Abstract
Objective
Examine whether more advanced kidney failure is associated with sedentary behavior and whether demographics, comorbidity, nutritional and inflammatory markers explain this association.
Design
Observational Study
Setting
Outpatients recruited from outpatient clinics and dialysis units
Subjects
160 patients with CKD or receiving MHD
Methods
Standardized questionnaires including Baecke physical activity questionnaire, standardized anthropometry examination and blood draw.
Main outcome measures
Sedentary behavior (defined as answering “very often” for “During leisure time I watch television” or answering “never” for “During leisure time I walk”) and being physically active (top 25th percentile of the total Baecke score)
Results
18.5% of CKD and 50.0% of MHD patients were sedentary (p <0.001) and 38.8% of CKD and 11.3% of MHD patients were physically active. In separate multivariable logistic regression models, compared to CKD patients, MHD patients were more sedentary (OR 3.84, 95% CI 1.18 to 12.51) and less physically active (OR 0.07, 95% CI 0.01 to 0.40) independent of demographics, comorbidity, smoking, body size, serum hsCRP and albumin. Congestive heart failure, peripheral vascular disease and higher BMI were independently associated with sedentary behavior whereas younger age, lower BMI, lower serum hsCRP and higher serum albumin were associated with being physically active.
Conclusions
Sedentary behavior is highly prevalent among diabetic CKD or MHD patients. The strong association of MHD status with sedentary behavior is not explained by demographics, smoking, comorbidity, nutritional and inflammatory markers. Interventions targeting obesity might improve sedentary behavior and physical activity whereas interventions targeting inflammation might improve physical activity in these populations.
Keywords: chronic kidney disease, maintenance hemodialysis, sedentary behavior, physical activity
Introduction
Basal metabolic rate (BMR) is the amount of energy expended while sitting quietly. Physical activity intensity could be defined using the metabolic intensity equivalent (MET), the ratio of the energy expended during an activity to the energy expended at rest1. Hence, 1 MET is the energy expenditure while sitting quietly. Sedentary behavior is engaging in “any waking activity characterized by an energy expenditure ≤ 1.5 metabolic equivalents and a sitting or reclining posture”2.
Much of the focus on physical activity in chronic kidney disease (CKD) and the dialysis population has been on decreased physical function3–5 and lower levels of moderate/ vigorous physical activities (MVPA)6–7. More recently, sedentary behavior (defined by sitting time8, television viewing time9, pedometer10 or accelerometer11) in CKD has been examined in cross-sectional studies. However, it is unclear whether uremia per-se is a risk factor for sedentary behavior. Furthermore, understanding the risk factors for sedentary behavior in CKD and dialysis patients would help in devising interventional trials targeting sedentary behavior in these populations.
Baecke questionnaire12, a measure of habitual physical activity includes questions about household activities, sports, and leisure time activities. It has been found to be fairly accurate in identifying individuals with low energy expenditure when compared to the gold standard of doubly labeled water method 13. We used the Baecke questionnaire to define sedentary behavior in CKD and maintenance hemodialysis (MHD) patients and examined the hypothesis that the greater prevalence of sedentary behavior in advanced kidney failure is independent of comorbidity, nutritional and inflammatory markers. We also examined the factors associated with sedentary behavior in CKD and maintenance hemodialysis (MHD) populations.
Methods
The current study is a secondary analysis of 80 CKD and 80 chronic MHD participants with type 2 diabetes recruited for three studies. The CKD participants were included from the “Effects of Febuxostat on Adipokines and Kidney Disease in Diabetic CKD” study (NCT01350388). In brief, that study was a randomized controlled trial to determine whether febuxostat therapy in overweight or obese, diabetic CKD patients and high serum uric acid levels impacts adipokines and markers of urinary fibrosis. The MHD population was comprised of 30 participants from the “Protein intake, nutrition, and cardiovascular disease in stage V CKD” study (NCT00566670), and 50 participants from the “Dialysis Registry” study (NCT02023528). The dialysis studies were observational studies. Details of these studies including the inclusion and exclusion criteria are available at clinicaltrials.gov. All studies were approved by the appropriate Institutional Review Board.
