Abstract
Background
Trimethylamine-N-oxide (TMAO) is a product of metabolism of phosphatidylcholine (lecithin) and carnitine by the intestinal microbiome. Elevated serum concentrations of TMAO have been linked to adverse cardiovascular outcomes in the general population. We examined correlates of serum TMAO and the relations among serum TMAO concentrations, all-cause mortality and cardiovascular mortality and hospitalizations in a nationally derived cohort of patients new to hemodialysis (HD).
Methods
We quantified serum TMAO by liquid chromatography and online tandem mass spectrometry and assessed nutritional and cardiovascular risk factors in 235 patients receiving hemodialysis and measured TMAO in pooled serum from healthy controls. We analyzed time to death and time to cardiovascular death or hospitalization using Cox proportional hazards regression.
Results
Serum TMAO concentrations (median 43, (25th – 75th percentile 28–67 µM/L) were elevated compared to persons with normal or near normal kidney function (1.41 ± 0.49 µM/L). TMAO was directly correlated with serum albumin (Spearman rank correlation 0.24, 95% CI 0.12, 0.35; P < 0.001), prealbumin (0.19, 95% CI 0.07, 0.31; P =0.003), and creatinine (0.21, 95% CI 0.08, 0.33; P =0.002), and inversely correlated with log CRP (−0.18, 95% CI −0.30, – 0.06; P =0.005). Higher serum concentrations of TMAO were not significantly associated with time to death (0.84, CI 0.65, 1.09 P=0.19) or time to cardiovascular hospitalization or cardiovascular death (0.88, CI 0.57, 1.35 P =0.55).
Conclusions
Serum TMAO concentrations were markedly elevated and correlated directly with biochemical markers of nutritional status and inversely with markers of inflammation in patients receiving hemodialysis. There was no significant association between serum TMAO concentrations and all-cause mortality or cardiovascular death or hospitalizations. In patients receiving dialysis – in contrast to the general population – adverse vascular effects of TMAO may be counterbalanced by associations with nutritional or inflammatory status.
Background
Meat consumption has been epidemiologically linked to cardiovascular disease. The traditional view that this association is mediated by fatty acid consumption has recently been questioned1. Foods rich in carnitine, choline and lecithin, including meat and eggs, have been found to be a source of trimethyl amine (TMA)1, which is synthesized by gut microbiotia2 and then oxidized to trimethyl amino oxide (TMAO) in the liver by flavin monooxygenase 3 (FMO3)3. TMAO has been demonstrated to augment macrophage scavenger activity and down-regulate bile acid synthetic enzymes Cyp7a1 and Cyp27a1, important but terminal components of reverse cholesterol transport1. Higher serum TMAO concentrations have been associated with higher risk of cardiovascular events in the general population4. TMA is elevated in patients with chronic kidney disease (CKD) and is responsible in part for uremic fetor5. TMAO has also been demonstrated to be significantly elevated in patients receiving dialysis relative to persons with normal or near normal kidney function and to be removed by dialysis6. More recently TMAO has been found to be high among patients with CKD not requiring dialysis, and higher concentrations in that population were associated with mortality and progressive loss of kidney function7. Alterations in gut permeability or the contents of the microbiome offer potential pathways for higher cardiovascular risk in the ESRD population,8,9, but TMAO is also excreted in the urine2,10–12, suggesting that impaired kidney function could contribute to higher levels in this population. The increased level of TMAO, its water solubility, and its strong association with cardiovascular disease among patients without kidney disease make it a potential target for therapy in patients receiving dialysis. Herein, we aimed to determine clinical correlates of, and outcomes associated with, the putative cardiovascular risk marker TMAO in a national cohort of patients new to dialysis.
