Abstract
The thisbe (ths) gene encodes a Drosophila fibroblast growth factor (FGF), and mutant females are viable but sterile suggesting a link between FGF signaling and fertility. Ovaries exhibit abnormal morphology including lack of epithelial sheaths, muscle tissues that surround ovarioles. Here we investigated how FGF influences Drosophila ovary morphogenesis and identified several roles. Heartless (Htl) FGF receptor was found expressed within somatic cells at the larval and pupal stages, and phenotypes were uncovered using RNAi. Differentiation of terminal filament cells was affected, but this effect did not alter ovariole number. In addition, proliferation of epithelial sheath progenitors, the apical cells, was decreased in both htl and ths mutants, while ectopic expression of the Ths ligand led to these cells’ over-proliferation suggesting that FGF signaling supports ovarian muscle sheath formation by controlling apical cell number in the developing gonad. Additionally, live imaging of adult ovaries was used to show that htl RNAi mutants, hypomorphic mutants in which epithelial sheaths are present, exhibit abnormal muscle contractions. Collectively, our results demonstrate that proper formation of ovarian muscle tissues is regulated by FGF signaling in the larval and pupal stages through control of apical cell proliferation and is required to support fertility.
Keywords: Drosophila, ovary morphogenesis, FGF signaling, Heartless, Pyramus, Thisbe, apical cells, proliferation, muscles, epithelial sheath, fertility
INTRODUCTION
The Drosophila melanogaster ovary is a highly studied developmental system that has already provided many important insights into the biology of organ development. In particular, the Drosophila adult ovary has served as an excellent model for the interaction of germ line stem cells (GSCs) with their somatic support cells known as the niche. In the ovary, the GSCs and niche facilitate egg production throughout the lifetime of the Drosophila female (Song et al., 2007; Xie and Spradling, 2000). Less is known regarding how the ovary is formed, but tight regulation of cell proliferation, differentiation, and survival by signaling pathways appears critical. Regulated signaling ensures that all cell types within this organ develop in a balanced manner during this period of major growth of the ovary at the larval and pupal stages.
Each ovary in the Drosophila adult consists of 15-20 ovarioles that contain GSCs, their associated niche, and a chain of oocytes at various stages of development. At the apical region of an ovariole, a unique structure called the germarium resides. It is within this structure that two to three GCSs reside at the apical tip next to their niche composed of terminal filament (TF) cells and cap cells (Eliazer and Buszczak, 2011). Much insight into the mechanisms controlling GSC maintenance and differentiation has been uncovered in Drosophila owing to the ease of accessibility of these cells within adult ovaries and because the system is amenable to genetic manipulation (Kirilly and Xie, 2007). In contrast, less is known regarding how GSCs, their somatic niche, and muscle tissues that encapsulate the ovarioles (the epithelial and peritoneal sheaths) are formed as these events occur earlier, at the larval and pupal stages, as ovaries develop.
Previous studies of ovary morphogenesis at the larval and pupal stages have focused on the role of signaling pathways in regulating cell number, proliferation, differentiation, and survival (rev. in Gilboa, 2015; Sarikaya and Extavour, 2015). EGFR, JAK/STAT, and Hippo signaling is important in mediating cellular homeostasis during the period of extreme cell growth of the gonad at the larval stage. Specifically, EGFR regulates the number of primordial germ cells (PGCs) as well as their somatic support cells, the interstitial cells (ICs) (Gilboa and Lehmann, 2006; Matsuoka et al., 2013). Ecdysone hormone also has been shown to trigger cell proliferation and to control growth of the ovary through effects on the insulin receptor (InR) and Target of rapamycin (Tor) pathway, as well (Gancz and Gilboa, 2013). Additionally, Bone morphogenetic protein (BMP) and Hedgehog (Hh) signaling pathways positively regulate PGC cell division at the larval stage (Sato et al., 2010). However, whether FGF signaling impacts ovary homeostasis and morphogenesis had not been previously investigated.
