Abstract
Background
It is unknown whether supplementation with calcium and vitamin D has an impact on menopause-related symptoms.
Methods
As part of the Women’s Health Initiative Calcium/Vitamin D Supplementation Trial (CaD), women were randomized at 40 clinical sites to elemental calcium carbonate 1,000 mg with vitamin D 400 IU daily or placebo. At the CaD baseline visit (year 1 or year 2) and during a mean follow-up of 5.7 years, participants provided data on menopause-related symptoms via questionnaires. Generalized linear mixed effects techniques were used to address research questions.
Results
After excluding participants with missing data (N=2,125), we compared menopause-related symptoms at follow-up visits of 11,584 women randomized to CaD with those of 11,436 women given the placebo. Women in the CaD arm did not have a different number of symptoms at follow-up compared to women taking the placebo (p=0.702). Similarly, there was no difference between sleep disturbance, emotional well-being, or energy/fatigue at follow-up in those who were randomized to CaD supplementation compared to those taking the placebo.
Conclusions
Our data suggest that supplementation with 1000 mg of calcium plus 400 IU of vitamin D does not influence menopause-related symptoms over an average of 5.7 years of follow-up among postmenopausal women with an average age of 64 at the WHI baseline visit.
Keywords: Vitamin D, calcium, menopause, hot flashes, mood, sleep
1.0 INTRODUCTION
Most women transitioning through menopause will experience symptoms including hot flashes, [1–3] mood disturbances, and muscle aches.[4, 5] In many women, these symptoms are severe enough to adversely affect their quality of life, work performance, and personal relationships.[6, 7] Current treatments for menopause-related symptoms, such as menopausal hormone therapy, antidepressants, and anticonvulsants, may have significant side effects and serious long-term adverse consequences.[8] In addition, after treatment is discontinued, these symptoms may recur or even develop de novo.[9–11] It is therefore important to investigate possible determinants of menopause-related symptoms so that new therapies can be developed.
There are several mechanisms whereby vitamin D could potentially improve menopausal symptoms. A menopausal decline in serotonin, a neurotransmittor with known effects on thermoregulation, could contribute to hot flashes.[12–14] In animal models, vitamin D prevents this serotonin decline.[15] Alternatively, estrogen increases the activity of the enzyme responsible for activating vitamin D.[16] The fall in estrogen that occurs during the menopausal transition could uncover previously subclinical vitamin D deficiency.[17–21] Vitamin D supplementation can improve mood and muscle aches in nonmenopausal populations, [22, 23] but its effects on a menopausal population have not been well studied.
Conversely, calcium may stimulate the production of a vasodilator neuropeptide, calcitonin gene-related peptide (CGRP), which has been positively linked to occurrence of menopausal hot flashes.[24–28] Indeed, taking calcium supplements has been linked to a higher likelihood of having hot flashes in breast cancer survivors.[29]
We examined the effect of 1,000 mg of elemental calcium carbonate plus 400 IU of vitamin D3 (CaD) on menopause-related symptoms in women who participated in the Women’s Health Initiative randomized, placebo-controlled Calcium/Vitamin D Supplementation Trial (CaD). We hypothesized that the potential favorable effects of vitamin D on menopause-related symptoms would outweigh the potential negative effects of calcium. Therefore, we believed that women given calcium and vitamin D would experience fewer menopausal symptoms during follow-up than women given the placebo.
2.0 MATERIALS AND METHODS
2.1 Study population and intervention
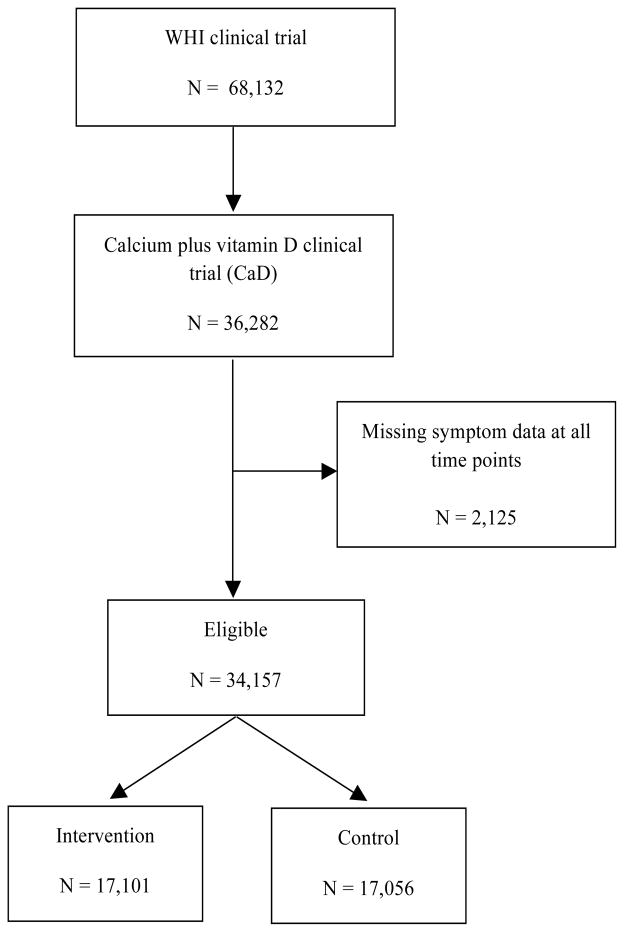
The Women’s Health Initative (WHI) Randomized Clinical Trial (CT) enrolled postmenopausal women aged 50 to 79 years at baseline into a hormone therapy (HT) [30] and/or dietary modification [31] (DM) trial (N=68,132). Between years 1 and 3, participants in both trials were also asked to join a randomized clinical trial investigating calcium plus vitamin D (CaD) compared with placebo (N36,282 women).[32] Women were randomized at 40 clinical sites to calcium carbonate 1000 mg plus vitamin D 400 IU daily, given as one tablet in two divided doses to be taken two times per day with meals, versus an identical-appearing placebo. Concurrent calcium supplementation was permitted, as was vitamin D supplementation up to 600 IU daily (increased to 1000 IU daily from 1999 through the end of the trial). Details of the study design [33] and baseline characteristics [32] have been presented previously. The primary outcome for the CaD trial was hip fracture. Secondary outcomes included total fractures and colorectal cancer. Included in our final analytic cohort were 34,157 women for whom we had data on menopause-related symptoms at some time point, with 17,101 in the intervention and 17,056 in the control arm. All participating women provided written informed consent.
