Abstract
Procyanidins are available in the diet from sources such as cocoa and grapes. Procyanidins are unique in that they are comprised of repeating monomeric units and can exist in various degrees of polymerization. The degree of polymerization plays a role in determining the biological activities of procyanidins. However, generalizations cannot be made regarding the correlation between procyanidin structure and bioactivity, because the size-activity relationship appears to be system-dependent. Our aim was to screen fractions of procyanidins with differing degrees of polymerization in vitro for anti-inflammatory activities in models of colonic inflammation. Monomeric, oligomeric, and polymeric cocoa procyanidin fractions were screened using cell models of disrupted membrane integrity and inflammation in human colon cells. High molecular weight polymeric procyanidins were the most effective at preserving membrane integrity and reducing secretion of interleukin-8 in response to inflammatory stimuli. Conversely, oligomeric procyanidins appeared to be the least effective. These results suggest that polymeric cocoa procyanidins may be the most effective for preventing loss of gut barrier function and epithelial inflammation, which are critical steps in the pathogenesis of metabolic endotoxemia, inflammatory bowel disease, and colon cancer. Therefore, further investigations of the potential health-protective benefits of cocoa procyanidins with distinct degrees of polymerization, particularly high molecular weight procyanidins, are warranted.
Keywords: Cocoa, procyanidins, degree of polymerization, inflammation, colon, permeability
1. INTRODUCTION
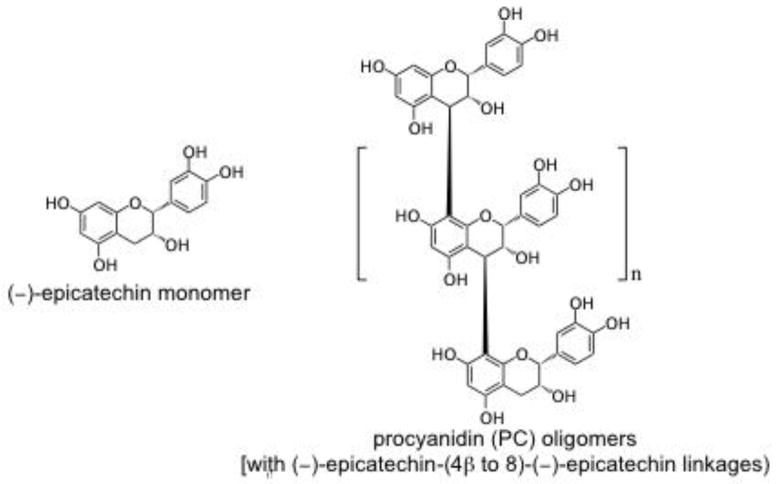
Flavan-3-ols, also referred to as flavanols or procyanidins (PCs), are a subclass of flavonoids comprised of monomers [(±)-catechin (C), (−)-epicatechin (EC), etc.], oligomers, and polymers. Flavanols are characterized by their degree of polymerization (DP, the number of monomeric residues in an oligomer or polymer) [1] and the mean DP (mDP), the average DP of all flavanols in a matrix. PCs are widely available in the diet, from sources such as cocoa, grapes, apples, and berries [2]. Examples of flavan-3-ols found in cocoa are shown in Figure 1. PCs have been widely studied for their biological activities related to prevention or amelioration of acute and chronic diseases. DP appears to play a role in determining PC efficacy in models of disease including inflammation [3–7] and cancer [8–14]. Unfortunately, no broad generalizations can be made regarding the correlation between PC structure and bioactivity because the DP-activity relationship appears to be system-dependent. In some cases, activity is directly proportional to DP; in other cases the reverse is true [15]. Moreover, in some systems, there appears to be an “optimum DP”, above and below which activity is reduced.
Figure 1.
Structures of selected representative flavan-3-ols found in cocoa: (−)-epicatechin monomer and procyanidin oligomers comprised of (−)-epicatechin monomers linked (4β → 8).
Bioavailability, which is roughly inversely proportional to DP, represents one of the factors further complicating the interpretation of PC biological activity. Reported oral bioavailability is generally <10% for monomers (although up to 55% has been reported for cocoa catechins) [16, 17], much lower for small procyanidins, and essentially 0% for large procyanidins [17–20]. In vitro assays that predict a positive DP-activity relationship may not translate to in vivo feeding studies, where poor bioavailability may severely limit tissue exposure and subsequent bioactivity of large PCs. Interestingly, however, the relative bioactivity of PCs in vivo does not necessarily correspond to their relative bioavailability [21, 22].