FGF signaling is involved in a multitude of important biological processes. FGF receptors (FGFRs) are a family of receptor tyrosine kinases. Upon receptor activation by ligand binding, various intracellular signaling pathways are induced (Feldman et al., 1995; Powers et al., 2000; Rottinger et al., 2008). To define a role for FGF signaling or to identify the specific molecular mechanisms involved can be challenging due to the complexity of the pathway. In humans and mice, for instance, twenty-four FGF and four FGFR genes have been discovered (Ornitz and Itoh, 2001), which support over one hundred possible FGF-FGFR complexes (Ornitz et al., 1996). Conversely, invertebrate systems have much simpler FGF signaling systems (Tulin and Stathopoulos, 2010a). In the case of Drosophila, three FGF and two FGFR genes have been discovered, supporting only three functional FGF-FGFR combinations (Kadam et al., 2009). The role of FGFR signaling in Drosophila as well as ligand choice varies and is context-specific (rev. in Bae et al., 2012).
A role for fibroblast growth factor (FGF) signaling pathway in supporting ovarian development has been suggested in vertebrates, but no previous study has directly examined the role of FGF signaling in the Drosophila ovary. In vertebrates, it has been shown that both FGF ligands and receptors are expressed within follicular cells of vertebrate ovaries, including human (Berisha et al., 2006; Buratini et al., 2007). Furthermore, FGF addition to cultured ovarian tissues leads to cells’ proliferation, and high levels of FGF signaling is correlated with many cancers including that of the ovary (Basu et al., 2014; Ropiquet et al., 2000). In particular, vertebrate FGF-8 exhibits gonad-specific expression, within the ovary and testes, suggesting this signaling pathway plays an important, yet currently uncharacterized role in supporting gonad development (Valve et al., 1997). Keeping FGF signaling properly regulated is important for normal ovary development, but its exact role in supporting gonad development is unclear. Furthermore, FGF signaling is conserved as its biological roles and structural properties appear similar in Drosophila and higher vertebrates (Huang and Stern, 2005; Tulin and Stathopoulos, 2010b). Studies of how FGF signaling impacts Drosophila ovary morphogenesis have the potential to provide novel insights into conserved functions and/or regulatory mechanisms acting in other organisms, including vertebrates.
In the current study, we investigated the role of FGF signaling in supporting Drosophila ovary morphogenesis and found that this signaling pathway has several roles spanning multiple stages of development. At the larval stage, our results demonstrate a role for the Htl FGFR in controlling specification of the adult stem cell niche through regulation of TF cell differentiation; in the larval and pupal stages, this pathway also supports migration of a somatic cell population in the ovary, the apical cells, through regulation of these cells’ proliferation. These earlier functions are necessary for the proper specification of the epithelial sheaths that surround individual ovarioles to support proper oocyte development and, thus, fertility.
MATERIALS AND METHODS
Fly stocks
Drosophila stocks were kept at 25°C, unless otherwise noted. yw stock was used as wildtype. To generate ths mutant viable flies, thse02026/Cyo ftz-lacZ (CFLZ) (Stathopoulos et al., 2004) and Df(2R)ths238/CFLZ (Kadam et al., 2009) were crossed to generate transheterozygotes. GAL4 lines used for genetic analysis were: c587.GAL4 (Kai and Spradling, 2003) and nos.GAL4vp16 (BDSC). UAS lines utilized for genetic analysis were: UAS.htl.RNAi40627 [Vienna Drosophila Research Center (VDRC); reported to have one off target]; (Dietzl et al., 2007; Kadam et al., 2012)], UAS.htl.RNAi6692 (VDRC; reported to have no off targets), UAS.ths.RNAi24538/CyO (VDRC, reported to have one off target), UAS.pyr.RNAi36523 (VDRC, reported to have over two hundred off targets), UAS.ths [AMS289-22; (Stathopoulos et al., 2004)], UAS.htl.lambda [#5367, Bloomington Drosophila Stock Center (BDSC)]. For temporal control, UAS.htl.RNAi40627 was crossed with w+; Sco/Cyo;tub-GAL80ts (#7018, BDSC). As necessary, If/CyO,actin-gfp; MKRS/Tm3,Ser,actin-gfp (from Dr. Kai Zinn, California Institute of Technology, US) was used as a marked balancer at the larval stage.
To examine expression patterns of htl or ths, htl.GAL4 or ths.GAL4 lines (Pfeiffer et al., 2008) were crossed with UAS.GFP. The following GAL4 lines from BDSC were assayed but only a subset (underlined) drove expression in the ovary: htl.GAL4 lines 47240, 40668, 40669, 48004, 40706, 47277, 40707, 40708, 48431, 47278, 47279; and ths.GAL4 lines 40051, 47051, 40049, 40050, 40052, 48624, 48355.
