Abstract
Background
Since 2008, synthetic cannabinoids are major new designer drugs of abuse. They are extensively metabolized and excreted in urine, but limited human metabolism data are available. As there are no reports on the metabolism of RCS-8, a scheduled phenylacetylindole synthetic cannabinoid with an N-cyclohexylethyl moiety, we investigated metabolism of this new designer drug by human hepatocytes and high resolution MS.
Methods
After human hepatocyte incubation with RCS-8, samples were analyzed on a TripleTOF 5600+ mass spectrometer with time-of-flight survey scan and information-dependent acquisition triggered product ion scans. Data mining of the accurate mass full scan and product ion spectra employed different data processing algorithms.
Results and Conclusion
More than 20 RCS-8 metabolites were identified, products of oxidation, demethylation, and glucuronidation. Major metabolites and targets for analytical methods were hydroxyphenyl RCS - 8 glucuronide, a variety of hydroxycyclohexyl-hydroxyphenyl RCS-8 glucuronides, hydroxyphenyl RCS-8, as well as the demethyl-hydroxycyclohexyl RCS-8 glucuronide.
Background
Synthetic cannabinoids are CB1 and/or CB2 receptor ligands mimicking Δ9-tetrahydrocannabinol effects, and are marketed initially as legal alternatives to cannabis [1,2]. Since 2008, these drugs have emerged on the new designer drug market and challenged clinicians, toxicologists, law enforcement officials and legislators - and there is no end to this trend in sight. In the United States whole structural classes and many single compounds are now scheduled as Class I compounds according to the 2012 Synthetic Drug Abuse Prevention Act [3]. Constant scheduling efforts to stem consumption of these not sufficiently tested, arbitrarily dosed and potentially harmful substances led to immediate introduction of new compounds. The pharmacology and toxicology of these drugs are unknown, preventing controlled administration studies in humans. Complicating the problem, synthetic cannabinoids are generally extensively metabolized in the human body, requiring rapid metabolite identification and incorporation into screening assays, as metabolites are usually the only compounds present in urine.
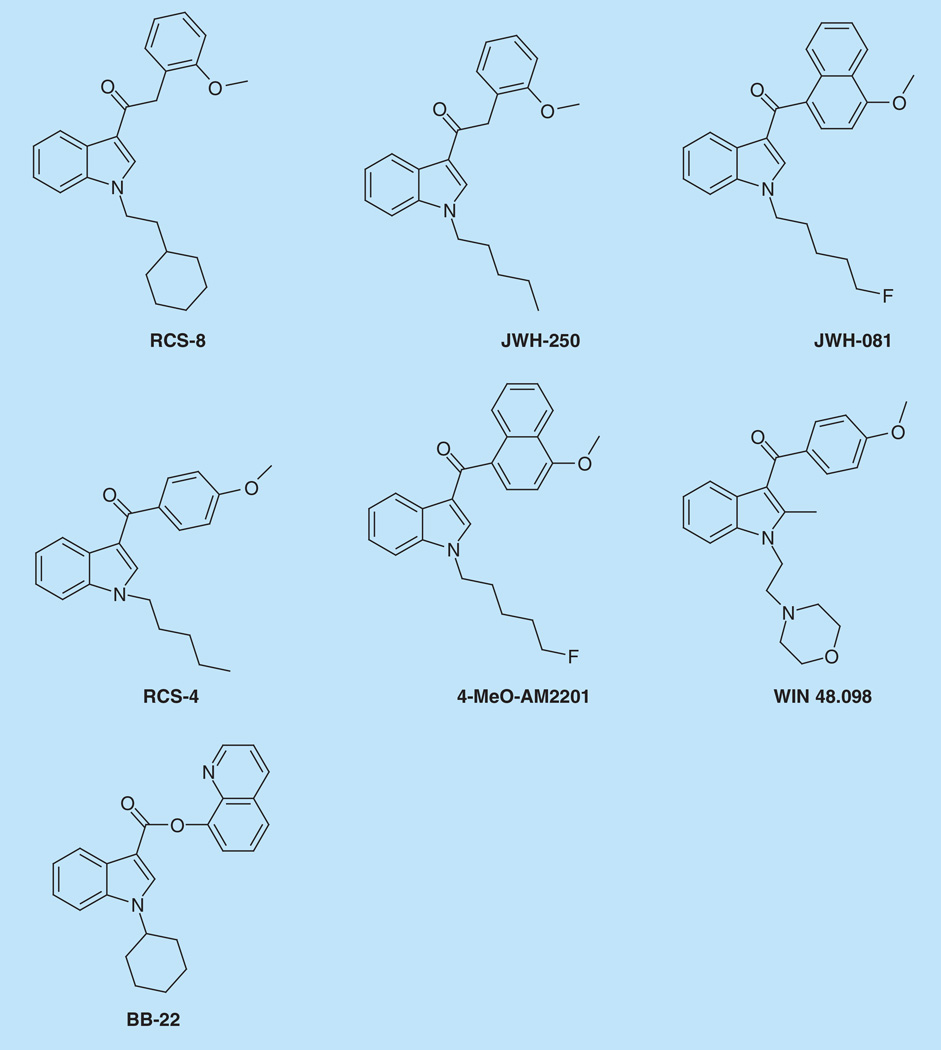
RCS-8 (1-(2-cyclohexylethyl)-3-(2-methoxyphenylacetyl)indole) is a phenylacetylin-dole whose synthesis is unpublished but similar structures were synthesized by Huffman et al. [4]. RCS-8 was identified in the US in 2012 [5,6], and was initially named after the website that sold it, together with RCS-4, a benzoylindole without structural similarity [7]. RCS-8 is more closely related to JWH-250, sharing the phenylacetylin-dole structure and 2′-methoxy group. The methoxy group also is found in JWH-081, 4-methoxy-AM2201, RCS-4, and WIN 48,098 (Figure 1). RCS-8 to date is the only seized synthetic cannabinoid to contain a cyclohexylethyl moiety attached to the indole nitrogen.