In vitro screening assays may be most useful for predicting PC activities in the lumen or epithelium of the gastrointestinal tract, where direct contact with higher concentrations of PCs is possible. Such activities include, but are not limited to, inhibition of luminal and brush border digestive enzymes, modulation of intestinal barrier function and endotoxin uptake, and modulation of intestinal inflammation, proliferation, and apoptosis. These activities are critical mediators of obesity, diabetes, cancer, and inflammatory diseases that can be modulated by PCs [23, 24].
Cocoa is one of the most flavanol-rich food products and contains PCs of a wide range of DP [1, 25–29]. Recently, we successfully fractionated a cocoa PC extract into monomer-, oligomer-, and polymer-rich fractions and reported that the oligomeric fraction appears to provide enhanced protection against diet-induced obesity and type-2 diabetes compared to monomeric or polymeric procyanidins [22]. These data prompted us to screen these fractions of similarly sized cocoa procyanidins in vitro for anti-inflammatory activities in models of colonic inflammation. We hypothesized that distinct cocoa procyanidin fractions (i.e. groups of similar PCs) containing distinct mDPs would exhibit distinct anti-inflammatory activities.
2. MATERIALS AND METHODS
2.1 Cocoa Procyanidin Fractions
A procyanidin-rich cocoa extract (CE) and fractions with distinct flavanol compositions (monomer-, oligomer-, and polymer-rich fractions) were produced from commercially available cocoa powder as described previously [22]. The concentrations and enrichment of specific cocoa procyanidins in each fraction is presented in Supplementary Table 1. The normal-phase HPLC flavanol profiles of CE and cocoa PC fractions are shown in Supplementary Figure 1. Additionally, a cocoa polymer fraction with greater enrichment of high MW PCs (92% by weight of DP7+) was a generous gift from The Hershey Co. (Hershey, PA the fraction was originally prepared for Hershey by Planta Analytica, Danbury, CT). The composition of this fraction is shown in Table 1.
Table 1.
Composition of a cocoa extract fraction enriched for high molecular weight polymeric procyanidins (DP 7+)
| DP | % (w/w) |
|---|---|
| 7 | 21 |
| 8 | 20 |
| 9 | 20 |
| 10 | 15 |
| 11 | 9 |
| 12 | 7 |
| other | 8 |
2.2 Normal-phase HPLC Analysis
The PC composition of the DP7+ fraction was evaluated by normal-phase HPLC profiling [22, 30]. Analyses were performed on an Agilent Technologies (Santa Clara, CA) 1260 Infinity HPLC equipped with a solvent degasser, quaternary pump, an autosampler with temperature control, a thermostat column compartment, and a fluorescence detector (FLD). Separations were carried out using a Develosil Diol column (100 Å, 250 × 4.6 mm, 5 μm particle size) equipped with a Luna HILIC guard column (4 × 3.0 mm ID SecurityGuard cartridge and cartridge holder) (both from Phenomenex, Torrance, CA). The column temperature was 35°C. Binary gradient elution employing 2% acetic acid (v/v) in ACN (phase A) and 2% acetic acid (v/v) and 3% ddH2O (v/v) in MeOH (phase B) was performed at a flow rate of 1 mL/min. The gradient was as follows: 93% A at 0 min, 93% A at 3 min, 62.4% A at 60 min, 0.0% A at 63 min, 0.0% A at 70 min, 93.0% A at 76 min, 7.0% B at 0 min, 7.0% B at 3 min, 37.6% B at 60 min, 100.0% B at 63 min, 100.0% B at 70 min, and 7.0% B at 76 min. FLD excitation and emission wavelengths were 230 nm and 321 nm, respectively. The DP7+ fraction was prepared at 10 mg/mL in acetone: water: acetic acid (70:28:2, v/v/v) immediately prior to analysis. Samples were held at 5 °C in the autosampler before injection. Injection volume was 5 μL. Mixtures of authentic standards consisting of monomers (DP 1: C, EC, ECG), PC oligomers (dimers-hexamers), and PC polymers (heptamers-decamers) were prepared and used as a reference for comparison of elution profiles as described previously [22].