METHODS
Study Design, Participants, and Data Collection
Design and Participants
The Comprehensive Dialysis Study (CDS) was a United States Renal Data System (USRDS) prospective cohort study of adults with end-stage renal disease (ESRD) who initiated hemodialysis or peritoneal dialysis between June 2005 and June 2007 in dialysis facilities throughout the US. The CDS was designed to examine associations among nutritional status, physical activity, and health-related quality of life in patients new to dialysis13. The CDS has been previously described in detail, including sampling of dialysis facilities, recruitment, and measures13,14. In brief, participants were successfully recruited from 297 (89%) of 335 selected dialysis facilities. Fifty six of 73 (77%) facilities subsampled to participate in the nutrition substudy agreed to participate and collected serum samples. Facilities were selected a priori by systematic probability sampling proportional to estimated size to participate in the nutrition substudy. Participants (n=266) provided serum samples at enrollment. We did not include 22 patients who were on peritoneal dialysis in our analyses because the small number of participants would not have allowed us to make valid inferences, and we were unable to obtain TMAO measurements on nine participants. Our analysis cohort included 235 participants receiving hemodialysis with baseline TMAO measurements. USRDS hospitalization data is available only for Medicare beneficiaries, so analyses of cardiovascular mortality or cardiovascular hospitalization included 152 patients.
The CDS was approved by the Institutional Review Boards of the University of California, San Francisco and Emory University. All patients provided informed consent for participation, including analysis of stored serum samples.
Data collection
We collected outcome data through linkage to the USRDS. Data on demographics, body composition (height and weight), dialysis modality and access, co-morbidities, and serum creatinine at dialysis initiation were collected from the Center for Medicare and Medicaid Services (CMS) Medical Evidence Form (CMS 2728) and a telephone interview administered by DataBanque Research Services (Pittsburgh, PA). Information on the date and cause of death was based on the ESRD Death Notification Form (CMS Form 2746). Cause-specific hospitalization was ascertained from the principal discharge diagnosis or from procedure codes, with hospitalization captured through Medicare claims (see Appendix for the International Classification of Diseases - 9 diagnosis and procedure codes used to ascertain cardiovascular hospitalizations of interest and codes selected from the CMS 2746 form to ascertain cardiovascular death).
Laboratory Methods
We quantitated TMAO using a Waters Acuity UPLC in tandem with an ABSciex 5600 Triple TOF mass spectrometer. A Waters Acuity BEH Amide column (2.1 i.d. ×150mm length, 1.8um particle size) with attached guard-column held at 45°C was used for separation. The mass spectrometer was operated using an electrospray source in positive mode with a voltage of 5.5kV, temperature of 300°C, source gas at 40 psi and curtain gas at 25 psi. Data were acquired both in full scan mode as well as in product ion mode with enhanced sensitivity for the products of native TMAO (76.1 → 58.1 Da) and the deuterated internal standard (85.1 → 66.1 Da). A declustering potential of 80V and collision energy of 22V was used for the product ion scans. We performed data quantification in MultiQuant 2.1 (ABSciex) using peak area from product ions of TMAO against the known quantity of its deuterated internal standard.
We measured albumin, prealbumin, and C-reactive Protein (CRP) concentrations in duplicate on each serum sample using a Beckman Array 360 nephelometer (Beckman Instructions 015–248545–F; Beckman Instruments, Inc., Brea, CA). Coefficients of variation (CoV) albumin: interassay CoV, 8.3%; intra-assay CoV 0.2%; prealbumin: interassay CoV, 4.3%; intra-assay CoV, 1.1%; and CRP: interassay CoV, 9.2%; intra-assay CoV 3.6%. If the two measures on any given sample differed within the duplicates by >1%, we repeated the testing.
Control samples included two commercially available pooled normal control samples, one consisting of only male participants (Valley BioMedical Winchester, Virginia.) and one of unspecified sex (UTAK Laboratories Inc Valencia, CA), as well as an internal laboratory normal control standard.
Statistical methods
Baseline characteristics of our study cohort were described using proportions for categorical variables and either means and standard deviations or medians and 25th to 75th percentiles for continuous variables. We calculated Spearman’s rank correlation coefficients among TMAO measurements and concurrent measurements of age, BMI, and other laboratory values.
