Abstract
Clinical studies demonstrate frequent co-existence of nicotine and alcohol abuse and suggest that this may result, in part, from the ready access to and intake of fat-rich diets. Whereas animal studies show that high-fat diet intake in adults can enhance the consumption of either nicotine or ethanol and that maternal consumption of a fat-rich diet during pregnancy increases operant responding for nicotine in offspring, little is known about the impact of dietary fat on the co-abuse of these two drugs. The goal of this study was to test in Long-Evans rats the effects of perinatal exposure to fat on the co-use of nicotine and ethanol, using a novel paradigm that involves simultaneous intravenous (IV) self-administration of these two drugs. Fat- vs. chow-exposed offspring were characterized and compared, first in terms of their nicotine self-administration behavior, then in terms of their nicotine/ethanol self-administration behavior, and lastly in terms of their self-administration of ethanol in the absence of nicotine. The results demonstrate that maternal consumption of fat compared to low-fat chow during gestation and lactation significantly stimulates nicotine self-administration during fixed-ratio testing. It also increases nicotine/ethanol self-administration during fixed-ratio and dose-response testing, with BEC elevated to 120 mg/dL, and causes an increase in breakpoint during progressive ratio testing. Of particular note is the finding that rats perinatally exposed to fat self-administer significantly more of the nicotine/ethanol mixture as compared to nicotine alone, an effect not evident in the chow-control rats. After removal of nicotine from the nicotine/ethanol mixture, this difference between the fat- and chow-exposed rats was lost, with both groups failing to acquire the self-administration of ethanol alone. Together, these findings suggest that perinatal exposure to a fat-rich diet, in addition to stimulating self-administration of nicotine, causes an even greater vulnerability to the excessive co-use of nicotine and ethanol.
Keywords: co-abuse, gestation, intravenous, offspring, operant responding, self-administration
Introduction
There is considerable evidence linking the consumption of dietary fat to the use and abuse of drugs. Clinical studies reveal a close relationship between dietary fat and the use of nicotine, with obese individuals showing a two-fold greater risk for developing nicotine addiction (Hussaini, Nicholson, Shera, Stettler, & Kinsman, 2011) and, conversely, with cigarette smokers consuming a significantly greater amount of high calorie, fat-rich foods than non-smokers (Dallongeville, Marécaux, Fruchart, & Amouyel, 1998). In addition, studies demonstrate a close relationship between dietary fat and alcohol abuse, with obese individuals exhibiting a greater rate of alcohol use (Sansone & Sansone, 2013), the intake of fat strongly correlated with the amount of alcohol consumed (Kesse, Clavel-Chapelon, Slimani, van Liere, & E3N Group, 2001), and a higher BMI associated with a higher familial risk for alcohol dependence (Lichenstein et al., 2014). Whereas animal studies relating adult dietary fat to nicotine consumption are lacking, a close positive relationship between fat and ethanol consumption has been demonstrated in rodents, with fat intake or administration of lipids causing an increase in ethanol intake (Carrillo, Leibowitz, Karatayev, & Hoebel, 2004) and the administration of ethanol, in turn, increasing the consumption of and preference for a fat-rich diet (Barson et al., 2009). Together, these studies support the idea that dietary fat is closely related to the abuse of drugs, such as nicotine and alcohol.
The possibility that exposure to dietary fat early in life can also affect later drug use is suggested by clinical evidence that unhealthy eating patterns and weight problems in childhood are associated with a lower age of onset of cigarette smoking and an increase in withdrawal symptoms during abstinence (Saules, Levine, Marcus, & Pomerleau, 2007). Also, maternal consumption of a fat-rich diet and being overweight during pregnancy (Dietz, 1998; Hannon, Rao, & Arslanian, 2005), which increase later consumption of and preference for fatty foods in the offspring (Fisher & Birch, 1995), is found to program other behaviors, such as anxiety, novelty seeking, and depression, which themselves are likely to promote drug use and increase vulnerability to drug abuse later in life (Bilbo & Tsang, 2010; Morganstern, Ye, Liang, Fagan, & Leibowitz, 2012; Rizzo, Silverman, Metzger, & Cho, 1997). Similar findings have been obtained in animal studies, demonstrating that in utero exposure to a fat-rich diet increases both alcohol drinking (Bocarsly et al., 2012; Cabanes, de Assis, Gustafsson, & Hilakivi-Clarke, 2000) and nicotine self-administration and seeking (Morganstern et al., 2013), while also promoting the consumption of and responding for palatable foods (Chang, Gaysinskaya, Karatayev, & Leibowitz, 2008; Naef et al., 2011; Ong & Muhlhausler, 2011). Together, these studies support a close positive relationship, in humans and animals, between early or prenatal exposure to dietary fat and the abuse of nicotine or ethanol.
The question to be addressed in this study is whether dietary fat affects the simultaneous consumption or co-use of nicotine and alcohol, a phenomenon frequently observed in humans. Clinical studies show that 70–80% of alcoholics smoke cigarettes (DiFranza & Guerrera, 1990; Falk, Yi, & Hiller-Sturmhofel, 2006), that smokers drink twice as much alcohol as nonsmokers (Falk et al., 2006) and are four times more likely to develop alcoholism (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Larsson & Engel, 2004), and that exposure to nicotine via smoking or transdermal patch increases the consumption of alcohol (Acheson, Mahler, Chi, & de Wit, 2006; Barrett, Tichauer, Leyton, & Pihl, 2006; Kandel, Chen, Warner, Kessler, & Grant, 1997). This evidence leads us to question whether exposure to dietary fat early in life might increase the co-use of nicotine and alcohol and the risk of their co-abuse. There are no studies to date, in humans or animals, directly testing the influence of dietary fat on simultaneous consumption of these two substances. There is only the suggestion that a greater availability in recent decades of palatable, fat-rich foods may be one factor that is increasing vulnerability to the co-abuse of nicotine and alcohol (WHO, 2003).
Studies examining the phenomenon of nicotine and ethanol co-use in animals have revealed mixed results. Earlier reports have shown that chronic peripheral administration of nicotine can increase both home-cage ethanol drinking and operant self-administration (Bito-Onon, Simms, Chatterjee, Holgate, & Bartlett, 2011; Lê, Corrigall, Harding, Juzytsch, & Li, 2000; Lê, Wang, Harding, Juzytsch, & Shaham, 2003; Olausson, Ericson, Löf, Engel, & Söderpalm, 2001) and, conversely, that alcohol-preferring P rats compared to non-preferring NP rats self-administer more nicotine and exhibit more robust nicotine-seeking behavior (Lê et al., 2006). More recent studies, which have combined intravenous (IV) self-administration of nicotine with oral self-administration of ethanol, have obtained different results, depending on the particular training paradigm used. Whereas rats trained to self-administer IV nicotine and oral ethanol sequentially show that nicotine increases subsequent ethanol self-administration and ethanol reduces nicotine self-administration (Lê, Funk, Lo, & Coen, 2014), rats trained to self-administer these drugs concurrently show no effect of nicotine on ethanol intake or of ethanol on nicotine intake (Lê et al., 2010). Further, when both drugs are given orally, outbred rats that readily drink these substances together consume them in similar amounts as when they are offered separately (Marshall, Dadmarz, Hofford, Gottheil, & Vogel, 2003), and P rats lever-press for a solution containing both nicotine and ethanol at the same level as when ethanol is provided alone (Hauser, Getachew, et al., 2012). While these studies lead one to question the phenomenon of co-use in animals, there is a recent report that allowed alcohol-preferring rats to self-administer a nicotine/ethanol mixture directly in the posterior ventral tegmental area (VTA) and obtained evidence for an interaction between the two drugs in a way that increased their co-use (Truitt et al., 2014). There is also a study in human subjects which describes an interactive effect of IV ethanol and IV nicotine on behavior and cognition (Ralevski et al., 2012). Together with the clinical studies, this finding provides support for a close, positive relationship between nicotine and ethanol intake. It suggests that another method, involving IV self-administration of both drugs together, may be used to demonstrate their co-use, which then will allow us to take the next step to test whether prenatal exposure to fat increases the risk for the co-abuse of these substances.
Thus, the present study examined two major experimental manipulations. First, we tested a new paradigm which involves IV self-administration of both nicotine and ethanol, separately and together, and permits us to examine changes in their co-use while avoiding certain aversive properties and interactions associated with oral self-administration of ethanol as well as nicotine (Gyekis et al., 2012; Kiefer & Dopp, 1989; Oliveira-Maia et al., 2009; Thuerauf, Kaegler, Renner, Barocka, & Kobal, 2000). Second, with this paradigm that allowed significant simultaneous IV self-administration, we then examined the effect of maternal consumption of a fat-rich diet, during gestation and lactation, on different aspects of nicotine/ethanol self-administration behavior in the offspring. These include the self-administration of nicotine alone as revealed by fixed-ratio (FR) testing, the self-administration of nicotine/ethanol solution as revealed by FR testing, dose-response and breakpoint analysis using progressive ratio testing, and then the self-administration of ethanol alone after removal of nicotine. With these measures and the use of a paradigm that involves simultaneous IV self-administration of both nicotine and ethanol, this study was designed to test the specific hypothesis that vulnerability of the offspring to excessive co-use of nicotine and ethanol is increased by perinatal exposure to dietary fat.