2.3 Cell Culture Conditions
Caco-2 and HT-29 human colon cancer cells (American Type Culture Collection, Manassas, VA) were maintained in sub-confluence in Dulbecco’s Modification of Eagle’s medium (DMEM) or McCoy’s 5A medium, respectively. All medium was supplemented with 10% fetal bovine serum, 100 IU/mL penicillin and 100 μg/mL streptomycin at 37°C under 5% CO2 atmosphere. Cells were subcultured by trypsinization.
2.4 Colon Permeability Assay
The ability of cocoa fractions to mitigate colon permeability in vitro was examined by measuring the apical to basolateral flux of fluorescein isothiocyanate–dextran (FITC-D) across differentiated Caco-2 cell monolayers as described previously [31]. In brief, Caco-2 cells were seeded in polycarbonate transwell inserts (0.33cm2 area and 0.4 μm pore size, Corning Life Sciences, Tewksbury, MA) and allowed to reach confluence and differentiate for 21 d. Only monolayers with a transepithelial electrical resistance (TEER) of greater than 500 Ω cm2 were used for experiments [32]. Differentiated monolayers were treated with final concentrations of 100 μg/mL cocoa extract, 100 μg/mL cocoa procyanidin fractions, 10–25 μg/mL DP7+ cocoa fraction, or vehicle only (dimethylsulfoxide, DMSO) 2 h prior to addition of 2% dextran sodium sulfate (DSS, avg. MW = 40,000 Da, MP Biomedicals, Solon, OH) to the media to induce loss of epithelial membrane integrity [33]. Cells were then co-incubated for an additional 48 h. Fluorescein isothiocyanate (FITC)-labeled dextran (MW = 4000 Da, Sigma-Aldrich, St. Louis, MO) was added to the apical compartment at a final concentration of 1 mg/mL, and cells were incubated for 6 h. Basolateral media (50 μL) were removed every 30 min and fluorescence determined using a Fluoroskan Ascent FL fluorescent plate reader (λex = 493 nm, λem= 517 nm, Thermo Scientific, Waltham, MA). The rate of increase in basolateral fluorescence was determined and normalized to the values derived from monolayers not treated with DSS. Representative FITC flux rates in cells are shown in Supplementary Figure 2.
2.5 Colon Inflammation Assay
HT-29 cells (1 × 106/well) were plated in 24-well plates and allowed to reach 80% confluence. Cocoa extract or PC fractions were added to each well at final concentrations of 100 μg/mL, or 10–25 μg/mL for the DP7+ cocoa fraction. After pretreatment for 24 h, cells were stimulated with 5 ng/mL, tumor necrosis factor (TNF)-α (Peprotech, Inc., Rocky Hill, NJ) for 6 h. Interleukin (IL)-8 levels in the medium were determined by ELISA (R&D Systems, Minneapolis, MN).
2.6 Data and Statistical Analysis
Statistical analyses were performed using Prism (v 6.0, GraphPad Software, La Jolla, CA). Data were analyzed by one-way ANOVA with Tukey’s HSD post-hoc test to compare all treatment means. Statistical significance was defined as P < 0.05.
3. RESULTS
3.1 DP7+ Fraction Composition
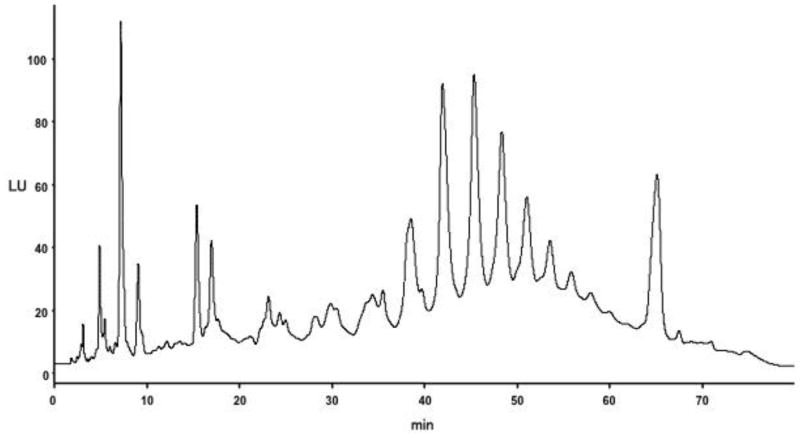
The PC profile of the DP7+ fraction was analyzed by normal-phase HPLC, and is shown in Figure 2. This fraction is highly enriched for high MW (late-eluting) PCs compared to low MW (early-eluting) PCs [22]. This fraction is even more highly enrich for high MW PCs compared to the polymer-rich fraction prepared from cocoa extract (see Supplementary Figure 1), which we characterized previously [22].