We modeled time to death and time to cardiovascular death or cardiovascular hospitalization using Cox proportional hazards regression models with TMAO as the primary predictor of interest and covariates including age, sex, race, diabetes, BMI, CRP as an inflammatory marker, and prealbumin and albumin as nutritional markers. We conducted unadjusted and multivariable adjusted analyses. The pre-specified model for the Cox analysis of time to death included all the predictors detailed above. Because of the smaller number of participants and events for the analysis of time to CV death or hospitalization, all predictors were not evaluated at once. We first examined TMAO as a univariate predictor, and evaluated whether the hazard ratio for TMAO changed with addition of additional predictors individually or in groups of up to three. Our final model consisted of prealbumin, race and diabetes, which were all significant in univariate models. We ran additional models including each non-included predictor with this set of four. None of the additional predictors were significant, and the hazard ratios for TMAO remained stable. We conducted all analyses using SAS 9.2 (Cary, NC, USA)
RESULTS
Patient characteristics and correlates of TMAO
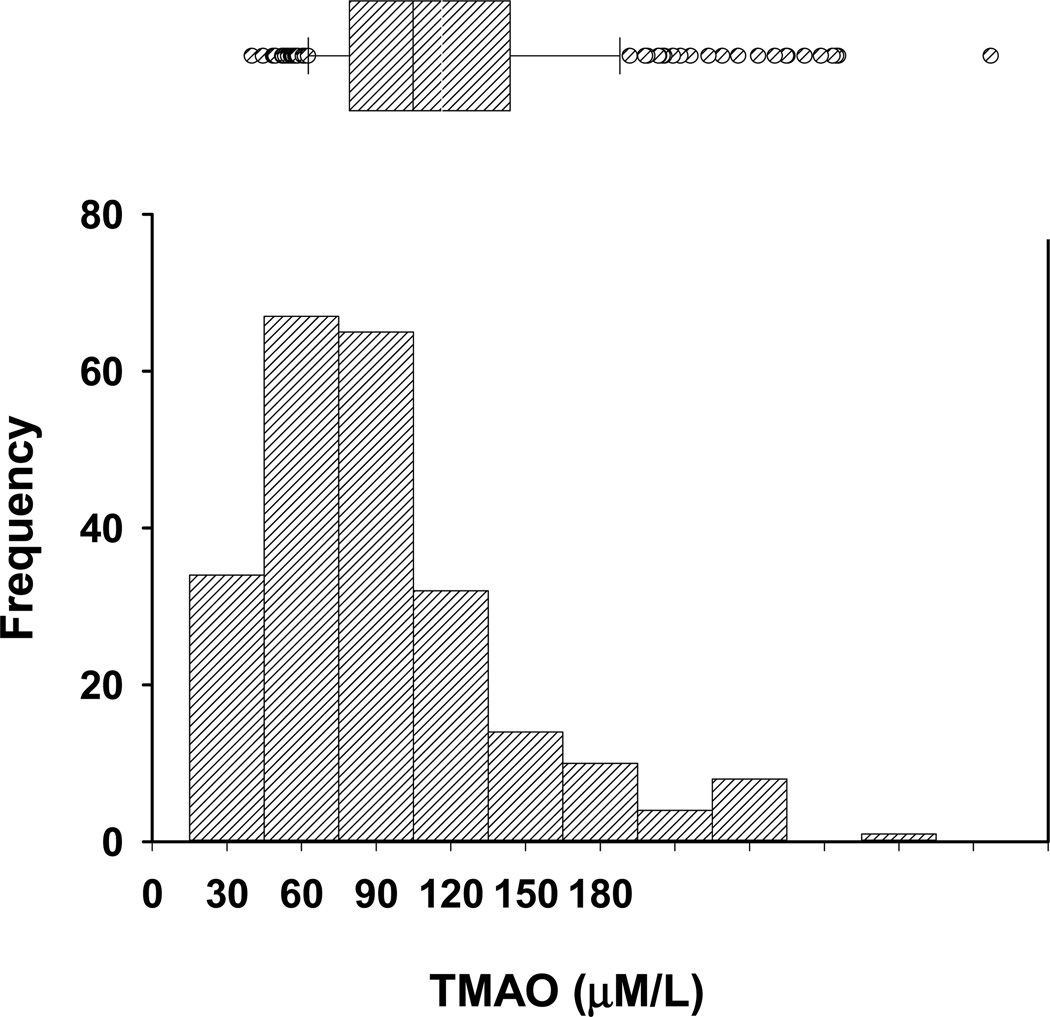
The mean age of our cohort of 235 patients on hemodialysis was 62±14 years (mean ± SD), 28% were black, and 59% had diabetes mellitus. TMAO values appeared non-normally distributed, i.e., skewed to the right (Figure 1) and were markedly elevated (mean 50±32 (Median 43, 25th –75th percentile – (28–67 µM/L)) (Table 1) when compared to our pooled control samples (1.41±0.49 µM/L). TMAO was directly correlated with albumin (Spearman rank correlation 0.24 [95% CI 0.11, 0.35]; p <0.001), prealbumin (0.19 [0.07, 0.31]; p =0.003), and creatinine (0.21 [0.08, 0.33]; p =0.002), and inversely with log CRP (−0.18 [−0.30, −0.06]; p=0.005). There were no significant associations between TMAO and age or body mass index (BMI) (Table 2).