Materials and methods
Animals
Timed-pregnant, Long-Evans rats (220–240 g) from Harlan Laboratories (Indianapolis, IN, USA) were delivered to the animal facility on embryonic day 4 (E4). The dams were individually housed in plastic cages in a fully accredited AAALAC facility (22 °C, with a 12:12 h light-dark cycle, with lights off at noon), according to institutionally approved protocols as specified in the NIH Guide to the Care and Use of Animals and also with approval of the Rockefeller University Animal Care and Use Committee. The rats were maintained ad libitum from E5 on either a fat-rich diet with 50% fat or a standard, low-fat chow diet, with the fat-rich diet group having lab chow available for three additional days (until E8) as the dams became fully adapted to the mixed fat-rich diet. Over the course of the experiments, food intake was measured daily during gestation and two times per week during lactation, and the body weights of dams and pups were recorded weekly. While the dams maintained on a fat-rich diet consumed more calories than the chow dams (90.1 ± 3.2 vs. 79.1 ± 1.9 kcal, p < 0.05), the groups were similar in their measures of daily weight gain (25.1 ± 4.5 vs. 23.7 ± 3.9 g, ns). Also, the fat-exposed and chow litters were similar in their size (10.3 ± 1.7 vs. 10.9 ± 1.3), body weight at birth (9.4 ± 0.2 vs. 9.7 ± 0.5 g), and female/male ratio (7:9 vs. 5:8), with no spontaneous abortions observed in either diet group. On postnatal day 1 (P1), the litters were culled to n = 8, primarily by eliminating the females. At weaning (P22), all male rats were pair-housed and switched to ad libitum lab chow diet for the duration of the experiment. They were single-housed following jugular catheterization surgery, as described below.
Diets
During the experimental period, the rats were maintained ad libitum either on standard rodent chow (13.2% fat, 3.4 kcal/g; PicoLab Rodent Diet 20 5053, Lab Diet, St. Louis, MO, USA) or the fat-rich diet (50% fat, 5.2 kcal/g) as described in prior publications (Dourmashkin et al., 2006; Leibowitz et al., 2004). Specifically, this fat-rich diet consisted of: fat from 75% lard (Armour, Omaha, NE, USA) and 25% vegetable oil (Wesson vegetable oil, Omaha, NE, USA); carbohydrate from 30% dextrin, 30% cornstarch (ICN Pharmaceuticals, Costa Mesa, CA, USA), and 40% sucrose (Domino, Yonkers, NY, USA); and protein from casein (Bioserv, Frenchtown, NJ, USA) with 0.03% L-cysteine hydrochloride added (ICN Pharmaceuticals). This diet was supplemented with minerals (USP XIV Salt Mixture Briggs; ICN Pharmaceuticals) and vitamins (Vitamin Diet Fortification Mixture; ICN Pharmaceuticals). The macronutrient composition of this semi-solid fat diet, calculated as percentage of total kilocalories, was 50% fat, 25% carbohydrate, and 25% protein. It was stored at 4 °C until use, and on a daily basis, fresh diet was weighed out in metal dishes and placed in the appropriate cages. This fat-rich diet is nutritionally complete and found to have no detrimental effects on the health of the animals.
Apparatus
Nicotine and ethanol self-administration testing was performed in 10 standard operant-conditioning chambers (Coulbourn Instruments, Allentown, PA, USA). Each chamber was equipped with two levers located 10 cm above the floor, with one lever defined as active and able to stimulate the infusion pump for 4 s and the other lever defined as inactive and having no scheduled consequences. The chambers also contained a LED house light placed on the wall opposite to the levers, a triple-cue light placed directly above the active lever, and a tone cue (4 kHz, 86 dB @ 10 cm) placed outside the chamber. The catheters were attached to an infusion pump (Harvard Apparatus, Natick, MA, USA) through a swivel system and protective metal spring tether. The operant chambers were controlled by a computer using the Graphic State Software package.
Jugular catheterization surgery
Following successful sucrose training (see Experimental Procedures below), the rats (P52–P55) were implanted with a jugular catheter using aseptic techniques. They were anesthetized with a combination of ketamine (80 mg/kg, intra-peritoneally [i.p.]) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary, and were prepared surgically with SILASTIC catheters in the right jugular vein as previously published (Corrigall & Coen, 1989; Morganstern et al., 2013). The catheter exited in the intrascapular region and was connected to a 22-gauge cannula attached to a mesh piece, which was implanted subcutaneously (s.c.) to hold the catheter in place. Immediately after surgery, the animals were given 0.03 mg/kg of the pain medication buprenorphine (s.c.) and 5 mg/kg of the antibiotic baytril (IV) and were then allowed 7–10 days to recover. The catheters were flushed daily with 0.9% saline containing heparin (50 units) and baytril antibiotic (2.27 mg/kg) and were then locked with a solution of 50% dextrose containing heparin (200 units).
Drugs
(−)Nicotine tartrate (Sigma-Aldrich Corp., St. Louis, MO, USA) was dissolved in isotonic saline, and the pH was adjusted to 7.0 with dilute NaOH. The unit dose for the intravenous nicotine self-administration (100 µL over 4 s) was 0.03 mg/kg (expressed as free base). For the nicotine/ethanol mixture, we first made the appropriate ethanol solution to be administered at 100 µL over 4 s at 0.02, 0.03, 0.04, or 0.05 g/kg/infusion and then added (−)nicotine tartrate, with the final dose of nicotine being 0.03 mg/kg/infusion.
Experimental Procedures
Acquisition of self-administration of nicotine
Prior to surgery, the fat- and chow-exposed offspring (n = 17/group) underwent operant training (from ages P42 to P50) for 45 mg sucrose pellets (Bioserv, Frenchtown, NJ, USA). Briefly, rats were food-deprived overnight and then restricted to 20 g of daily chow, given immediately after the training session, for the remainder of the sucrose training period, during which no visual stimuli were presented. Measurements of daily chow intake showed that the rats on most days consumed essentially all of the 20 g of chow provided. All rats were first trained on an FR1 reinforcement schedule, and once the rats earned 100 pellets within an hour, they were switched to an FR2 schedule and then to an FR3 schedule until they were able to earn 100 rewards under each schedule of reinforcement. The fat- and chow-exposed rats exhibited no measured differences in their responding during the sucrose-training period [active lever presses: FR1 (25 ± 3.8 vs. 21 ± 4.1), FR2 (124 ± 14.1 vs. 139 ± 14.7)], and FR3 (306 ± 31.4 vs. 321 ± 39.3] or in the number of days taken to progress from FR1 to FR2 schedules (6.4 ± 1.4 vs. 7.7 ± 2.8) or from FR2 to FR3 schedules (1.1 ± 0.2 vs. 1.3 ± 0.2). Following successful training using this protocol, the rats (from ages P52 to P55) were surgically implanted with a jugular catheter and allowed to recover for 7–8 days prior to the start of the experiment. During the acquisition phase, animals were trained for 1 h each day during the dark cycle to self-administer nicotine (0.030 mg/kg/infusion over 4 s in a volume of 100 µL). The training dose of 0.03 mg/kg/infusion was chosen based on published evidence (Donny, Caggiula, Knopf, & Brown, 1995; Paterson & Markou, 2004; Suto, Austin, & Vezina, 2001) showing that the peak rates of responding and the number of infusions on a fixed-ratio schedule are typically obtained at doses ranging from 0.01–0.03 mg/kg/infusion. The 4-s duration was chosen based on published evidence (Fowler & Kenny, 2011; Morganstern et al., 2013) suggesting that 3–4-s infusion times yield optimal responding for nicotine in adult rats and more closely mimic natural smoking conditions which entail a gradual transit of nicotine from the lungs to the brain (Sorge & Clarke, 2009). As described in our recently published paper (Morganstern et al., 2013), the animals at the beginning of the session received two infusions of nicotine to fill the lines with 200 µL nicotine solution with the house light on, and afterward the house light remained off throughout the training session. This volume of nicotine was precisely calculated based on the length of the catheter and connecting tubing to be filled without exposing the rats to the nicotine. Each active lever press simultaneously activated the cue light (4 s) and tone cue (1 s) as well as the infusion pump. After the 4-s infusion, the cue light remained on for an additional 20 s, which was defined as the time-out period during which responses were recorded but had no scheduled consequences as previously described (Clemens, Caillé, & Cador, 2010; Morganstern et al., 2013; Yan et al., 2012). After the time-out period, the cue light was turned off. During the first part of acquisition training (10 days), responding was reinforced on an FR1 schedule (one infusion for each active press), with the reinforcement schedule subsequently increasing to FR2 for 4 days and then to FR3 for 3 days.