Figure 2.
Normal-phase HPLC chromatogram (with fluorescence detection) of a DP7+ cocoa PC fraction.
3.2 Epithelial Permeability Assay
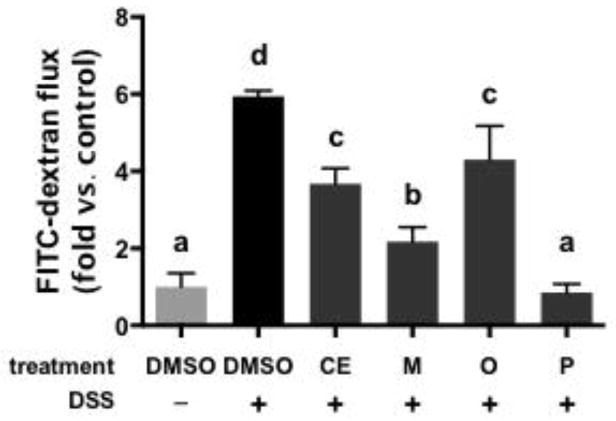
The ability of cocoa PCs to protect intestinal epithelial cells from DSS-induced loss of membrane integrity is shown in Figure 3. DSS significantly increased apical → basolateral flux of FITC-dextran compared to vehicle (DMSO) treated cells. CE and all cocoa PC fractions significantly inhibited DSS-induced loss of barrier function. The polymer-rich fraction possessed the greatest protective activity, followed by the monomer-rich fraction. CE and the oligomer-rich fraction were least protective. The results for CE and the monomer-rich fraction correlate with our previous study, in which we found that these were the least and most effective at reducing serum levels of endotoxin, respectively, in high fat-fed mice [22]. By contrast, the data from the oligomer- and polymer-rich fractions do not correlate well with our previous study.
Figure 3.
Impact of cocoa extract (CE) and cocoa procyanidin fractions (monomer-, oligomer- and polymer-rich fractions: M, O, and P, respectively) (100 μg/mL) on the barrier integrity of differentiated Caco-2 cells exposed to dextran sodium sulfate (DSS), as measured by transmembrane permeability to FITC-dextran. Data represent the mean of two independent experiments (n = 3 per experiment) ± SEM. Different superscript letters indicate P < 0.05 by one way ANOVA with Tukey’s HSD post-test.
3.3 Inflammation Assay
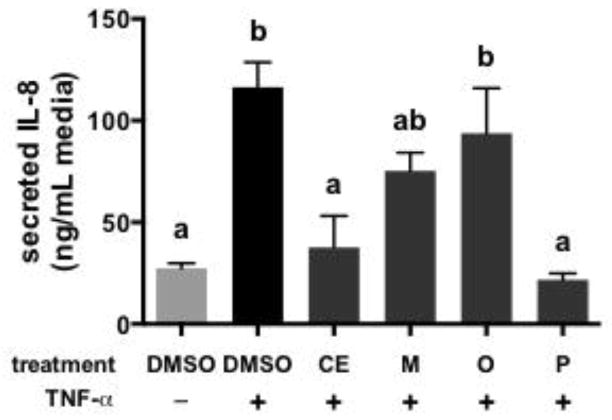
The anti-inflammatory activities of cocoa PCs are shown in Figure 4. Stimulation of cells with TNF-α induced a significant increase in IL-8 production compared to unstimulated cells. CE reduced IL-8 secretion to levels that were similar to unstimulated cells. Among the individual cocoa PC fractions, only the polymer-rich fraction significantly reduced IL-8 secretion compared to vehicle. This result is consistent with data showing that polymeric PCs more effectively inhibited NF-κB activation and secretion of pro-inflammatory eicosanoids (PGE2) and cytokines (TNF-α) in endotoxin-stimulated mouse macrophages compared to oligomeric PCs [3]. This also agrees with data showing that only cocoa PCs with DP ≥ 4 were able to stimulate secretion of anti-inflammatory IL-4 in human peripheral blood mononuclear cells [6]. In terms of inflammatory-associated cancer, the present result is consistent with in vitro studies showing that cytotoxicity and anti-cancer activities of PCs are positively correlated with DP [10–12, 14].
Figure 4.