Figure 1. Distribution of trimethylamine N Oxide levels in hemodialysis patients.
Distribution of trimethylamine N Oxide (TMAO) by decile (lower figure) mean ± 2 standard deviations (upper figure) in 235 hemodialysis patients.
Table 1.
Baseline Demographic Characteristics of analyzed cohort (N=235)*
| Characteristic | Value |
|---|---|
| Age, y | 61.8 ± 14.2 |
| Sex, male | 130 (55.3%) |
| Race, black | 65 (27.7%) |
| BMI, kg/m2 | 29.7 ± 7.28 |
| Albumin, g/dl | 3.47 ± 0.48 |
| Prealbumin, mg/dl | 30.7 ± 9.02 |
| TMAO, µM/L | 43 (27.5–66.6) |
| Creatinine, mg/dl | 7.15 ± 3.86 |
| CRP, mg/L | 7.5 (3.95–13.0) |
| Atherosclerosis | 85 (36.2%) |
| Congestive heart failure | 79 (33.6%) |
| Diabetes Mellitus | 138 (58.7%) |
Values are mean ± sd or median (interquartile range). Categorical data are shown as N (%).
Abbreviations: BMI, body mass index; TMAO, trimethyl amino oxide; CRP, C-reactive protein.
Table 2.
Correlations between TMAO and clinical predictors (N=235)*
| Variable | Spearman Rank Correlation |
95% Confidence Interval |
P-Value |
|---|---|---|---|
| BMI | −0.02 | −0.15 to 0.11 | 0.76 |
| Albumin | 0.24 | 0.11 to 0.35 | <0.001 |
| Prealbumin | 0.19 | 0.07 to 0.31 | 0.003 |
| Log CRP | −0.18 | −0.30 to −0.06 | 0.005 |
| Creatinine | 0.21 | 0.08 to 0.33 | 0.002 |
| Age | −0.09 | −0.22 to 0.04 | 0.17 |
Abbreviations: BMI, body mass index; TMAO, trimethyl amine N oxide; CRP, C-reactive protein.
Positive control samples using commercial blood plasma as well as NIST SRM 1950 standardized blood plasma yielded TMAO levels similar to TMAO levels published previously2,4. Reproducibility of the assays was better than 5% relative standard deviation using these standard blood plasma samples under the extraction conditions detailed above.
Association of TMAO with all-cause mortality
In the Cox model for mortality, 235 patients were followed from enrollment date through December 31, 2011 for a median follow-up time of 4.0 (25th – 75th percentile 1.8, 5.9) years, and 132 (56%) died. Median time to death was 2.5 (25th – 75th percentile 1.6, 4.6) years. The reasons for censoring included 38 (16%) for transplant, 2 (0.9%) for loss to follow-up, and 63 (27%) for study end.
Contrary to our hypothesis, higher serum concentrations of TMAO were not significantly associated with higher mortality either by univariate or multivariable Cox model analysis (Tables 3 and 4). In univariate analysis (Table 3), log TMAO was not significantly associated with death (HR 0.84, 95% [0.65, 1.09] per one unit (p=0.19)). When analyzed by quartiles, patients in the highest quartile of TMAO had a forty percent lower hazard of mortality (HR 0.61, 95% [0.38, 0.97]; p=0.04) than those in the lowest quartile (Table 3).
Table 3.
