
No Open-Set Missing 5 Y (n=12) |
| Male, n (%) | 89 (48) | 75 (48) | 8 (44) | 6 (50) |
| Non-white†, n (%) | 49 (26) | 33 (21) | 10 (56) | 6 (50) |
| Maternal Education ≤ high school, n (%) | 39 (21) | 30 (19) | 4 (24) | 5 (42) |
| Income ≤ $50,000 n (%) | 77 (42) | 57 (37) | 11 (61) | 9 (75) |
| Combined Maternal Sensitivity, Baseline, mean (SD) | 5.3 (.7) | 5.3 (.65) | 4.8 (.96) | 4.9 (.75) |
| Combined IQ, Baseline, mean (SD) | 96.5 (21) | 96.7 (21) | 95.9 (21) | 95.1 (25) |
| Complicated Perinatal History (Premature/NICU), n (%) | 26 (15) | 18 (12) | 5 (31) | 3 (25) |
| Age at hearing loss, months, mean (SD) | 2.8 (7) | 2.3 (7) | 4.6 (8) | 5 (9) |
| Age at amplification, months, mean (SD) | 13.4 (11) | 12.5 (10) | 15.5 (10) | 21.7 (13) |
| Unaided 4-frequency average, better ear, Baseline, dB HL, mean (SD) | 105 (16) | 104.3 (16.5) | 109.6 (14) | 109.9 (19) |
| Aided 4-frequency average, better ear, Baseline, dB HL, mean (SD) | 70.8 (21.8) | 68.5 (22) | 85.8 (16) | 79.4 (21) |
| Radiology Report: | ||||
| No abnormalities, n (%) | 138 (76) | 114 (74) | 14 (78) | 10 (83) |
| EVAS, n (%) | 20 (11) | 18 (12) | 2 (11) | 0 |
| Meningitis‡, n (%) | 7 (4) | 6 (4) | 0 | 1 (8) |
| Other abnormalities, n (%) | 17 (9) | 14 (9) | 2 (11) | 1 (8) |
| Age at CI, mean (SD) | 28.3 (14) | 27.4 (14) | 34 (15) | 30.8 (14) |
Non-white included the categories: black, Asian, other, no response.
Meningitis noted on imaging or history
Y = years. n = count. % is percentage. SD is standard deviation. CI is cochlear implantation. dB HL is decibels hearing loss. NICU is neonatal intensive care unit. EVAS is Enlarged Vestibular Aqueduct Syndrome.
Highlighted rows indicate significant difference by Performance Group (categorical variable = significant chi-square, continuous variable = significant one-way ANOVA).
Child Characteristics
Child race was classified on the basis of parent report and were grouped as white or non-white. Parents also provided their child’s age at onset of deafness, and the age the child first received amplification. A 4-frequency average of unaided pure-tone thresholds (500, 1000, 2000, and 4000 Hz) in the better hearing ear was collected, as it is typically used to represent residual hearing prior to implantation (unaided PTA) [32]. Aided 4-frequency pure-tone threshold average (aided PTA) was collected and is here used to represent the ability to access any level of sound prior to implantation.
The child’s cognitive status at baseline was assessed using the BSID for children under 2 years of age and the Leiter-R for children aged 2 years and older. For the purpose of analysis, we used a standardized cognitive function variable that combined the scores from these 2 scales from all children.
Perinatal complications were self-reported by the families as normal birth or premature birth and whether the child was admitted to neonatal intensive care unit (NICU) at birth. The variable was coded into normal birth or some complication in perinatal history (premature birth, a NICU admission, or both).
Family Characteristics
Household income per year was categorized, based on parent report, as either above the median United States family income or below the median. Mothers reported their level of education, which was categorized as either a maximum of high school or any post-secondary education.
Maternal sensitivity (MS) was coded from video-taped interactions of the parent and child engaging in free play, puzzles and art in 3 standardized 20 minute sessions [2]. Coding was based on the 7-point scale used in the National Institute of Child Health and Human Development’s Early Childcare Study, [33, 34], across 4 subscales: sensitivity/responsivity, respect for child’s autonomy, positive regard, and hostility. These subscales evaluate the degree to which the mother expresses emotional support and positive feelings to the child and whether the mother respects the child’s individuality, motives and perspectives during the session. The composite, or combined, MS score was used for the analysis (below).
Radiology
All available radiology reports were reviewed, 3 subjects were missing imaging reports (n=182 reports reviewed). The dates of acquisition varied from 2002 to 2005. The standard of care at that time was usually a CT scan (n=143, 78%), but magnetic resonance imaging (MRI) had been ordered for 39 (21%) children. The scan quality was quite variable. For example, in 4 children, imaging reports were completed after the first CI surgery. Four CT reports (2%) did not include a specific comment regarding the cochlea or any inner ear structure. The 4 reports were coded as normal under the assumption that if an abnormal finding was observed, the radiologist would have made a comment. Just 2 of the 143 CT reports noted the scans as “high resolution”.
Radiologic findings were classified into 4 non-overlapping categories: “Normal inner ear”, “Enlarged Vestibular Aqueduct Syndrome (EVAS)”, “Meningitis”, and “Abnormal cochlear morphology”. “EVAS” included reports of enlarged or dilated vestibular aqueducts. Meningitis included children with an etiology of meningitis reported in their medical history or imaging findings. “Abnormal” included findings of cystic cochlea, hypotrophic cochlea, cochlear dysplasia, dilation of the cochlea and vestibule and all other findings indicating abnormal inner ear anatomy [35].