Participants included in the current study were: >18 years of age; diagnosed with either CKD (eGFR < 60mL/min/1.73m2 or eGFR 60 to <90 mL/min/1.73m2 with albuminuria), or stage 5 CKD on MHD; diagnosed with type 2 diabetes; completed the Baecke Physical Activity Questionnaire and had other relevant data available for this analysis. All participants underwent standardized study procedures conducted by the same team of trained study personnel.
Physical activity was assessed with the Baecke questionnaire12, a reliable and validated13–14 measure of habitual physical activity. It was administered at baseline in the interventional CKD study and the observational dialysis registry study. Television viewing time has been used as a measure of sedentary behavior9, 15. As sedentary behavior is engaging in activities that barely raise energy expenditure above the BMR, we defined sedentary behavior as answering “very often” for “During leisure time I watch television” or answering “never” for “During leisure time I walk”. In additional analyses, we also examined the occupation/work, sports, and leisure time activity indices derived from the Baecke questionnaire12.
Statistical Analysis
There were 160 participants included in the analysis. Descriptive statistics for continuous variables are shown as mean ± standard deviation or medians with 25th and 75th percentiles and categorical variables are presented as percentages. Baseline characteristics between diabetic CKD and MHD participants were compared by two-tailed Student’s t test or Wilcoxon rank-sum test for continuous variables and Chi-squared test or Fisher exact test for categorical variables.
In order to examine whether the risk of sedentary behavior is higher in MHD participants compared to CKD patients, unadjusted associations of MHD status with sedentary behavior was first examined in a logistic regression model. The extent to which this association was further attenuated by demographics (age, gender, race and education), comorbidity (history of coronary artery disease, coronary heart failure, peripheral vascular disease, stroke and hypertension), smoking, and body size and laboratory parameters (hsCRP and serum albumin) was examined by adding serially these groups of factors into the above logistic model. Serum hsCRP was heavily skewed and therefore, hsCRP was log transformed and then divided by the logarithm of 2; the results are expressed as the increase in the odds ratios for every 2-fold increase in hsCRP.