Acquisition of self-administration of nicotine/ethanol solution
After nicotine training was completed on the FR3 schedule, the reinforcement schedule was returned to FR1, and a mixture of nicotine and ethanol was offered, respectively, at a dose of 0.03 mg/kg/infusion and 0.04 g/kg/infusion and administered over 4 s in a volume of 100 µL as described above for nicotine alone. The rats were again trained under this dose of drug combination on the FR1 schedule (4 days), with the reinforcement schedule increasing to FR2 for 3 days and then to FR3 for 4 days.
Dose-response testing
After the last session on the FR3 schedule, a dose-response curve was determined on the FR1 schedule of reinforcement using a single dose of nicotine (0.03 mg/kg/infusion) (Guillem & Peoples, 2011) and the four following doses of ethanol, 0.02, 0.03, 0.04, and then 0.05 g/kg/infusion (DeNoble, Mele, & Porter, 1985; Ikegami et al., 2002). There were 4 sessions at each dose and a 1-day return to the training dose (0.04 g/kg/infusion) before each new dose of ethanol.
Progressive ratio testing
After these dose-response measures were collected, all animals were returned to the training dose (0.03 mg/kg/infusion of nicotine and 0.04 g/kg/infusion of ethanol) for 5 days prior to progressive ratio (PR) testing. The PR testing was conducted over a 4-day period, according to procedures previously described (Caille, Clemens, Stinus, & Cador, 2012). The PR schedule was determined using the exponential formula 5Xexp (0.2 × infusion), such that the required responses per infusion were as follows: 3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, and so forth. The PR conditions were identical to the FR sessions, except that the duration of each PR session was extended to 2 h. Breakpoint was determined when >20 min of inactivity on the active lever passed, according to procedures described in prior studies of nicotine self-administration (Brunzell & McIntosh, 2012; Forget, Coen, & Le Foll, 2009; Garcia, Lê, & Tyndale, 2014; Morganstern et al., 2013). During PR testing, all animals reached breakpoint within the 2-h session and thus were all included in the data analysis.
Self-administration of ethanol after removing nicotine from the solution
After completion of the PR training, we next determined whether the rats lever-pressed for ethanol alone once the nicotine had been removed from the infusion. Rats were returned to FR1 and allowed to lever-press for ethanol only (0.04 g/kg/infusion). They were maintained on the FR1, FR2, and FR3 schedules, with 12 days for each FR schedule.
Acquisition of ethanol self-administration in nicotine-naïve rats
To examine the acquisition of ethanol self-administration alone in rats that had never been exposed to nicotine or ethanol, a separate set of rats (n = 12) was treated as described above, first trained to lever-press for sucrose and then surgically implanted with a jugular catheter and allowed to recover for 7–8 days. The rats were then trained to lever-press for ethanol using the method identical to that described above for nicotine acquisition on the FR1 (12 days) and FR2 (12 days) schedules.
Blood collection and measurements of blood alcohol concentration
Blood was collected from the tail vein on the 4th day of nicotine/ethanol self-administration on FR3 schedule of reinforcement. Blood serum was assayed for blood ethanol concentration (BEC) using an Analox GM8 Alcohol Analyzer (Lunenberg, MA, USA).
Statistical analysis
Differences in the effects of perinatal diet on drug self-administration at different FR schedules and differences in response to nicotine/ethanol vs. nicotine alone within each diet group were tested with either a repeated-measures ANOVA (when FR was a within-subject factor) followed up by pairwise comparisons using Tukey’s HSD or a one-way ANOVA followed by Tukey’s post hoc test as appropriate. For progressive ratio testing, measures of the number of rewards earned and breakpoint (last ratio completed) were analyzed separately using unpaired Student’s t tests (p < 0.05). Data were determined to be distributed normally using the Shapiro-Wilk test. Significance was determined at p < 0.05, and data are reported as mean ± standard error of the mean (S.E.M.).
Results
Co-administration of nicotine and ethanol
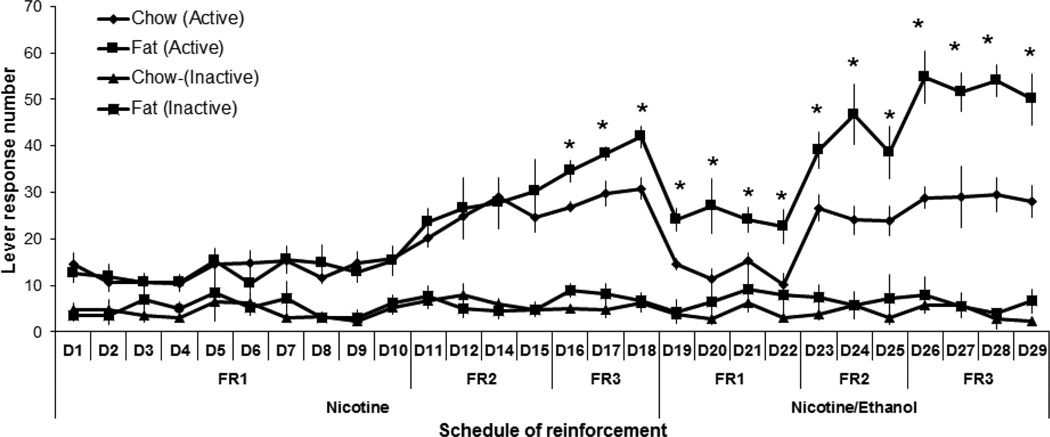
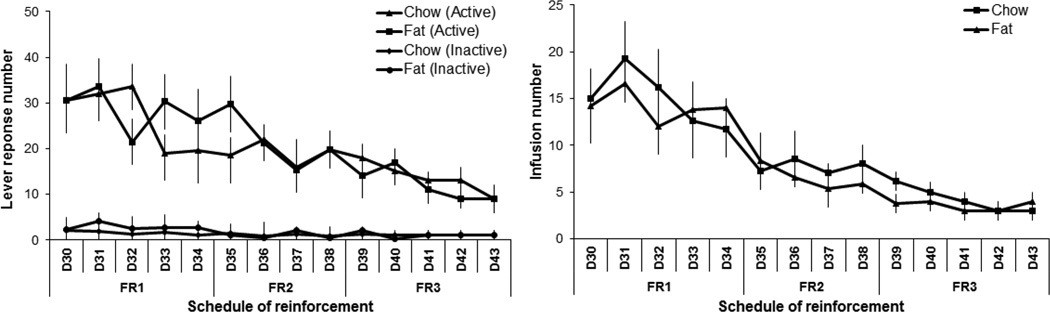
This experiment tested whether the offspring from Long-Evans dams that consumed a fat-rich vs. chow diet during pregnancy and lactation exhibit differences, first in their self-administration of nicotine and then in their co-administration of nicotine and ethanol. Analysis of nicotine self-administration by itself (0.03 mg/kg/infusion) revealed an overall main effect (with all FR schedules combined) of perinatal fat vs. chow exposure on active lever pressing (F[1,18] = 11.82, p < 0.01), with no effect on the overall number of infusions earned (F[1,21] = 1.40, ns) or inactive lever pressing (F[1,18] = 1.42, ns) (Fig. 1 and 2). There was a significant main effect of the schedule of reinforcement on active lever pressing (F[3,54] = 44.83, p < 0.01) and the number of infusions earned (F[3,63] = 7.48, p < 0.01), with a significant interaction between perinatal treatment and these measures of lever pressing (F[3,54] = 7.06, p < 0.01) and number of infusions earned (F[3,63] = 7.47, p < 0.01). Specifically, active lever pressing and infusion number were significantly increased in the fat- compared to chow-exposed offspring on the FR3 schedule (+31%, p < 0.02 and +30%, p < 0.05, respectively), but not the FR1 or FR2 schedule of reinforcement (ns). Analysis of the lever pressing for nicotine in the chow group across the FR1, FR2, and FR3 schedules of reinforcement revealed that responding for nicotine during FR2 and FR3 was significantly greater than during FR1 (p < 0.01) and that lever pressing during the FR2 and FR3 schedules did not differ from each other (ns). The significant linear trend for within-subject contrasts (F[1,12] = 32.10, p < 0.01) indicates that responding on each FR schedule becomes progressively greater when compared to the preceding one, as described previously (Morganstern et al., 2013).
Fig. 1.
