Abstract
Background
An induced-pain paradigm has been used in back-healthy people to understand risk factors for developing low back pain during prolonged standing.
Objectives
The purposes of this study were to (1) compare baseline lumbar lordosis in back-healthy participants who do (Pain Developers) and do not (Non-Pain Developers) develop low back pain during 2 hours of standing, and (2) examine the relationship between lumbar lordosis and low back pain intensity.
Design
Cross-Sectional
Method
First, participants stood while positions of markers placed on the lumbar vertebrae were recorded using a motion capture system. Following collection of marker positions, participants stood for 2 hours while performing light work tasks. At baseline and every 15 minutes during standing, participants rated their low back pain intensity on a visual analog scale. Lumbar lordosis was calculated using marker positions collected prior to the 2 hour standing period. Lumbar lordosis was compared between pain developers and non-pain developers. In pain developers, the relationship between lumbar lordosis and maximum pain was examined.
Results/findings
There were 24 (42%) pain developers and 33 (58%) non-pain developers. Lumbar lordosis was significantly larger in pain developers compared to non pain developers (Mean difference=4.4°; 95% Confidence Interval=0.9° to 7.8°, Cohen’s d=0.7). The correlation coefficient between lumbar lordosis and maximum pain was 0.46 (P=0.02).
Conclusion
The results suggest that standing in more lumbar lordosis may be a risk factor for low back pain development during prolonged periods of standing. Identifying risk factors for low back pain development can inform preventative and early intervention strategies.
Keywords: Lumbar lordosis, low back pain, prolonged standing, induced-pain paradigm
INTRODUCTION
Low back pain (LBP) accounts for 40% of worker’s compensation claims in the U.S. (Guo et al., 1995) and results in the loss of over 100 million workdays each year (Atlas et al., 2004). Prolonged, low load, static postures such as standing during work and everyday activities have been associated with increased risk for developing LBP (Picavet and Schouten, 2000). Although standing for prolonged periods of time is common, not all people who are exposed to prolonged standing will develop LBP. Factors that lead an individual to be susceptible to LBP development during prolonged standing are not well understood.
In previous studies investigators have used an induced pain paradigm to examine factors that may contribute to LBP symptoms during prolonged standing (Gregory and Callaghan, 2008; Gregory et al., 2008; Gallagher et al., 2011; Nelson-Wong et al., 2008; Nelson-Wong et al., 2009; Nelson-Wong and Callaghan, 2010c; Nelson-Wong et al., 2010; Nelson-Wong and Callaghan, 2010b; Nelson-Wong and Callaghan, 2010a; Nelson-Wong et al., 2012; Nelson-Wong and Callaghan, 2014; Marshall et al., 2011). The paradigm requires back-healthy people to stand for 2 hours while performing simulated, light work tasks. The people rate their LBP at baseline and every 15 minutes throughout the 2 hours of standing. People who develop LBP during standing are classified as pain developers (PDs) and those who do not develop LBP are classified as non-pain developers (NPDs). Across the prior studies, 40 to 70% of back-healthy people developed LBP symptoms during standing (Nelson-Wong and Callaghan, 2014). Some differences between PDs and NPDs in clinical and biomechanical variables have been identified (Nelson-Wong et al., 2009; Nelson-Wong et al., 2012; Marshall et al., 2011). No studies, however, have examined differences in curvature of the lumbar spine, specifically the amount of lumbar lordosis, at baseline in PDs compared to NPDs.