Figure 1. Structures of RCS-8, the phenylacetylindole JWH-250, four other methoxy group synthetic cannabinoids JWH-081, RCS-4, 4-methoxy AM2201 and WIN 48,098, and the recently detected BB-22 that has a cyclohexyl ring.
Cannabinoid receptor RCS-8 affinities are unknown; however, Huffmann et al. state that in general, compounds with a 2-substituted phenylacetyl group have good affinity for both CB1 and CB2 receptors [4]. Because some studies demonstrated that the n-pentyl chain provides maximum affinity for CB1 receptors [8], we assume that RCS-8 binds less to this receptor, but it remains unclear to what extent the cyclohexylethyl moiety alters receptor interactions. In internet drug forums, users describe RCS-8 as less potent than JWH-250, lasting twice as long without causing anxiety or paranoia [9].
There are few or no data on these new drugs’ pharmacokinetics. Metabolism studies demonstrate that many synthetic cannabinoids follow analogous pathways [10–14]; however, predictions are not always accurate, making it imperative to find strategies for elucidating major metabolites of new synthetic cannabinoids. A promising in vitro approach for comprehensive determination of human metabolite profiles is combining human hepatocyte incubation, analysis of samples with high-resolution MS (HRMS), and software-assisted data mining. This is routine practice in pharmaceutical research, where an early understanding of human metabolism for a large number of potential drug candidates is needed. Compared to other biological systems, for example, human liver microsomes and in vivo rats, human hepatocytes are better suited for predicting authentic metabolite profiles because they contain human phase I and II enzymes in concentrations that mimic the in vivo liver environment. HRMS offers significantly enhanced specificity over conventional MS. Compared to the common metabolite identification procedure, which consisted of running several experiments on triple quadrupole mass spectrometers including precursor ion, neutral loss and product ion scans, a single injection on a quadrupole-time-of-flight instrument can often identify all relevant expected and unexpected metabolites. Recently developed, user-friendly software also expedites HRMS data mining for identifying metabolites.
It is crucial to target synthetic cannabinoid metabolites in urine, with recent successful elucidation of abused synthetic cannabinoids including JWH-018 [11,13,15–17], JWH-073 [11,15,17,18], JWH-081 [11], JWH-122 [11], JWH-210 [11], JWH-250 [10,11], AM694 [19], AM2201 [14,16,20], RCS-4 [11,12], AB-001 [21] and UR-144 [14,22]. In some of these studies, drug users’ samples were analyzed, less often authors smoked the compounds themselves, others incubated drugs with human liver microsomes or administered drugs to rats. We recently published metabolism studies on AKB48 [23] and XLR-11 [24] after human hepatocyte incubation. However, JWH-250 is the only phenylacetylindole synthetic cannabinoid whose metabolism was investigated to date. Hydroxylation was the predominant metabolic transformation, with either one or multiple hydroxylations at different substructures, that is, on the alkyl side chain, indole moiety or phenyl ring. Dealkylation at the indole nitrogen was observed, whereas no O-demethylated metabolites were detected [10,11]. However, the findings for metabolic reactions on the aromatic methoxy group are not consistent between RCS-4 and JWH-081, two other synthetic cannabinoids carrying this functional group. Hutter et al. did not find O-demethylation for JWH-081 [11], whereas Kavanagh et al. could detect an O-demethylated RCS-4 metabolite [12]. Notably, RCS-8 is the only currently abused synthetic cannabinoid carrying a cyclohexylethyl ring. This is the first study investigating the metabolism of a synthetic cannabinoid with this substructure, which will inform metabolism of other emerging synthetic cannabinoids. Uchiyama recently reported the appearance of a new compound, named QUCHIC or BB-22, which contains a cyclohexylmethyl ring [25].
Methods
Chemicals & reagents
1 mg RCS-8 in powder form was obtained from Cayman Chemicals (Ann Arbor, MI, USA), 10 g of diclofenac in powder form was purchased from Toronto Research Chemicals (Toronto, Canada). LC-MS grade water and formic acid were acquired from Fisher Scientific (Fair Lawn, New Jersey, USA), and LC–MS grade acetonitrile from Sigma Aldrich (St. Louis, Montana, USA).
Incubation with human hepatocytes
RCS-8 was dissolved in methanol and incubated at a final concentration of 10 µmol/l with 1 ml solution containing 1.3 million pooled cryopreserved human hepatocytes from three different donors (purchased from BioreclamationIVT, Westbury, NY, USA) with a viability >80% under constant shaking in an incubator set at 37°C. Samples collected after 0, 1 and 3 h incubation were immediately mixed with an equal volume of acetonitrile to stop the reaction. Diclofenac (10 µmol/l) was incubated along with RCS-8 and analyzed for expected metabolites by HRMS confirming hepatocyte metabolic activity under these conditions. Samples were stored frozen at −80 °C until analysis.
Sample preparation
Samples were clarified via centrifugation at 4°C for 5 min at 15,000 g. The supernatant was diluted 1:4 with mobile phase (0.1% formic acid in water, solvent A; 0.1% formic acid in acetonitrile, solvent B; 50:50, v/v). No further sample preparation was required before injecting 10 µl into the MS system. Three controls (mobile phase, RCS-8 neat standard at 50 ng/ml and the incubation sample at 0 h) also were analyzed. Samples were centrifuged and diluted to reduce signal to avoid saturation of the detector and potentially inaccurate mass measurement. Although no liquid or solid-phase extraction of human hepatocyte samples was performed, we did not experience more contamination of the instrument than usual. Hepatocyte in vitro samples were relatively clean compared with blood and urine samples that contain much more matrix, samples were diluted, and eluent from the beginning of the gradient and during the washing step was also diverted to waste.