Furthermore, unadjusted and adjusted associations of the above factors with sedentary behavior were examined in logistic regression models.
Summation of the Baecke household score, sport score, and leisure time activity score resulted in a continuous overall unitless activity score. We classified those in the top 25th percentile of the overall score as physically active and examined the associations of MHD vs. CKD status with Baecke overall activity score ≥ 7.375 (75th percentile) in logistic regression models similar to those described above for sedentary behavior. All statistical analyses were conducted by using Stata (version 12).
Results
Demographic and clinical characteristics of the entire cohort and the CKD and MHD subgroups are described in Table 1. Compared to the MHD participants, those with CKD were older and had higher BMI. However, the MHD participants still had higher prevalence of cardiovascular conditions, higher CRP levels and lower albumin levels.
Table 1.
Demographics and clinical characteristics of the diabetic CKD and MHD participants
| All (n = 160) | CKD (n = 80) | MHD (n = 80) | p value | |
|---|---|---|---|---|
| Age (years) | 63.7 ± 12.5 | 67.6 ±10.9 | 59.6 ±12.9 | <0.001 |
| Male (%) | 56.3% | 53.8% | 58.8% | 0.52 |
| Black race (%) | 4.4% | 3.8% | 5.0% | 0.70 |
| Education less than college (%) | 31.9% | 17.5% | 46.3% | <0.001 |
| Body mass index (kg/m2) | 33.0 ± 7.7 | 34.4 ± 6.8 | 31.6 ± 8.4 | 0.02 |
| Smoking (%) | 31.9% | 36.3% | 27.5% | 0.24 |
| Coronary artery disease (%) | 28.1% | 17.5% | 38.8% | 0.003 |
| Congestive heart failure (%) | 21.3% | 10.0% | 32.5% | 0.001 |
| Peripheral vascular disease (%) | 20.0% | 3.8% | 36.3% | <0.001 |
| Stroke (%) | 11.9% | 3.8% | 20.0% | 0.001 |
| Hypertension (%) | 80.0% | 76.3% | 83.8% | 0.24 |
| Serum hsCRP (mg/L) | 3.0 (1.5, 6.4) | 2.2 (1.2, 4.5) | 4.6 (2.0, 8.6) | 0.001 |
| Serum albumin (g/dL) | 3.9 ± 0.4 | 4.1 ± 0.3 | 3.8 ± 0.5 | <0.001 |
| eGFR (ml/min/1.73 m2) | 54.7 ± 18.7 | NA |
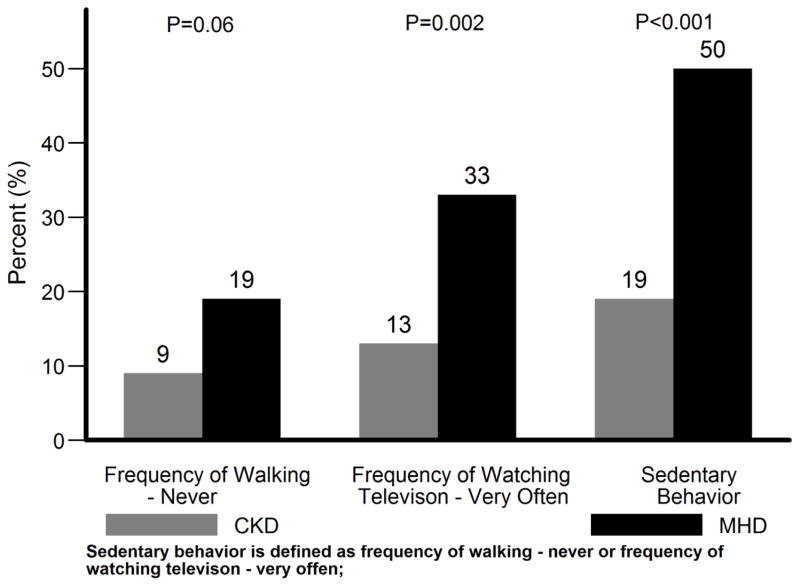
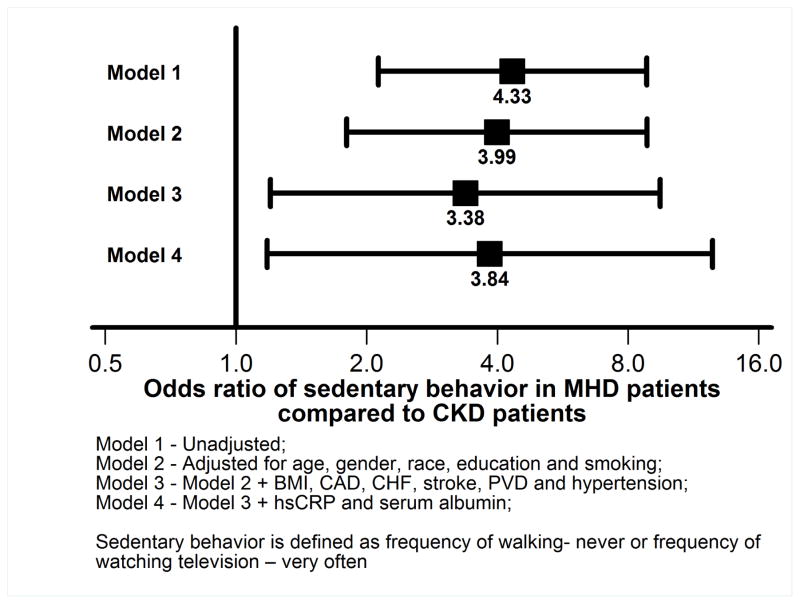
As shown in Figure 1, the frequency of sedentary behavior was greater in the MHD population. Compared to the CKD subgroup, MHD subgroup had 4.33 fold higher odds (95% CI, 2.13 to 8.83) of being classified as sedentary unadjusted for other factors (Figure 2). Even after further adjustment for demographics (model 2 in Figure 2) or comorbidity and BMI (model 3 in Figure 2) or serum albumin and CRP (model 4 in Figure 2), MHD patients had greater than 3.8 fold higher odds of being sedentary (OR 3.84, 95% CI 1.18 to 12.51 in model 4 in Figure 2).
Figure 1.