Lumbar lordosis potentially is an important characteristic to examine for two reasons. First, prior studies of cadaveric spines have shown that small changes in the orientation of one vertebra relative to another (e.g. a lordotic orientation) can result in large changes in the distribution of loading on the posterior elements of the vertebra (e.g., facet joints, neural arch) (Adams and Hutton, 1980). The force concentrated over a relatively small area can lead to high concentrations of stress (Dunlop et al., 1984; Shirazi-Adl, 1991). The high concentrations of stress on innervated spine tissues may contribute to development of symptoms even in the absence of tissue damage (Adams, 2004). Second, in our prior work, we have shown that people with non-specific LBP can be classified into homogeneous subgroups (Van Dillen et al., 2003b). People are subgrouped based on the consistency of symptom responses and signs during standardized clinical tests that place different types of loads (flexion, extension, rotation) on the lumbar spine (Van Dillen et al., 1998; Van Dillen et al., 2003b; Harris-Hayes and Van Dillen, 2009). The subgroups are named for the directions of movements and postures that consistently increase the person’s symptoms and are improved when the movements and postures are systematically modified to change the specific loading on the lumbar spine (Van Dillen et al., 2003a; Van Dillen et al., 2009). We have reported that people in one specific LBP subgroup, lumbar extension-rotation, stand in more lumbar lordosis than (1) people in other LBP subgroups, and (2) back-healthy people (Norton et al., 2004). People in this subgroup report increased symptoms with movements and postures during clinical tests that result in extension or rotation loading on the lumbar spine (Maluf et al., 2000; Van Dillen et al., 2003b) and report decreased symptoms when the extension or rotation loading is modified (Van Dillen et al., 2003b; Van Dillen et al., 2003a; Van Dillen et al., 2009). Of particular importance to the current study, the people in the lumbar extension-rotation subgroup also report a shorter interval before LBP symptoms increase during standing compared to other LBP subgroups (unpublished data). Thus, standing appears to be more symptom-provoking for people in the lumbar extension-rotation subgroup compared to other LBP subgroups. The reduced time for LBP symptoms to increase in standing in this LBP subgroup may be due to the effects of loading on the posterior elements of the vertebrae associated with standing in lumbar lordosis.
Given the findings from the studies of stress concentrations and the study of posture in subgroups of people with LBP identified based on consistent responses to different types of spine loading (Van Dillen et al., 2003b; Van Dillen et al., 2003a; Van Dillen et al., 2009), it is reasonable to consider that back-healthy people who develop LBP symptoms during the standing paradigm may stand in more lumbar lordosis than back-healthy people who do not develop LBP symptoms. The purpose of the current study, therefore, was to examine if (1) PDs displayed larger lumbar lordosis at baseline compared to NPDs, and (2) there was a relationship between lumbar lordosis and LBP symptoms developed during standing in PDs. We hypothesized that (1) PDs would display larger lumbar lordosis at baseline compared to NPDs, and (2) lumbar lordosis would be positively related to LBP symptom intensity in PDs.
METHODS
Participants
Fifty seven back-healthy people (28 female, 29 male) were recruited. Participants were recruited by posting flyers on campuses of local universities and the St. Louis metropolitan area. Participants also were recruited through 2 community-based, university operated, recruitment organizations. Inclusion criteria included no lifetime history of an episode of LBP that resulted in (1) seeking some type of health intervention (e.g., physician, physical therapist, chiropractor), (2) 3 or more consecutive days of missed work or school, or (3) 3 or more days of altered activities of daily living. Exclusion criteria included employment in a job that involved standing in one place for more than 1 hour per day during the last 12 months, not able to stand for > 4 hours, a body mass index > 30, or report of LBP symptoms at the beginning of the standing task. If a participant reported any symptoms (any value above 0 mm on the VAS) at the start of standing, he or she was excluded from the study. All participants read and signed an informed consent form that was approved by the Human Research Protection Office at Washington University School of Medicine.
Equipment and Procedures
After signing the informed consent document, all participants remained seated to report their current LBP symptom intensity level on a visual analog scale (VAS). The VAS is a 100 mm horizontal line with the anchors of “no pain” and “worst pain imaginable.” Participants placed an ‘X’ through the line at the point that best represented their perception of their current LBP symptom intensity. Intensity was quantified by measuring the distance of the ‘X’ from the left end of the scale with a ruler. Greater distances indicated higher symptom intensities. Participants also completed the Baecke Questionnaire of Habitual Physical Activity (Baecke et al., 1982).
At baseline, prior to the 2 hour standing period, retro-reflective markers were placed on the participant’s skin superficial to the spinous process of the first (L1), third (L3) and fifth (L5) lumbar vertebrae. Participants then stood in the middle of the capture volume. Marker positions were captured using an 8 camera, 3-dimensional motion capture system (Vicon Motion Systems, Denver, CO) with a sampling rate of 120 Hz. The marker positions captured prior to the 2 hours of standing were used to calculate baseline lumbar lordosis.
Following collection of the marker positions, markers were removed and participants were positioned in front of a work table in a 2 feet by 4 feet confined workspace. The table was adjusted to 5 cm below the participant’s wrist while his or her elbows were flexed to 90° (Kromer and Grandjean, 1997). Participants then stood for 2 hours performing simulated light work tasks. Participants were allowed to shift their weight as often as desired but were told to keep both feet on the ground the majority of the time, and were not allowed to rest their feet on the legs of the table or arms on the surface of the table.