Instrumentation
The Shimadzu Prominence HPLC system (Shimadzu Corp, Columbia, Maryland, USA) consisted of two LC-20AD XR pumps, a DGU-20A5R degasser, a SIL-20AC XR autosampler and a CTO-20AC column oven. MS data were acquired on an AB SCIEX Triple TOF 5600+ (AB SCIEX, Redwood City, CA, USA) instrument, which was controlled with AB SCIEX Analyst TF (version 1.6) software. The instrument calibration is maintained with an automated external calibration that was performed every fifth injection via infusion through the Calibrant Delivery System.
Chromatographic conditions
Chromatographic conditions were as follows: column, Kinetex C18 (100 mm × 2.1 mm ID, 2.6 µm) fitted with a KrudKatcher Ultra HPLC in-line cartridge (0.5 µm × 0.1 mm ID, Phenomenex, Torrance, California, USA); oven temperature 40°C; mobile phases, A and B; flow rate 0.3 ml/min; gradient, initial concentration of mobile phase B 10%, held until 0.3 min, increased to 25% at 0.5 min, to 85% at 20.0 min and to 95% at 20.1 min, held until 21.9 min, decreased back to 10% at 22.0 min, re-equilibration for 3.0 min; total run time 25.0 min; autosampler temperature 4°C. The diverter valve switched to MS at 2.0 min and back to waste at 20.0 min.
MS
An information-dependent acquisition (IDA) method was used for identifying RCS-8 metabolites. Mass spectrometric conditions were as follows: interface, positive electrospray ionization (ESI); gas 1 and 2, nitrogen 50 psi; curtain gas, nitrogen 40 psi; source temperature 500°C; ion spray voltage 5500 V; declustering potential 100 V. Conditions for the time-of-flight (TOF) survey scan were: scan range 100–950 Da, accumulation time 0.1 s, collision energy 10 eV. Information-dependent acquisition experiment criteria were: MS/MS monitoring for the four most intense ions exceeding 500 cps; no exclusion of former target ions; mass tolerance 50 mDa; dynamic background subtraction (DBS) on. Based on RCS-8 structure, the following mass defect filtering templates were applied: 375.2198 Da (RCS-8 parent), 551.2519 Da (glucuronidation), 682.3036 Da (glutathione conjugation), 455.1766 Da (sulfatation), 265.1103 Da (dealkylation), 112.1252 Da (N-deal-kylation, loss of C17H13NO2), 345.2093 Da (loss of methoxy group), 361.2042 Da (O-demethylation) and 441.1424 Da (N-dealkylation and glucuronidation); mass tolerance ±40 mDa; width around molecular weight ± 50 Da. Product ion scan conditions were: accumulation time 0.075 s; scan range 60–950 Da; collision energy spread 38 ± 10 eV.
Data processing
Data processing and evaluation of candidates was performed with MetabolitePilot™ (version 1.5, AB SCIEX). Mass defect filter (MDF), neutral loss filter (NLF) and product ion filter (PIF) were used for metabolite search. In addition, a set of theoretically possible biotransformations containing 50 phase I and II reactions was created for extracted ion chromatography analysis of metabolites. Among these biotransformations were oxidation, dioxidation, trioxidation, carboxylation, demethylation, dealkylation, desaturation, glucuronidation, sulfate conjugation and combinations. It should be noted that unexpected metabolites, not generated by any biotransformation prediction, are not missed if their intensity is high enough. In each raw list of potential metabolites the 10 most intense ‘unexpected metabolite’ signals are listed, which are carefully checked and may be identified as real metabolites. Processing parameters were: retention time window 2.0–20.0 min; peak finding algorithms, predicted mass, generic, mass defect, based on characteristic product ions/neutral losses (at least 2); intensity thresholds, XIC peak 1500 cps, TOFMS peak 400 cps, MS/MS peak 100 cps; minimum peak width 2.5 sec. The identified peaks were confirmed as metabolites based on mass accuracy as well as plausible retention time and fragmentation. Based on MS peak areas in the 1 and 3 h sample, the ten most intense metabolites at each time point were determined.
Results
4′-Hydroxydiclofenac and diclofenac acyl β-d-glucuronide metabolites were detected in the diclofenac hepatocyte samples, which served as a positive control for hepatocyte metabolic activity. Accurate full scan and product ion spectra were obtained and thoroughly investigated identifying more than 20 RCS-8 metabolites (Table 1) with mass accuracy <2.9 ppm. Metabolites eluted between 5.45 and 14.58 min with RCS-8 parent at 17.76 min (Figure 2). The chromatographic conditions were adjusted to adequately separate polar metabolites, and also prevent hydrophobic metabolites like the N-oxides from eluting during column washing and re-conditioning. Metabolites resulted from monooxidation (M13, M15, M16, M18 and M20–23), dioxidation (M1, M3, M4, M7, M9, M11, and M14), demethylation (M19), and combinations of these biotransformations (M2, M5, M6, M8, M10, M12 and M17). Most metabolites underwent further glucuronidation (M1–8, M11–13, M15, M16, M19 and M21). Main sites of oxidation were on the cyclohexyl and phenyl ring. Glucuronidation preferably occurred on the aromatic hydroxyl group. No N-dealkylation of the cyclohexylethyl moiety was observed. The major metabolites were found to be hydroxyphenyl RCS-8 glucuronide (M21), a variety of hydroxycyclohexyl-hydroxyphenyl RCS-8 glucuronides (M1, M3, M4, M7 and M11), unconjugated hydroxyphenyl RCS-8 (M18), two demethyl-hydroxycyclohexyl RCS-8 glucuronides (M2, M6) as well as the demethyl RCS-8 glucuronide (M19). RCS-8 peak areas decreased during incubation, while in general, metabolite peak areas increased from 1 h to 3 h incubation. Some phase I metabolites showed decreasing intensities (M14, M17, M18 and M20) or were not found in the 3 h sample (M10, M22 and M23). These findings are consistent with ongoing metabolism of the parent compound to phase I metabolites and subsequent generation of phase II conjugates from phase I metabolites. No metabolites in the T0 sample or atypical biotransformations or sulfate conjugation were observed.