Univariate associations of TMAO with outcomes
| TMAO variable | HR (95% CI) | p-value |
|---|---|---|
| All-cause mortality | ||
| Continuous, log-transformed | 0.84 (0.65 – 1.09) | 0.19 |
| Quartiles | ||
| Q1: ≤27.5 | 1.00 | |
| Q2:> 27.5 – 43.0 | 0.69 (0.38 – 1.11) | 0.12 |
| Q3: >43.0 – 66.6 | 0.69 (0.42 – 1.11) | 0.12 |
| Q4: >66.6 – 184 | 0.61 (0.38 – 0.97) | 0.04 |
| Cardiovascular death or hospitalization | ||
| Continuous, log-transformed | 0.88 (0.57 – 1.35) | 0.55 |
| Quartiles | ||
| Q1: ≤ 26.8 | 1.00 | |
| Q2: >26.8 – 42.0 | 0.93 (0.43 – 2.00) | 0.86 |
| Q3: >42.0 – 62.0 | 0.86 (0.39 – 1.92) | 0.71 |
| Q4: >62.0 | 0.71 (0.32 – 1.59) | 0.40 |
Table 4.
Multivariable predictors of all-cause mortality.
| Variable | HR (95% CI) | p-value |
|---|---|---|
| TMAO, µM/L | ||
| Q1: ≤27.5 | 1.00 | |
| Q2: >27.5 – 43.0 | 0.86 (0.53 – 1.40 | 0.54 |
| Q3: >43.0 – 66.6 | 1.20 (0.71 – 2.00) | 0.50 |
| Q4: >66.6 – 184 | 1.14 (0.67 – 1.93) | 0.62 |
| Age, per 10 years | 1.46 (1.25 – 1.70) | <0.001 |
| Sex, female | 1.07 (0.74 – 1.54) | 0.73 |
| Race, black | 0.54 (0.33 – 0.87) | 0.01 |
| BMI, kg/m2 | ||
| <20 | 0.75 (0.20 – 2.90) | 0.68 |
| 20 – 24.99 | 1.00 | |
| 25 – 29.99 | 1.07 (0.68 – 1.70) | 0.77 |
| ≥30 | 0.82 (0.50 – 1.36) | 0.45 |
| Albumin, g/dL | 0.74 (0.45 – 1.22) | 0.66 |
| Prealbumin, mg/dL | 0.94 (0.91 – 0.97) | <0.001 |
| Log CRP | 1.04 (0.87 – 1.24) | 0.66 |
| Diabetes mellitus | 0.97 (0.66 – 1.43) | 0.87 |
In multivariable analyses, the adjusted association between serum TMAO and mortality was in the hypothesized direction, but not statistically significant with a wide confidence interval (HR 1.14, [95% CI 0.67 – 1.93]; p=0.62, comparing the fourth to the first quartile) (Table 4). Older age, non-Black race, and lower serum prealbumin concentrations were associated with higher mortality (Table 4).
Association of TMAO with cardiovascular death or hospitalization
In the Cox model for time to cardiovascular death or first cardiovascular hospitalization, 152 Medicare beneficiaries were followed from enrollment date through December 31, 2011 for a median follow-up time of 2.5 (IQR 1.4, 5.0) years. There were 48 (32%) cardiovascular deaths or hospitalizations during follow-up with median time to event of 1.7 (IQR 0.67, 2.5) years. The presence of diabetes mellitus was associated with higher risk (HR 2.20 CI 1.14 – 4.40 P = 0.02), and prealbumin was associated with lower risk (HR 0.96 CI 0.92 – 0.99) per mg/dL, P = 0.01) of cardiovascular death or hospitalization when each were used as single predictors for analysis of risk. Higher log CRP was associated with higher risk but failed to reach statistical significance (HR 1.19 95% CI 0.93 – 1.52, P = 0.17). TMAO was not statistically significantly associated with cardiovascular death or hospitalizations, either as a continuous variable or by quartile (Table 3).
The relation between TMAO and time to cardiovascular death or hospitalization was not statistically significant in multivariable models after adjusting for race, diabetes, and prealbumin (Table 5). Adjusted hazard ratios comparing the forth quartile with the first quartile had large confidence intervals ranging from 60% lower risk of cardiovascular death or hospitalization to a 2-fold higher risk.
Table 5.
Multivariable predictors of cardiovascular death or hospitalization.
| Variable | HR (95% CI) | p-value |
|---|---|---|
| TMAO, µM/L | ||
| Q1: ≤27.5 | 1.00 | |
| Q2: >27.5 – 43.0 | 1.37 (0.61 – 3.10) | 0.44 |
| Q3: >43.0 – 66.6 | 1.23 (0.53 – 2.80) | 0.63 |
| Q4: >66.6 – 184 | 0.92 (0.40 – 2.10) | 0.84 |
| Race, black | 0.49 (0.23 – 1.04) | 0.06 |
| Prealbumin, mg/dL | 0.96 (0.92 – 0.99) | 0.02 |
| Diabetes mellitus | 2.10 (1.05 – 4.10) | 0.04 |
DISCUSSION
We found that serum TMAO concentrations were markedly higher in our cohort of patients receiving hemodialysis compared with control serum we obtained and compared with levels previously reported in the literature among healthy individuals1,2,4.