Outcome Measures
Speech Recognition Test Battery
Auditory development was assessed using a hierarchical battery of speech recognition measures using age- and functionally-appropriate instruments [36], which progressively increased in the complexity of auditory skill required for task completion. The measures comprising the battery are: the Meaningful Auditory Integration Scales and its Infant Toddler version (IT/MAIS)[37, 38]; the Early Speech Perception Test (ESP)[39]; the Pediatric Speech Intelligibility Test (PSI)[40]; Lexical Neighborhood Test and its multi-talker version (LNT, MLNT)[41]; Phonetically Balanced Word Lists - Kindergarten (PBK)[42] and the Hearing in Noise Test for Children (HINT-C)[43]. In moving through the hierarchy, the child was required to attain a criterion level of performance on an earlier, easier test before advancing to the next, more difficult test, irrespective of age, thus eliminating floor and ceiling effects. Tests were discontinued once a child demonstrated ceiling performance over 2 consecutive follow-up intervals.
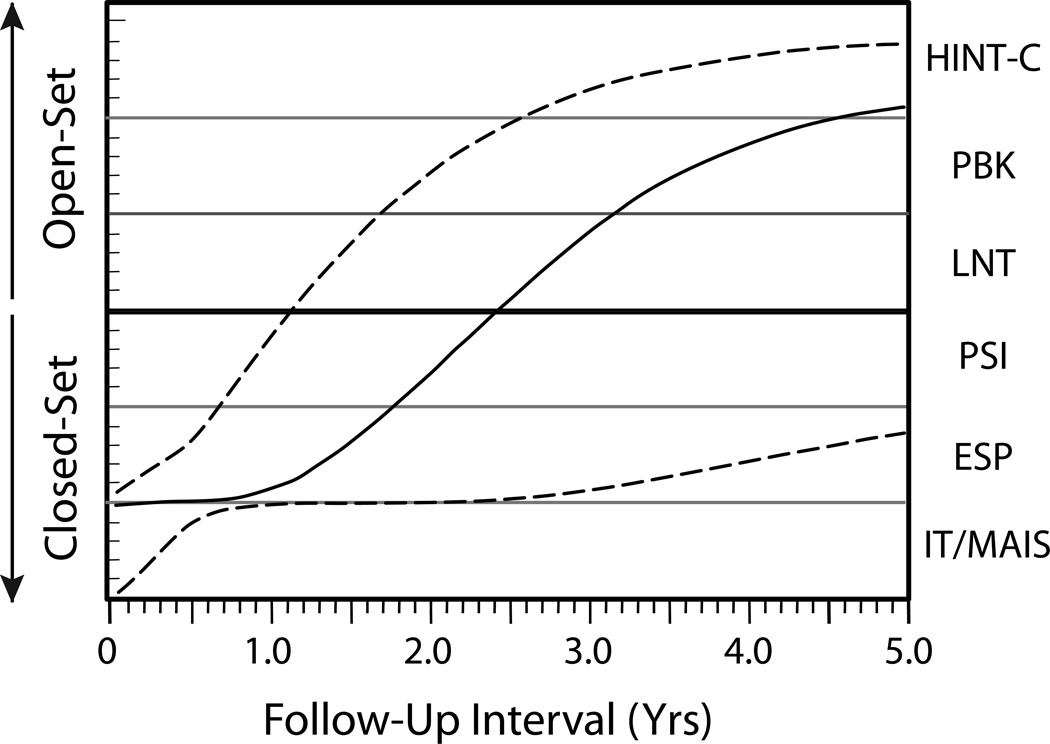
Child performance was summarized by a cumulative index, the Speech Recognition Index in Quiet (SRI-Q) [24] that descriptively captured progression through the hierarchy each post-CI interval (For a complete description of the SRI-Q [44], Figure 1 shows the median (solid line) SRI-Q on this hierarchical battery from baseline through 5 years post-CI across our entire CI sample [45]). Also shown are estimates of the 90th and 10th percentiles of performance (dashed lines).
Figure 1.
Median (solid line) and 90th and 10th percentiles (dashed lines) of performance on hierarchical speech recognition test battery by CI recipients as a function of follow-up interval post-implant in the CDaCI study. See text for a description of the measures. The bold horizontal line between the PSI and LNT highlights the boundary between closed-set and open-set speech recognition tasks. (Adapted from Johnson et al, 2012) [45]
For the analysis, children with 5-year data were grouped according to whether or not they had achieved open-set speech recognition on the CDaCI test battery by that interval. Subjects with a 5-year evaluation were grouped by whether they could be tested on an open-set test (“open-set group”) or not (“no open-set group”). Within the no-open-set group, the children demonstrated improved auditory detection with the implant, but no consistent ability to identify syllabic pattern or stress. From a communication standpoint, the inability of a child with a CI to advance in auditory skill development would be expected to have an adverse effect on spoken language development. All but one of the children in the no open-set group (94%) relied on some level of sign support to communicate. In contrast, 84% of the open-set group communicated exclusively by spoken language (i.e., no sign support).