Table 1.
RCS-8 metabolites identified after incubation with human hepatocytes, sorted by retention time. Rank was based on MS peak areas.
| ID | Name | m/z | Formula | Retention time | Characteristic fragments | MS peak area |
Rank |
||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 1 h | 3 h | ||||||
| M1 | Di-Oxidation + Glucuronidation |
584.2493 | C31H37NO10 | 5.45 | 137, 226, 313, 390, 408 | 1.04E+05 | 2.69E+05 | 3 | 1 |
| M2 | O-demethylation + Oxidation +Glucuronidation |
554.2388 | C30H35NO9 | 5.60 | 107, 130, 135, 226, 244, 264, 360, 378 |
4.84E+04 | 1.40E + 05 | 6 | 5 |
| M3 | Di-Oxidation + Glucuronidation |
584.2491 | C31H37NO10 | 5.62 | 137, 226, 313, 390, 408 | 6.21E+04 | 1.46E+05 | 5 | 3 |
| M4 | Di-Oxidation + Glucuronidation |
584.2492 | C31H37NO10 | 5.69 | 137, 226, 313, 390, 408 | 7.22E+04 | 1.43E+05 | 4 | 4 |
| M5 | O-demethylation + Oxidation + Glucuronidation |
554.2384 | C30H35NO9 | 5.82 | 107, 130, 226, 244, 264, 360, 378 |
1.66E + 04 | 4.38E + 04 | 9 | |
| M6 | O-demethylation + Oxidation + Glucuronidation |
554.2385 | C30H35NO9 | 5.98 | 107, 130, 226, 244, 264, 360, 378 |
1.99E + 04 | 3.67E + 04 | ||
| M7 | Di-Oxidation + Glucuronidation |
584.2487 | C31H37NO10 | 6.14 | 137, 226, 313, 390, 408 | 2.49E + 04 | 5.24E+04 | 8 | 8 |
| M8 | O-demethylation + Oxidation + Glucuronidation |
554.2383 | C30H35NO9 | 6.39 | 130, 226, 244, 264, 360, 378 | 6.72E + 03 | 1.34E+04 | ||
| M9 | Di-Oxidation | 408.2173 | C25H29N04 | 6.43 | 137, 226, 270, 390, 408 | 1.74E+04 | 2.71E+04 | ||
| M10 | O-demethylation + Di-Oxidation |
394.2020 | C24H27N04 | 6.48 | 123, 130, 151, 226, 270, 376 | 1.04E + 04 | |||
| M11 | Di-Oxidation + Glucuronidation |
584.2487 | C31H37NO10 | 6.54 | 137, 226, 313, 390, 408 | 2.47E + 04 | 4.35E+04 | 9 | 10 |
| M12 | O-demethylation + Oxidation + Glucuronidation |
554.2381 | C30H35NO9 | 6.76 | 130, 226, 244, 360, 378 | 8.68E+03 | |||
| M13 | Oxidation + Glucuronidation |
568.2540 | C31H37N09 | 7.57 | 121, 226, 374, 392 | 1.14E + 04 | 3.29E + 04 | ||
| M14 | Di-Oxidation | 408.2170 | C25H29N04 | 7.68 | 137, 144, 226, 244, 390 | 2.08E+04 | 7.08E + 03 | ||
| M15 | Oxidation + Glucuronidation |
568.2531 | C31H37N09 | 7.80 | 121, 226, 244, 392 | 1.33E + 04 | 2.96E+04 | ||
| M16 | Oxidation + Glucuronidation |
568.2524 | C31H37N09 | 8.41 | 121, 226, 278, 392 | 9.36E+03 | 1.74E + 04 | ||
| M17 | O-demethylation + Oxidation |
378.2061 | C24H27N03 | 9.07 | 107, 130, 135, 226, 264, 360 | 1.79E + 04 | 4.13E+03 | ||
| M18 | Oxidation | 392.2228 | C25H29N03 | 10.45 | 91, 121, 144, 226, 374 | 1.17E+05 | 5.63E + 04 | 2 | 7 |
| M19 | O-demethylation + Glucuronide Conjugation |
538.2429 | C30H35NO8 | 10.68 | 107, 132, 135, 228, 254, 344, 362 |
4.57E + 04 | 6.26E + 04 | 7 | 6 |
| M20 | Oxidation | 392.2225 | C25H29N03 | 10.78 | 91, 121, 149, 226, 278 | 2.11E + 04 | 6.51E + 03 | 10 | |
| M21 | Oxidation + Glucuronidation |
568.2534 | C31H37N09 | 10.81 | 137, 228, 254, 313, 392 | 1.31E+05 | 1.51E + 05 | 1 | 2 |
| M22 | Oxidation | 392.2227 | C25H29N03 | 12.16 | 91, 121, 130, 226, 278 | 9.95E + 03 | |||
| M23 | Oxidation (at phenyl ring) |
392.2226 | C25H29N03 | 14.58 | 107, 137, 144, 228 | 1.28E + 04 | |||
| Parent | 376.2284 | C25H29N02 | 17.76 | 2.11E+06 | 9.14E+05 | ||||
Figure 2. A combined extracted ion chromatogram of all RCS-8 metabolites found in the 1 h sample.
The metabolites were detected by extracted ion chromatography analysis, mass defect filter, neutral loss filter and/or product ion filter. Metabolite M12 was only detected in the 3 h sample and is given in parentheses at the corresponding retention time.