TMAO was directly correlated with albumin and prealbumin and inversely correlated with CRP. In our cohort, serum TMAO concentrations were not statistically significantly associated with all-cause mortality or cardiovascular mortality or hospitalization.
The levels of TMAO that we have reported are lower than those reported in a smaller cohort of patients receiving dialysis previously reported by Bain et al.6. The assays used in our laboratory and in use by others not exploring patients with dialysis dependent kidney disease1,2,4,7 differ from that used by Bain et al.6 in that we used liquid mass spectroscopy utilizing liquid extraction and internal standards instead of gas chromatography – mass spectroscopy (GC-MS) with solid phase microextraction6, a method that could not add an internal standard. Their results from healthy individuals were approximately an order of magnitude higher than we report here from normal controls as well as values previously reported by others1,2,4,7 using liquid chromatography. Thus, because of these fundamental methodological differences, the levels of TMAO reported here should not be compared to previous studies of patients with ESRD6.
TMAO has recently been described as a risk factor for cardiovascular disease and has been shown in animal models to up regulate macrophage receptors associated with atherosclerosis2, providing a link between dietary nutrients, the gut microbiome, and vascular injury. TMAO has been found to decrease expression of a number of bile acid transport genes in the liver (Cyp7a1 and Cyp27a1) adversely affecting cholesterol elimination1 blocking reverse cholesterol transport through this mechanism, providing another potential link to vascular disease. Bile acids are also increased in CKD and ESRD, although no disruption in the Cyp7a1 pathway has been identified as the mechanism in ESRD15,16. Although mortality risk in the general population is also associated with precursors of TMA, such as L-carnitine1, when TMAO and L-carnitine are considered together, only TMAO is associated with higher risk. Patients on dialysis have been reported to have functional L-carnitine deficiency17–19. Despite the potential for L-carnitine conversion to TMAO, supplemental L-carnitine has been studied for use in intradialytic hypotension, left ventricular dysfunction and to correct or ameliorate resistance to erythropoietin17–19. A recently published meta-analysis examined the results of a total of 49 trials on L-carnitine in patients on dialysis that enrolled 1734 patients, finding a reduction in LDL cholesterol and CRP, but no increase or decrease in cardiovascular risk. Each of the studies were relatively small, but the doses of L-carnitine administered were well in excess of what would be encountered nutritionally20 with no increase in adverse events noted.
We did not observe a statistically significant association between serum TMAO concentrations and mortality or cardiovascular outcomes despite levels that were several-fold higher than values reported to be associated with the highest risk of adverse cardiovascular outcomes in the general population4. These results parallel those of other biomarkers, such as total and LDL cholesterol, which are either unassociated with risk or are associated with lower risk of adverse outcomes in patients receiving dialysis in contrast to associations observed in the general population. It is not entirely clear why risk factors, such as total20 and LDL cholesterol22,23 – and TMAO in our study – do not find a significant association with outcomes in dialysis patients. It is possible that by the time patients reach ESRD, damage to the vascular endothelium has reached a point where further exposure can no longer affect outcome. TMAO has been shown to cause injury in experimental models, at least in part, by interfering in reverse cholesterol transport. However, this effect is distal to sites that are impaired in ESRD, where there are multiple structural, functional and maturational abnormalities affecting HDL24–26. It is possible that reverse cholesterol transport is so significantly disrupted in patients receiving dialysis that additional downstream effects on bile acid transport contribute no additional risk. Alternatively, cholesterol and other injurious compounds may still be exerting deleterious effects on the vasculature, but competing risks from other factors associated with these biomarkers, such as inflammation and/or malnutrition, may overwhelm any deleterious effects. The correlations between TMAO and nutritional and inflammatory biomarkers we observed would be consistent with the latter possibility. We have previously shown that both serum albumin and creatinine concentrations are associated with measures of protein intake (normalized Protein Catabolic Rate (nPCR) and inflammation (CRP) cross-sectionally and longitudinally27,28. Similarly, prealbumin concentration was associated also with nPCR and inflammatory biomarkers29.