Thirty-three children (17.6%) were lost to follow-up prior to the 5-year interval and the speech recognition skill level at 5 years post-implantation was unknown. Of these 33 children, 18 had progressed to open-set speech recognition at his/her last evaluation. For the purpose of the analysis, these 18 subjects were included within the open-set group, as it is unlikely that a child would regress to closed-set speech recognition ability (total n=155). Twelve of the 33 children (6.5%) had not achieved open-set speech recognition performance as of the last evaluation and were grouped into the “No open-set, missing” group. This is an important group to include with respect to pre-implant characteristics, as their lack of progress in skill development during the first few years following implantation may have contributed to dropping out of the study without communication or notification [46].
Statistical Analyses
Chi-square was used to examine group differences (open set, no-open set, no-open-set, missing) by categorical variables (sex, race, mother’s education, family income, complicated perinatal history, and imaging result). For continuous variables (maternal sensitivity, combined IQ, ages, 4-frequency averages), one-way ANOVA was used to examine for group differences. Exploratory logistic regressions in SPSS 21.0 were conducted, examining for variables that reliably differed by performance group, independent of the other variables. The first set of tests (chi-square, ANOVA) was to examine for performance group differences at baseline and the logistic regressions examine for group differences in the presence of other variables. Only those variables with significant differences on either chi-square or ANOVA were included in the logistic regressions. Significance for all statistical tests was set at p<0.05.
Results
Race was significantly associated with group (chi-square = 17.5, p = .025). Non-white children represented a higher percentage of cases in the lower performing groups (33%) relative to white children (10%). Income was also associated with group (chi-square = 29.8, p = .008). A higher percentage of children from families earning ≤$50,000 annually tended to be in the lower performing groups (26%) relative to the percentage of children from families earning greater than $50,000 annually (9.3%). Finally, complicated perinatal history was associated with group (chi-square = 4.2, p=.04). Children in the no-open-set group experienced a complicated perinatal history more often (n=5, 31%) than those achieving open-set (n=18, 12%). (See Table 1).
The groups differed on combined maternal sensitivity at baseline (F=8.1, p<.0001). The open-set group had a higher average maternal sensitivity relative to the two no-open-set groups. Average age at amplification differed by group (F=4.7, p<.011), with the open-set group averaging 12 months at age of amplification and the no-open-set groups averaging a greater number of months. Finally, the baseline aided PTA was significantly different by group (F=7.0, p=.001), with the open-set group averaging 68.5 dB HL and the other 2 groups averaging considerably higher (See Table 1).