2.2 Data Collection
2.2.1 Demographic and health characteristics
Participants self-reported data on demographics (i.e., age, race, education, years since menopause), lifestyle factors (i.e., physical activity, smoking), and UV exposure (i.e., Langley’s measure of UV exposure). They underwent physical measurements (i.e., height and weight to calculate BMI).[32] A standardized, in-person, interviewer-administered form was used to collect information on the dose, frequency, and duration of current supplements (i.e., calcium, vitamin D) and medication use [i.e., menopausal hormone therapy (HT)]. Dietary vitamin D and calcium intakes during the previous 3 months were estimated from a self-administered food-frequency questionnaire (FFQ) specifically designed for WHI.[32, 34, 34]
2.2.2. Menopausal symptoms
Participants provided data on menopause-related symptoms via self-report questionnaires at the CaD baseline visit (year 1 to year 2 of overall trial) and during follow-up. Questionnaires included a checklist of menopause-related symptoms based on the Postmenopausal Estrogen/Progestin Interventions (PEPI) symptom tool [35] and other national surveys and clinical trials.[36, 37] The psychosocial forms containing these symptom items were reviewed for content validity by nationally recognized behavioral and clinical experts and were pretested extensively on age-appropriate women from diverse racial/ethnic groups.[10] For this analysis, the following symptoms were analyzed: hot flashes, night sweats, dizziness, heart racing or skipping beats, tremors, feeling restless or fidgety, feeling tired, difficulty concentrating, forgetfulness, mood swings, vaginal dryness, breast tenderness, headaches or migraines, waking up several times at night, waking earlier than planned, trouble falling back to sleep after waking earlier than planned, overall typical sleep pattern and quality, and loss of energy. Previous research indicates that these are the typical symptoms associated with menopause.
For most of the symptoms, participants rated symptom severity on a 4-point scale: symptom did not occur, mild (did not interfere with usual activities), moderate (interfered somewhat with usual activities), or severe (so bothersome that usual activities could not be performed). For sleep symptoms, [38] participants rated how often they had occurred in the previous 4 weeks (from none to five or more times per week).
Our primary outcome was total number of symptoms of any severity (from mild to severe). Secondary outcomes included the energy/fatigue and emotional well-being subscales of the Short Form 36 Health Survey (SF-36). For each of these subscales, higher scores indicated better health (range from 0–100).[39] We also examined a sleep disturbance construct, the Women’s Health Initiative Insomnia Rating Scale, [40] created from five questions on trouble falling asleep, waking up several times at night, waking earlier than planned, trouble falling back to sleep after waking earlier than planned, and overall typical sleep. The summary score ranged from 0 to 20, with a higher score indicating greater sleep disturbance. Finally, we examined the effect of CaD vs. placebo over time on each symptom.
2.3 Retention, Adherence, and Follow-up
We defined adherence as use of 80% or more of the study medication and assessed it by weighing returned pill bottles at annual visits. In year 1, the proportion of women in the CaD trial taking 80% or more of the study medication was 60% overall. That remained stable through year 7, ranging between 56% and 63%, with small differences between treatment groups, [41] At the end of the study, 76% of participants were still taking study medications and 59% were taking 80% or more of their daily pills.[42] The CaD trial ended as planned in March 2005. Over the course of the study, approximately 3% of participants withdrew or were lost to follow-up, and 4% died.[42] Women who withdrew, were lost to follow-up, or died before the end of the trial were included in the analyses if they had reported menopause-related symptoms at least once during the CaD trial.
2.4 Statistical Methods
This is a secondary analysis of the CaD clinical trial. We evaluated changes in menopause-related symptoms during an average of 5.7 years of follow-up in those randomized to CaD compared to placebo.