Nicotine and nicotine/ethanol lever response in perinatal fat-exposed vs. chow-fed rats. Lever responses to nicotine (0.03 mg/kg/infusion) or nicotine/ethanol (0.03 mg/kg/0.04 g/kg/infusion) increase during the first 29 days under FR1, FR2, and FR3 reinforcement schedules in fat-exposed vs. chow-exposed offspring (n = 17/group). *p < 0.05
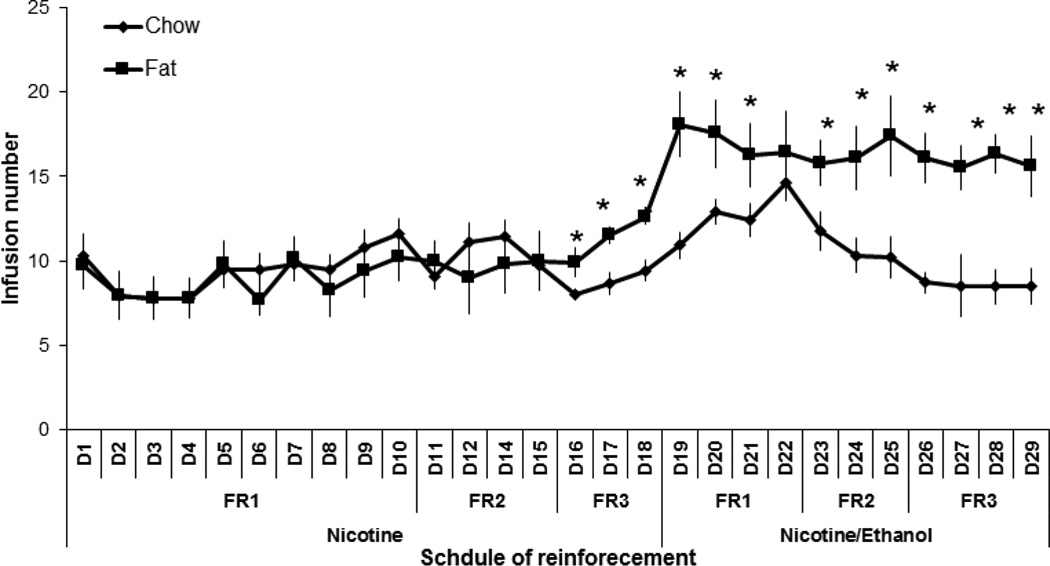
Fig. 2.
Nicotine and nicotine/ethanol infusion number in perinatal fat vs. chow-fed rats. Infusion number of nicotine (0.03 mg/kg/infusion) or nicotine/ethanol (0.03 mg/kg/0.04 g/kg/infusion) increases during the first 29 days under FR1, FR2, and FR3 reinforcement schedules in fat-exposed vs. chow-fed offspring (n = 12/group). *p < 0.05
Analysis of the self-administration of the combined nicotine/ethanol solution, with doses respectively of 0.03 mg/kg/infusion and 0.04 g/kg/infusion, also revealed a significant main effect of perinatal diet treatment on active lever pressing (F[1,17] = 57.95, p < 0.01) and number of infusions (F[1,18] = 16.91, p < 0.01), with no effect on inactive lever pressing (F[1,18] = 0.69, ns) (Fig. 1 and 2). There was also a main effect of schedule of reinforcement on active lever pressing (F[2,34] = 44.83, p < 0.01) and infusion number (F[2,36] = 46.55, p < 0.01), with no significant interaction with perinatal treatment for both of these measures (F[2,34] = 2.29, ns) and (F[2,36] = 1.16, ns), respectively. The fat-exposed offspring had a 35–90% (p < 0.05) higher lever pressing and infusion number than chow-exposed offspring. Together, these data demonstrate that perinatal exposure to fat significantly increases the self-administration not only of nicotine alone but also of nicotine and ethanol mixed together. Self-administration of nicotine/ethanol led to a significant elevation of BEC (p < 0.05) in the fat-exposed (120 ± 10.2 mg/dL) vs. chow-exposed (85 ± 19.3 mg/dL) rats when measured at 30 min after the start of the self-administration session.
Further analyses were performed to compare the lever-pressing behavior of the fat- and chow-exposed offspring on the nicotine/ethanol solution (0.03 mg/kg nicotine/0.04 g/kg ethanol) to that of nicotine alone (0.03 mg/kg). Within-group analysis revealed an overall main effect of perinatal diet treatment on drug self-administration, as measured by the total number of active lever responses (F[1,14] = 19.36, p < 0.01) and infusions earned (F[1,15] = 15.09, p < 0.01), with no difference in inactive lever presses (F[1,14] = 2.31, ns) (Fig. 1 and 2). There was also a significant main effect of the type of drug solution, nicotine/ethanol vs. nicotine, on active lever pressing (F[1,14] = 4.10, p < 0.05), with a significant interaction between perinatal diet treatment and drug solution (F[1,24] = 9.30, p < 0.01). The total number of infusions was also significantly affected by the drug solution (F[1,15] = 15.23, p < 0.01), with a significant interaction again observed between perinatal treatment and drug solution (F[1,15] = 10.01, p < 0.01). Further within-group analyses showed that, while the chow-exposed rats exhibited no difference in their lever pressing for the nicotine/ethanol solution compared to nicotine alone (F[1,14] = 0.74, ns), the fat-exposed offspring exhibited a difference in their lever pressing (F[1,16] = 16.89, p < 0.01), which was significantly greater for the nicotine/ethanol solution than for nicotine alone (+90%, p < 0.01). There was also a main effect of FR reinforcement schedule on active lever pressing (F[2,28] = 48.67, p < 0.01), with no interaction between perinatal diet treatment and reinforcement schedule (F[2,28] = 2.60, ns), but there was no effect of reinforcement schedule on infusion number (F[2,30] = 2.30, ns). These findings demonstrate that perinatal exposure to fat compared to chow, which stimulates the IV self-administration of nicotine as well as of nicotine in combination with ethanol, has a significantly stronger, stimulatory effect on the co-administration of nicotine/ethanol together, a difference not evident in the chow-exposed rats.
Dose-response determination to nicotine/ethanol
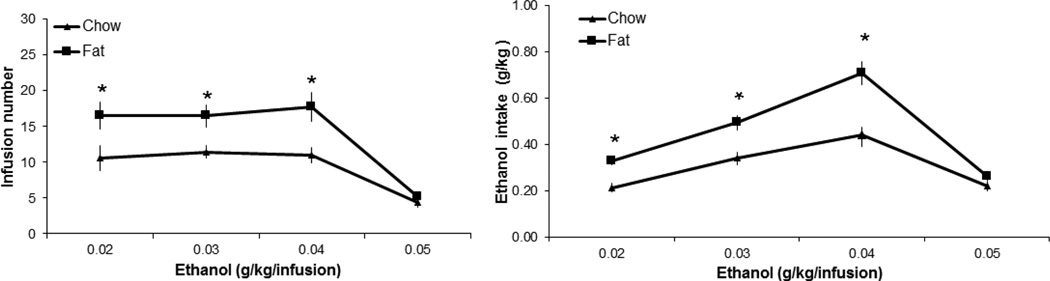
To evaluate the sensitivity of the offspring to ethanol in the nicotine/ethanol solution, this experiment tested increasing doses of ethanol (from 0.2 to 0.5 g/kg/infusion) in combination with a single dose of nicotine (0.03 mg/kg/infusion) and compared the dose-response curves for the fat- and chow-exposed rats. Analysis of infusion number and ethanol intake as the ethanol dose increased revealed in the fat-exposed rats an overall significant increase compared to chow-exposed offspring in both the number of infusions earned (F[1,25] = 24.94, p < 0.01) and the intake of ethanol (F[1,25] = 26.98, p < 0.01) (Fig. 3). In addition, there was a significant main effect of ethanol dose on the number of infusions (F[3,75] = 43.45, p < 0.01), with a significant interaction between ethanol dose and perinatal diet treatment (F[3,75] = 3.39, p = 0.01). Further analyses revealed a significant main effect of ethanol dose on rewards earned in both the fat-exposed (F[3,24] = 24.83, p < 0.01) and chow-exposed (F[3,39] = 18.29, p < 0.01) rats. Pairwise comparisons showed that the number of rewards earned, while similar within each treatment group across the three lower doses of ethanol (0.02, 0.03, 0.04 g/kg/infusion, ns), was markedly reduced (p < 0.01) at the highest ethanol dose (0.05 g/kg/infusion). These results show that, as the dose of ethanol increases to 0.04 g/kg/infusion, the fat-exposed rats continue to self-administer significantly more of the ethanol/nicotine mixture than the chow-exposed rats. This effect, however, is lost at the highest dose, where both groups reduced their intake. These results suggest that the rising concentration of ethanol increases the rewarding potential of the ethanol/nicotine mixture.
Fig. 3.