The performance groups did not differ by male/female composition, mother’s education, age at activation, unaided PTA, or combined IQ score. The imaging results also did not differ by group, with 78% of the no-open set group and 83% of the no-open-set, missing group showing a normal inner ear CT or MRI imaging results relative to 74% in the open-set group (see Table 1).
Logistic Regression
Two logistic regressions were carried out, one comparing the open-set group with the no-open-set group, and a second comparing the two no-open-set groups. The first examines for variables that differentiate those children who were able to develop open-set speech recognition and the second examines for variables that differentiate the children and families who were able to stay in the study relative to those who were lost to follow-up, all in children who did not progress to open-set speech recognition at the same rate as the preponderance of the sample.
Markers of socio-economic status (race, income), age at initial amplification, pre-implant best aided hearing, pre-implant combined maternal sensitivity, complicated perinatal history, and imaging results were submitted to an exploratory logistic regression analysis. The analysis comparing the open-set and no-open-set groups showed that income, imaging results, and age at amplification did not show a significant independent contribution to group differences and so were removed from the analysis reported here. The final model for both logistic regressions included best aided PTA, combined mother’s sensitivity, race, and complicated perinatal history (see Table 2).
Table 2.
Logistic Regression Results
| Predicting Attaining Open-Set by 5 years post-activation | ||||
|---|---|---|---|---|
| Variable | B | Wald χ2 | p | Odds |
| Baseline aided four frequency average, better ear | .04 | 6.047 | .014 | 1.04 |
| Baseline Combined Maternal Sensitivity | −1.48 | 9.99 | .002 | .228 |
| Complicated Perinatal History (Y,N) | 1.51 | 4.33 | .037 | 4.51 |
| Race (White, Nonwhite) | 1.33 | 4.33 | .038 | 3.79 |
| Predicting No-Open-Set non-missing by missing at 5 years post-activation | |||
|---|---|---|---|
| Variable | B | Wald χ2 | p |
| Baseline aided four frequency average, better ear | −.026 | .91 | ns |
| Baseline Combined Maternal Sensitivity | .94 | 1.9 | ns |
| Complicated Perinatal History (Y,N) | −.50 | .26 | ns |
| Race (White, Nonwhite) | .73 | .67 | ns |
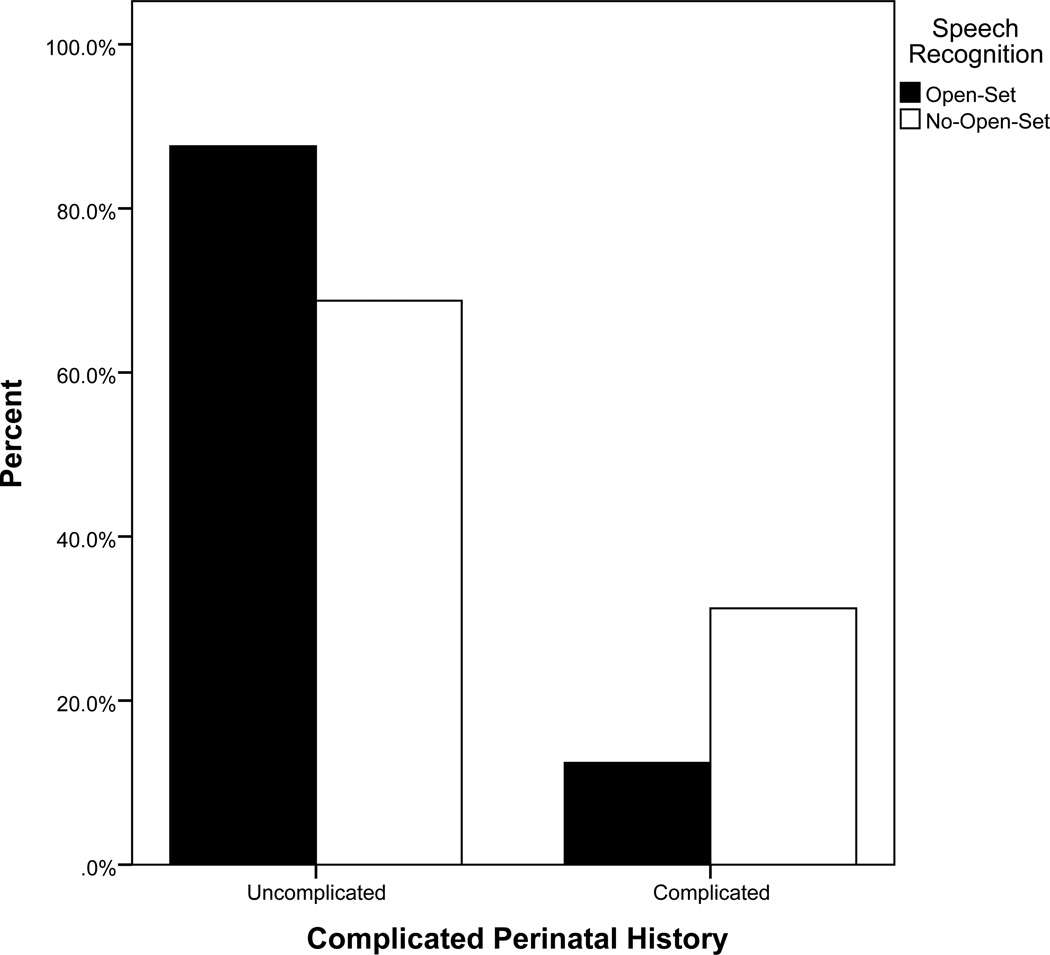
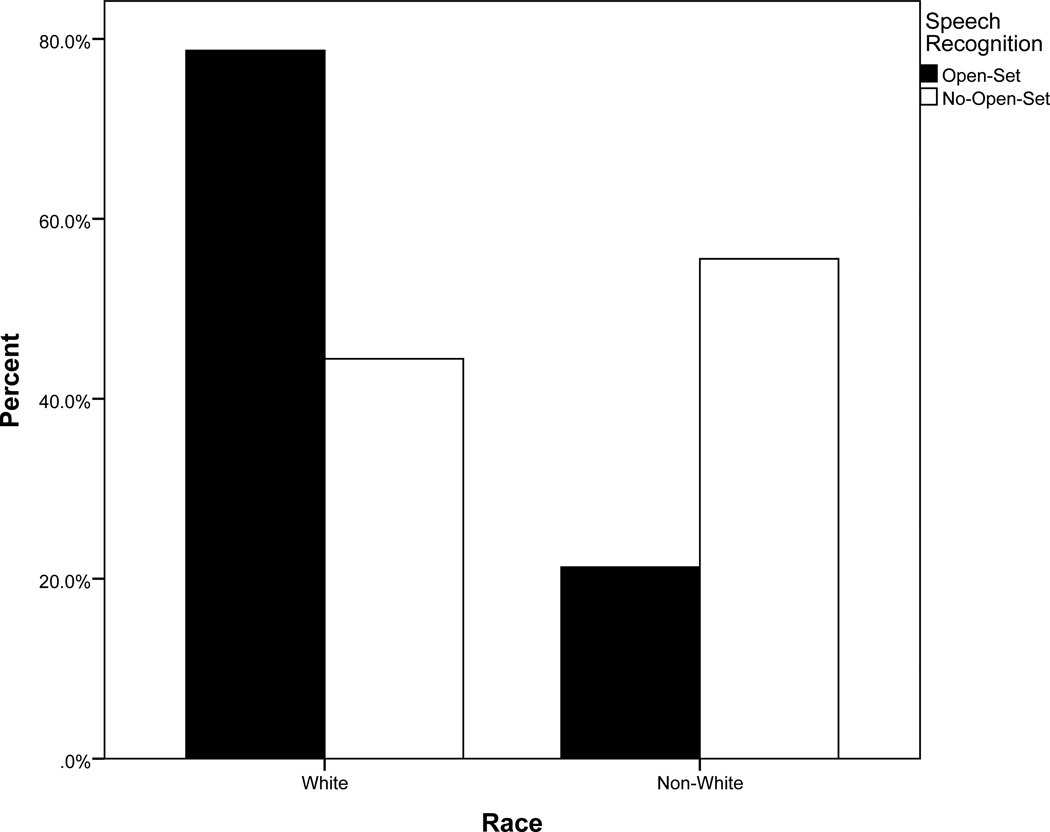
Overall, baseline better ear aided PTA, baseline maternal sensitivity, and complicated perinatal history differentiated between children who were able to attain open-set speech recognition and those who did not (See Table 2). We see that for every 1 dB increase in better ear aided PTA resulted in a 4% increase in the odds of not progressing to open-set speech recognition. Low maternal sensitivity, independent of hearing, contributed significantly to the increased odds of not developing open-set speech recognition. For those children experiencing a complicated perinatal history, the odds of not developing open set were 4.5 times higher than those experiencing a normal birth (see Figure 2a). Finally, non-white children had 3.8 times higher odds of not developing open-set speech recognition relative to white children (see Figure 2b).