Frequency of sedentary behavior in diabetic CKD and MHD patients
Figure 2.
Associations of MHD vs. CKD status with sedentary behavior in logistic regression models
The associations of other factors with sedentary behavior in the entire cohort are summarized in Table 2. Reflecting the younger age and lower education level of the MHD sub-group, unadjusted, these two factors were associated with sedentary behavior. However, they were no longer significant in the adjusted models. Congestive heart failure and peripheral vascular disease were strongly associated with sedentary behavior in unadjusted and adjusted models (Table 2), albeit the confidence intervals were wider in the adjusted models. Higher BMI was associated with greater odds of sedentary behavior in both unadjusted and adjusted models. On the other hand, hypertension and higher serum albumin was associated with lower odds of sedentary behavior in only the unadjusted model.
Table 2.
Factors associated with sedentary behavior in univariate and multivariate logistic regression models in diabetic CKD and MHD patients
| Univariate models OR (95%CI) |
p | Multivariate model OR (95%CI) |
p | |
|---|---|---|---|---|
| MHD vs. CKD | 4.33 (2.13, 8.83) | <0.001 | 3.84 (1.18, 12.51) | 0.03 |
| Each SD ↑ in age* | 0.73 (0.52, 1.01) | 0.06 | 0.82 (0.52, 1.29) | 0.38 |
| Male gender | 0.64 (0.33, 1.24) | 0.19 | 0.54 (0.22, 1.34) | 0.18 |
| Black race | 1.46 (0.31, 6.75) | 0.63 | 0.77 (0.11, 5.26) | 0.79 |
| Education less than college | 2.53 (1.27, 5.06) | 0.01 | 1.37 (0.54, 3.50) | 0.51 |
| Smoking | 1.20 (0.60, 2.41) | 0.60 | 1.62 (0.62, 4.19) | 0.32 |
| Coronary artery disease | 1.23 (0.60, 2.52) | 0.57 | 0.57 (0.18, 1.75) | 0.32 |
| Congestive heart failure | 4.37 (1.97,9.69) | <0.001 | 4.08 (1.27, 13.07) | 0.02 |
| Stroke | 1.86 (0.71, 4.89) | 0.21 | 0.73 (0.17, 3.13) | 0.67 |
| Peripheral vascular disease | 4.43 (1.96,10.00) | <0.001 | 2.76 (0.88, 8.65) | 0.08 |
| Hypertension | 2.69 (1.03, 7.00) | 0.04 | 2.72 (0.78, 9.46) | 0.12 |
| Each SD↑ in BMI* | 1.33 (0.96, 1.84) | 0.09 | 1.78 (1.15, 2.75) | 0.009 |
| Each doubling of hsCRP | 1.16 (0.96, 1.39) | 0.13 | 0.88 (0.68, 1.13) | 0.32 |
| Each SD ↑ in serum albumin* | 0.56 (0.38, 0.82) | 0.003 | 0.79 (0.50, 1.26) | 0.33 |
Each SD of age = 12.5 (years); Each SD of BMI = 7.7 (kg/m2); Each SD of serum albumin = 0.44 (g/dL)
The possible range of scores for each of the Baecke physical activity indices is from 1 to 5. Baecke physical activity indices by the CKD and MHD subgroups are summarized in Table 3. The differences in physical activity between the CKD and MHD participants were highest in the work index followed by the sports index. The MHD group also reported lower levels of leisure time activities as well.
Table 3.
Summary of Baecke activity scores*
| CKD | MHD | P value** | |
|---|---|---|---|
| Work index score | 2.75 (2.38, 3.00) | 1.00 (1.00, 1.94) | <0.001 |
| Sports index score | 1.75 (1.50, 2.25) | 1.50 (1.25, 2.00) | 0.02 |
| Leisure index score | 2.50 (2.00, 3.00) | 2.25 (1.75, 2.75) | 0.008 |
Presented as median, 25th and 75th percentile.
P-value by Wilcoxon rank-sum test.