Dose-response to ethanol in perinatal fat-exposed vs. chow-fed rats self-administering nicotine/ethanol. Rats perinatally exposed to fat vs. chow exhibit greater lever pressing for nicotine/ethanol and greater intake of ethanol as the dose of ethanol increases (0.02–0.04 g/kg/infusion) and nicotine dose stays the same (0.03 mg/kg/infusion) (n = 12/group).*p < 0.05
Progressive ratio responding to nicotine/ethanol
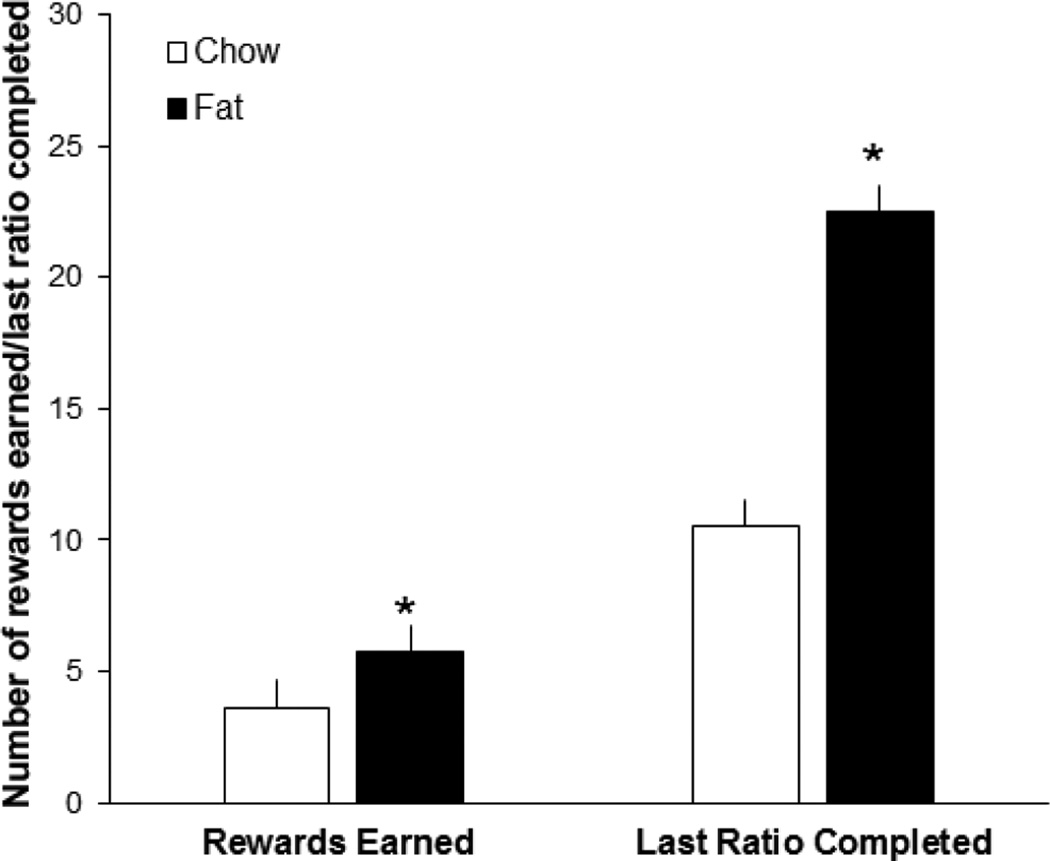
Progressive ratio responding for the nicotine/ethanol solution was also measured in the rats perinatally exposed to the fat or chow diet, to determine whether they exhibit a difference in their reinforcement from this solution. As depicted in Fig. 4, progressive ratio analysis revealed significant differences between the diet groups, with the fat-exposed rats earning significantly more rewards than the chow-exposed rats (t[20] = −2.70, p < 0.05). Further analysis of the breakpoint showed that the fat- vs. chow-exposed rats exhibit a significant increase in the median last ratio completed (t[19] = −4.18, p < 0.05) (Fig. 4). These results, revealing a greater number of rewards earned and greater breakpoints in the fat-exposed offspring, suggest that these rats are more motivated than chow-control rats to attain the nicotine/ethanol solution and thus that they attribute a greater value and reward to this drug mixture.
Fig. 4.
Progressive ratio testing in perinatal fat-exposed vs. chow-fed rats self-administering nicotine/ethanol (0.03 mg/kg/0.04 g/kg/infusion). Fat-exposed compared to chow-fed rats earn more rewards and demonstrate an increase in the median last ratio completed (n = 12/group). *p < 0.05
Ethanol self-administration
The final experiment examined the rat’s lever-pressing behavior for IV ethanol alone, after removal of nicotine, at the FR1, FR2, and FR3 schedules of reinforcement. The overall assessment revealed no differences between the fat- and chow-exposed rats in their number of active presses (F[1,19] = 0.01, ns) and number of infusions (F[1,19] = 0.04, ns), as well as their number of inactive presses (F[1,16] = 0.01, ns) (Fig. 5). While there was a significant main effect of FR reinforcement schedule on active lever presses (F[2,38] = 9.40, p < 0.01), there was no interaction between this measure and perinatal treatment (F[2,38] = 0.58, ns). There was also a significant main effect on the number of infusions (F[2,38] = 21.69, p < 0.01) but no interaction with this measure and perinatal treatment (F[2,38] = 2.34, ns) and no main effect on inactive lever presses (F[2,38] = 1.48, ns). This lack of a difference between the fat- and chow-exposed rats may reflect the fact that the offspring in both groups failed to acquire ethanol self-administration once nicotine was removed from the solution, as indicated by the absence of an increase in the number of lever presses or infusions as the rats proceeded from FR1 to FR2 to FR3 schedules. To determine whether the lack of lever presses for ethanol in these animals was due to the fact that they were examined at the end of the test series involving nicotine as well as ethanol, we examined a separate group of naïve rats (N = 12) with no prior exposure to the drugs and found using our test paradigm that neither fat- nor chow-exposed rats acquired ethanol IV self-administration, showing no escalation in their lever pressing as they advanced from the FR1 (12 ± 2.81) to FR2 (8 ± 1.45) ratio of reinforcement (F[1,110] = 2.66, ns). These findings in both tests demonstrate that rats fail to respond to IV ethanol alone, whether after nicotine has been removed from the nicotine/ethanol mixture or when trained first to administer ethanol with no prior exposure to nicotine.
Fig. 5.
Ethanol self-administration in perinatal fat-exposed vs. chow-fed rats. Both fat-exposed and chow-fed rats fail to acquire ethanol self-administration (0.04 g/kg/infusion) after nicotine is removed from the solution, as demonstrated by a lack of escalation in the number of lever responses and infusions during a 14-day period under the FR1, FR2, and FR3 schedules of reinforcement (n = 11/group). *p < 0.05
Discussion
The results of this study in Long-Evans rats show that maternal consumption of fat during pregnancy and lactation has profound effects on later drug self-administration in adult offspring. It causes an increase in the self-administration not only of nicotine but also of a combination of nicotine and ethanol when the two drugs are presented together. It also increases responding for nicotine/ethanol during dose-response testing and leads to greater motivation for nicotine/ethanol as demonstrated by a higher breakpoint using progressive ratio testing. Of particular note is the additional finding that maternal fat consumption causes the offspring to self-administer significantly more nicotine plus ethanol than they do nicotine alone, a difference not seen in the offspring of dams maintained on a low-fat, chow diet.
The new model used in the present study provides the first evidence for IV self-administration of nicotine and ethanol mixed together in a single solution. Prior studies developing methods for administering a combination of nicotine and ethanol have involved either oral self-administration of the two drugs separately or together (Hauser, Getachew, et al., 2012; Marshall et al., 2003), IV self-administration of nicotine combined with oral self-administration of ethanol sequentially or concurrently (Hauser, Katner, et al., 2012; Lê et al., 2010, 2014), or the self-infusion of a mixture of nicotine and ethanol directly into the posterior VTA (Truitt et al., 2014). While these paradigms have been successful in getting rats to self-administer both drugs, in some cases at pharmacologically relevant levels (Hauser, Katner, et al., 2012), the results obtained relating these two drugs may in some cases be determined by their specific properties or the specific paradigms of administration. When given orally, both substances have orosensory properties that include a bitter taste, aversive odor, or irritating effect (Gyekis et al., 2012; Oliveira-Maia et al., 2009; Thuerauf et al., 2000) which by themselves may influence drug-taking behavior (Crabbe, Harris, & Koob, 2011; Gyekis et al., 2012; Kulkosky, 1985). Also, a specific paradigm involving sequential administration of the two drugs may yield results reflecting the effect of one drug on intake of the other, rather than the co-use of these drugs. This is suggested by studies showing that the injection of nicotine can stimulate the drinking of ethanol and that rats prone to drinking excess ethanol are subsequently found to self-administer more nicotine (Bito-Onon et al., 2011; Lê et al., 2000, 2003, 2006; Olausson et al., 2001). Our model, which involves the IV method of self-administration of both drugs and their simultaneous administration, eliminates both of these possible confounding factors while showing greater self-administration of the nicotine/ethanol mixture as compared to nicotine or ethanol alone. This result is consistent with a recent report showing that rats self-administer the ethanol/nicotine mixture directly into the posterior VTA (Truitt et al., 2014).