Figure 2.
a. Percent of sample experiencing birth trauma by speech recognition at 5 years post cochlear implant activation.
b. Percent of sample who self-identified as white or non-white by speech recognition at 5 years post cochlear implant activation.
Discussion
Results obtained for this longitudinal, prospective cohort of 185 children identified a number of characteristics associated with an inability achieve open-set speech recognition after 5 years of implant experience. Poorer aided access to sound in the better ear, lower maternal sensitivity to communication needs, complicated perinatal history, and minority status were all associated with inability to develop open set speech recognition after 5 years of cochlear implant use. These measures, crudely perhaps, assess the constellation of factors enabling a deaf child and his/her family to take advantage of implantable technology to develop auditory skills.
When the same set of variables were examined for differences between the no-open-set group and no-open-set, missing group, there were no differences between the groups. It appears that these variables that are associated with lack of open-set speech recognition development do not contribute to dropping out of the study.
The variables that did not show an association with open-set speech recognition acquisition were somewhat surprising. There was no association between radiology reports and failure to progress to open-set testing. The performance groups did not differ by categorization of radiological findings into normal inner ear and abnormal inner ear [35]. Since the initial design of the CDaCI study, researchers and clinicians alike have recommended high-resolution CT and thin slice MRI for clearer identification of inner ear abnormalities [19, 47–53]. Cochlear nerve hypoplasia is one inner ear abnormality visible on pre-implant imaging. Hypoplasia has been linked to abnormal electrically evoked auditory brainstem responses, even after consistent CI use [54]. The opportunities for hypoplastic cochlear nerve morphology to result in good speech recognition are rare, indeed, if nerve conduction signals are not detected at the brainstem. Currently, the ability of hypoplastic cochlear nerve morphology to produce benefit is an area of ongoing controversy (e.g., [22]).
Our ability to detect the relationship between anatomy and CI performance was reduced due to the quality of the images and lack of access to the original images. Thus, we were not able to confirm the radiological findings showing either normal or abnormal anatomy. Additionally, the study inclusion criteria excluded children with notable cochlear morphology, restricting the sample to perhaps the best CI candidates anatomically. Nonetheless, our results support the previous finding of no relationship between abnormal inner ear morphology and change in IT-MAIS/MAIS and Reynell verbal comprehension scores at 24 months post implantation [35].
Age at implantation and at amplification have been identified as key factors in a host of post-CI outcomes, including spoken language [1, 55] and parental ratings of child development [56], we did not find those variables to be significant predictors of who will achieve open-set speech recognition after 5 years of implant use. In addition, unaided pre-implant hearing did not appear to be associated with speech recognition group. Rather, auditory access to sounds in the speech spectrum with amplification prior to implantation significantly differentiated between the open-set and no-open set groups.
The significance of better ear aided PTA as a factor differentiating the open-set and no-open-set groups points to the contribution of pre-implant auditory access and experience to the continued growth in auditory-based skills following implantation. While not considered sufficient for the development of age-appropriate spoken language skills, the earlier access to sound provided by hearing amplification devices in the pre-implant period appears to bolster children’s ability to take advantage of the acoustic cues important to speech in the post-CI period. Although age at amplification and aided thresholds provide a rudimentary and imperfect snapshot of the amount and quality of a child’s auditory access to speech using their amplification, it does capture some of the variability observed in hearing aid “benefit” among children with severe and profound hearing loss.
It should be noted that the children in this cohort were implanted a decade ago, when hearing loss criteria were more stringent and statewide early identification programs had recently been implemented in the United States. Thus, these findings may not be generally representative for the population of children being implanted today, including those who: have been identified and fit with amplification at earlier ages, implanted under more relaxed hearing loss criteria, and been able to take advantage of early intervention programs. Nevertheless, there continues to be a subgroup of children who may have not been able to take advantages of these contemporary advances and thus continue to be at risk for poor speech recognition outcomes.
Maternal Sensitivity
In the present study, combined maternal sensitivity scores at baseline were associated with speech recognition outcomes at 5 years post-CI. That is, those children whose mothers who scored higher on a cluster of subscales rating emotional responsiveness, positive regard and respect for child’s autonomy in structured play sessions were more likely to be in the group with open set recognition by 5 years post-CI. Similarly, Quittner et al. [2] have shown positive associations between maternal sensitivity and children’s language development.
Research in infant-directed speech (IDS) in pre-lingual hearing infants suggests the prosody and high variation in pitch facilitates syllabic discrimination and word recognition [57, 58]. Furthermore, IDS contributed to the infant’s emotional, cognitive and language development [58]. In pediatric CI recipients, associating meaning with words may facilitate auditory development. The association between maternal sensitivity and speech recognition may be in part because higher ratings of maternal sensitivity are associated with better IDS tailored to meet their child’s needs. The role of IDS and maternal sensitivity in language and auditory development in pre-lingually deafened CI recipients warrants further investigation. Recent research into the quality and quantity of parental linguistic interactions have identified a number of effective strategies that support spoken language development post-implant [59–61] laying the foundation for future interventions.