Discussion
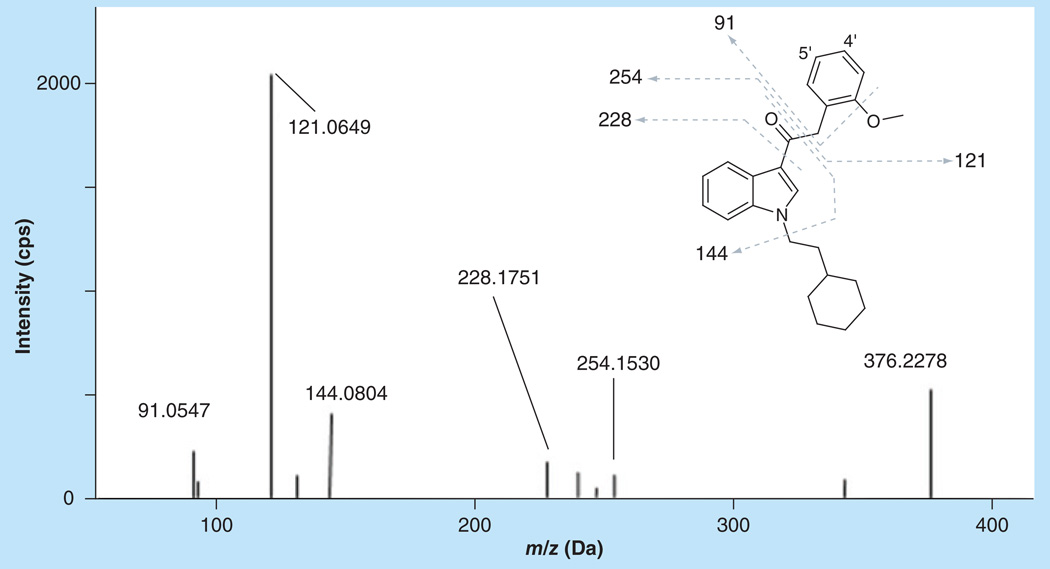
RCS-8 showed a variety of different product ions (Figure 3), with m/z 121 being the most intense fragment ion at the given collision energy conditions. This fragment is generated by cleavage of the bond between the carbonyl group and the α-carbon, corresponding to the methoxy benzyl structure. The less abundant m/z 254 ion is the remaining fragment after breaking the same bond. Fragment m/z 144 is the indole structure with the carbonyl group, which was found for many synthetic cannabinoids with similar structure. The benzyl structure yields m/z 91. Almost all RCS-8 metabolites result from oxidation, dioxidation and demethylation along with oxidation, which can subsequently undergo glucuronidation.
Figure 3. MS/MS spectrum and structure of RCS-8 with assigned fragmentation pattern, labeling of metabolic sites as used throughout the text.
Oxidized & possibly glucuronidated metabolites
We observed eight monooxidized metabolites (M13, M15, M16, M18, M20–23); four underwent further glucuronidation. Figure 4A depicts all metabolites with unconjugated metabolites in grey. Product ion spectra for M18 and M21 are depicted in Figure 5. Early retention times of some of these monohydroxylated metabolites indicated that they might be glucuronides (m/z 568.253) that underwent in-source fragmentation to give a signal at m/z 392.222. However, we did not observe any m/z 568.2538 ions in the TOF MS spectra for M18, M20, M22 and M23 suggesting that in-source glucuronide fragmentation did not occur. Instead, M18 (RT 10.45 min) and M20 (RT 10.78 min) were confirmed as unconjugated monooxidized metabolites, although the earlier retention time compared with M21, which is monooxidized and glucuronidated (RT 10.81 min), is surprising. A possible reason might be different location sites of the hydroxyl group.
Figure 4. Extracted ion chromatograms for the three major groups of RCS-8 metabolites.
Aglycone signals are shown in gray. (A) Oxidation and glucuronide conjugation; (B) dioxidation and glucuronide conjugation; (C) O-demethylation, oxidation and glucuronidation.
*Corresponding product ion spectra are depicted in Figure 5.
Figure 5. Product ion spectra and proposed structures for the ten most abundant RCS-8 metabolites after human hepatocyte incubation after 1 and 3 h of incubation.
The M21 and M23 product ion spectra display a fragment at m/z 137, related to a hydroxylation on the phenyl ring, and the m/z 228 fragment, which confirmed that the indole cyclohexylalkyl substructure remained unchanged. It is unclear where the oxidation occurs. Since both an alkyl and a methoxy group direct secondary substituents towards ortho/para positions, the hydroxyl group is most likely attached to the 4′ or 5′ position that are sterically the most favorable. The remaining six metabolites had m/z 121 and m/z 226 fragments, indicating hydroxylation occurred on the cyclohexylalkyl substructure. Probably, the cyclohexyl ring that is less hindered than the ethyl chain is preferably oxidized. In contrast to aromatic systems, primary substituents at the cyclohexyl ring do not play a key role for steric preferences of secondary substituents. Hence, the different positions are similarly targeted. Based on calculated logP (clogP) values [26], which serve as a guidance for polarity of the molecule, polarity increases in the order ortho < meta < para suggesting the following assignment for the three glucuronidated and oxidized metabolites: M13 (RT 7.57 min, para, clogP 2.78), M15 (RT 7.80 min, meta, clogP 3.02) and M16 (RT 8.41, ortho, clogP 3.07). Similarly, the three unconjugated metabolites would follow the same pattern: M18 (RT 10.45 min, para, clogP 4.61), M20 (RT 10.78 min, meta, clogP 4.85) and M22 (RT 12.16 min, ortho, clogP 4.90). This hypothesis is consistent with M18 being the most intense peak since the para position is the most accessible hydroxylated site. ‘Early-eluting’ conjugated and ‘late-eluting’ unconjugated oxidized metabolites can have overlapping retention times since oxidation at a phenyl ring makes a molecule less polar than oxidation at an aliphatic system.