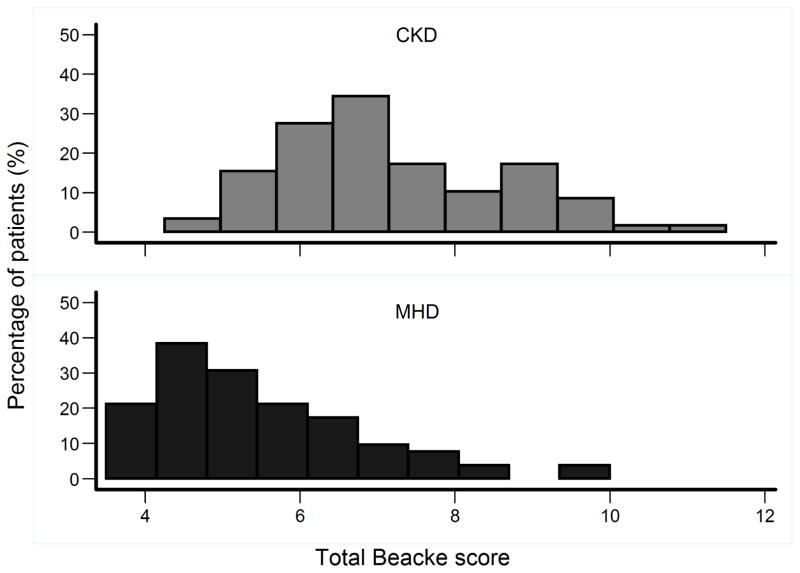
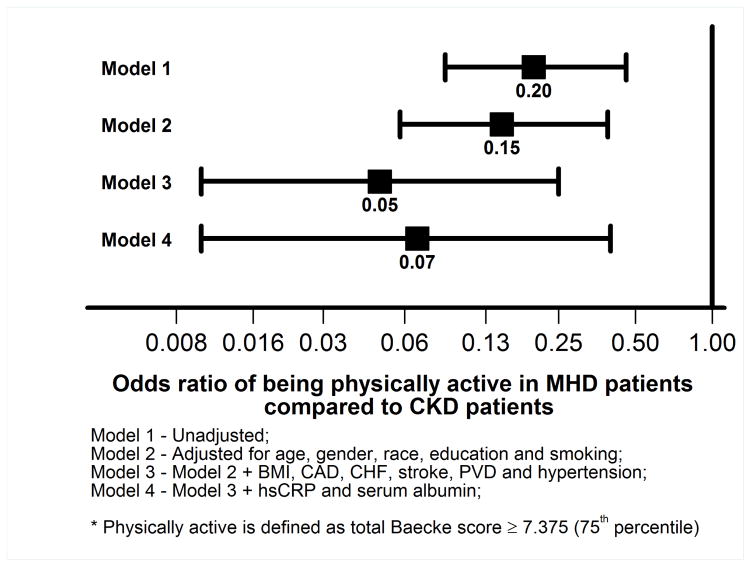
As shown in Figure 3, the distribution of the total Baecke score in the non-CKD and CKD participants were significantly different (median (25th – 75th percentiles) 6.88 (6.13 –8.25) vs. 5.25 (4.50 –6.25), p <0.001). Unadjusted, the MHD group had 80% lower odds of being physically active (defined as ≥ 75 percentile of the total Baecke activity score) as shown in Figure 4. This relationship was further strengthened when adjusted for demographics, comorbidity, serum hsCRP and albumin (Figure 4). In the multivariable logistic regression model, younger age, lower BMI, lower serum hsCRP and higher serum albumin were associated with higher odds of being physically active (Table 4).
Figure 3.
Distribution of the total Baecke score in diabetic CKD and MHD patients
Figure 4.
Associations of MHD vs. CKD status with being physically active in logistic regression models
Table 4.