Auditory-Visual Speech Perception Battery
The children enrolled in the CDaCI study not only represent research subjects, but also are part of the clinical caseload. In particular, the children we highlight in this study demonstrate little to no progress in their auditory skill development during a time when the benchmark of success is auditory-only, open-set speech recognition. In an attempt to capture a more accurate reflection of the child’s everyday functioning with the CI, and enable the child to experience some degree of success on speech recognition tasks, an auditory-visual test (A-V) test battery was compiled for this subset of children participating in the CDaCI study. That is, those children who do not meet criteria for progressing further in the auditory-only modality are assessed using a parallel hierarchy of speech recognition measures presented live-voice, face-to-face, allowing the child to see the speaker’s face. Many of the children within this group have advanced to more difficult tests when evaluated in the auditory-visual modality. In fact, some children have successfully transitioned back into the standard auditory-only test battery with continued follow-up.
Conclusions
The effects of maternal sensitivity, functional hearing prior to implantation (better ear aided PTA), minority status, and a complicated perinatal history all were associated with the inability to progress to open-set speech recognition at 5 years post-cochlear implantation. Taken together, these variables indicate the broad range of influences on a profoundly deaf child, influences that can slow the acquisition of auditory skill development and slow the development of language, reading, and academic skills.
Acknowledgments
Funded by National Institute for Deafness and Other Communication Disorders (R01 DC04797)
References
- 1.Niparko JK, Tobey E, Thal D, et al. Spoken Language Development in Children Following Cochlear Implantation. JAMA. 2010;303(15):1498–1506. doi: 10.1001/jama.2010.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quittner AL, Cruz I, Barker DH, et al. Effects of maternal sensitivity and cognitive and linguistic stimulation on cochlear implant users' language development over four years. J Pediatr. 2013;162(2):343–348. e3. doi: 10.1016/j.jpeds.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geers AE, Strube MJ, Tobey EA, et al. Epilogue: Factors Contributing to Long-Term Outcomes of Cochlear Implantation in Early Childhood. Ear Hear. 2011;32:84S–92S. doi: 10.1097/AUD.0b013e3181ffd5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunn CC, Walker E, Oleson J, et al. Longitudinal Speech Perception and Language Performance in Pediatric Cochlear Implant Users: The Effect of Age at Implantation. Ear Hear. 2014;35:148–160. doi: 10.1097/AUD.0b013e3182a4a8f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisoni DB, Cleary M, Geers AE, et al. Individual differences in effectiveness of cochlear implants in children who are prelingually deaf: new process measures of performance. Volta Review. 1999;101(3):111–164. [PMC free article] [PubMed] [Google Scholar]
- 6.Pisoni DB. Information-processing skills of deaf children with cochlear implants: some new process measures of performance. Int Congr Ser. 2004;1273:283–287. doi: 10.1016/j.ics.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisoni DB, Cleary M. Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear Hear. 2003;24(1 Suppl):106S–120S. doi: 10.1097/01.AUD.0000051692.05140.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwolan TA, Zimmerman-Phillips S, Ashbaugh CJ, et al. Cochlear implantation of children with minimal open-set speech recognition skills. Ear Hear. 1997;18(3):240–251. doi: 10.1097/00003446-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Meyer TA, Svirsky MA, Kirk KI, et al. Improvements in speech perception by children with profound prelingual hearing loss: effects of device, communication mode, and chronological age. J Speech Lang Hear Res. 1998;41(4):846–858. doi: 10.1044/jslhr.4104.846. [DOI] [PubMed] [Google Scholar]
- 10.Berliner KI, Tonokawa LL, Dye LM, et al. Open-set speech recognition in children with a single-channel cochlear implant. Ear Hear. 1989;10(4):237–242. doi: 10.1097/00003446-198908000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Geers AE, Moog JS. Predicting long-term benefits from single-channel cochlear implants in profoundly hearing-impaired children. Am J Otol. 1988;9(2):169–176. [PubMed] [Google Scholar]
- 12.Boothroyd A. Auditory Development of the Hearing Child. Scand Audiol. 1997;26(Suppl 46):9–16. [PubMed] [Google Scholar]
- 13.Hnath-Chisolm T, Laipply E, Boothroyd A. Age-related changes on a children's test of sensory-level speech percpetion capacity. Journal of Speech, Language, and Hearing Research. 1998;41:94–106. doi: 10.1044/jslhr.4101.94. [DOI] [PubMed] [Google Scholar]
- 14.O'Donoghue GM, Nikolopoulos TP, Archbold SM. Determinants of speech perception in children after cochlear implantation. Lancet. 2000;356(9228):466–468. doi: 10.1016/S0140-6736(00)02555-1. [DOI] [PubMed] [Google Scholar]