Most studies to date have shown that rats either fail to acquire IV self-administration of ethanol alone (DeNoble et al., 1985; Numan, Naparzewska, & Adler, 1984) or achieve very low levels of daily ethanol intake (Gass & Olive, 2007; Sinden & Le Magnen, 1982), with additional procedures such as a fixed-time food delivery schedule, a forced infusion of high ethanol doses, or a sucrose reinforcer found to potentiate ethanol IV self-administration (Numan, 1981; Oei & Singer, 1979; Windisch, Kosobud, & Czachowski, 2014). The model used in the present study, which allows rats to self-administer IV ethanol and nicotine together, gave us the opportunity to determine whether rats trained to self-administer first nicotine alone and then ethanol in combination with nicotine might then continue to self-administer ethanol alone after nicotine has been removed. Our tests, however, failed to reveal ethanol self-administration in the absence of nicotine. This confirms the general notion that IV administration of ethanol in rats is a relatively weak, positive reinforcer that does not support self-administration behavior (DeNoble et al., 1985; Numan, 1981; Numan et al., 1984; Sinden & Le Magnen, 1982). The only other study that used this paradigm of combining ethanol with another drug of abuse examined IV self-administration of a mixture of ethanol with cocaine (Ikegami et al., 2002). This report showed that rats trained to self-administer the ethanol/cocaine mixture continue to self-administer ethanol alone (up to 2 g/kg/session/day) over a several-week period. This indicates that the combination of ethanol with cocaine, as compared to ethanol with nicotine, may have a more potent and sustaining effect on IV self-administration of ethanol. It is possible, however, that the lack of ethanol self-administration after removal of nicotine may reflect a general decline in behavior across the long series of tests. We examined this possibility in an additional group of rats that were drug-naïve and found using our test paradigm that they similarly failed to acquire IV ethanol self-administration, consistent with the results of other published studies (DeNoble et al., 1985; Grupp, 1981; Sinden & Le Magnen, 1982). With multiple reports showing that rats readily selfadminister ethanol in both oral operant and two-bottle choice paradigms (Simms, Bito-Onon, Chatterjee, & Bartlett, 2010; Simms et al., 2008), this evidence substantiates the general notion that ethanol when administered IV by itself is not reinforcing.
Our results also demonstrate that exposure to dietary fat during gestation and lactation produces significant changes in later drug self-administration behavior. The fat-exposed adult offspring as compared to chow-exposed rats exhibit a general increase in their nicotine responding during the training period. This result substantiates our recent study of nicotine self-administration in Sprague-Dawley rats (Morganstern et al., 2013), and it is also consistent with other reports indicating that early exposure to a palatable, fat-rich diet increases ethanol drinking and enhances sensitivity to the locomotor-stimulating effects of another drug, amphetamine (Bocarsly et al., 2012; Cabanes et al., 2000). While somewhat lower than those described in certain studies (Bardo, Green, Crooks, & Dwoskin, 1999; Lynch, 2009; Sanchez, Moore, Brunzell, & Lynch, 2014), the levels of nicotine IV self-administration obtained in the present study are similar to those in other reports (Cohen, Koob, & George, 2012; Gilpin et al., 2014) as well as our previous publication (Morganstern et al., 2013). Our main new finding is that fat-exposed offspring exhibit increased responding to a mixture of nicotine and ethanol, an effect evident during the training period, the dose-response testing, and the progressive ratio testing. Whereas the baseline responding during progressive ratio testing is similar to that in our prior study (Morganstern et al., 2013), it is somewhat lower than that described in other publications (Lynch, 2009; Shram, Funk, Li, & Lê, 2008), perhaps due to the fact that the rats in our studies underwent almost 50 days of training prior to the progressive ratio test. Although both IV nicotine and IV ethanol are generally thought to be weak reinforcers and may even be aversive (Grupp, 1981; Palmatier et al., 2007; Stairs, Neugebauer, & Bardo, 2010), our findings here showing prenatal fat exposure to stimulate responding for the nicotine/ethanol mixture while increasing the breakpoint values indicate that the nicotine/ethanol mixture is reinforcing and the fat-exposed offspring are more motivated by the rewarding properties of this nicotine/ethanol mixture.
The present study is the first to report BEC levels after IV self-administration of a mixture of ethanol and nicotine. During IV self-administration of nicotine and ethanol together, BEC levels were significantly higher (p < 0.01) in the fat-stimulated (120 ± 10.2 mg/dL) compared to chow (85 ± 9.3 mg/dL) offspring. These BEC levels in the chow group, which exceed those commonly achieved in Sprague Dawley rats (Barson, Fagan, Chang, & Leibowitz, 2013; Bito-Onon et al., 2011), are comparable to those found in selectively bred alcohol-preferring P rats (Bell, Rodd, Lumeng, Murphy, & McBride, 2006). Furthermore, BEC levels in the fat-exposed rats are equivalent to those found after an alcohol binge or during alcohol relapse in the P rats (Bell et al., 2006; Rodd-Henricks et al., 2001). Although nicotine levels were not measured in this study, extrapolation from data obtained in other nicotine self-administration studies (LeSage, Keyler, Collins, & Pentel, 2003; Shoaib & Stolerman, 1999) suggests that blood nicotine levels in the chow group are likely to be around 65 ng/mL, which is comparable to nicotine levels in smokers (Benowitz & Jacob, 1984). A recent study, showing that ethanol can affect nicotine accumulation in the brain, is consistent with the idea that ethanol is involved in nicotine addiction (Katner et al., 2015).
The result obtained using combined IV self-administration of these two drugs provides the first evidence that exposure to fat during an early period of development leads to an increased motivation for the nicotine and ethanol combination. With the rats increasing their responding to the mixture while the ethanol concentration was increased and the nicotine concentration remained constant, this suggests that the presence of ethanol in the nicotine/ethanol mixture is enhancing the rewarding potential of this mixture. This is supported by the evidence that ethanol, at doses up to 2 g/kg/day, exerts a dominant discriminative control over nicotine in an nicotine/ethanol mixture (Ford, McCracken, Davis, Ryabinin, & Grant, 2012). Together, this supports other studies showing frequent co-abuse of these two drugs in humans as well as animals (DiFranza & Guerrera, 1990; Falk et al., 2006; Hauser, Katner et al., 2012; Lê et al., 2010, 2014).
Neurochemical systems that mediate this increase in nicotine self-administration in fat-exposed offspring may involve the common brain pathways affected by fat and nicotine intake in adult animals as well as by prenatal exposure to fat. These include the hypothalamic and mesolimbic systems involving dopamine (DA) and acetylcholine (ACh), the opioid peptide enkephalin (ENK), and the orexigenic peptides, orexin/hypocretin (OX), galanin (GAL) and melanin-concentrating hormone (MCH) (Barson, Morganstern, & Leibowitz, 2011, 2012; Chang, Gaysinskaya, Karatayev, & Leibowitz, 2008; Morganstern et al., 2013; Picciotto, Higley, & Mineur, 2012; Vucetic, Kimmel, Totoki, Hollenbeck, & Reyes, 2010). Prenatal exposure to fat is found to stimulate the expression of these peptides in the hypothalamus and nucleus accumbens (Chang et al., 2008; Vucetic et al., 2010) and produce changes in the mesolimbic dopaminergic reward system (Naef et al., 2011; Ong & Muhlhausler, 2011; Vucetic et al., 2010) and in cholinergic activity in mesostriatal and hypothalamic areas (Morganstern et al., 2013). The possibility that these neurochemical changes in the offspring can, in turn, promote later consumption of nicotine is supported by pharmacological evidence in rats, showing that nicotine self-administration is reduced by hypothalamic administration of an OX receptor 1 antagonist or peripheral administration of an opioid antagonist (Ismayilova & Shoaib, 2010; LeSage, Perry, Kotz, Shelley, & Corrigall, 2010), by dopaminergic blockade through peripheral injection of receptor antagonists or lesions in the mesolimbic dopaminergic system (Corrigall & Coen, 1991; Corrigall, Franklin, Coen, & Clarke, 1992; Kutlu et al., 2013), and by systemic injection of a specific α7 nAChR antagonist, methyllycaconitine (Markou & Paterson, 2001). With additional evidence showing that nicotine injection can stimulate these orexigenic peptides and the dopaminergic and cholinergic systems (Houdi, Dasgupta, & Kindy, 1998; Loughlin et al., 2006; Rada, Jensen, & Hoebel, 2001), it is likely that nicotine self-administration and these neurochemicals function within a positive feedback loop. In addition to having direct effects on nicotine self-administration, these neurochemical systems are also involved in stimulating emotional behaviors, including anxiety and locomotor activity (Bilbo & Tsang, 2010; Kang, Kurti, Fair, & Fryer, 2014; Raygada, Cho, & Hilakivi-Clarke, 1998), which are closely associated with an increased propensity for drug abuse (Kabbaj, 2006; Piazza, Deminiere, Le Moal, & Simon, 1989). Thus, when stimulated by perinatal exposure to fat, these different brain systems may be involved in promoting IV nicotine self-administration.