Factors associated with being physically active* in univariate and multivariate logistic regression models in diabetic CKD and MHD patients
| Univariate models OR (95%CI) |
p | Multivariate model OR (95%CI) |
p | |
|---|---|---|---|---|
| MHD vs. CKD | 0.20 (0.09, 0.46) | <0.001 | 0.07 (0.01, 0.40) | 0.002 |
| Each SD ↑ in age** | 1.02 (0.71, 1.46) | 0.93 | 0.57 (0.32, 1.03) | 0.06 |
| Male gender | 1.41 (0.68, 2.94) | 0.36 | 1.33 (0.49, 3.59) | 0.58 |
| Black race | 2.35 (0.50, 10.99) | 0.28 | 9.09 (1.06, 78.09) | 0.04 |
| Education less than college | 0.73 (0.49, 1.09) | 0.12 | 1.51 (0.50, 4.51) | 0.46 |
| Smoking | 0.89 (0.41,1.94) | 0.77 | 0.53 (0.19, 1.50) | 0.23 |
| Coronary artery disease | 0.54 (0.23, 1.24) | 0.15 | 0.80 (0.22, 2.92) | 0.74 |
| Congestive heart failure | 0.56 (0.24, 1.33) | 0.19 | 0.91 (0.20, 4.07) | 0.90 |
| Stroke | 0.58 (0.22, 1.52) | 0.27 | 1.07 (0.15, 7.92) | 0.94 |
| Peripheral vascular disease | 0.32 (0.07, 1.45) | 0.14 | 1.63 (0.35, 7.72) | 0.54 |
| Hypertension | 0.49 (0.18, 1.38) | 0.18 | 0.53 (0.17, 1.62) | 0.27 |
| Each SD↑ in BMI** | 0.56 (0.24, 1.29) | 0.17 | 0.42 (0.22, 0.80) | 0.008 |
| Each doubling of hsCRP | 0.69 (0.54, 0.87) | <0.001 | 0.74 (0.55, 1.00) | 0.052 |
| Each SD ↑ in serum albumin** | 2.75 (1.65, 4.59) | <0.001 | 1.89 (1.07, 3.34) | 0.03 |
Defined as total Baecke score ≥ 7.375 (75th percentile)
Each SD of age = 12.5 (years); Each SD of BMI=7.7 (kg/m2); Each SD of serum albumin=0.44 (g/dL)
Discussion
The results of this study indicate that compared to diabetic CKD patients, sedentary behavior is more prevalent in diabetic MHD patients and this is independent of comorbidity and markers of nutrition and inflammation. Furthermore, congestive heart failure, peripheral vascular disease and higher BMI are associated with sedentary behavior in this diabetic CKD and MHD cohort. While MHD patients are also less likely to be physically active, the factors that are associated with being physically active are different from sedentary behavior; younger age, absence of coronary artery disease, lower serum hsCRP and higher serum albumin are associated with higher odds of being physically active.
The Physical Activity Guidelines for Americans recommends at least 150 min/week of moderate intensity activity or 75 min/week of vigorous intensity activity16. However, 2.5 hrs/ week of moderate/ vigorous physical activity (MVPA) is only about 2% of total awake time (assuming awake time of 16 hrs/day or 112 awake hrs/ week). Hence, even if the weekly MVPA goals are achieved, one might still spend the majority of wake hours in sedentary activities. Indeed, in 91 healthy women, aged 40–75 years, in whom physical activity was measured objectively with an accelerometer for 1 week, total time spent sitting was not different between those who reached the MVPA goals versus those who did not17. Moreover, in 4066 Australian adults who reported at least 2.5 hrs per week of MVPA, significant, detrimental dose-response associations of television-viewing time were observed with waist circumference, systolic blood pressure, and 2-h plasma glucose 22. Furthermore, increase in sedentary activity duration was associated with increased mortality independent of MVPA in the US general population 19. Hence, sedentary behavior is not merely the absence of MVPA and achieving MVPA goals does not make one non-sedentary. Therefore, addressing sedentary behavior likely needs a different approach than simply prescribing MVPA goals.
Higher levels of physical activity and lower levels of sitting time as assessed by a questionnaire were associated with a lower prevalence of CKD independently of each other and other risk factors in a cross-sectional study 8. In another study of community dwelling adults, total and light physical activities, measured objectively with an accelerometer, were found to be positively associated with kidney function 11. Television viewing time was associated with albuminuria and low eGFR in another study9. The current study results indicate that compared to non-dialysis dependent diabetic CKD patients, diabetic MHD patients are > 3 fold higher risk of being sedentary.