- 15.Waltzman SB, Cohen NL, Gomolin RH, et al. Open-set speech perception in congenitally deaf children using cochlear implants. Am J Otol. 1997;18(3):342–349. [PubMed] [Google Scholar]
- 16.Geers AE. Predictors of reading skill development in children with early cochlear implantation. Ear and Hearing. 2003;24(1):59S–68S. doi: 10.1097/01.AUD.0000051690.43989.5D. [DOI] [PubMed] [Google Scholar]
- 17.Sarant JZ, Blamey PJ, Dowell RC, et al. Variation in speech perception scores among children with cochlear implants. Ear Hear. 2001;22(1):18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Pyman B, Blamey P, Lacy P, et al. The development of speech perception in children using cochlear implants: effects of etiologic factors and delayed milestones. Am J Otol. 2000;21(1):57–61. [PubMed] [Google Scholar]
- 19.Buchman C, Teagle HFB, Roush PA, et al. Cochlear implantation in children with labyrinthine anomalies and cochlear nerve deficiency: implications for auditory brainstem implantation. Laryngoscope. 2011;121(9):1979–1988. doi: 10.1002/lary.22032. [DOI] [PubMed] [Google Scholar]
- 20.Gordon KA, Daya H, Harrison RV, et al. Factors contributing to limited open-set speech perception in children who use a cochlear implant. Int J Pediatr Otorhinolaryngol. 2000;56:101–111. doi: 10.1016/s0165-5876(00)00400-6. [DOI] [PubMed] [Google Scholar]
- 21.Black J, Hickson L, Black B, et al. Paediatric cochlear implantation: Adverse prognostic factors and trends from a review of 174 cases. Cochlear Implants International. 2014;15(2):62–77. doi: 10.1179/1754762813Y.0000000045. [DOI] [PubMed] [Google Scholar]
- 22.Young NM, Kim FM, Ryan ME, et al. Pediatric cochlear implantation of children with eighth nerve deficiency. Int J Pediatr Otorhinolaryngol. 2012;76(10):1442–1448. doi: 10.1016/j.ijporl.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Black J, Hickson L, Black B, et al. Prognostic indicators in paediatric cochlear implant surgery: a systematic literature review. Cochlear Implants Int. 2011;12(2):67–93. doi: 10.1179/146701010X486417. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberg LS, Johnson KC, Martinez AS, et al. Speech recognition at 1-year follow-up in the childhood development after cochlear implantation study: Methods and preliminary findings. Audiol Neurootol. 2006;11(4):259–268. doi: 10.1159/000093302. [DOI] [PubMed] [Google Scholar]
- 25.Geers AE. Factors Affecting the Development of Speech, Language, and Literacy in Children With Early Cochlear Implantation. Language, Speech and Hearing Services in Schools. 2002;33:172–183. doi: 10.1044/0161-1461(2002/015). [DOI] [PubMed] [Google Scholar]
- 26.Geers AE, Brenner CA, Tobey EA. Long-Term Outcomes of Cochlear Implantation in Early Childhood: Sample Characteristics and Data Collection Methods. Ear Hear. 2011;32:2S–12S. doi: 10.1097/AUD.0b013e3182014c53. [DOI] [PubMed] [Google Scholar]
- 27.Quittner AL, Barker DH, Cruz I, et al. Parenting Stress among Parents of Deaf and Hearing Children: Associations with Language Delays and Behavior Problems. Parent Sci Pract. 2010;10(2):136–155. doi: 10.1080/15295190903212851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fink NE, Wang NY, Visaya J, et al. Childhood Development after Cochlear Implantation (CDaCI) study: Design and baseline characteristics. Cochlear Implants International. 2007;8(2):92–116. doi: 10.1179/cim.2007.8.2.92. [DOI] [PubMed] [Google Scholar]
- 29.Bayley N. Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: The Psychological Corp; 1993. [Google Scholar]
- 30.Roid G, Miller L. Leiter International Performance Scale--Revised. Wood Dale, IL: Stoelting Co; 2002. [Google Scholar]
- 31.Reynell J, Gruber C. Reynell Developmental Language Scales: US Edition. Los Angeles, CA: Western Psychological Services; 1990. [Google Scholar]
- 32.Stelmachowicz PG, Lewis DE. Some theoretical considerations concerning the relation between functional gain and insertion gain. J Speech Hear Res. 1988;31(3):491–496. doi: 10.1044/jshr.3103.491. [DOI] [PubMed] [Google Scholar]
- 33.National Institute of Child, H.H.D.E.C.C.R., Network. Child care and mother–child interaction in the first three years of life. Developmental Psychology. 1999;35(6):1399–1413. [PubMed] [Google Scholar]
- 34.National Institute of Child, H.H.D.E.C.C.R., Network. The Relation of Child Care to Cognitive and Language Development. Child Development. 2000;71(4):960–980. doi: 10.1111/1467-8624.00202. [DOI] [PubMed] [Google Scholar]
- 35.Francis HW, Buchman CA, Visaya J, et al. Surgical Factors in Pediatric Cochlear Implantation and Their Early Effects on Electrode Activation and Functional Outcomes. Otol Neurotol. 2008;29:502–508. doi: 10.1097/MAO.0b013e318170b60b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenberg LS, Johnson KC, Martinez AS, et al. Studies in pediatric hearing loss at the House Research Institute. J Am Acad Audiol. 2012;23(6):412–421. doi: 10.3766/jaaa.23.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins AM, Renshaw JJ, Berry SW. Evaluating meaningful auditory integration in profoundly hearing-impaired children. Am J Otol. 1991;12(Suppl):144–150. [PubMed] [Google Scholar]
- 38.Zimmerman-Phillips S, Robbins AM, Osberger MJ. Assessing cochlear implant benefit in very young children. Ann Otol Rhinol Laryngol Suppl. 2000;185:42–43. doi: 10.1177/0003489400109s1217. [DOI] [PubMed] [Google Scholar]