The high levels of TMAO we detected also raise a third possibility – that any negative effects of TMAO on the vasculature occur when concentrations exceed levels observed among healthy individuals and do not increase further when levels rise to the extraordinary levels seen in this dialysis cohort. In this scenario, all patients would experience the negative effects associated with high TMAO, possibly contributing to the exceedingly high cardiovascular risk observed in this population.
Our study has several strengths. First, the cohort is drawn from a national sample and patients were treated in many facilities across the country. TMAO concentrations are significantly elevated in hemodialysis patients and clearly would provide a target to reduce cardiovascular risk, were one conferred by this oxidized amine.
However, our study has several limitations. First, given the modest sample size, we cannot conclude definitively that there is no association between the markedly elevated serum TMAO concentrations and mortality (or cardiovascular disease). In addition, we did not collect information on dietary intake or antibiotic use, which are known to affect serum TMAO concentrations. Therefore we could not determine whether these factors were associated with serum TMAO concentrations in our population.
Practical applications
Diets rich in carnitine and phosphatidylcholine; red meats and eggs for example, have the potential to increase the risk of cardiovascular disease by interaction with bacteria in the gut and serving as a precursor for trimethyl amine oxide (TMAO). While elevated serum TMAO concentrations are associated with higher cardiovascular risk in patients who are not receiving dialysis, we could not find evidence linking TMAO concentrations with mortality or cardiovascular risk in patients new to dialysis. For now, there is no specific rationale to restrict from the diet foods rich in TMAO precursors.
Supplementary Material
ACKNOWLEDGEMENTS
Support:
Financial Disclosure: The authors declare that they have no other relevant financial interests. The results presented in this paper have not been published previously in whole or part, except in abstract format
Support: This work was supported by the National Institutes of Health (contract N01 DK-1-2450 to Dr. Chertow, contract N01-DK-0005 to Dr Johansen and K23DK093584 to Dr Dalrymple. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koeth RA, Wang Z, Levinson BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013 May;19(5):576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert DM, Mamer OA, Akerman BR, Choinière L, Gaudet D, Hamet P, Treacy EP. In vivo variability of TMA oxidation is partially mediated by polymorphisms of the FMO3 gene. Mol Genet Metab. 2001 Jul;73(3):224–229. doi: 10.1006/mgme.2001.3189. [DOI] [PubMed] [Google Scholar]
- 4.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013 Apr 25;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simenhoff ML, Burke JF, Saukkonen JJ, Ordinario AT, Doty R. Biochemical profile or uremic breath. N Engl J Med. 1977 Jul 21;297(3):132–135. doi: 10.1056/NEJM197707212970303. [DOI] [PubMed] [Google Scholar]
- 6.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant. 2006 May;21(5):1300–1304. doi: 10.1093/ndt/gfk056. [DOI] [PubMed] [Google Scholar]
- 7.Tang WW, Wang Z, Kennedy DJ, Wu Y, Buffa J, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut Microbiota-Dependent Trimethylamine N-oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ Res. 2014 Nov 5; doi: 10.1161/CIRCRESAHA.116.305360. pii: CIRCRESAHA.114.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, abnormal immunity in kidney disease. Kidney Int. 2013 Jun;83(6):1010–1016. doi: 10.1038/ki.2012.440. Epub 2013 Jan 16. [DOI] [PubMed] [Google Scholar]
- 9.Vitetta L, Gobe G. Uremia and chronic kidney disease: the role of the gut microflora and therapies with pro- and prebiotics. Mol Nutr Food Res. 2013 May;57(5):824–832. doi: 10.1002/mnfr.201200714. Epub 2013 Mar 1. [DOI] [PubMed] [Google Scholar]
- 10.de la Huerga J, Popper H. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobiliary diseases. J Clin Invest. 1951;30:463–470. doi: 10.1172/JCI102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihle BU, Cox RW, Dunn SR, Simenhoff ML. Determination of body burden of uremic toxins. Clin Nephrol. 1984;22:82–89. [PubMed] [Google Scholar]