Dioxidized & possibly glucuronidated metabolites
Seven metabolites were dioxidized (M1, M3, M4, M7, M9, M11 and M14), and five further glucuronidated. Figure 4B shows a chromatogram with unconjugated metabolites depicted in gray, Figure 5 the product ion spectra of M1 and M11. All metabolites showed a fragment at m/z 137 related to oxidation at the phenyl ring and a fragment at m/z 226 associated with oxidation at the cyclohexylalkyl structure (after water loss). All glucuronides gave a product ion at m/z 313, which is indicative for glucuronidation at the oxidized phenyl ring. No further assignment of the position of the hydroxyl groups can be reasonably made. As seen before, retention time ranges of glucuronides and aglycones can overlap, which is the case for M9 (RT 6.43 min, dioxidized metabolite) and M11 (RT 6.54 min, dioxidized and glucuronidated metabolite).
Demethylated & further biotransformed metabolites (oxidation & glucuronidation)
The demethylated metabolite was not found, but several combined metabolites derived from it. We identified eight metabolites that underwent demethylation of the methoxy group, followed by oxidation (M17), dioxidation (M10), glucuronidation (M19) or oxidation plus glucuronidation (M2, M5, M6, M8 and M12). Figure 4C shows the chromatogram for the metabolites of the last group (aglycones are depicted in gray).
M19 is generated by demethylation and glucuronidation producing characteristic fragments at m/z 107 and 135, indicating demethylation, and at m/z 228 and 254 consistent with unchanged cyclohexylalkyl and indole structure. The structure of this metabolite can be unambiguously assigned (Figure 5).
m/z 226 is a characteristic fragment found in the product ion spectra of all oxidized and O-demethylated metabolites (M2, M5, M6, M8, M12 and M17) suggesting oxidation on the cyclohexylalkyl structure. This could be further supported by the presence of other fragments (m/z 107, 130, 135, 244 and 264) when they co-occurred with m/z 226. The product ions at m/z 135 and 107 are related to the demethylated and otherwise unchanged phenyl ring with and without the carbonyl group, respectively. The fragment at m/z 130 is associated with the indole moiety while m/z 264 corresponds to the indole moiety and demethylated phenyl ring. Cleavage of the bond between the carbonyl group and indole structure produces a fragment at m/z 244. The product ion spectra of M2, M5 and M6 are shown in Figure 5.
M10 is a demethylated and dioxidized RCS-8 metabolite. The MS/MS spectrum displays fragment ions at m/z 123 and 151 suggesting that one oxidation occurred on the demethylated phenyl ring as well as m/z 226 that is related to one oxidation at the cyclohexylalkyl structure. This oxidation pattern is consistent with that observed for the other dioxidized metabolites.
Major metabolites
Although matrix effects can affect MS peak areas, we determined the most intense metabolites for each time point. Most likely, these are the major RCS-8 human metabolites. Peak areas and ranks for these metabolites are given in Table 1 while structures, fragmentation pattern and product ion spectra are depicted in Figure 5.
The most dominant metabolite at 1 h and second most dominant after 3 h was hydroxyphenyl RCS-8 glucuronide (M21, RT 10.81 min); hydroxylation on the phenyl ring likely occurred at the 4′ or 5′ position. The most abundant metabolite at 3 h and third most abundant at 1 h was M1 belonging to the most dominant group, the dihydroxy RCS-8 glucuronides (M1, RT 5.45 min; M3, RT 5.62 min; M4, RT 5.69; M7, RT 6.14 min; M11, RT 6.54 min). Within this group the early-eluting metabolites M1, M3 and M4 were the most dominant isomers (Figure 4B). Spectra looked similar showing characteristic fragments at m/z 137, 226 and 313. All metabolites carried a glucuronidated hydroxyl group on the phenyl ring and a hydroxyl group at the cyclohexylalkyl structure. Two examples are depicted in Figure 5. Though exact assignment is impossible with HRMS alone, M1 and M3 hydroxylation likely occurs on the para position of the cyclohexyl ring. Sterically this is the most favorable site producing the most polar, early eluting metabolites. The only major metabolites that are unconjugated are the two hydroxycyclohexyl RCS-8 metabolites M18 (RT 10.45 min) and M20 (RT 10.78 min). Four demethylated metabolites also were highly abundant, three demethyl hydroxycyclohexyl RCS-8 glucuronides (M2, RT 5.60 min; M5, RT 5.82 min; M6, RT 5.98 min), and the demethyl RCS-8 glucuronide (M19, RT 10.68 min). In contrast to M2, M5 and M6, whose oxidation and glucuronidation sites remain unclear, structure assignment for M19 is unambiguous.
Comparison with other synthetic cannabinoids
Although incubation with hepatocytes reflects liver metabolism, it cannot simulate additional processes involved in substance elimination, for example. extrahepatic metabolism, enterohepatic circulation, renal filtration, re-absorption and secretion. Shifts of metabolic patterns due to parent and metabolite concentration changes also are not simulated. Therefore, metabolites found in these experiments may be present in human blood and urine after RCS-8 intake but must be confirmed with authentic specimens.
In general, RCS-8 followed the metabolic pattern previously identified for other synthetic cannabinoids, that is, oxidation(s) followed by glucuronidations and O-demethylation of the methoxy group. The latter finding is in contrast to Grigoryev et al. [10] and Hutter et al. [11] for JWH-250, another phenacetylindole with a methoxy group. While one could argue that Hutter et al. reported only a few major metabolites, Grigoryev identified over 22 compounds, none O-demethylated. Results are inconsistent for other synthetic cannabinoids with a methoxy group.
N-Dealkylation at the indole nitrogen was not observed for RCS-8. This biotransformation seems to occur with alkyl chains, but not with the RCS-8 cyclohexylethyl ring. This hypothesis is supported by studies that investigated the metabolism of JWH-250 [10], RCS-4 [12], JWH-018 [13], UR-144 and AM2201 [14], all of which documented N-dealkylated metabolites.