The primary outcome is the total number of symptoms (of any severity) at a given time point. Secondary outcomes include the energy/fatigue and emotional well-being subscales of the SF-36 [39] and a sleep disturbance construct. To estimate the effect of CaD supplementation on outcomes, we employed generalized linear mixed effects techniques. More specifically, for the primary continuous outcome of number of symptoms, a linear mixed effects model with a subject-specific random effect that assumes Gaussian errors was fit. The model included year — represented by indicator terms to allow for potential non-linearity between symptoms and time — and interaction terms between the indicators for year and randomized arm, which represent the parameters of interest: the difference in symptom trajectory between arms. Inclusion of the subject-specific random effect accounts for the correlation of outcomes within a subject over time. Similar methods were used when examining the secondary continuous outcomes.
To determine whether randomization to the CaD supplementation arm had a significant effect on individual symptoms of any severity (i.e., mild, moderate, or severe presence), we utilized these same approaches, regressing the binary outcome on treatment assignment in a model that utilized a logit link and assumed binomial errors. As in the primary model, a subject-specific random intercept was included to account for the correlation of outcomes. As in the primary analysis, to assess differences in trajectory over time by CaD trial arm on specific symptoms, we explored the interaction between trial arm and year.
We also examined whether hormone therapy was an effect modifier by assessing the interaction between trial arm, year, and hormone use.
We performed two sensitivity analyses, one that evaluates differences in overall symptom trajectory by treatment arm among those who were adherent, and another where a linear relationship between time and symptoms is assumed.
As multiple secondary hypotheses were examined, we controlled the false-discovery rate to be no more than 5% using the Benjamini-Hochberg method.[43] In all, hypotheses for 22 secondary outcomes were tested (three continuous and 19 binary). We used the same approach for the 22 tests in both the sensitivity analysis considering effect modification by hormone use and in the analysis treating time as linear.
Analyses were performed using SAS v9.3 (data cleaning and descriptive analyses), Stata 13.1 (modeling), and R 3.0.3 (graphs and multiple correction of p-values).
3.0 RESULTS
3.1 Descriptive Analyses and Baseline Characteristics
Participants were, on average, 63.7 ± 6.5 (SD) years old at WHI baseline. Characteristics that could be related to menopausal symptom prevalence and/or vitamin D status did not differ for women in both arms at WHI baseline.(Table 1). Data on menopause-related symptoms were collected over an average of 5.7 ± 3.2 years after the baseline CaD visit (year 1 of overall clinical trial).
Table 1.
Selected demographic and health characteristics of women by CaD arm of eligible cohort (N=34,157) at WHI baseline
| Intervention N=17,101 |
Control N=17,056 |
P-value | |
|---|---|---|---|
| Age | |||
| 50–59 | 6383 (37.3) | 6360 (37.3) | 0.98 |
| 60–60 | 7832 (45.8) | 7803 (45.7) | |
| 70+ | 2886 (16.9) | 2893 (17.0) | |
|
| |||
| Time since menopause | |||
| <5 | 3710 (21.7) | 3727 (21.9) | 0.86 |
| 5 – <10 | 3108 (18.2) | 3064 (18.0) | |
| ≥10 | 10283 (60.1) | 10265 (60.2) | |
|
| |||
| Race/ethnicity, N (%) | Missing N=60 | ||
| White | 14339 (84.0) | 14411 (84.6) | 0.29 |
| Hispanic/Latino | 657 (3.9) | 600 (3.5) | |