This strong association is not explained by demographics, comorbidity, body size, serum albumin or CRP levels. External barriers such as lack of money and access to transport as well as internal barriers such as lack of motivation and time might play important role in sedentary behavior20. Furthermore, in MHD patients, post-dialysis fatigue21 as well as anemia might be important contributors to sedentary behavior. It is also conceivable that inactivity and watching television during dialysis sessions might further induce sedentary behavior. It is also possible that factors such as peripheral vascular disease might also be mediators of the associations of uremia with sedentary behavior. Further studies are warranted to understand the relative role of these factors in sedentary behavior in MHD patients.
We noted significant associations of congestive heart failure, peripheral vascular disease, and higher BMI with sedentary behavior. It is possible that these associations are bi-directional, i.e., they are probably the causes and consequences of sedentary behavior. Therefore, interventions that target sedentary behavior might impact on cardiovascular disease in CKD and MHD patients.
In this study, based on the Baecke questionnaire, we defined sedentary behavior as “very often” watching television or “never” walking during leisure time, as prolonged television viewing or prolonged sitting time has been associated with increased risk of obesity22–23, metabolic syndrome23–24, diabetes15, 23, 25–26, cardiovascular and all-cause mortality23, 27–28. In the current study, we observed an association of sedentary behavior with congestive heart failure, peripheral vascular disease and higher BMI suggesting internal face validity of our definition of sedentary behavior. This simple two question tool based on the frequency of watching television and the frequency of walking during leisure time is easily adaptable in busy clinical practice for screening for sedentary behavior.
It is of interest that the factors associated with sedentary behavior (Table 2) are different from the factors associated with being physically active (Table 4). Of note, higher BMI is associated with both sedentary behavior and lower physical activity whereas lower CRP and higher serum albumin were associated with being physically active. We speculate that anti-inflammatory interventions might improve physical activity in CKD and MHD patients.
The clinical implications of these findings are that as sedentary behavior is much more common in hemodialysis patients, clinicians should take advantage of the frequent interactions with these patients and emphasize the importance of increased physical activity.
The limitations of the current study include the cross-sectional nature of the study that limits causal inferences. No data on physical activities before the onset of dialysis were available. Furthermore, objective measurements of physical activity were not obtained.
In summary, MHD patients are at higher risk of sedentary behavior independent of comorbidity and markers of nutrition and inflammation. Furthermore, sedentary behavior is associated with higher BMI and cardiovascular disease in CKD and dialysis populations. A simple two question tool of frequency of watching television and frequency of leisure time walking could potentially identify sedentary behavior in this high risk population. Further studies are warranted to determine whether modifying sedentary behavior reduces cardiovascular risk in CKD and MHD patients.
Practical Application.
Sedentary behavior is much more common in hemodialysis patients
Clinicians should take advantage of the frequent interactions with these patients and emphasize the importance of increased physical activity.
Acknowledgments
Supported by grants RO1- DK091437 and RO1 – DK078112 to SB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.U.S. Department of Health & Human Services. [Accessed October 6th, 2013];2008 Physical Activity Guidelines for Americans. 2008 at http://www.health.gov/paguidelines/guidelines/default.aspx#toc.