The tasks used included shuffling cards, sorting poker chips, and a simple assembly task. There also was a quiet standing condition. The work tasks and quiet standing were completed in 15 minute blocks of time with the order of tasks randomized prior to the start of standing. Randomization of the tasks was performed using random.org/lists. Each task and the period of quiet standing were completed twice during the two hours, with the added constraint that the same task could not be performed consecutively. Following randomization, if any of the 3 work tasks or quiet standing was to be performed consecutively, randomization was repeated. At baseline and every 15 minutes during the standing test, participants reported the intensity of their LBP symptoms on the VAS.
Alignment and VAS variables
All kinematic data were processed using customized programs written in MATLAB (MathWorks Inc., Natick MA). Marker coordinate data were low pass filtered with a cutoff of 3 Hz using a 4th order dual pass Butterworth filter. Lumbar curvature angle was the index of lumbar lordosis. The curvature angle was calculated by (1) finding the distance of a vector (l) from L1 to L5, (2) finding the distance of the vector (d) that is perpendicular from l to L3, (3) using the formula: 2arctan(0.5l/d) (Norton et al., 2004), (4) subtracting the obtained value from 180° so that a larger angle would equal larger lumbar lordosis (Figure 1).
Figure 1.
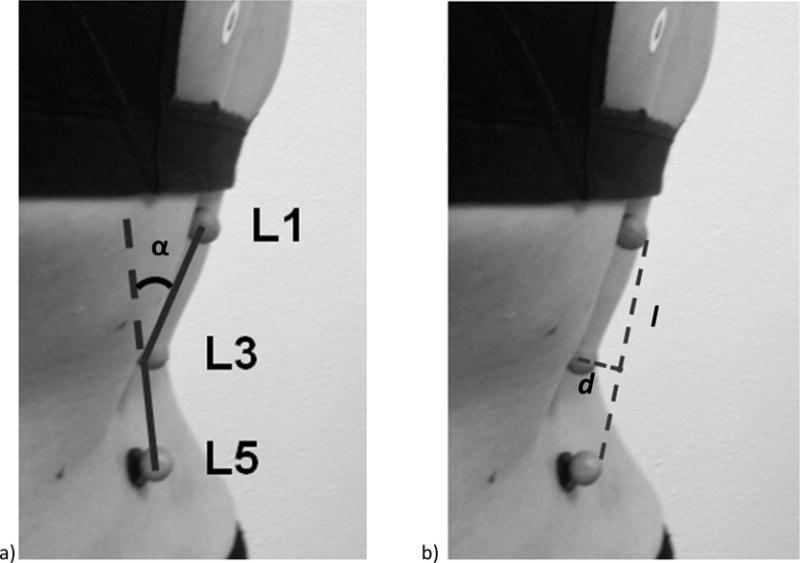
Participant standing with retroreflective markers superficial to the spinous processes of the first, third, and fifth vertebrae (L1, L3, and L5, respectively). (a) Lumbar curvature angle (α) was calculated as the angle of a vector from L1 to L3 relative to a vector from L3 to L5. (b) The distance of a vector from L1 to L5 (l) and the distance of a vector perpendicular from l to L3 (d).
Lumbar curvature angle was calculated for each frame captured and then 11 frames were averaged. For each participant, the maximum VAS (Max VAS) score that was reported during the 2 hours of standing was identified.
Statistical Analyses
Back-healthy participants were separated into PDs and NPDs. PDs were participants that reported any symptoms after baseline and maintained the symptoms throughout the standing test. NPDs were people who reported 0 on the VAS throughout the standing task. A Chi-square analysis was conducted to test for differences in the distribution of sex in PDs and NPDs. Independent groups t-tests were conducted to test for differences in demographics, activity level, and baseline lumbar curvature angle between PDs and NPDs. A Cohen’s d statistic was calculated to estimate effect size of the difference in lumbar curvature angle between groups. In PDs, a Pearson correlation coefficient was calculated for lumbar curvature angle and Max VAS scores. A simple linear regression analysis then was performed to examine the degree to which Max VAS scores could be predicted by the degree of lumbar curvature angle at baseline. The significance level for all analyses was set at P ≤ 0.05. Statistical analyses were performed in SPSS version 21.0 (IBM, Armonk, NY).