| Black or African American | 1489 (8.7) | 1434 (8.4) | |
| Other | 586 (3.4) | 581 (3.4) | |
|
| |||
| Education, N (%) | Missing N=211 | ||
| 0–8 years | 220 (1.3) | 197 (1.2) | 0.63 |
| Some high school | 626 (3.7) | 602 (3.6) | |
| High school | 3105 (18.3) | 3171 (18.7) | |
| School after high school | 6774 (39.9) | 6725 (39.7) | |
| College degree or higher | 6269 (36.9) | 6257 (36.9) | |
|
| |||
| Body mass index, kg/m2, N (%) | Missing N=169 | ||
| <25 | 4494 (26.4) | 4615 (27.2) | 0.10 |
| 25 – <30 | 6119 (35.9) | 6141 (36.2) | |
| ≥30 | 6408 (37.7) | 6211 (36.6) | |
|
| |||
| Smoking, N (%) | Missing N=340 | ||
| Never smoked | 8779 (51.8) | 8901 (52.7) | 0.26 |
| Past smoker | 6847 (40.4) | 6709 (39.7) | |
| Current smoker | 1309 (7.7) | 1272 (7.5) | |
|
| |||
| UV exposure | |||
| Low | 8809 (51.5) | 8770 (51.4) | 0.86 |
| High | 8292 (48.5) | 8286 (48.6) | |
|
| |||
| HT use | |||
| Never used | 8215 (48.1) | 8053 (47.3) | 0.08 |
| Past user | 3018 (17.7) | 2955 (17.3) | |
| Current user | 5855 (34.3) | 6036 (35.4) | |
|
| |||
| Dietary Modification Arm | |||
| Not randomized to DM | 5194 (30.4) | 5125 (30.0) | 0.45 |
| Intervention | 4508 (26.4) | 4598 (27.0) | |
| Control | 7399 (43.3) | 7333 (43.0) | |
|
| |||
| History of cancer | Missing N=292 | ||
| No | 16250 (95.9) | 16268 (96.1) | 0.24 |
| Yes | 695 (4.1) | 652 (3.9) | |
|
| |||
| Overactive thyroid | Missing N=2939 | ||
| Never | 15259 (97.60) | 15238 (97.78) | 0.14 |
| Past | 303 (1.94) | 296 (1.90) | |
| Current | 72 (0.46) | 50 (0.32) | |
|
| |||
| Underactive thyroid | Missing N=2418 | ||
| Never | 13773 (86.63) | 13784 (87.01) | 0.06 |
| Past | 741 (4.66) | 784 (4.95) | |
| Current | 1384 (8.71) | 1273 (8.04) | |
|
| |||
| Physical activity, total MET hours/week | Missing N=3101 | ||
| 10.64 (12.61) | 10.62 (12.36) | 0.88 | |
|
| |||
| Dietary calcium, mg | Missing N=106 | ||
| 822.42 (455.19) | 826.13 (449.67) | 0.45 | |
|
| |||
| Dietary vitamin D, mcg | Missing N=106 | ||
| 4.37 (3.01) | 4.39 (2.97) | 0.49 | |
|
| |||
| Supplemental calcium, mg | Missing N=1 | ||
| 295.51 (501.53) | 294.17 (483.22) | 0.80 | |
|
| |||
| Supplemental vitamin D, mcg | Missing N=1 | ||
| 4.29 (5.72) | 4.37 (5.74) | 0.20 | |
|
| |||
| Days from clinical trial to CaD randomization | |||
| 401.52 (102.82) | 401.54 (102.67) | 0.99 | |
|
| |||
| Total number of menopause-related symptoms (Year 1) | Missing N=3568 | ||
| 6.06 (3.27) | 6.10 (3.32) | 0.27 | |
|
| |||
| Sleep disturbance construct (Year 1) | Missing N=3568 | ||
| 6.39 (4.44) | 6.39 (4.44) | 0.93 | |
|
| |||
| Emotional well-being construct (Year 1) | Missing N=3568 | ||
| 80.94 (14.05) | 80.78 (14.27) | 0.31 | |
|
| |||
| Energy/fatigue construct (Year 1) | Missing N=3568 | ||
| 65.10 (19.34) | 64.91 (19.41) | 0.39 | |
3.2 Primary Analyses
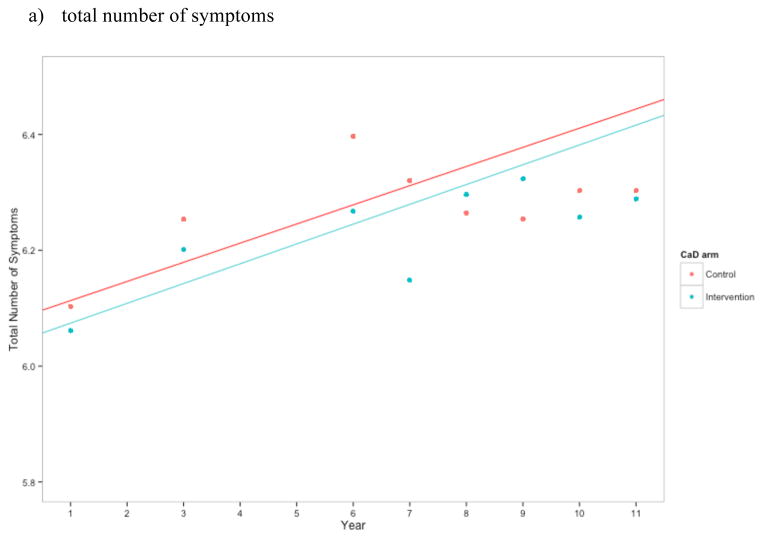
Women in the CaD arm had a similar number of symptoms to those in the placebo group (p = 0.702; Figure 2a). Over the course of follow-up, the mean total number of symptoms for women in the placebo arm was 6.32, and the mean for women in the intervention arm was 6.26. Despite a slight increase around year 6, the estimated mean difference between the two arms remained relatively stable over time. Compared to those in the placebo arm, women in the intervention arm had 0.06 fewer (95% CI for intervention – placebo: −0.29, 0.17) symptoms at year 3, 0.15 fewer (−0.38, 0.08) at year 6, 0.03 more (−0.10, 0.15) at year 9, and 0.02 fewer (−0.26, 0.21) at year 11; these differences were not statistically significant (Table 2). Results were unchanged when we excluded those who were not adherent (data not shown).