- 2. [Accessed October 17, 2013];What is Sedentary Behaviour? 2013 http://www.sedentarybehaviour.org/what-is-sedentary-behaviour/
- 3.Padilla J, Krasnoff J, Da Silva M, et al. Physical functioning in patients with chronic kidney disease. J Nephrol. 2008 Jul-Aug;21(4):550–559. [PubMed] [Google Scholar]
- 4.Anand S, Chertow GM, Johansen KL, et al. Association of self-reported physical activity with laboratory markers of nutrition and inflammation: the Comprehensive Dialysis Study. J Ren Nutr. 2011 Nov;21(6):429–437. doi: 10.1053/j.jrn.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eidemak I, Feldt-Rasmussen B, Kanstrup IL, Nielsen SL, Schmitz O, Strandgaard S. Insulin resistance and hyperinsulinaemia in mild to moderate progressive chronic renal failure and its association with aerobic work capacity. Diabetologia. 1995 May;38(5):565–572. doi: 10.1007/BF00400725. [DOI] [PubMed] [Google Scholar]
- 6.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T. Physical Activity and Mortality in Chronic Kidney Disease (NHANES III) Clin J Am Soc Nephrol. 2009 Oct 9; doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stengel B, Tarver-Carr ME, Powe NR, Eberhardt MS, Brancati FL. Lifestyle factors, obesity and the risk of chronic kidney disease. Epidemiology. 2003 Jul;14(4):479–487. doi: 10.1097/01.EDE.0000071413.55296.c4. [DOI] [PubMed] [Google Scholar]
- 8.Bharakhada N, Yates T, Davies MJ, et al. Association of sitting time and physical activity with CKD: a cross-sectional study in family practices. Am J Kidney Dis. 2012 Oct;60(4):583–590. doi: 10.1053/j.ajkd.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 9.Lynch BM, White SL, Owen N, Healy GN, Dunstan DW. Television viewing time and risk of chronic kidney disease in adults: the AusDiab Study. Annals of Behavioral Medicine. 2010;40(3):265–274. doi: 10.1007/s12160-010-9209-1. [DOI] [PubMed] [Google Scholar]
- 10.Akber A, Portale AA, Johansen KL. Pedometer-assessed physical activity in children and young adults with CKD. Clin J Am Soc Nephrol. 2012 May;7(5):720–726. doi: 10.2215/CJN.06330611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins MS, Sevick MA, Richardson CR, Fried LF, Arena VC, Kriska AM. Association between physical activity and kidney function: National Health and Nutrition Examination Survey. Medicine and science in sports and exercise. 2011;43(8):1457–1464. doi: 10.1249/MSS.0b013e31820c0130. [DOI] [PubMed] [Google Scholar]
- 12.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982 Nov;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 13.Hertogh EM, Monninkhof EM, Schouten EG, Peeters PH, Schuit AJ. Validity of the modified Baecke questionnaire: comparison with energy expenditure according to the doubly labeled water method. The international journal of behavioral nutrition and physical activity. 2008;5:30. doi: 10.1186/1479-5868-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono R, Hirata S, Yamada M, Nishiyama T, Kurosaka M, Tamura Y. Reliability and validity of the Baecke physical activity questionnaire in adult women with hip disorders. BMC musculoskeletal disorders. 2007;8:61. doi: 10.1186/1471-2474-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003 Apr 9;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health & Human Services. [Accessed October 6th, 2013];2008 Physical Activity Guidelines for Americans. 2008 at http://www.health.gov/paguidelines/guidelines/default.aspx#toc.
- 17.Craft LL, Zderic TW, Gapstur SM, et al. Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. The international journal of behavioral nutrition and physical activity. 2012;9:122. doi: 10.1186/1479-5868-9-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008 Apr;31(4):661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 19.Koster A, Caserotti P, Patel KV, et al. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One. 2012;7(6):e37696. doi: 10.1371/journal.pone.0037696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinn DJ, White M, Harland J, Drinkwater C, Raybould S. Barriers to physical activity and socioeconomic position: implications for health promotion. Journal of Epidemiology and Community Health. 1999;53(3):191. doi: 10.1136/jech.53.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon P, Doyle J, Johansen K. Postdialysis fatigue is associated with sedentary behavior. Clinical nephrology. 2011;75(5) [PubMed] [Google Scholar]
- 22.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. med sci sports exerc. 2008;40(4):639–645. doi: 10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- 23.Thorp AA, Owen N, Neuhaus M, Dunstan DW. Sedentary behaviors and subsequent health outcomes in adults: a systematic review of longitudinal studies, 1996–2011. American journal of preventive medicine. 2011;41(2):207–215. doi: 10.1016/j.amepre.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Gardiner PA, Healy GN, Eakin EG, et al. Associations between television viewing time and overall sitting time with the metabolic syndrome in older men and women: the Australian Diabetes, Obestity, and Lifestyle study. J Am Geriatr Soc. 2011;59(5):788–796. doi: 10.1111/j.1532-5415.2011.03390.x. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001 Jun 25;161(12):1542–1548. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007 Nov;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 27.Dunstan DW, Barr EL, Healy GN, et al. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010 Jan 26;121(3):384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- 28.Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Medicine and science in sports and exercise. 2009 May;41(5):998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]