In Garrison Keillor’s fictional mid-Western town of Lake Wobegon, “all the women are strong, all the men are good looking, and all the children are above average”. It might also seem deliberately paradoxical to say that “most patients are at below average risk” or that “most patients within a trial experience less than the average level of benefit from a drug”. But it turns out that these statements are true more often than not.1
To start with a simple example, the lifetime risk that an American will develop lung cancer is about 7.5%. But because smoking strongly increases the risk of lung cancer, the average risk of 7.5% can be stratified into smokers, who have a risk of 15 – 20%, and non-smokers, with a risk of around 1%. As 60–70% of the population have never smoked, most Americans are substantially below the average risk developing of lung cancer.
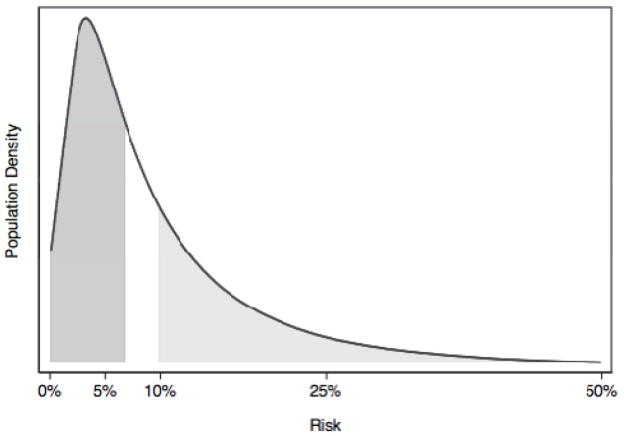
This is naturally a simplification, because the probability of lung cancer is not the same for all smokers. Statisticians have created prediction models giving lung cancer risk in terms of age, gender, pack years and current smoking status.2 The math underlying these prediction models is such that the risk distribution will almost always be skewed to the right when the overall outcome rate is <50%. The figure shows the distribution of risk from a typical risk prediction model. In this hypothetical example, 10% of patients develop disease, and so the mean risk is 10%. The median risk is closer to 7% and in fact about two-thirds of patients have a risk less than the mean.
Figure.
The distribution of risk for a typical risk prediction model. The mean risk is 10%, the median risk is 7%. The dark grey area constitutes the 50% of patients with risk less than the median; the light grey represents the patients with risk greater than the mean, about a third. Approximately two-thirds of patients have risks less than the mean.
The implications of this “Lake Wobegon effect” are far from trivial. Take a typical randomized trial taught in an introductory evidence-based medicine class, with cardiovascular event rates of 10% in the control arm compared to 5% in the drug group. This is a 5% absolute risk reduction and a number-needed-to-treat of 20. Let us assume that most clinicians think that the burdens, costs, side-effects and risks of the drug are low enough that it would, in fact, be worth treating 20 patients, although no more than 20, in order to prevent one cardiovascular event. As such, the trial is deemed a success and the drug widely prescribed. But let us further assume that a prediction model is available that can be used to determine the risk of an individual patient based on risk factors such as blood pressure and cholesterol, and that the prediction model has similar properties to the one shown in the figure. If relative risk is roughly constant across different levels of absolute risk (50%), and absolute risk of drug harms is also approximately constant, then it can be seen that only about a third of patients benefit sufficiently from the drug to outweigh its costs, burdens and harms. Or, to put it another way, although the treatment is worthwhile on average, it is not worthwhile in the average patient.
We have examined this effect empirically in patients with ST-elevation myocardial infarction. Using a previously developed risk model, we found that patients in the highest risk quartile have about 16-fold the risk of mortality compared to the lowest risk quartile. The typical patient, on the other hand, has a risk only about half the average. When we reanalyzed the GUSTO trial using this risk model, we found that the more potent and expensive thrombolytic therapy (tPA) was indeed effective on average, but for most patients the degree of benefit likely did not warrant the extra risks and costs compared to the less effective, but safer and less expensive alternative, streptokinase.3 In a similar study, we found that primary angioplasty saves lives on average compared to thrombolytic therapy, but not in typical risk patients: up to 75% of ST-elevation MI patients derive no mortality benefit from angioplasty.1,4,5 Because such risk-stratified analyses are rare, the emphasis placed on the average summary results can lead to low value care and overtreatment in many, particularly for treatments that carry substantial risks or costs.