RESULTS
Twenty four of the 57 participants (42%) were classified as PDs. All participants had a VAS of 0 mm at the beginning of standing. There were no significant differences between groups for sex, age, height, mass, BMI, or activity level (Table 1).
Table 1.
Participant characteristics and lumbar curvature angle in pain developers (PDs) and non pain developers (NPDs).
| Characteristic | Group
|
Statistical Value | P-value | |
|---|---|---|---|---|
| NPDs (n = 33) | PDs (n = 24) | |||
| Sex (% female)a | 39% | 62% | X2 = 3.0 | 0.09 |
| Age (years) | 23.9 (3.5) | 24.7 (3.3) | t = −0.9 | 0.37 |
| Height (cm) | 171.4 (8.7) | 171.8 (7.1) | t = −0.2 | 0.84 |
| Mass (kg) | 67.1 (9.1) | 69.2 (12.8) | t = −0.7 | 0.48 |
| BMI (kg/m2) | 22.8 (2.3) | 23.3 (2.8) | t = −0.7 | 0.50 |
| Baecke Questionnaire of Habitual Physical Activity (3–15) [23] | 8.2 (1.2) | 8.1 (1.3) | t = 0.2 | 0.91 |
| Lumbar Curvature Angle (°) | 21.0 (6.6) | 25.4 (6.3) | t = 2.5 | 0.02* |
Sex is the percentage of females in each group; all other values are the mean (standard deviation)
Represents a significant difference (P ≤ 0.05)
Compared to NPDs, PDs displayed a larger lumbar curvature angle (mean difference = 4.37°, P = 0.02, Cohen’s d = 0.68; medium effect size (Cohen, 1988). In PDs, there was a significant relationship between lumbar curvature angle and Max VAS (r = 0.46, P = 0.02, Figure 2), indicating that larger lumbar curvature angles were associated with larger Max VAS scores.
Figure 2.
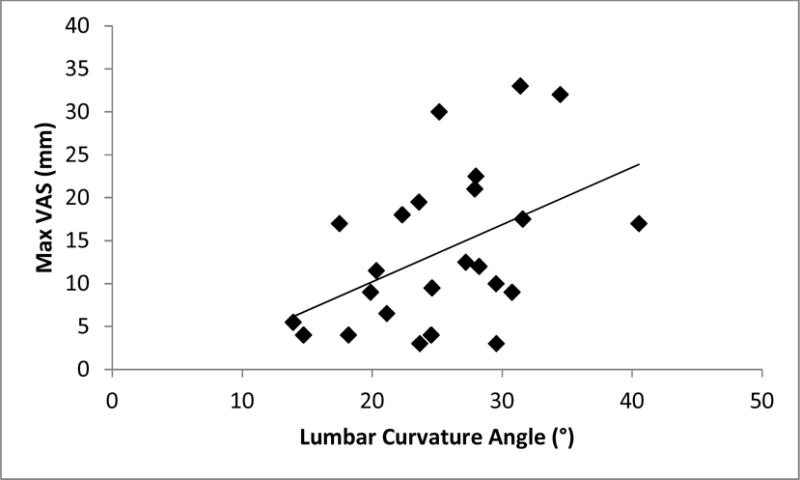
Scatterplot of lumbar curvature angle and maximum visual analog scale (Max VAS) values in PDs (r = 0.46, P =0.02).
In PDs, 22% of the variance in Max VAS scores was predicted by lumbar curvature angle (Max VAS = −3.10 + (0.67 × lumbar curvature angle), R2 = 0.22, P = 0.02).
DISCUSSION
The purpose of this study was to test the hypotheses that lumbar lordosis in back-healthy people classified as PDs would be (1) larger compared to back-healthy people classified as NPDs, and (2) related to LBP symptom intensity during prolonged standing. We found that lumbar lordosis in PDs was (1) larger compared to NPDs, and (2) positively related to maximum LBP intensity during standing. These data provide evidence that in back-healthy people lumbar spine alignment appears to interact with the demands put on the spine to increase a person’s risk for developing LBP symptoms. Our conclusions about lumbar spine alignment are reinforced by the fact that even with the acute, transient symptoms induced during the standing paradigm, there was a significant positive relationship between symptom intensity and the degree of lordosis; the larger the lordosis the higher the LBP symptom intensity.