- 12.Simenhoff ML, Saukkonen JJ, Burke JF, Wesson LG, Schaedler RW. Amine metabolism and the small bowel in uraemia. Lancet. 1976;2:818–821. doi: 10.1016/s0140-6736(76)91207-1. [DOI] [PubMed] [Google Scholar]
- 13.Kutner NG, Johansen KL, Kaysen GA, Pederson S, Chen SC, Agodoa LY, Eggers PW, Chertow GM. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009 Mar;4(3):645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutner NG, Johansen KL, Kaysen GA, Pederson S, Chen SC, Agodoa LY, Eggers PW, Chertow GM. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009 Mar;4(3):645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gai Z, Chu L, Hiller C, Arsenijevic D, Penno CA, Montani JP, Odermatt A, Kullak-Ublick GA. Effect of chronic renal failure on the hepatic, intestinal, and renal expression of bile acid transporters. Am J Physiol Renal Physiol. 2014 Jan 1;306(1):F130–F137. doi: 10.1152/ajprenal.00114.2013. [DOI] [PubMed] [Google Scholar]
- 16.Vaziri ND, Sato T, Liang K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int. 2003 May;63(5):1756–1763. doi: 10.1046/j.1523-1755.2003.00911.x. [DOI] [PubMed] [Google Scholar]
- 17.Hedayati SS. Dialysis-related carnitine disorder. Semin Dial. 2006;19:323–328. doi: 10.1111/j.1525-139X.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S. L-Carnitine in dialysis patients. Semin Dial. 2001;14:209–217. doi: 10.1046/j.1525-139x.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 19.Hurot JM, Cucherat M, Haugh M, Fouque D. Effects of l-carnitine supplementation in maintenance hemodialysis patients: A systematic review. J Am Soc Nephrol. 2002;13:708–714. doi: 10.1681/ASN.V133708. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Abbate M, Tang L, Cai G, Gong Z, Wei R, Zhou J, Chen X. L-Carnitine supplementation for adults with end-stage kidney disease requiring maintenance hemodialysis: a systematic review and meta-analysis. Am J Clin Nutr. 2014 Feb;99(2):408–422. doi: 10.3945/ajcn.113.062802. [DOI] [PubMed] [Google Scholar]
- 21.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990 May;15(5):458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004 Jan 28;291(4):451–459. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 23.Kwan BC1, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol. 2007 Apr;18(4):1246–1261. doi: 10.1681/ASN.2006091006. Epub 2007 Mar 14. [DOI] [PubMed] [Google Scholar]
- 24.Mekki K, Bouchenak M, Lamri M, Remaoun M, Belleville J. Changes in plasma lecithin: cholesterol acyltransferase activity, HDL(2), HDL(3) amounts and compositions in patients with chronic renal failure after different times of hemodialysis. Atherosclerosis. 2002 Jun;162(2):409–417. doi: 10.1016/s0021-9150(01)00728-6. [DOI] [PubMed] [Google Scholar]
- 25.Mekki K, Bouchenak M, Remaoun M, Belleville JL. Effect of long-term hemodialysis on plasma lecithin: cholesterol acyltransferase activity and the amounts and compositions of HDL2 and HDL3 in hemodialysis-treated patients with chronic renal failure: a 9-year longitudinal study. Med Sci Monit. 2004 Aug;10(8):CR439–CR446. [PubMed] [Google Scholar]
- 26.Mangé A, Goux A, Badiou S, Patrier L, Canaud B, Maudelonde T, Cristol JP, Solassol J. HDL proteome in hemodialysis patients: a quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0034107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, Levin NW, Yan G HEMO Study Group. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis. 2003 Dec;42(6):1200–1211. doi: 10.1053/j.ajkd.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 28.Kaysen GA, Chertow GM, Adhikarla R, Young B, Ronco C, Levin NW. Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int. 2001 Jul;60(1):333–340. doi: 10.1046/j.1523-1755.2001.00804.x. [DOI] [PubMed] [Google Scholar]
- 29.Molfino A, Heymsfield SB, Zhu F, Kotanko P, Levin NW, Dwyer T, Kaysen GA. Prealbumin is associated with visceral fat mass in patients receiving hemodialysis. J Ren Nutr. 2013 Nov;23(6):406–410. doi: 10.1053/j.jrn.2013.02.007. Epub 2013 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.