Perhaps the “ground zero” of overtreatment in contemporary medicine is screening for prostate cancer. Population data show that, following the introduction of screening with prostate-specific antigen (PSA), mortality fell somewhat but incidence increased dramatically. We believe that typical approaches to prostate cancer screening, which assume all men are at average risk, are a major cause of overdiagnosis. In fact, risk can be very strongly separated depending on PSA. Men in the top quartile of PSA at age 60, equivalent to a PSA of 2 ng/ml or above, have a risk of prostate cancer mortality more than 20 times greater than those with lower PSAs, and 90% of deaths by age 85 occur in this group,6 a clear example of the Lake Wobegon effect. We recently demonstrated that screening only men at high risk rather than screening all men drastically reduced screening harms - in terms of overdiagnosis - but retained 100% of the screening benefits - in terms of mortality reductions - because screening did not reduce prostate cancer deaths in the low PSA group 7
Of course, the distribution of risk is a model-dependent property. While risk models are available for many clinically important outcome, their implementation in research and clinical domains has been limited, and the barriers to their wider use are not insubstantial. The relationship between harms and benefits might also be complex, and it is likely that in some cases, the risk of harms is somewhat correlated with the degree of benefit.
Nevertheless, it is clear that the Lake Wobegon effect – the apparently paradoxical finding that most patients are at below average risk and expect to experience less than average benefit from treatment – is a common and underappreciated phenomenon in medicine, with important clinical implications: in many cases, too many patients are screened, diagnosed and treated. A better understanding of this effect, and better use of risk prediction in both our research and in clinical practice will be essential to ensure that we focus our attention on those patients who stand most to gain.
Acknowledgments
Funding: Supported in part by funds from David H. Koch provided through the Prostate Cancer Foundation, the Sidney Kimmel Center for Prostate and Urologic Cancers, P50-CA92629 SPORE grant from the National Cancer Institute to Dr. H Scher, and the P30-CA008748 NIH/NCI Cancer Center Support Grant to MSKCC. Additional support was provided by the National Institutes of Health (U01 NS086294), the Patient-centered Outcomes Research Institute (1IP2PI000722) and CTSA U-award Grant number is UL1 TR001064.
Reference List
- 1.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298(10):1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB, Gould MK. When the Average Applies to No One: Personalized Decision Making About Potential Benefits of Lung Cancer Screening. Ann Intern Med. 2012;157(8):571–573. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]
- 3.Kent DM, Hayward RA, Griffith JL, et al. An independently derived and validated predictive model for selecting patients with myocardial infarction who are likely to benefit from tissue plasminogen activator compared with streptokinase. Am J Med. 2002;113(2):104–111. doi: 10.1016/s0002-9343(02)01160-9. [DOI] [PubMed] [Google Scholar]
- 4.Kent DM, Schmid CH, Lau J, Selker HP. Is primary angioplasty for some as good as primary angioplasty for all? J Gen Intern Med. 2002;17(12):887–894. doi: 10.1046/j.1525-1497.2002.11232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thune JJ, Hoefsten DE, Lindholm MG, et al. Simple risk stratification at admission to identify patients with reduced mortality from primary angioplasty. Circulation. 2005;112(13):2017–2021. doi: 10.1161/CIRCULATIONAHA.105.558676. [DOI] [PubMed] [Google Scholar]
- 6.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341:c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson S, Assel M, Sjoberg D, et al. Influence of blood prostate specific antigen levels at age 60 on benefits and harms of prostate cancer screening: population based cohort study. BMJ. 2014;348:g2296. doi: 10.1136/bmj.g2296. [DOI] [PMC free article] [PubMed] [Google Scholar]