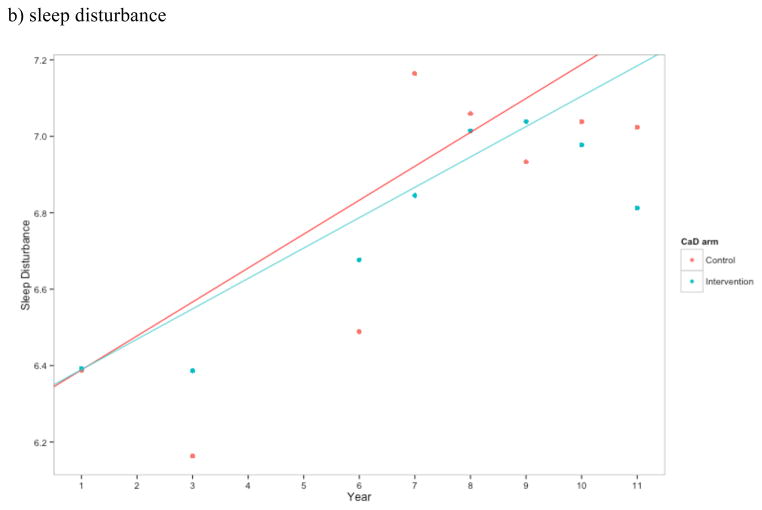
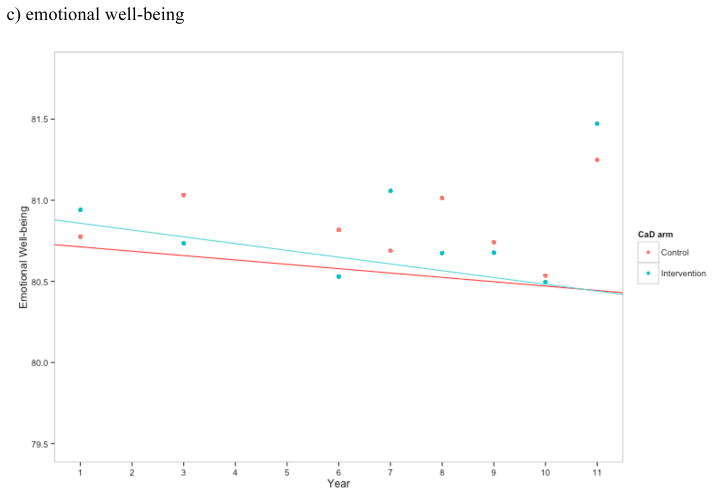
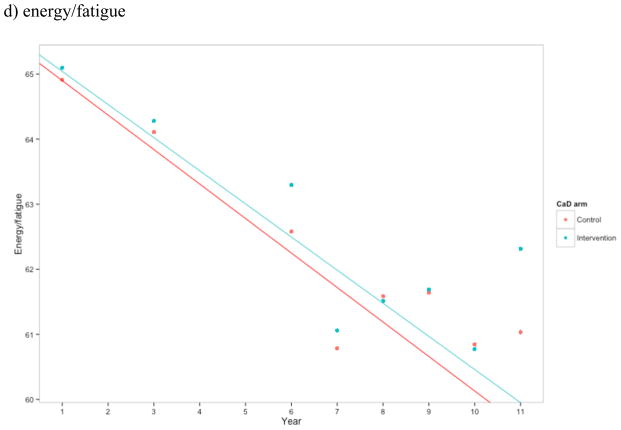
Figure 2.
Estimates of a) total number of symptoms, b) sleep disturbance construct, c) emotional wellness, and d) energy/fatigue for CaD and placebo arm at each time point. Higher scores in b) and c) indicate better health, while higher scores in d) indicate greater sleep disturbance. Points represent the mean at each time point for the control and intervention CaD trial arms. Linear regression estimates were generated treating year as linear and with an interaction between trial arm and year.
Table 2.
Comparison of menopausal symptoms in participants assigned to placebo vs. those assigned to calcium and Vitamin D
| Symptoms | P-value comparing CaD trial arms | Adjusted p-value* |
|---|---|---|
| Symptom total | 0.702 | -- |
| Sleep disturbance | 0.120 | 0.909 |
| Emotional well-being | 0.813 | 0.909 |
| Energy/fatigue | 0.764 | 0.909 |
Results from linear regression treating time as non-linear. Models included CaD arm, year, and interaction between arm and year
P-values adjusted after controlling the false-discovery rate to be no more than 5%.
3.3 Secondary and Sensitivity Analyses
Women randomized to CaD supplementation did not have a significantly different level of sleep disturbance (adjusted p = 0.909), emotional well-being (adjusted p = 0.909), or energy/fatigue (adjusted p = 0.909) during follow up compared to those given placebo (Figures 2b–d). Furthermore, there was no evidence that hormone therapy was an effect modifier.
We also examined whether randomization to the placebo arm was associated with a higher likelihood of suffering from individual symptoms during follow-up. We did not find this to be the case for any individual symptom in the placebo group compared to the CaD group at follow-up visits (Table 3). There was also no evidence that hormone therapy was an effect modifier of the association between CaD arm and individual symptoms after adjustment for multiple testing.
Table 3.