Our hypothesis that PDs would display more lumbar lordosis than NPDs was based, in part, on an earlier study of alignment in people with non-specific LBP. In particular, Norton et al. (Norton et al., 2004) reported that when people with LBP were subgrouped based on symptom responses and signs during clinical tests that place different loads on the lumbar spine, the lumbar extension-rotation subgroup displayed larger lumbar lordosis in standing compared to back-healthy people and people in other LBP subgroups. People in the lumbar extension-rotation subgroup also reported a shorter interval before LBP symptoms increased during standing compared to other subgroups of people with LBP (unpublished data). The fact that a shorter interval was needed to provoke LBP symptoms in this subgroup compared to other LBP subgroups suggests that lumbar alignment may contribute to the increase in symptoms. Combined with the results from the current study, it is reasonable to propose that in back-healthy people the degree of lumbar lordosis may contribute to an increase in susceptibility for LBP symptoms during prolonged standing.
In previous studies investigators have reported that compared to a neutral position, lordotic postures cause increased compressive loading on the posterior spinal structures and increased stress peaks in the intervertebral disc. For example, using cadaver spines investigators have examined loading in neutral and lordotic postures. In these studies neutral alignment was defined as 2 vertebrae having an angle of 0° relative to each other. Adams and Hutton (Adams and Hutton, 1980) reported that a lumbar spinal segment placed in 2° of lordosis caused the facet joints to support 16% of the compressive load compared to only 1% when the segment was in a neutral alignment. The increase in force concentrated over the relatively small area of the facet joints resulted in high stress concentrations on the facet joint tissue (Dunlop et al., 1984). An increase of 2° of lumbar lordosis also has been shown to result in large stress peaks in the posterior annulus of the intervertebral disc, rather than an even distribution of stress across the disc in a neutral position (Shirazi-Adl, 1991; Adams, 2004). High stress concentrations in specific innervated spine tissues have the potential to contribute to development of acute LBP symptoms (Adams, 2004).
There are two plausible mechanisms for the development of LBP symptoms due to high stress concentrations. The first is stimulation of nociceptors (Marras, 2003). Nociceptors have been identified in facet joint capsules (McLain and Pickar, 1998), the articular processes of the facet joints (Bogduk, 1983), and the outer layers of the annulus of the lumbar intervertebral disc (Edgar, 2007). Thus, high stress concentrations due to posterior loading could cause symptoms even in the absence of mechanical damage to the tissues (Adams, 2004). The second is mechanical damage. Mechanical damage will occur if the posterior loading associated with the lumbar lordosis exceeds the load tolerance of the tissues. In addition, if a lordotic posture is maintained over time this could lead to insufficient rest time for normal tissue adaptation and recovery (Sahrmann, 2002; McGill, 1997), subsequently accelerating the rate of mechanical damage to the tissue.
A limitation of the current study is that the lumbar curvature angle was measured at baseline and not throughout the standing task. Thus, we do not know whether the lumbar curvature angle changed over time during standing. In a previous study, the standing paradigm was used and the results indicated that all participants tended to increase the degree of flexion in which they stood over time (Gregory and Callaghan, 2008). However the investigators did not examine either (1) differences in lumbar curvature angle between PDs and NPDs, or (2) the amount of lumbar curvature participants assumed at the beginning of the test. Measurement of lumbar spine posture in standing over time warrants further examination. Another limitation is that the people who participated in the study were between 18 and 30 years old. Consequently, our results may not be generalizable to people of all ages.
CONCLUSIONS
Lumbar lordosis in PDs was (1) larger compared to NPDs, and (2) related to LBP symptom intensity during standing. Lumbar lordosis may be a risk factor for LBP symptom development in back-healthy people who participate in activities that require prolonged periods of standing. Identifying characteristics that increase the risk for LBP symptom development can inform strategies for preventative and early intervention strategies. Future work should focus on differences in lumbar posture throughout the standing paradigm between PDs and NPDs and include back-healthy participants across a wider range of ages.
Highlights.
We measured lumbar lordosis during normal standing in back-healthy participants
Participants then stood for 2 hours while rating their LBP intensity throughout
Twenty four of 57 participants developed LBP during standing
People who developed LBP had larger lumbar lordosis than people who did not
Baseline lumbar lordosis was related to LBP intensity in people who developed LBP
Acknowledgments
This publication was supported by (1) the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) to CJS and (2) by NIH grant R01 HD047709-04 to LVD. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest. The authors would like to acknowledge Katherine Baxter and Sara Bohall for contributions with participant recruitment and data collection and analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
“X” and “X” developed the study purpose. “X”, “X”, and “X” collected and analyzed the data. All authors contributed to interpretation of the data. “X” and “X” drafted the original article. All authors contributed to critical revision of the article for intellectual content and provided final approval of the version to be submitted.