We also generated five pyr-GAL4 lines by cloning 1-3 kB fragments of non-coding DNA sequence flanking the pyr gene into the Gateway donor vector and pBGUw vector (Pfeiffer et al., 2008 #1883) to create GAL4 drivers HV01-05; of these, only HV03 and HV04 supported expression in the developing ovary. Primer sequences are provided (Table S1).
To examine Htl localization, an inframe insertion of the Cherry reporter was inserted into a construct “htl-mcherry” able to rescue the htl mutant. The 52 kb htl P[acman] construct was generated using recombineering-mediated gap repair performed as described (Venken et al., 2006). Insertion of the cherry gene just before the stop codon of htl was performed by standard recombineering techniques, using the Cherry-SV40-frt-kan-frt plasmid modified from the GFP-SV40-frt-kan-frt plasmid kindly provided by Dr. Eric Davidson (Caltech). The kan cassette was flipped out by arabinose induction of Flp in the SW105 cells (Warming et al., 2005).
The Ths rescue construct contains 33 kB of sequence spanning the ths gene and is able to rescue the ths mutant. Primer sequences for htl and ths rescue constructs are provided (Table S1).
Collection and aging
Eggs were collected in fresh vials for two hours to prevent overcrowding. Once flies were removed, vials were incubated at 25°C for various lengths of time: 72 hours (h) for early-larval third instar, 96h for mid-larval third instar, 120h for late-larval third instar, 144h for early pupae, 168h for middle pupae, and 192 h for late pupae. For UAS.htl.RNAi;GAL80ts, after a two-hour-egg collection, vials were incubated at 18°C until development to adult. The adult flies were transferred to 29°C, and incubated for an additional, appropriate length of time. Before dissection, adult flies were well fed with yeast paste for one day.
For fertility assays, five female flies of each genotype were crossed with two yw male flies. The eggs were collected on apple juice plates, and number of eggs deposited counted after 24 hours.
Fixation, immunocytochemistry, and in-situ hybridization
Dissected ovaries were fixed in 33% paraformaldehyde in PBT solution for 20 minutes (min) at room temperature (RT). The fixed ovaries were washed with PBS three times and incubated in blocking solution (10% BSA in PBT) for 1h. After the blocking, samples were incubated with primary antibodies for ~18 h at 4°C and, subsequently, were washed with 1:10 diluted blocking solution 4x, with 30 min incubation for each wash. Secondary antibodies diluted 1:100 in blocking solution were added to the sample, and incubated further for ~18h at 4°C. The samples were washed with PBT 3x and mounted in Vecta-shield mounting medium (Vector Laboratories). Images were captured with a Pascal confocal microscope (Zeiss).
The primary antibodies utilized were rabbit anti-GFP (1:400; Molecular Probes), rabbit anti-RFP (1:1000, Rockland), rat anti-Vasa (1:40; Developmental Studies Hybridoma Bank (DSHB)), mouse anti-α-Spectrin (1:133; DSHB), mouse anti-Fasciclin (Fas) III (1:60; DSHB), mouse anti-Engrailed (En) (1:400; DSHB), rabbit anti-Phospho-Histone-H3 (PH3) (1:1000; Rockland), rabbit anti-dual-phosphorylated ERK (dpERK) (1:200; Cell Signaling) and rabbit anti-Downstream of FGF (Dof) (gift of Dr. Maria Leptin, Univ. of Cologne, Germany). Appropriate secondary antibodies (Invitrogen) were used.
Rhodamine phalloidin-Alexa488 (Invitrogen) and TO-PRO3 (Life Technologies) were used to detect ovarian muscle tissues and nuclei, respectively. For these detections, standard protocols provided by the manufactures were used.
For detection of htl, ths, or pyr transcripts, in-situ hybridization using gene specific riboprobes were used as previously described (Stathopoulos et al., 2004). Ovaries were mounted in Permount (Fisher Scientific).
Counting number of TF cells per stack and TF stacks per ovary
Ovaries at the 3LL stage were stained with rhodamine phalloidin and TO-PRO3 to outline TF cells and nuclei, respectively. At this stage, TF cells are blunt-edge, disc-shaped, and ~7-9 TF cells are present in each TF stack, which can be identified by rhodamine phalloidin staining. Furthermore, TF cells have flattened nuclear shape, which can be distinguished by TO-PRO 3 staining. With these aids, the number of TF cells per stack and the number of TF stack per ovary were counted by eye through analysis of a stack of confocal images capturing the entire ovary.