The same neurochemical systems involved in increasing nicotine self-administration may also have a role in stimulating the consumption of ethanol. Similar to nicotine, ethanol drinking is increased by hypothalamic administration of OX (Lawrence, Cowen, Yang, Chen, & Oldfield, 2006; Schneider, Rada, Darby, Leibowitz, & Hoebel, 2007) and MCH (Duncan, Proulx, & Woods, 2005; Morganstern et al., 2010), by an ENK analogue in the hypothalamus, accumbens, and VTA (Morganstern, Barson, & Leibowitz, 2011), or by central cholinergic stimulation (Hauser et al., 2014). In addition, it is reduced by inactivation of DA receptors in the accumbens and by peripheral administration of a DA receptor antagonist (Bahi & Dreyer, 2012; Di Chiara & Imperato, 1988). The most significant finding in the present study is that offspring perinatally exposed to a fat-rich diet self-administer significantly more of the nicotine plus ethanol mixture than they do of nicotine alone, a difference not evident in chow-fed offspring that lever-pressed similarly for both nicotine and nicotine/ethanol. This evidence suggests that exposure to fat may affect the rewarding properties of the solution containing nicotine and ethanol together, leading these two drugs to interact in some manner that enhances their combined rewarding effects. While the different peptide systems may be involved, the strongest available evidence is provided by studies of the mesolimbic dopaminergic system showing nicotine and ethanol to have both additive and synergistic effects on mesocorticolimbic DA signaling. This has been revealed using systemic administration of nicotine plus accumbal administration of ethanol, which together increase DA levels in the accumbens to a greater extent than administration of either drug alone (Tizabi, Bai, Copeland, & Taylor, 2007; Tizabi, Copeland, Louis, & Taylor, 2002). Nicotine and ethanol are also found to act synergistically when administered directly into the posterior VTA, where the mixture of these two drugs as compared to either alone produces a greater change in gene expression in mesolimbic DA neurons (Truitt et al., 2014). Further, pretreatment with nicotine in the VTA increases the stimulatory effect of ethanol on DA, indicating that repeated exposure to nicotine stimulates the responsiveness of DA neurons to ethanol (Ding et al., 2012). These findings suggest that rats exposed to dietary fat early in life, which are found here to be more behaviorally sensitive to the combined effects of nicotine and ethanol than they are to either drug alone, are likely to experience a greater activation of the dopaminergic system that increases the rewarding properties of the drug combination. This fat-induced effect, possibly mediated by DA, may help to explain the proposal (WHO, 2003) that an increased availability of fat-rich diets in recent decades may be a factor that contributes to the co-use/abuse of ethanol and nicotine.
Acknowledgments
The authors would like to thank Dr. Jessica R. Barson for her guidance with the statistical analysis and manuscript preparation. This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number 1R21 AA020593-01A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology (Berl) 2012;222:141–153. doi: 10.1007/s00213-011-2630-8. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug and Alcohol Dependence. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Barson JR, Fagan SE, Chang GQ, Leibowitz SF. Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers. Alcoholism: Clinical and Experimental Research. 2013;37(Suppl 1):E141–E151. doi: 10.1111/j.1530-0277.2012.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Karatayev O, Chang GQ, Johnson DF, Bocarsly ME, Hoebel BG, et al. Positive relationship between dietary fat, ethanol intake, triglycerides, and hypothalamic peptides: counteraction by lipid-lowering drugs. Alcohol. 2009;43:433–441. doi: 10.1016/j.alcohol.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiology & Behavior. 2011;104:128–137. doi: 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Neurobiology of consummatory behavior: mechanisms underlying overeating and drug use. ILAR Journal. 2012;53:35–58. doi: 10.1093/ilar.53.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., 3rd Daily intake of nicotine during cigarette smoking. Clinical Pharmacology and Therapeutics. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB Journal. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addiction Biology. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly ME, Barson JR, Hauca JM, Hoebel BG, Leibowitz SF, Avena NM. Effects of perinatal exposure to palatable diets on body weight and sensitivity to drugs of abuse in rats. Physiology & Behavior. 2012;107:568–575. doi: 10.1016/j.physbeh.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabanes A, de Assis S, Gustafsson JA, Hilakivi-Clarke L. Maternal high n-6 polyunsaturated fatty acid intake during pregnancy increases voluntary alcohol intake and hypothalamic estrogen receptor alpha and beta levels among female offspring. Developmental Neuroscience. 2000;22:488–493. doi: 10.1159/000017480. [DOI] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods in Molecular Biology. 2012;829:243–256. doi: 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- Carrillo CA, Leibowitz SF, Karatayev O, Hoebel BG. A high-fat meal or injection of lipids stimulates ethanol intake. Alcohol. 2004;34:197–202. doi: 10.1016/j.alcohol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. The Journal of Neuroscience. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Caillé S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37:2153–2160. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallongeville J, Marécaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. The Journal of Nutrition. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC, Porter JH. Intravenous self-administration of pentobarbital and ethanol in rats. Pharmacology, Biochemistry, and Behavior. 1985;23:759–763. doi: 10.1016/0091-3057(85)90068-1. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–525. [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. Journal of Studies on Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Katner SN, Rodd ZA, Truitt W, Hauser SR, Deehan GA, Jr, et al. Repeated exposure of the posterior ventral tegmental area to nicotine increases the sensitivity of local dopamine neurons to the stimulating effects of ethanol. Alcohol. 2012;46:217–223. doi: 10.1016/j.alcohol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, Leibowitz SF. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiology & Behavior. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcoholism: Clinical and Experimental Research. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi HY, Hiller-Sturmhofel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fisher JO, Birch LL. Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. Journal of the American Dietetic Association. 1995;95:759–764. doi: 10.1016/S0002-8223(95)00212-X. [DOI] [PubMed] [Google Scholar]
- Ford MM, McCracken AD, Davis NL, Ryabinin AE, Grant KA. Discrimination of ethanol-nicotine drug mixtures in mice: dual interactive mechanisms of overshadowing and potentiation. Psychopharmacology (Berl) 2012;224:537–548. doi: 10.1007/s00213-012-2781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–624. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia KL, Lê AD, Tyndale RF. Effect of food training and training dose on nicotine self-administration in rats. Behavioural Brain Research. 2014;274:10–18. doi: 10.1016/j.bbr.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Reinstatement of ethanol-seeking behavior following intravenous self-administration in Wistar rats. Alcoholism: Clinical and Experimental Research. 2007;31:1441–1445. doi: 10.1111/j.1530-0277.2007.00480.x. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Whitaker AM, Baynes B, Abdel AY, Weil MT, George O. Nicotine vapour inhalation escalates nicotine self-administration. Addiction Biology. 2014;19:587–592. doi: 10.1111/adb.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grupp LA. Ethanol as the negative reinforcer in an active avoidance paradigm. Progress in Neuropsychopharmacology. 1981;5:241–244. doi: 10.1016/0364-7722(81)90075-8. [DOI] [PubMed] [Google Scholar]
- Guillem K, Peoples LL. Acute effects of nicotine amplify accumbal neural responses during nicotine-taking behavior and nicotine-paired environmental cues. PLoS One. 2011;6:e24049. doi: 10.1371/journal.pone.0024049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyekis JP, Dingman MA, Revitsky AR, Bryant BP, Vandenbergh DJ, Frank ME, et al. Gustatory, trigeminal, and olfactory aspects of nicotine intake in three mouse strains. Behavior Genetics. 2012;42:820–829. doi: 10.1007/s10519-012-9546-x. [DOI] [PubMed] [Google Scholar]
- Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA, Jr, Toalston JE, Ding ZM, Truitt WA, et al. Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addiction Biology. 2014;19:800–811. doi: 10.1111/adb.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, et al. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcoholism: Clinical and Experimental Research. 2012;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr, Ding ZM, Toalston JE, Scott BJ, et al. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcoholism: Clinical and Experimental Research. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdi AA, Dasgupta R, Kindy MS. Effect of nicotine use and withdrawal on brain preproenkephalin A mRNA. Brain Research. 1998;799:257–263. doi: 10.1016/s0006-8993(98)00454-5. [DOI] [PubMed] [Google Scholar]
- Hussaini AE, Nicholson LM, Shera D, Stettler N, Kinsman S. Adolescent obesity as a risk factor for high-level nicotine addiction in young women. The Journal of Adolescent Health. 2011;49:511–517. doi: 10.1016/j.jadohealth.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Ikegami A, Olsen CM, Fleming SM, Guerra EE, Bittner MA, Wagner J, et al. Intravenous ethanol/cocaine self-administration initiates high intake of intravenous ethanol alone. Pharmacology, Biochemistry, and Behavior. 2002;72:787–794. doi: 10.1016/s0091-3057(02)00738-4. [DOI] [PubMed] [Google Scholar]