- 39.Moog J, Geers A. Early Speech Perception Test for profoundly hearing-impaired children. St. Louis, MO: Central Institute for the Deaf; 1990. [Google Scholar]
- 40.Jerger S, Jerger J. Pediatric Speech Intelligibility Test. St. Louis, MO: Auditec; 1984. [Google Scholar]
- 41.Kirk KI, Pisoni DB, Osberger MJ. Lexical effects on spoken word recognition by pediatric cochlear implant users. Ear Hear. 1995;16(5):470–481. doi: 10.1097/00003446-199510000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haskins H. A phonetically balanced test of speech discrimination for children. Northwestern University; 1949. [Google Scholar]
- 43.Gelnett D, Sumida A, Nilsson M, et al. Annual Meeting: Am Acad Audiology. Dallas, TX: 1995. Development of the hearing in noise test for children (HINT-C) [Google Scholar]
- 44.Wang NY, Eisenberg LS, Johnson KC, et al. Tracking Development of Speech Recognition: Longitudinal Data From Hierarchical Assessments in the Childhood Development After Cochlear Implantation Study. Otol Neurotol. 2008;29:240–245. doi: 10.1097/MAO.0b013e3181627a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson KC, Eisenberg LS, Visser-Dumont L, et al. 12th International Conference on Cochlear Implants and Other Implantable Auditory Technologies. Baltimore, MD: 2012. Rate of growth in speech recognition over five years of follow-up in the CDaCI Study. [Google Scholar]
- 46.Little RJA, Rubin DB. Series in Probability and Statistics. 2nd ed. NY: Wiley; 2002. Statistical Analysis with Missing Data. [Google Scholar]
- 47.Casselman J, Offeciers E, De Foer B, et al. CT and MR imaging of congential abnormalities of the inner ear and internal auditory canal. European journal of radiology. 2001;40(2):94–104. doi: 10.1016/s0720-048x(01)00377-1. [DOI] [PubMed] [Google Scholar]
- 48.Casselman J, Offeciers F, Govaerts P, et al. Aplasia and hypoplasia of the vestibulocochlear nerve: diagnosis with MR imaging. Radiology. 1997;202(3):773–781. doi: 10.1148/radiology.202.3.9051033. [DOI] [PubMed] [Google Scholar]
- 49.Komatsubara S, Haruta A, Nagano Y, et al. Evaluation of cochlear nerve imaging in severe congenital sensorineural hearing loss. ORL J Otorhinolaryngol Relat Spec. 2007;69(3):198–202. doi: 10.1159/000099231. [DOI] [PubMed] [Google Scholar]
- 50.Buchman C, Roush PA, Teagle HFB, et al. Auditory Neuropathy Characteristics in Children with Cochlear Nerve Deficiency. Ear Hear. 2006;27:399–408. doi: 10.1097/01.aud.0000224100.30525.ab. [DOI] [PubMed] [Google Scholar]
- 51.Roche JP, Huang BY, Castillo M, et al. Imaging Characteristics of Children With Auditory Neuropathy Spectrum Disorder. Otol Neurotol. 2010;31:780–788. doi: 10.1097/mao.0b013e3181d8d528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adunka OF, Roush PA, Teagle HF, et al. Internal Auditory Canal Morphology in Children with Cochlear Nerve Deficiency. Otol Neurotol. 2006;27:793–801. doi: 10.1097/01.mao.0000227895.34915.94. [DOI] [PubMed] [Google Scholar]
- 53.Adunka OF, Jewells V, Buchman CA. Value of Computed Tomography in the Evaluation of Children With Cochlear Nerve Deficiency. Otol Neurotol. 2007;28:597–604. doi: 10.1097/01.mao.0000281804.36574.72. [DOI] [PubMed] [Google Scholar]
- 54.Valero J, Blaser S, Papsin B, et al. Electrophysiologic and Behavioral Outcomes of Cochlear Implantation in Children With Auditory Nerve Hypoplasia. Ear Hear. 2012;33:3–18. doi: 10.1097/AUD.0b013e3182263460. [DOI] [PubMed] [Google Scholar]
- 55.Tobey EA, Thal D, Niparko JK, et al. Influence of implantation age on school-age language performance in pediatric cochlear implant users. Int J Audiol. 2013;52(4):219–229. doi: 10.3109/14992027.2012.759666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark JH, Wang NY, Riley AW, et al. Timing of cochlear implantation and parents' global ratings of children's health and development. Otol Neurotol. 2012;33(4):545–552. doi: 10.1097/MAO.0b013e3182522906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estes KG, Hurley K. Infant-directed prosody helps infants map sounds to meanings. Infancy. 2013;18(5) doi: 10.1111/infa.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saint-Georges C, Chetouani M, Cassel R, et al. Motherese in interaction: at the cross-road of emotion and cognition? (A systematic review) PLoS One. 2013;8(10):e78103. doi: 10.1371/journal.pone.0078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DesJardin JL, Eisenberg LS. Maternal contributions: supporting language development in young children with cochlear implants. Ear Hear. 2007;28(4):456–469. doi: 10.1097/AUD.0b013e31806dc1ab. [DOI] [PubMed] [Google Scholar]
- 60.Ratner NB. Why talk with children matters: clinical implications of infant- and child-directed speech research. Semin Speech Lang. 2013;34(4):203–214. doi: 10.1055/s-0033-1353449. quiz C1. [DOI] [PubMed] [Google Scholar]
- 61.Cruz I, Quittner AL, Marker C, et al. Identification of effective strategies to promote language in deaf children with cochlear implants. Child Dev. 2013;84(2):543–559. doi: 10.1111/j.1467-8624.2012.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]