Live Imaging and image analysis
For live imaging, ovaries of 3-4 day old flies fed with yeast paste for one day were dissected in culture medium as described previously (Prasad et al., 2007). Dissected ovaries were placed on a cavity microscope slide with culture medium. Ovaries were imaged using a Zeiss Pascal confocal microscope at 488nm wavelength using a 10X objective lens with continuous scan of a single frame for no more than 20 minutes. Once a time series was completed, the file was converted to .avi file using Fiji imaging software.
RESULTS
ths mutants are sterile and have defective ovarian muscle sheaths
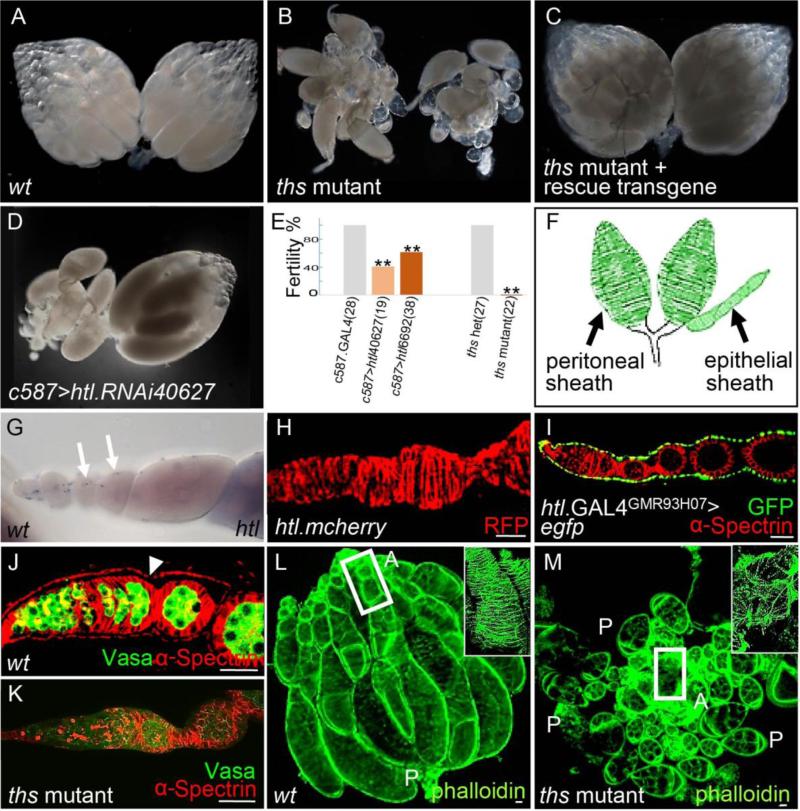
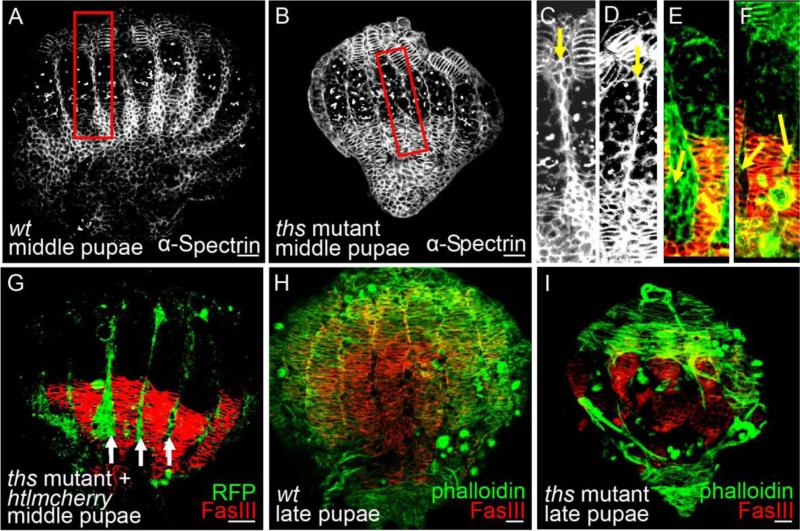
A role for FGF signaling in female reproduction was suggested by the finding that hypomorphic mutants for one Htl-receptor ligand, Ths, are viable but sterile. ths mutant adult females were obtained by crossing thse02026, a piggyback mutation that is semi-lethal, to Df(2R)ths238, a small deficiency that deletes the region containing the ths gene (Kadam et al., 2009). Each of the two ovaries present in the abdomen of wildtype Drosophila melanogaster females consists of ~15 ovarioles (Fig. 1A). However, in FGF mutant females [i.e., thse02026/Df(2R)ths238], two ovaries are present, but their overall structure appears disorganized (Fig. 1B). Furthermore, ths mutant females rarely deposit eggs (Fig. 1E), and yet oocytes do not accumulate within their abdomens suggesting a block to oogenesis (data not shown). To confirm that the observed phenotypes relate to loss of FGF signaling, a ths rescue transgene was introduced into the ths mutant background (Fig. 1C; see Materials & Methods). Both the morphology and fertility phenotypes were rescued (Fig. 1C; data not shown), supporting the view that FGF signaling through the Ths FGF ligand is required to support ovary function.