Impact of cocoa extract (CE) and cocoa procyanidin fractions (monomer-, oligomer- and polymer-rich fractions: M, O, and P, respectively) on the secretion of IL-8 by HT-29 human colon cancer cells stimulated with TNF-α. Data represent the mean of two independent experiments (n = 2 per experiment) ± SEM. Different superscript letters indicate P < 0.05 by one-way ANOVA with Tukey’s HSD post-test.
3.4 Epithelial Permeability Assay (DP 7+)
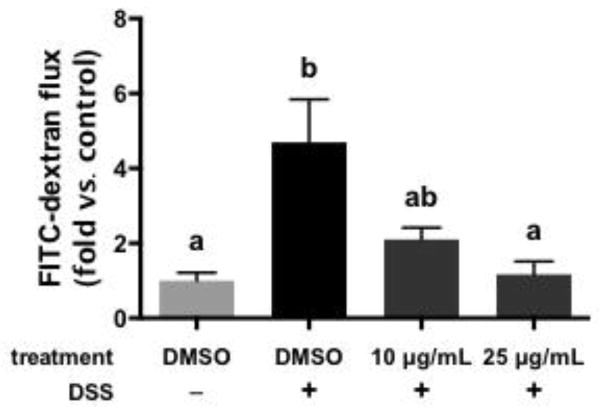
In order to further confirm the impacts of high MW cocoa PCs in these models, a more concentrated extract (92% by weight PCs with DP 7+) was assayed for activity using the same models. Due to the greater enrichment for DP 7-12 compared to the polymer fraction previously assayed, lower concentrations (10–25 μg/mL) were employed for the DP 7+ fraction. The ability of this fraction to protect intestinal epithelial cells from DSS-induced loss of membrane integrity is shown in Figure 5. Similar to the previous experiment, the DP 7+ enriched fraction inhibited the effects of DSS on membrane integrity in a dose dependent-fashion (10 μg/mL partly inhibited the effects of DSS, while 25 μg/mL completely blocked the effects of DSS).
Figure 5.
Impact of a high molecular weight polymeric cocoa procyanidin extract (DP 7+, 10 or 25 μg/mL) on the barrier integrity of differentiated Caco-2 cells exposed to dextran sodium sulfate (DSS), as measured by transmembrane permeability to FITC-dextran. Data represent the mean ± SEM (n = 3). Different superscript letters indicate P < 0.05 by one way ANOVA with Tukey’s HSD post-test.
3.5 Inflammation Assay (DP 7+)
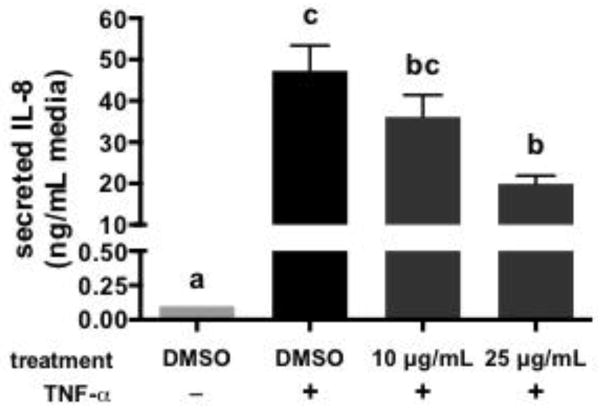
The anti-inflammatory activities of the DP 7+ fraction are shown in Figure 6. The DP 7+ enriched fraction blunted increases in IL-8 secretion induced by TNF-α stimulation. The effects of the DP 7+ fraction were dose-dependent: 10 μg/mL did not significantly decrease IL-8 secretion compared to vehicle, while 25 μg/mL significantly decreased IL-8 secretion compared to vehicle control but not to the level of IL-8 in unstimulated cells. It should be noted that the basal (unstimulated) and stimulated vehicle control levels of IL-8 in the cells were very different between the first and second experiments (25 vs. 0.1 ng/mL for unstimulated, 120 vs. 45 ng/mL for stimulated vehicle control, Figure 4 vs. Figure 6). These differences may be due to differences in cell passage number and/or degradation of the TNF-α stock used for stimulation. Nevertheless, TNF-α induced a significant increase in IL-8 in both experiments, which was inhibited to varying degrees by the cocoa extract or cocoa fractions.
Figure 6.
Impact of a high molecular weight polymeric cocoa procyanidin extract (DP 7+, 10 or 25 μg/mL) on the secretion of IL-8 by HT-29 human colon cancer cells stimulated with TNF-α. Data represent the mean ± SEM (n = 3). Different superscript letters indicate P < 0.05 by one-way ANOVA with Tukey’s HSD post-test.