Future perspective
In 2008, synthetic cannabinoids detection in herbal blends was described in the scientific literature for the first time [27,28]. Over the last 5 years, the number and diversity of available synthetic cannabinoids steadily increased displaying an as yet unknown level of commercialization of new designer drugs. To date, governments are addressing the problem by scheduling synthetic cannabinoids that show high prevalence and produce serious adverse effects, or by scheduling whole structural classes of cannabimimetic agents. However, given that Cannabis is a scheduled substance, that purchase of new, unscheduled synthetic cannabinoids over the internet is possible worldwide and that users are willing to experiment with these compounds, we assume that synthetic cannabinoids abuse will continue. Hence, forensic and clinical laboratories will have to deal with intoxications and the challenges of detecting these substances in biological matrices. In case the classical scheduling procedure is maintained, constantly changing targets can be expected so that rapid identification of newly marketed compounds and their metabolites retains importance. In the past, emergence of new technologies made analyses faster and easier, and with greater sensitivity and automation. HRMS is becoming more available, and offers the advantage of high specificity and retrospective analysis, certainly leading to analytical improvements. Due to the public health threat, combined laboratory efforts might be expected. For instance, databases to quickly monitor new substances or establishing online reference spectra libraries could be possible.
Executive summary.
Introduction
Due to constant changes in designer drug availability, metabolism of newly marketed synthetic cannabinoids must be quickly elucidated to enable major metabolite data in different biological matrices to be rapidly available to federal, state and local laboratories.
Human hepatocyte drug incubation, analysis with high-resolution MS and software-assisted data analysis is a fast and realistic approach for metabolite identification.
Results
We generated a metabolic profile for RCS-8, a synthetic cannabinoid with phenylacetylindole structure and a cyclohexylethyl side chain, and found more than 25 metabolites.
Main biotransformations were monooxidation, dioxidation and demethylation or combination of those, often followed by glucuronidation. No dealkylation, sulfation or atypical biotransformations were observed.
The most intense RCS-8 metabolites were hydroxyphenyl RCS-8 glucuronide, a variety of hydroxycyclohexyl-hydroxyphenyl RCS-8 glucuronides, unconjugated hydroxyphenyl RCS-8, two demethyl-hydroxycyclohexyl RCS-8 glucuronides, as well as the demethyl RCS-8 glucuronide. We propose that these metabolites likely are primary targets of RCS-8 metabolism in human urine.
Acknowledgments
This research was funded by AB SCIEX and the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health. A Wohlfarth, A Gandhi, K Scheidweiler and M Huestis are employees at the National Institute on Drug Abuse, National Institutes of Health. S Pang is employed by AB SCIEX, M Zhu is employed by Bristol-Myers Squibb.
Key terms
- Synthetic cannabinoids
Synthetic cannabinoids are structurally nonhomogeneous compounds that act as agonists at the CB1 and/or CB2 receptors. Many marketed compounds contain a pentylindole core structure with varying substituents in the 3-position, for example, naphthoyl, phenylacetyl, benzoyl, adamantyl, tetramethylcyclopropyl, quinoline systems or aliphatic structures. The linker between the indole system and the substituent often is a carbonyl group, but carboxamide and ester linkers recently appeared.
- CB1 and CB2 receptors
Cannabinoid receptors are G-protein-coupled receptors discovered in the 1990s. To date, two receptor subtypes, CB1 and CB2 were cloned with different distribution patterns in the body. CB1 receptors are primarily located in the central nervous system, with high concentrations in the cortex, basal ganglia, cerebellum, hypothalamus and hippocampus, and modulate psychoactive effects while CB2 receptors are primarily located in immune tissues, but also mediate analgesic effects.
- Information-dependent acquisition (IDA)
In an IDA method, a full-scan occurs first in each MS cycle. The mass spectrum is evaluated in real time, based on defined criteria and precursor ions for subsequent product ion scans are selected based on a the variety of selection criteria, for example, ion intensity, defined masses in an inclusion list, a characteristic isotope pattern, a characteristic neutral loss or with mass defect filtering.
- Mass defect filtering
The mass defect is the non-integral portion of the mass-to-charge-ratio of a compound and is usually specified in mDa. Summing up the exact masses of each atom (e.g., C, 12.0000 Da; H, 1.0072 Da; O, 15.9960 Da; N and 14.0047 Da) will result in a unique mass defect. When applying a mass defect window, all compounds with a mass defect within this window will be detected, no matter the nominal mass.