Figure 1. ths mutant females are viable but sterile, and exhibit ovarian muscle defects.
(A-D) Images of adult ovaries isolated from the following genetic backgrounds: wildtype (A), ths mutant (B), ths mutant rescue (C) or htl-knockdown (D) using a light microscope. In this and all other figures, “ths mutant” refers to the ths02026/Df(2R)ths238 transheterozygous combination of alleles, which is viable.
(E) Graph of fertility assays comparing FGF mutants to controls. The number of deposited eggs was counted for mutants (i.e. two different htl KD RNAi constructs and ths mutant) and the values normalized relative to respective “wildtype” controls (i.e. c587.GAL4 or ths heterozygote, respectively). Sample size (i.e. number of flies) indicated within bracket. In this and all other figures, “ths heterozyote” refer to ths02026/Cyo,actin-GFP or Df(2R)ths238/Cyo,actin-GFP. For statistical analysis, two-tailed Student's t-test was used, and ρ<0.05 was considered significant (**).
(F) Schematic showing two types of ovarian muscle tissues: peritoneal and epithelial sheaths.
(G) In situ hybridization of wildtype adult ovarioles using an antisense riboprobe to detect htl transcripts. Arrows mark htl transcripts identified in the epithelial sheath.
(H-K) Stainings to examine epithelial sheaths associated with ovarioles isolated from flies of the following genetic backgrounds: transgenic htl-mcherry line (H), UAS.GFP driven by htl.GAL4GMR93H07(I), wildtype (J), and ths mutant (K). Antibodies recognizing RFP (H, red), GFP (I, green), Vasa (J, K, green), and α-Spectrin (I, J, K, red) were used. Arrow in J marks the epithelial sheath.
(L-M) Views of wildtype (L) and ths mutant (M) ovaries stained with rhodamine phalloidin (green) used to detect the actin cytoskeleton. In panels (L) and (M), “A” denotes the apical region, whereas “P” denotes the posterior region. Boxed regions are shown within insets at magnification.
In this and all other figures, scale bars denotes 20um.
Next, we examined the Htl-receptor loss-of-function phenotype and compared it with that of the Ths ligand. htl null mutants are zygotically lethal, therefore RNAi was used. htl levels were reduced by driving a UAS.RNAi hairpin construct with a somatic cell specific driver, c587.GAL4. Upon htl knock-down (KD), the organization of the ovary was disrupted (Fig. 1D), and the egg/embryo deposit percentage was significantly decreased compared to the control (i.e. driver alone; Fig. 1E). The htl RNAi phenotype was not as severe as ths mutants and may relate to the observation that few c587>htl.RNAi progeny make it to the adult stage. Survival may select for weak htl KD. The similarity of ths and htl mutant phenotypes suggested that FGF signaling through the Htl receptor and Ths ligand regulates ovary morphogenesis.
To provide insight into these defects, we examined the expression of the FGF receptor, htl, to infer the location of FGF signaling action. In ovarioles isolated from the adult ovary, htl transcripts were identified, specifically, within cells of the epithelial sheath using in situ hybridization with gene-specific riboprobes (Fig. 1G). The sheath covers each ovariole and is composed of a layer of squamous epithelium surrounded by bands of muscle (Hudson et al., 2008). The epithelial sheath secretes a thick basement membrane and provides structural support to the ovariole (Fig. 1F; Cummings, 1974).