Contributor Information
Christopher J. Sorensen, Email: sorensenc@wusm.wustl.edu.
Barbara J. Norton, Email: nortonb@wustl.edu.
Jack P. Callaghan, Email: jack.callaghan@uwaterloo.ca.
Ching-Ting Hwang, Email: hwangch@wusm.wustl.edu.
Linda R. Van Dillen, Email: vandillenl@wustl.edu.
Reference List
- Adams MA. Biomechanics of back pain. Acupunct Med. 2004 Dec;22(4):178–88. doi: 10.1136/aim.22.4.178. [DOI] [PubMed] [Google Scholar]
- Adams MA, Hutton WC. The effect of posture on the role of the apophysial joints in resisting intervertebral compressive forces. J Bone Joint Surg Br. 1980 Aug;62(3):358–62. doi: 10.1302/0301-620X.62B3.6447702. [DOI] [PubMed] [Google Scholar]
- Atlas SJ, Wasiak R, van den Ancker M, Webster B, Pransky G. Primary care involvement and outcomes of care in patients with a workers’ compensation claim for back pain. Spine. 2004 May 1;29(9):1041–8. doi: 10.1097/00007632-200405010-00017. [DOI] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982 Nov;36(5):936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bogduk N. The innervation of the lumbar spine. Spine (Phila Pa 1976) 1983 Apr;8(3):286–93. doi: 10.1097/00007632-198304000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Dunlop RB, Adams MA, Hutton WC. Disc space narrowing and the lumbar facet joints. J Bone Joint Surg Br. 1984 Nov;66(5):706–10. doi: 10.1302/0301-620X.66B5.6501365. [DOI] [PubMed] [Google Scholar]
- Edgar MA. The nerve supply of the lumbar intervertebral disc. J Bone Joint Surg Br. 2007 Sep;89(9):1135–9. doi: 10.1302/0301-620X.89B9.18939. [DOI] [PubMed] [Google Scholar]
- Gallagher KM, Nelson-Wong E, Callaghan JP. Do individuals who develop transient low back pain exhibit different postural changes than non-pain developers during prolonged standing? Gait Posture. 2011 Oct;34(4):490–5. doi: 10.1016/j.gaitpost.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Gregory DE, Brown SH, Callaghan JP. Trunk muscle responses to suddenly applied loads: do individuals who develop discomfort during prolonged standing respond differently? J Electromyogr Kinesiol. 2008 Jun;18(3):495–502. doi: 10.1016/j.jelekin.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gregory DE, Callaghan JP. Prolonged standing as a precursor for the development of low back discomfort: an investigation of possible mechanisms. Gait Posture. 2008 Nov 27;28(1):86–92. doi: 10.1016/j.gaitpost.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Guo HR, Tanaka S, Cameron LL, Seligman PJ, Behrens VJ, Ger J, et al. Back pain among workers in the United States: national estimates and workers at high risk. Am J Ind Med. 1995 Nov;28(5):591–602. doi: 10.1002/ajim.4700280504. [DOI] [PubMed] [Google Scholar]
- Harris-Hayes M, Van Dillen LR. Inter-tester reliability of physical therapists classifying low back pain problems based on the movement system impairment classification system. Phys Med Rehabil. 2009 Feb;1(2):117–26. doi: 10.1016/j.pmrj.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer KHE, Grandjean E. The design of workstations Fitting the Task to the Human: a Textbook of Occupational Ergonomics. Fifth. Bristol, PA: Taylor & Francis Inc.; 1997. pp. 53–99. [Google Scholar]
- Maluf KS, Sahrmann SA, Van Dillen LR. Use of a classification system to guide nonsurgical management of a patient with chronic low back pain. Phys Ther. 2000 Nov;80(11):1097–111. [PubMed] [Google Scholar]
- Marras WS. The case for cumulative trauma in low back disorders. Spine J. 2003 May;3(3):177–9. doi: 10.1016/s1529-9430(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Marshall PW, Patel H, Callaghan JP. Gluteus medius strength, endurance, and co-activation in the development of low back pain during prolonged standing. Hum Mov Sci. 2011 Feb;30(1):63–73. doi: 10.1016/j.humov.2010.08.017. [DOI] [PubMed] [Google Scholar]
- McGill SM. The biomechanics of low back injury: implications on current practice in industry and the clinic. J Biomech. 1997 May;30(5):465–75. doi: 10.1016/s0021-9290(96)00172-8. [DOI] [PubMed] [Google Scholar]
- McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine. 1998 Jan 15;23(2):168–73. doi: 10.1097/00007632-199801150-00004. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Alex B, Csepe D, Lancaster D, Callaghan JP. Altered muscle recruitment during extension from trunk flexion in low back pain developers. Clin Biomech. 2012 Dec;27(10):994–8. doi: 10.1016/j.clinbiomech.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Callaghan JP. Changes in muscle activation patterns and subjective low back pain ratings during prolonged standing in response to an exercise intervention. J Electromyogr Kinesiol. 2010a Dec;20(6):1125–33. doi: 10.1016/j.jelekin.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Callaghan JP. Is muscle co-activation a predisposing factor for low back pain development during standing? A multifactorial approach for early identification of at-risk individuals. J Electromyogr Kinesiol. 2010b Apr;20(2):256–63. doi: 10.1016/j.jelekin.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Callaghan JP. Repeatability of clinical, biomechanical, and motor control profiles in people with and without standing-induced low back pain. Rehabil Res Pract. 2010c:1–9. doi: 10.1155/2010/289278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Wong E, Callaghan JP. Transient low back pain development during standing predicts future clinical low back pain in previously asymptomatic individuals. Spine. 2014 Mar 15;39(6):E379–E383. doi: 10.1097/BRS.0000000000000191. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Flynn T, Callaghan JP. Development of active hip abduction as a screening test for identifying occupational low back pain. J Orthop Sports Phys Ther. 2009 Sep;39(9):649–57. doi: 10.2519/jospt.2009.3093. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Gregory DE, Winter DA, Callaghan JP. Gluteus medius muscle activation patterns as a predictor of low back pain during standing. Clin Biomech. 2008 Feb 16;23(5):545–53. doi: 10.1016/j.clinbiomech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Nelson-Wong E, Howarth SJ, Callaghan JP. Acute biomechanical responses to a prolonged standing exposure in a simulated occupational setting. Ergonomics. 2010 Sep;53(9):1117–28. doi: 10.1080/00140139.2010.500400. [DOI] [PubMed] [Google Scholar]
- Norton BJ, Sahrmann SA, Van Dillen FL. Differences in measurements of lumbar curvature related to gender and low back pain. J Orthop Sports Phys Ther. 2004 Sep;34(9):524–34. doi: 10.2519/jospt.2004.34.9.524. [DOI] [PubMed] [Google Scholar]
- Picavet HS, Schouten JS. Physical load in daily life and low back problems in the general population-The MORGEN study. Prev Med. 2000 Nov;31(5):506–12. doi: 10.1006/pmed.2000.0737. [DOI] [PubMed] [Google Scholar]
- Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. 1. St. Louis: Mosby, Inc.; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi-Adl A. Finite-element evaluation of contact loads on facets of an L2–L3 lumbar segment in complex loads. Spine. 1991 May;16(5):533–41. doi: 10.1097/00007632-199105000-00009. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Maluf KS, Sahrmann SA. Further examination of modifying patient-preferred movement and alignment strategies in patients with low back pain during symptomatic tests. Man Ther. 2009 Feb;14(1):52–60. doi: 10.1016/j.math.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, Fleming DA, McDonnell MK, et al. Reliability of physical examination items used for classification of patients with low back pain. Phys Ther. 1998 Sep;78(9):979–88. doi: 10.1093/ptj/78.9.979. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom N. The effect of modifying patient-preferred spinal movement and alignment during symptom testing in patients with low back pain: a preliminary report. Arch Phys Med Rehabil. 2003a Mar;84(3):313–22. doi: 10.1053/apmr.2003.50010. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, McDonnell MK, Bloom NJ. Movement system impairment-based categories for low back pain: stage 1 validation. J Orthop Sports Phys Ther. 2003b Mar;33(3):126–42. doi: 10.2519/jospt.2003.33.3.126. [DOI] [PubMed] [Google Scholar]