- Calculated logP (clogP)
The octanol-water partition coefficient log P is a well-established measure of molecular hydrophobicity that affects the drug’s behavior in the body (absorption, bioavailability, receptor interactions, metabolism and toxicity), as well as during analysis (sample preparation, elution). Values for logP are determined by experiment, but also can be calculated for unknown compounds (clogP) by adding contributions of every non-overlapping group and considering electronic and steric effects.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Sedefov R, Gallegos A, Kind L, Lopez D, Auwarter V, Hughes B. Understanding the ‘Spice’ Phenomenon. EMCDDA 2009 Thematic paper, Lisbon, Portugal. 2009. [Google Scholar]
- 2.Seely KA, Lapoint J, Moran JH, Fattore L. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):234–243. doi: 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Government. Section 1151: Synthetic Drug Abuse Prevention Act of 2012. The Food and Drug Administration Safety and Innovation Act (S.3187) 2012 [Google Scholar]
- 4.Huffman JW, Szklennik PV, Almond A, et al. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg. Med. Chem. Lett. 2005;15(18):4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5. Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J. Forensic Sci. 2012;57(5):1168–1180. doi: 10.1111/j.1556-4029.2012.02207.x.. • Comprehensive report of various analytical techniques used for identifying several synthetic cannabinoids in herbal incense blends
- 6.Shanks KG, Dahn T, Behonick G, Terrell A. Analysis of first and second generation legal highs for synthetic cannabinoids and synthetic stimulants by ultra-performance liquid chromatography and time of flight mass spectrometry. J. Anal. Toxicol. 2012;36(6):360–371. doi: 10.1093/jat/bks047. [DOI] [PubMed] [Google Scholar]
- 7.Berrier A. Classes and Structures of Emerging Cannabimimetics and Cathinones. Emerging Trends in Synthetic Drugs Workshop; Gaithersburg, MD, USA. 2013. [Google Scholar]
- 8.Wiley JL, Compton DR, Dai D, et al. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J. Pharmacol. Exp. Ther. 1998;285(3):995–1004. [PubMed] [Google Scholar]
- 9.Bluelight Drug User Forum. Thread: 1-(1-(2-cyclohexylethyl)-1H–indol-3-yl)-2-(2-methoxyphenyl)ethanone. 2010 www.bluelight.org/vb/threads/510125--1-%281-%282-cyclohexylethyl%29--1H–indol-3-yl%29--22-%282-methoxyphenyl%29ethanone. [Google Scholar]
- 10.Grigoryev A, Melnik A, Savchuk S, Simonov A, Rozhanets V. Gas and liquid chromatography-mass spectrometry studies on the metabolism of the synthetic phenylacetylindole cannabimimetic JWH-250, the psychoactive component of smoking mixtures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879(25):2519–2526. doi: 10.1016/j.jchromb.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Hutter M, Broecker S, Kneisel S, Auwarter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J. Mass Spectrom. 2012;47(1):54–65. doi: 10.1002/jms.2026. [DOI] [PubMed] [Google Scholar]
- 12. Kavanagh P, Grigoryev A, Melnik A, Simonov A. The identification of the urinary metabolites of 3-(4-methoxybenzoyl)-1-pentylindole (RCS-4), a novel cannabimimetic, by gas chromatography-mass spectrometry. J. Anal. Toxicol. 2012;36(5):303–311. doi: 10.1093/jat/bks032.. • Study reported an O demethylated metabolite of a structurally similar synthetic cannabinoid, RCS-4
- 13.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci. Int. 2010;200(1–3):141–147. doi: 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Sobolevsky T, Prasolov I, Rodchenkov G. Detection of urinary metabolites of AM-2201 and UR-144, two novel synthetic cannabinoids. Drug Test. Anal. 2012;4(10):745–753. doi: 10.1002/dta.1418. [DOI] [PubMed] [Google Scholar]
- 15. Chimalakonda KC, Moran CL, Kennedy PD, et al. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal. Chem. 2011;83(16):6381–6388. doi: 10.1021/ac201377m.. • The authors identified CYP2C9 and CYP1A2 as major CYP450s involved in the oxidation of the JWH-018 and AM2201
- 16.Chimalakonda KC, Seely KA, Bratton SM, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/spice: identification of novel cannabinoid receptor ligands. Drug Metab. Dispos. 2012;40(11):2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigoryev A, Savchuk S, Melnik A, et al. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011;879(15–16):1126–1136. doi: 10.1016/j.jchromb.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 18.Moran CL, Le VH, Chimalakonda KC, et al. Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal. Chem. 2011;83(11):4228–4236. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigoryev A, Kavanagh P, Melnik A. The detection of the urinary metabolites of 1-[(5-fluoropentyl)-1H–indol-3-yl]-(2-iodophenyl)methanone (AM-694), a high affinity cannabimimetic, by gas chromatography-mass spectrometry. Drug Test. Anal. 2012;5(2):110–115. doi: 10.1002/dta.1336. [DOI] [PubMed] [Google Scholar]
- 20.Hutter M, Moosmann B, Kneisel S, Auwarter V. Characteristics of the designer drug and synthetic cannabinoid receptor agonist AM-2201 regarding its chemistry and metabolism. J. Mass Spectrom. 2013;48(7):885–894. doi: 10.1002/jms.3229. [DOI] [PubMed] [Google Scholar]
- 21.Grigoryev A, Kavanagh P, Melnik A. The detection of the urinary metabolites of 3-[(adamantan-1-yl)carbonyl]-1-pentylindole (AB-001), a novel cannabimimetic, by gas chromatography-mass spectrometry. Drug Test. Anal. 2012;4(6):519–524. doi: 10.1002/dta.350. [DOI] [PubMed] [Google Scholar]
- 22.Grigoryev A, Kavanagh P, Melnik A, Savchuk S, Simonov A. Gas and liquid chromatography-mass spectrometry detection of the urinary metabolites of UR-144 and its major pyrolysis product. J. Anal. Toxicol. 2013;37(5):265–276. doi: 10.1093/jat/bkt028. [DOI] [PubMed] [Google Scholar]
- 23. Gandhi As, Zhu M, Pang S, et al. First Characterization of AKB-48 metabolism, a novel synthetic cannabinoid, using human hepatocytes and high-resolution mass spectrometry. AAPS J. 2013;15(4):1091–1098. doi: 10.1208/s12248-013-9516-0.. • First study to report metabolites of synthetic cannabinoids after incubation with human hepatocytes
- 24.Wohlfarth A, Pang S, Zhu M, et al. First metabolic profile of XLR-11, a novel synthetic cannabinoid, obtained by using human hepatocytes and high-resolution mass spectrometry. Clin. Chem. 2013;59(11):1638–1648. doi: 10.1373/clinchem.2013.209965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y. URB-754: a new class of designer drug and 12 synthetic cannabinoids detected in illegal products. Forensic Sci. Int. 2013;227(1–3):21–32. doi: 10.1016/j.forsciint.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 26.Molinspiration Cheminformatics. www.molinspiration.com.
- 27. Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J. Mass Spectrom. 2009;44(5):832–837. doi: 10.1002/jms.1558.. • First study reporting cannabinoid-like designer drugs were used as adulterants in commercially available products designed for inhalation
- 28.Steup C. Untersuchung des Handelsproduktes ‘SPICE’. THC Pharm GmbH. http://usualredant.de/downloads/analyse-thc-pharm-spice-jwh-018.pdf.