To confirm the expression domain of Htl protein within the epithelial sheath, we used several approaches. First, a fusion gene was created in which the monomeric Cherry fluorescent protein (FP) sequence was inserted into the htl gene in the context of a 54 kb rescue construct to create a C-terminal fusion of this FP to Htl (“Htl-mCherry”) so that the Cherry reporter could be used as a proxy for Htl FGFR protein expression. In ovaries isolated from Htl-mCherry transgenic females, strong anti-RFP staining was detected in the epithelial sheaths (Fig. 1H), as well as in the peritoneal sheaths, a distinct muscle tissue that surrounds the entire ovariole collective (data not shown; Fig. 1F). Next, we examined cis-regulatory sequences acting to support htl expression. In a previous genome-wide study of non-coding DNA sequences supporting expression in the brain (Pfeiffer et al., 2008), sequences flanking the htl gene were placed upstream of a heterologous gene, GAL4, encoding a transcription factor able to support ectopic expression through UAS sequences (Phelps and Brand, 1998). We found that a particular htl.GAL4 driver (htl.GAL4GMR93H07), only one out of eleven tested, supports expression in the ovary and that this expression was present in the epithelial sheath (Fig. 1I). Results from all three expression assays (in situ, FP fusion, and cis-regulatory activity) support the view that htl is expressed in the ovarian muscle sheaths, an ovarian tissue that has been little studied (Cummings, 1974; Hudson et al., 2008). We hypothesized that FGF signaling regulates some aspect of ovarian muscle sheaths function and/or morphogenesis that relates to the phenotypes observed in ths mutants, namely loss of overall structural organization within adult ovaries (Fig. 1B).
As a result, the epithelial sheaths of wildtype and ths mutant ovaries were examined. In ths mutant ovarioles, no epithelial sheath was observed as assayed by localization of α-Spectrin, a constituent of the sub-membrane cytoskeleton of epithelial cells (Fig. 1K, compare with Fig 1J). Moreover, while organized actin filaments are clearly visible within epithelial sheaths of wildtype ovaries (Fig. 1L), in ths mutant ovaries, only a limited amount of actin staining was identified and it was confined to a few disorganized actin-rich masses (Fig. 1M). No evidence of peritoneal sheaths was present in ths mutants (data not shown). Previous studies have shown that apical cells (ACs), a type of somatic gonadal cell residing at the anterior of the developing ovary, are responsible for specification of both epithelial and peritoneal sheaths during gonadal development (Cohen et al., 2002; King, 1970). Therefore, we investigated whether these identified muscle tissue malformations in the adult ovary of ths mutants might relate to defects at earlier stages during ovary morphogenesis.
Htl FGF receptor is expressed within apical cells throughout the course of their migration
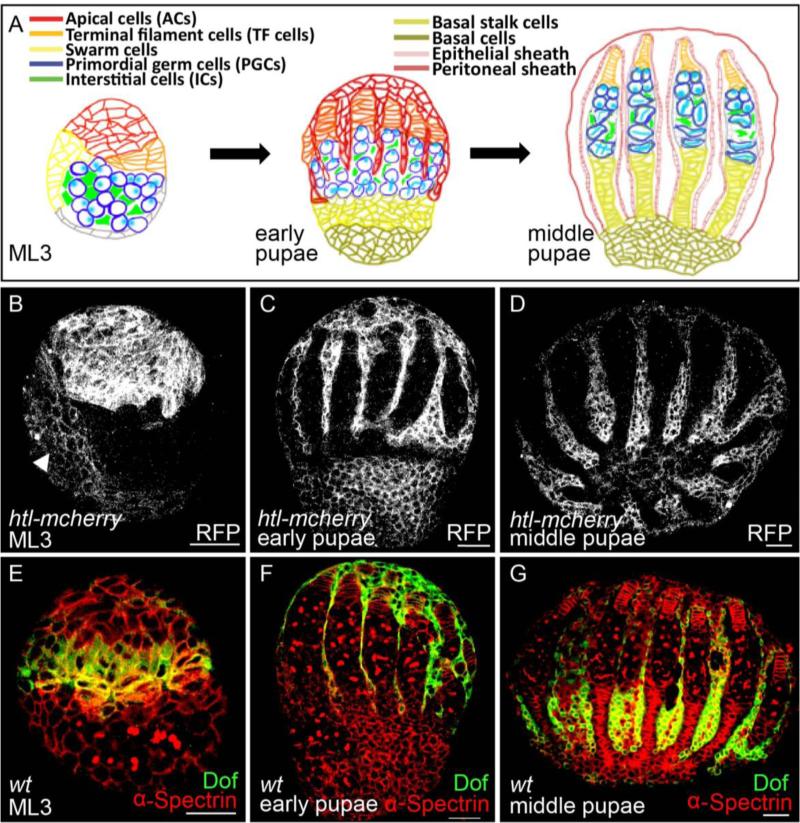
Morphogenesis of the adult ovary starts in the embryo and continues throughout the larval and pupal stages and involves many changes (Fig. 2A; rev. in Gilboa, 2015). To start, in embryos, primordial germ cells (PGCs) undergo a migration to the somatic gonadal mesoderm. These somatic gonadal mesoderm cells serve as the precursor population for several types of cells present in the developing ovary, later, at the larval stage. Specifically, at mid-larval third instar (ML3), somatic cells include ACs (discussed above), ICs, and swarm cells (Fig. 2A, left). These cell types all actively undergo mitotic cell divisions throughout larval and