P-values comparing individual menopausal symptoms in participants assigned to placebo compared to those assigned to Calcium and Vitamin D at a selected year (Year 8)
| Symptoms | P-value comparing CaD trial arms | Adjusted p-value* |
|---|---|---|
| Hot flashes or night sweats | 0.787 | 0.909 |
| Hot flashes | 0.491 | 0.909 |
| Night sweats | 0.892 | 0.909 |
| Dizziness | 0.544 | 0.909 |
| Heart racing | 0.594 | 0.909 |
| Tremors | 0.656 | 0.909 |
| Restless | 0.171 | 0.909 |
| Feeling tired | 0.102 | 0.909 |
| Difficulty concentrating | 0.623 | 0.909 |
| Forgetfulness | 0.364 | 0.909 |
| Mood swings | 0.569 | 0.909 |
| Vaginal dryness | 0.909 | 0.909 |
| Breast tenderness | 0.909 | 0.909 |
| Headache | 0.703 | 0.909 |
| Wake at night | 0.243 | 0.909 |
| Trouble sleeping | 0.424 | 0.909 |
| Trouble going back to sleep | 0.567 | 0.909 |
| Quality of sleep | 0.289 | 0.909 |
| Restless sleep | 0.241 | 0.909 |
Results from logistic regression treating time as non-linear. Models included CaD arm, year, and interaction between arm and year.
P-values adjusted after controlling the false-discovery rate to be no more than 5%.
Finally, treating year as linear instead of allowing it to be nonlinear did not change our findings (Tables S1 and S2).
4.0 DISCUSSION
Among postmenopausal women with a mean age of 64 at WHI baseline, we did not find that supplementation with 1000 mg of calcium plus 400 IU of vitamin D significantly influenced menopause-related symptoms over 5.7 years of follow-up. Our results do not suggest that menopausal women should take calcium plus vitamin D at these doses to improve vasomotor, mood, or sleep complaints.
We had hypothesized that vitamin D would improve postmenopausal women’s well-being. There are several reasons why we might not have found an effect. First, vitamin D and calcium may not, in fact, play any role in the development or amelioration of menopause-related symptoms. Alternatively, vitamin D could have a favorable effect but calcium could have a negative effect, thus canceling out vitamin D’s beneficial effects. Calcium intake has been associated with worsening hot flashes.[29] Indeed, there was a suggestion of a higher likelihood of hot flashes or night sweats during follow-up in the CaD group, although the p-value was not significant, especially after controlling for multiple comparisons.
The dosage of vitamin D used in the trial may not have been sufficient to influence symptoms. The results of an unrelated dose-response study [44] suggest that 400 IU of vitamin D3 would likely have raised mean serum 25(OH)D levels by about 4 ng/ml, which may not be enough to see a clinical difference in menopause-related symptoms, especially in women who were vitamin D deficient. Also, participants were allowed off-protocol supplementation: up to 600 IU daily vitamin D initially and up to 1000 IU daily from 1999 on. There is, however, indirect evidence that CaD arm participants had higher overall CaD intake than those assigned to placebo: when analyzing the trial’s primary outcomes, women assigned to the CaD arm had significantly higher hip bone density, and women compliant with study pills had a significant reduction in hip fracture risk.[42] When we limited our analysis to participants who were compliant, our results were unchanged. We also did not measure the vitamin D sufficiency status of the population; because personal intake of both calcium and vitamin D intake was somewhat high, many of the women may have had sufficient vitamin D status. Examining a vitamin D-deficient group of women might have been able to demonstrate an effect.
There are several limitations to this study. Women were 64 years old on average at baseline, and hot flashes are most prevalent in women in their early 50s.[45] Also, women with severe menopausal symptoms that disrupted their lives were discouraged from participating in the trial. In our analysis, only 33% of women had hot flashes or night sweats of any severity at baseline, and the majority of them still had hot flashes at the end of the study. Also, consistent with our a priori analysis plan, we examined whether women had menopausal symptoms, regardless of symptom severity. We were therefore unable to determine if calcium and vitamin D influenced symptom severity such as daily number of hot flashes. In addition, we were not able to examine symptom change over short periods of time; hot flashes often resolve within just a few years.[46] In our study, the overall number of menopause-related symptoms actually increased slightly over the 10-year period due to increases in non-vasomotor symptoms such as decreased energy level and concentration. Therefore, an overall measure of symptom number, as was pre-specified in our a priori analysis plan, may have obscured the effects of vitamin D in certain subgroups of women.
In summary, we did not find evidence that over an average of 5.7 years calcium and vitamin D supplementation influence menopause-related symptoms including vasomotor, mood, or sleep complaints. There is no evidence from this study to suggest that calcium and vitamin D supplementation leads to improved well-being among postmenopausal women with an average age of 64.
Supplementary Material
Figure 1.
Flow chart displaying the number of women in eligible and analytic cohorts
Highlights.
Postmenopausal women randomized to daily calcium carbonate 1,000 mg with vitamin D 400 IU did not have a different number of menopause-related symptoms over an average of 5.7 years compared to women given placebo.
Similarly, there was no difference between sleep disturbance, emotional well-being, or energy/fatigue at follow-up in those who were randomized to calcium and vitamin D supplementation compared to those taking the placebo.
There is no evidence from this study to suggest that calcium and vitamin D supplementation leads to improved well-being among postmenopausal women with an average age of 64.