- Ismayilova N, Shoaib M. Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology (Berl) 2010;210:211–220. doi: 10.1007/s00213-010-1845-4. [DOI] [PubMed] [Google Scholar]
- Kabbaj M. Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS & Neurological Disorders Drug Targets. 2006;5:513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- Kandel D, Chen K, Warner LA, Kessler RC, Grant B. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug and Alcohol Dependence. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Kang SS, Kurti A, Fair DA, Fryer JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. Journal of Neuroinflammation. 2014;11:156. doi: 10.1186/s12974-014-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Toalston JE, Smoker MP, Rodd ZA, McBride WJ, Engleman EA. Time-course of extracellular nicotine and cotinine levels in rat brain following administration of nicotine: effects of route and ethanol coadministration. Psychopharmacology (Berl) 2015;232:551–560. doi: 10.1007/s00213-014-3681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse E, Clavel-Chapelon F, Slimani N, van Liere M E3N Group. Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC) The American Journal of Clinical Nutrition. 2001;74:322–327. doi: 10.1093/ajcn/74.3.322. [DOI] [PubMed] [Google Scholar]
- Kiefer SW, Dopp JM. Taste reactivity to alcohol in rats. Behavioral Neuroscience. 1989;103:1318–1326. doi: 10.1037//0735-7044.103.6.1318. [DOI] [PubMed] [Google Scholar]
- Kulkosky PJ. Brain-gut neuropeptides and the limitation of ethanol consumption. Neuroscience and Biobehavioral Reviews. 1985;9:179–190. doi: 10.1016/0149-7634(85)90044-2. [DOI] [PubMed] [Google Scholar]
- Kutlu MG, Burke D, Slade S, Hall BJ, Rose JE, Levin ED. Role of insular cortex D1 and D2 dopamine receptors in nicotine self-administration in rats. Behavioral Brain Research. 2013;256:273–278. doi: 10.1016/j.bbr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neuroscience and Biobehavioral Reviews. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcoholism: Clinical and Experimental Research. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Funk D, Lo S, Coen K. Operant self-administration of alcohol and nicotine in a preclinical model of co-abuse. Psychopharmacology (Berl) 2014;231:4019–4029. doi: 10.1007/s00213-014-3541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. The Journal of Neuroscience. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology (Berl) 2010;208:475–486. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berl) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Dourmashkin JT, Chang GQ, Hill JO, Gayles EC, Fried SK, et al. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Research. 2004;1008:168–178. doi: 10.1016/j.brainres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein SD, Jones BL, O'Brien JW, Zezza N, Stiffler S, Holmes B, et al. Familial risk for alcohol dependence and developmental changes in BMI: the moderating influence of addiction and obesity genes. Pharmacogenomics. 2014;15:1311–1321. doi: 10.2217/pgs.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. The Journal of Comparative Neurology. 2006;49:575–588. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacology, Biochemistry, and Behavior. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine & Tobacco Research. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marshall CE, Dadmarz M, Hofford JM, Gottheil E, Vogel WH. Self-administration of both ethanol and nicotine in rats. Pharmacology. 2003;67:143–149. doi: 10.1159/000067801. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Barson JR, Leibowitz SF. Regulation of drug and palatable food overconsumption by similar peptide systems. Current Drug Abuse Reviews. 2011;4:163–173. doi: 10.2174/1874473711104030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, et al. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiology & Behavior. 2010;101:428–437. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, et al. Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet. Psychopharmacology (Berl) 2013;230:509–524. doi: 10.1007/s00213-013-3178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Ye Z, Liang S, Fagan S, Leibowitz SF. Involvement of cholinergic mechanisms in the behavioral effects of dietary fat consumption. Brain Research. 2012;1470:24–34. doi: 10.1016/j.brainres.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Moquin L, Dal Bo G, Giros B, Gratton A, Walker CD. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Numan R. Multiple exposures to ethanol facilitate intravenous self-administration of ethanol by rats. Pharmacology, Biochemistry, and Behavior. 1981;15:101–108. doi: 10.1016/0091-3057(81)90346-4. [DOI] [PubMed] [Google Scholar]
- Numan R, Naparzewska AM, Adler CM. Absence of reinforcement with low dose intravenous ethanol self-administration in rats. Pharmacology, Biochemistry, and Behavior. 1984;21:609–615. doi: 10.1016/s0091-3057(84)80046-5. [DOI] [PubMed] [Google Scholar]
- Oei TP, Singer G. Effects of a fixed time schedule and body weight on ethanol self-administration. Pharmacology, Biochemistry, and Behavior. 1979;10:767–770. doi: 10.1016/0091-3057(79)90330-7. [DOI] [PubMed] [Google Scholar]
- Olausson P, Ericson M, Löf E, Engel JA, Söderpalm B. Nicotine-induced behavioral disinhibition and ethanol preference correlate after repeated nicotine treatment. European Journal of Pharmacology. 2001;417:117–123. doi: 10.1016/s0014-2999(01)00903-7. [DOI] [PubMed] [Google Scholar]
- Oliveira-Maia AJ, Stapleton-Kotloski JR, Lyall V, Phan TH, Mummalaneni S, Melone P, et al. Nicotine activates TRPM5-dependent and independent taste pathways. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1596–1601. doi: 10.1073/pnas.0810184106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong ZY, Muhlhausler BS. Maternal "junk-food" feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB Journal. 2011;25:2167–2179. doi: 10.1096/fj.10-178392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, et al. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug and Alcohol Dependence. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001;157:105–110. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perry EB, Jr, D'Souza DC, Bufis V, Elander J, Limoncelli D, et al. Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine & Tobacco Research. 2012;14:596–606. doi: 10.1093/ntr/ntr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raygada M, Cho E, Hilakivi-Clarke L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings' aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase C activity. The Journal of Nutrition. 1998;128:2505–2511. doi: 10.1093/jn/128.12.2505. [DOI] [PubMed] [Google Scholar]
- Rizzo TA, Silverman BL, Metzger BE, Cho NH. Behavioral adjustment in children of diabetic mothers. Acta Paediatrica. 1997;86:969–974. doi: 10.1111/j.1651-2227.1997.tb15181.x. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of concurrent access to multiple ethanol concentrations and repeated deprivations on alcohol intake of alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2001;25:1140–1150. [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH, Lynch WJ. Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology (Berl) 2014;231:1753–1762. doi: 10.1007/s00213-013-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone RA, Sansone LA. Obesity and substance misuse: is there a relationship? Innovations in Clinical Neuroscience. 2013;10:30–35. [PMC free article] [PubMed] [Google Scholar]
- Saules KK, Levine MD, Marcus MD, Pomerleau CS. Differences in smoking patterns among women smokers with childhood versus later onset of weight problems. Eating Behaviors. 2007;8:418–422. doi: 10.1016/j.eatbeh.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism: Clinical and Experimental Research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Plasma nicotine and cotinine levels following intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 1999;143:318–321. doi: 10.1007/s002130050954. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008;33:739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcoholism: Clinical and Experimental Research. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden JD, Le Magnen J. Parameters of low-dose ethanol intravenous self-administration in the rat. Pharmacology, Biochemistry, and Behavior. 1982;16:181–183. doi: 10.1016/0091-3057(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Clarke PB. Rats self-administer intravenous nicotine delivered in a novel smokingrelevant procedure: effects of dopamine antagonists. The Journal of Pharmacology and Experimental Therapeutics. 2009;330:633–640. doi: 10.1124/jpet.109.154641. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Bardo MT. Nicotine and cocaine self-administration using a multiple schedule of intravenous drug and sucrose reinforcement in rats. Behavioural Pharmacology. 2010;21:182–193. doi: 10.1097/FBP.0b013e32833a5c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–180. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Thuerauf N, Kaegler M, Renner B, Barocka A, Kobal G. Specific sensory detection, discrimination, and hedonic estimation of nicotine enantiomers in smokers and nonsmokers: are there limitations in replacing the sensory components of nicotine? Journal of Clinical Psychopharmacology. 2000;20:472–478. doi: 10.1097/00004714-200008000-00012. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol and Alcoholism. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2002;26:394–399. [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GA, Jr, Toalston JE, Wilden JA, Bell RL, et al. Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcoholpreferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology (Berl) 2014;232:639–649. doi: 10.1007/s00213-014-3702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases. Geneva: World Health Organization; 2003. [PubMed] [Google Scholar]
- Windisch KA, Kosobud AE, Czachowski CL. Intravenous alcohol self-administration in the P rat. Alcohol. 2014;48:419–425. doi: 10.1016/j.alcohol.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z, et al. Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology. 2012;37:685–696. doi: 10.1038/npp.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]