4. DISCUSSION
The results of the present study suggest that high MW polymeric cocoa PCs may be the most effective for preventing loss of gut barrier function and epithelial inflammation, which are critical steps in the pathogenesis of metabolic endotoxemia, inflammatory bowel disease, and colon cancer. Previous studies have shown that cocoa possesses anti-cancer and anti-inflammatory activities in the colon [34–37], but the present data indicate that the polymers are the most effective of the PC components of cocoa. These activities may be particularly translatable to in vivo situations, as they are epithelial activities that do not require systemic bioavailability for activity. Therefore, polymeric PCs may effectively modulate these key mechanistic targets, resulting in improved disease prevention or reduced disease severity. This may imply that enriching the polymeric PC content of cocoa (by altering horticultural practices, fermentation, drying, roasting, processing, etc.) or developing specific PC polymer-rich matrices may result in products with enhanced anti-inflammatory activities. Additional research is required in appropriate animal models of metabolic endotoxemia (high-fat feeding), colon inflammation (DSS-induced inflammation, or bacterial colitis in IL-10 null mouse models), and colon cancer [azoxymethane (AOM) or AOM+DSS models] in order to further elucidate the potential activities of polymeric PCs. However, such in vivo studies are difficult due to the time and cost associated with isolating sufficient quantities of individual PCs or purified PC fractions to conduct lengthy animal studies [22]. Efficient fractionation or other strategies are thus needed to facilitate these studies as well as translation to human clinical work.
Interestingly, although cocoa oligomers appeared to be the least effective at protecting epithelial membrane integrity and preventing inflammation in the present study, this fraction was the most effective at preventing weight gain and development of glucose intolerance and insulin resistance in mice fed a high fat diet in our previous study [22]. This highlights the fact that the activities of PCs, and the relationship between DP and activity, are highly context-dependent. Therefore, interventions using custom PC profiles to prevent or ameliorate chronic diseases should be carefully tailored to match composition to the desired activity. Nevertheless, this study and our previous work [38, 39] highlight the potential bioactivities of larger PCs, which have received little attention (compared to the monomeric and dimeric PCs) due to complexities associated with their qualitative and quantitative analysis, as well as scarcity of authentic standards. This work highlights the need for novel approaches to elucidate the activities of these complex compounds. Future work is needed to isolate, purify, characterize and test purified individual compounds within the high MW polymeric fraction, although this approach is difficult with existing technologies.
Supplementary Material
Acknowledgments
The authors acknowledge Caroline Ryan, Laura Griffin and Dr. Katherine Thompson-Witrick (Dept. of Food Science and Technology, Virginia Tech) for assistance with characterization of cocoa extract and fractions. Start-up research funding for A.P. Neilson from the Department of Food Science and Technology and the College of Agriculture and Life Sciences at Virginia Polytechnic Institute and State University was used to support this research. The work was also supported in part by NIH grant AT004678 to J.D. Lambert.
Abbreviations
- C
(±)-catechin
- EC
(−)-epicatechin
- AOM
azoxymethane
- CE
cocoa extract
- DP
degree of polymerization
- DSS
dextran sodium sulfate
- DMSO
dimethylsulfoxide
- FITC
fluorescein isothiocyanate
- LU
fluorescence units
- IL
interleukin
- mDP
mean degree of polymerization
- M
monomer-rich fraction
- O
oligomer-rich fraction
- P
polymer-rich fraction
- PCs
procyanidins
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robbins RJ, Leonczak J, Li J, Johnson JC, Collins T, Kwik-Uribe C, et al. Determination of flavanol and procyanidin (by degree of polymerization 1-10) content of chocolate, cocoa liquors, powder(s), and cocoa flavanol extracts by normal phase high-performance liquid chromatography: collaborative study. J AOAC Int. 2012;95:1153–60. doi: 10.5740/jaoacint.12-162. [DOI] [PubMed] [Google Scholar]