Acknowledgments
Funding source – The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C. The funding source has no involvement in the analysis and interpretation of data, in the writing of the report, nor the decision to submit the article for publication.
Thank you to Elizabeth Sheeley for help with manuscript preparation and Katherine Essick for editorial assistance. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Comments: Erin S. LeBlanc, I declare that I participated in the study conception and design, interpretation of data, drafting of manuscript, and critical revision: and that I have seen and approved the final version. I have the following conflicts of interest: my institution has received grant funding from Amgen, Bristol-Meyers Squibb, and Astrazeneca for unrelated research projects.
Haley K. Hedlin, I declare that I participated in the data analysis, interpretation of data, and critical revision: and that I have seen and approved the final version. I have no conflicts of interest.
FeiFei Qin, I declare that I participated in the data analysis, interpretation of data, and critical revision: and that I have seen and approved the final version. I have no conflicts of interest.
Manisha Desai, I declare that I participated in the data analysis, interpretation of data, and critical revision: and that I have seen and approved the final version. I have no conflicts of interest.
Jean Wactawski-Wende, I declare that I participated in the study conception and design, acquisition of data, interpretation of results, and critical revision. I have seen and approved the final version. I have no conflicts of interest.
Nancy A. Perrin, I declare that I participated in the study conception and design, data analysis, interpretation of data, drafting of manuscript, and critical revision: and that I have seen and approved the final version. I have the following conflicts of interest: my institution has received grant funding from Amgen, Bristol-Meyers Squibb, and Astrazeneca for unrelated research projects.
JoAnn E. Manson, I declare that I participated in the study conception and design, interpretation of data, and critical revision of the manuscript: and that I have seen and approved the final version. I have the following conflicts of interest: my institution has received investigator-initiated grant support from Mars Symbioscience for an unrelated research project.
Karen C. Johnson, I declare that I participated in the study conception and design, interpretation of data, drafting of manuscript, and critical revision: and that I have seen and approved the final version. I have no conflicts of interest
Kamal Masaki, I declare that I participated in the study conception and design, interpretation of data, critical revision of manuscript, and that I have seen and approved the final version. I have no conflicts of interest with commercial enterprises.
Frances A. Tylavsky, I declare that my contribution was with reviewing and contributing to manuscript development. I have no conflicts of interest.
Marcia L. Stefanick, I declare that I participated in the study conception and design, interpretation of data, drafting of manuscript, and critical revision: and that I have seen and approved the final version. I have no conflicts of interest.
Ethical approval: All participating women provided written informed consent. The study was approved by each institution’s Institutional Review Board.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5.0 REFERENCES
- 1.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992 Jan;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 2.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000 Sep;96(3):351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 3.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010 Sep;17(5):946–54. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman EW, Sammel MD, Lin H, et al. Symptoms associated with menopausal transition and reproductive hormones in midlife women. Obstet Gynecol. 2007 Aug;110(2 Pt 1):230–40. doi: 10.1097/01.AOG.0000270153.59102.40. [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health. State-of-the-Science Conference statement: Management of menopause-related symptoms. Ann Intern Med. 2005 Jun 21;142(12 Pt 1):1003–13. [PubMed] [Google Scholar]
- 6.Sarrel PM. Women, work, and menopause. Menopause. 2012 Mar;19(3):250–2. doi: 10.1097/gme.0b013e3182434e0c. [DOI] [PubMed] [Google Scholar]
- 7.Conde DM, Pinto-Neto AM, Santos-Sa D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006 Aug;22(8):441–6. doi: 10.1080/09513590600890306. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Lindh-Astrand L, Brynhildsen J, Hoffman M, Hammar M. Vasomotor symptoms usually reappear after cessation of postmenopausal hormone therapy: A Swedish population-based study. Menopause. 2009 Nov;16(6):1213–7. doi: 10.1097/gme.0b013e3181a53221. [DOI] [PubMed] [Google Scholar]
- 10.Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005 Jul 13;294(2):183–93. doi: 10.1001/jama.294.2.183. [DOI] [PubMed] [Google Scholar]
- 11.Brunner RL, Aragaki A, Barnabei V, et al. Menopausal symptom experience before and after stopping estrogen therapy in the Women’s Health Initiative randomized, placebo-controlled trial. Menopause. 2010 Sep;17(5):946–54. doi: 10.1097/gme.0b013e3181d76953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol. 2009 May;25(5):303–14. doi: 10.1080/09513590802632514. [DOI] [PubMed] [Google Scholar]
- 13.Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000 Oct 31;36(3):155–64. doi: 10.1016/s0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 14.Pinkerton JV, Zion AS. Vasomotor symptoms in menopause: Where we’ve been and where we’re going. J Womens Health (Larchmt) 2006 Mar;15(2):135–45. doi: 10.1089/jwh.2006.15.135. [DOI] [PubMed] [Google Scholar]