- 2.Hammerstone JF, Lazarus SA, Schmitz HH. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2000;130:2086S–92S. doi: 10.1093/jn/130.8.2086S. [DOI] [PubMed] [Google Scholar]
- 3.Gentile C, Allegra M, Angileri F, Pintaudi AM, Livrea MA, Tesoriere L. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. European Journal of Nutrition. 2012;51:353–63. doi: 10.1007/s00394-011-0220-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee YA, Kim YJ, Cho EJ, Yokozawa T. Ameliorative effects of proanthocyanidin on oxidative stress and inflammation in streptozotocin-induced diabetic rats. J Agric Food Chem. 2007;55:9395–400. doi: 10.1021/jf071523u. [DOI] [PubMed] [Google Scholar]
- 5.Lee YA, Cho EJ, Yokozawa T. Effects of Proanthocyanidin Preparations on Hyperlipidemia and Other Biomarkers in Mouse Model of Type 2 Diabetes. J Agric Food Chem. 2008;56:7781–9. doi: 10.1021/jf800639m. [DOI] [PubMed] [Google Scholar]
- 6.Mao T, Powell J, Van de Water J, Keen C, Schmitz H, Gershwin M. Effect of cocoa procyanidins on the secretion of interleukin-4 in peripheral blood mononuclear cells. Journal of Medicinal Food. 2000;3:107–14. [Google Scholar]
- 7.Tatsuno T, Jinno M, Arima Y, Kawabata T, Hasegawa T, Yahagi N, et al. Anti-inflammatory and Anti-melanogenic Proanthocyanidin Oligomers from Peanut Skin. Biological and Pharmaceutical Bulletin. 2012;35:909–16. doi: 10.1248/bpb.35.909. [DOI] [PubMed] [Google Scholar]
- 8.Zhou K, Raffoul JJ. Potential Anticancer Properties of Grape Antioxidants. Journal of Oncology. 2012;2012:8. doi: 10.1155/2012/803294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin MA, Goya L, Ramos S. Potential for preventive effects of cocoa and cocoa polyphenols in cancer. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;56:336–51. doi: 10.1016/j.fct.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt BM, Howell AB, McEniry B, Knight CT, Seigler D, Erdman JW, et al. Effective Separation of Potent Antiproliferation and Antiadhesion Components from Wild Blueberry (Vaccinium angustifolium Ait.) Fruits. J Agric Food Chem. 2004;52:6433–42. doi: 10.1021/jf049238n. [DOI] [PubMed] [Google Scholar]
- 11.Gosse F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, et al. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26:1291–5. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- 12.Ugartondo V, Mitjans M, Touriño S, Torres JL, Vinardell MaP. Comparative Antioxidant and Cytotoxic Effect of Procyanidin Fractions from Grape and Pine. Chemical Research in Toxicology. 2007;20:1543–8. doi: 10.1021/tx700253y. [DOI] [PubMed] [Google Scholar]
- 13.Torres JLs, Varela Ba, García MaT, Carilla J, Matito C, Centelles JJ, et al. Valorization of Grape (Vitis vinifera) Byproducts. Antioxidant and Biological Properties of Polyphenolic Fractions Differing in Procyanidin Composition and Flavonol Content. J Agric Food Chem. 2002;50:7548–55. doi: 10.1021/jf025868i. [DOI] [PubMed] [Google Scholar]
- 14.Lizarraga D, Lozano C, Briede JJ, van Delft JH, Tourino S, Centelles JJ, et al. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J. 2007;274:4802–11. doi: 10.1111/j.1742-4658.2007.06010.x. [DOI] [PubMed] [Google Scholar]
- 15.Andriambeloson E, Magnier C, Haan-Archipoff G, Lobstein A, Anton R, Beretz A, et al. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J Nutr. 1998;128:2324–33. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- 16.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. American Journal of Clinical Nutrition. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 17.Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Molecular Aspects of Medicine. 2010;31:446–67. doi: 10.1016/j.mam.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Prasain JK, Peng N, Dai YY, Moore R, Arabshahi A, Wilson L, et al. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–43. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serra A, Macià A, Romero M-P, Valls J, Bladé C, Arola L, et al. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br J Nutr. 2010;103:944–52. doi: 10.1017/S0007114509992741. [DOI] [PubMed] [Google Scholar]
- 20.Deprez S, Mila I, Huneau JF, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxidants & Redox Signaling. 2001;3:957–67. doi: 10.1089/152308601317203503. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita Y, Okabe M, Natsume M, Ashida H. Comparison of Anti-Hyperglycemic Activities Between Low- and High-Degree of Polymerization Procyanidin Fractions from Cacao Liquor Extract. J Food Drug Anal. 2012;20:283–7. [Google Scholar]
- 22.Dorenkott MR, Griffin LE, Goodrich KM, Thompson-Witrick KA, Fundaro G, Ye L, et al. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J Agric Food Chem. 2014;62:2216–27. doi: 10.1021/jf500333y. [DOI] [PubMed] [Google Scholar]
- 23.Goodrich KM, Fundaro G, Griffin LE, Grant A, Hulver MW, Ponder MA, et al. Chronic administration of dietary grape seed extract increases colonic expression of gut tight junction protein occludin and reduces fecal calprotectin in a secondary analysis of healthy Wistar Furth rats. Nutr Res. 2012;32:787–94. doi: 10.1016/j.nutres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Gu Y, Yu S, Park JY, Harvatine K, Lambert JD. Dietary cocoa reduces metabolic endotoxemia and adipose tissue inflammation in high-fat fed mice. The Journal of Nutritional Biochemistry. 2013:439–45. doi: 10.1016/j.jnutbio.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerstone JF. Procyanidin content and variation in some commonly consumed foods. J Nutr. 2000;130:2086S. doi: 10.1093/jn/130.8.2086S. [DOI] [PubMed] [Google Scholar]
- 26.Lee KW. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51:7292–5. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 27.Crozier SJ, Preston AG, Hurst JW, Payne MJ, Mann J, Hainly L, et al. Cacao seeds are a “Super Fruit”: A comparative analysis of various fruit powders and products. Chem Cent J. 2011;5 doi: 10.1186/1752-153X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wollgast J, Anklam E. Review on polyphenols in Theobroma cacao: changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int. 2000;33:423–47. [Google Scholar]
- 29.Gu LW, House SE, Wu XL, Ou BX, Prior RL. Procyanidin and catechin contents and antioxidant capacity of cocoa and chocolate products. J Agric Food Chem. 2006;54:4057–61. doi: 10.1021/jf060360r. [DOI] [PubMed] [Google Scholar]
- 30.Robbins RJ. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography–fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. Journal of chromatography. 2009;1216:4831–40. doi: 10.1016/j.chroma.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, Zhang H, Wu H, Li H, Liu L, Guo J, et al. Protective role of 1,25(OH)2vitamin D3 in the mucosal injury and epithelial barrier disruption in DSS-induced acute colitis in mice. BMC Gastroenterology. 2012;12:57. doi: 10.1186/1471-230X-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranaldi G, Consalvo R, Sambuy Y, Scarino ML. Permeability characteristics of parental and clonal human intestinal Caco-2 cell lines differentiated in serum-supplemented and serum-free media. Toxicology in vitro : an international journal published in association with BIBRA. 2003;17:761–7. doi: 10.1016/s0887-2333(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 33.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. BioMed Research International. 2012;2012 doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodríguez-Ramiro I, Ramos S, López-Oliva E, Agis-Torres A, Gómez-Juaristi M, Mateos R, et al. Cocoa-rich diet prevents azoxymethane-induced colonic preneoplastic lesions in rats by restraining oxidative stress and cell proliferation and inducing apoptosis. Mol Nutr Food Res. 2011;55:1895–9. doi: 10.1002/mnfr.201100363. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Ramiro I, Ramos S, López-Oliva E, Agis-Torres A, Bravo L, Goya L, et al. Cocoa polyphenols prevent inflammation in the colon of azoxymethane-treated rats and in TNF-α-stimulated Caco-2 cells. Br J Nutr. 2013;110:206–15. doi: 10.1017/S0007114512004862. [DOI] [PubMed] [Google Scholar]
- 36.Andujar I, Recio MC, Giner RM, Cienfuegos-Jovellanos E, Laghi S, Muguerza B, et al. Inhibition of Ulcerative Colitis in Mice after Oral Administration of a Polyphenol-Enriched Cocoa Extract Is Mediated by the Inhibition of STAT1 and STAT3 Phosphorylation in Colon Cells. J Agric Food Chem. 2011;59:6474–83. doi: 10.1021/jf2008925. [DOI] [PubMed] [Google Scholar]
- 37.Perez-Berezo T, Franch A, Castellote C, Castell M, Perez-Cano FJ. Mechanisms involved in down-regulation of intestinal IgA in rats by high cocoa intake 2012. 2012;23:838–44. doi: 10.1016/j.jnutbio.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Turton M, O’shea D, Gunn I, Beak S, Edwards C, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 39.Gu YY, Hurst WJ, Stuart DA, Lambert JD. Inhibition of Key Digestive Enzymes by Cocoa Extracts and Procyanidins. J Agric Food Chem. 2011;59:5305–11. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.