- 15.Cass WA, Smith MP, Peters LE. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann N Y Acad Sci. 2006 Aug;1074:261–71. doi: 10.1196/annals.1369.023. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan JR, Santen R, Cauffman S, Cavaliere A, Greer RB, Demers LM. The effect of endogenous estrogen fluctuation on metabolism of 25-hydroxyvitamin D. Calcif Tissue Int. 1986 Sep;39(3):139–44. doi: 10.1007/BF02555109. [DOI] [PubMed] [Google Scholar]
- 17.Motsinger S, Lazovich D, Maclehose RF, Torkelson CJ, Robien K. Vitamin D intake and mental health-related quality of life in older women: The Iowa Women’s Health Study. Maturitas. 2012 Mar;71(3):267–73. doi: 10.1016/j.maturitas.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoang MT, Defina LF, Willis BL, Leonard DS, Weiner MF, Brown ES. Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults: the Cooper Center longitudinal study. Mayo Clin Proc. 2011 Nov;86(11):1050–5. doi: 10.4065/mcp.2011.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Johnson KC, Lane D, et al. 25-hydroxyvitamin D concentration, vitamin D intake and joint symptoms in postmenopausal women. Maturitas. 2011 Jan;68(1):73–8. doi: 10.1016/j.maturitas.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003 Dec;78(12):1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 21.Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: The Invecchiare in Chianti study. J Am Geriatr Soc. 2008 May;56(5):785–91. doi: 10.1111/j.1532-5415.2008.01644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan QJ, Reddy PS, Kimler BF, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010 Jan;119(1):111–8. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J Intern Med. 2008 Dec;264(6):599–609. doi: 10.1111/j.1365-2796.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen JT, Hirai Y, Seimiya Y, Hasumi K, Shiraki M. Menopausal flushes and calcitonin-gene-related peptide. Lancet. 1993 Jul 3;342(8862):49. doi: 10.1016/0140-6736(93)91911-5. [DOI] [PubMed] [Google Scholar]
- 25.Valentini A, Petraglia F, De VD, et al. Changes of plasma calcitonin gene-related peptide levels in postmenopausal women. Am J Obstet Gynecol. 1996 Sep;175(3 Pt 1):638–42. doi: 10.1053/ob.1996.v175.a74287. [DOI] [PubMed] [Google Scholar]
- 26.Wyon YA, Spetz AC, Theodorsson GE, Hammar ML. Concentrations of calcitonin gene-related peptide and neuropeptide Y in plasma increase during flushes in postmenopausal women. Menopause. 2000 Jan;7(1):25–30. doi: 10.1097/00042192-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wyon Y, Frisk J, Lundeberg T, Theodorsson E, Hammar M. Postmenopausal women with vasomotor symptoms have increased urinary excretion of calcitonin gene-related peptide. Maturitas. 1998 Nov 16;30(3):289–94. doi: 10.1016/s0378-5122(98)00047-4. [DOI] [PubMed] [Google Scholar]
- 28.Wyon Y, Lindgren R, Lundeberg T, Hammar M. Effects of acupuncture on climacteric vasomotor symptoms, quality of life and urinary excretion of neuropeptides among postmenopausal women. Menopause. 1995;2:3–12. [Google Scholar]
- 29.Kimmick G, Broadwater G, Vitolins M. Hot flashes in breast cancer survivors and an association with calcium supplement use. Int J Womens Health. 2010;2:291–4. doi: 10.2147/IJWH.S12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 31.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women’s Health Initiative Dietary Modification trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 32.Jackson RD, LaCroix AZ, Cauley JA, McGowan J. The Women’s Health Initiative calcium-vitamin D trial: overview and baseline characteristics of participants. Ann Epidemiol. 2003 Oct;13(9 Suppl):S98–106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 33.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 34.Patterson RE, Levy L, Tinker LF, Kristal AR. Evaluation of a simplified vitamin supplement inventory developed for the Women’s Health Initiative. Public Health Nutr. 1999 Sep;2(3):273–6. doi: 10.1017/s1368980099000361. [DOI] [PubMed] [Google Scholar]
- 35.Greendale GA, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: Results from the Postmenopausal Estrogen/Progestin Interventions Trial. Obstet Gynecol. 1998 Dec;92(6):982–8. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- 36.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995 Sep 20;87(18):1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 37.Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994 Oct 24;154(20):2349–55. [PubMed] [Google Scholar]
- 38.Matthews KA, Shumaker SA, Bowen DJ, et al. Women’s health initiative. Why now? What is it? What’s new? Am Psychol. 1997 Feb;52(2):101–16. doi: 10.1037//0003-066x.52.2.101. [DOI] [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. [PubMed] [Google Scholar]
- 40.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003 Jun;15(2):137–48. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 41.Margolis KL, Ray RM, Van HL, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008 Nov;52(5):847–55. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006 Feb 16;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 43.Benjamini B, Hochner H. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. 1995;57(1):289–300. [Google Scholar]
- 44.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003 Jan;77(1):204–10. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 45.Rodstrom K, Bengtsson C, Lissner L, Milsom I, Sundh V, Bjorkelund C. A longitudinal study of the treatment of hot flushes: the population study of women in Gothenburg during a quarter of a century. Menopause. 2002 May;9(3):156–61. doi: 10.1097/00042192-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.