pupal development and further differentiate into more specialized cell types (Couderc et al., 2002). For example, by late-larval third instar (LL3) following ML3 but before pupation, some of the ACs differentiate into terminal filament (TF) cells to specify a component of the adult germline stem cell niche. In addition, cell movements support morphogenesis of the ovary. For instance, at the early pupal stage, a subset of ACs initiates migration from the anterior of the gonad towards the posterior (Fig. 2A, middle). By ~24 hours after puparium formation, at the middle pupal stage, the migration is complete. As a result of the migration, a subset of ACs make direct contact with the primordial ovarioles to specify the epithelial sheath, whereas others migrate to the periphery of the gonad to specify the peritoneal sheath (Fig. 2A, right).
Figure 2. FGF signaling acts in multiple somatic cell types within the developing gonad at the larval and pupal stages.
(A) Schematic showing ovarian morphogenesis at three stages: mid-larval third instar (ML3), early pupae, and middle pupae. In this an all other panels/figures, lateral views of the larval/pupal ovary are shown with anterior up and posterior down.
(B-D) Immunostainings of ovaries obtained from transgenic line htl-mcherry using anti-RFP antibody (white). Three different stages are shown: ML3 (B), early pupae (C), and middle pupae (D). Arrowhead in (B) marks migrating swarm cells at ML3.
(E-G) Immunostainings of wildtype ovaries using anti-Dof (green) and anti-α-Spectrin (red) antibodies. Three different stages are shown: ML3 (E), early pupae (F), and middle pupae (G).
With the aid of the htl-mcherry reporter, expression of Htl-mCherry protein was identified in somatic tissues of the developing gonad (Fig. 2B-D). Using an anti-RFP antibody, staining was detected in ACs present at the anterior of the gonad at ML3 (Fig. 2B). At later stages, expression remained detectable in the ACs as they migrate from the anterior toward the posterior end of the developing ovary at the early pupal stage (Fig. 2C) as well as at the end of their migration at the middle pupal stage (Fig. 2D). In addition, weak expression of Htl-mCherry was identified in other somatic cells, possibly the swarm cells, which also undergo a posteriorly-directed migration to form basal cells, precursors of basal stalk cells (Fig. 2B, arrowhead).
To provide additional insight into the role of FGF signaling in the developing ovary, other reporters of FGFR-activation were examined. Intracellular signaling downstream of FGFR-activation requires Downstream of FGFR (Dof, also known as Stumps/Heartbroken), an intracellular adaptor protein (Imam et al., 1999; Michelson et al., 1998; Vincent et al., 1998). Dof expression was examined using an anti-Dof antibody and localized to differentiating TF cells at the ML3 stage (Fig. 2E), migrating ACs at the early pupae stage (Fig. 2F), as well as ACs having completed their migration at the middle pupae stage (Fig. 2G). This pattern overlaps with the domain of Htl FGFR expression inferred using the Htl-mCherry fusion. However, the Dof expression domain encompassed only a subset of the receptor expression domain, suggesting that Dof might possibly differentially influence downstream signaling pathway activation.
To provide additional insight into the activation domains of Htl FGFR, a receptor tyrosine kinase, we assayed where MAPK signaling was active using an antibody against the dual-phosphorylated form of MAPK (dpERK) (Gabay et al., 1997). At the early larval third instar (EL3) stage, before TFs are specified, dpERK staining was found broadly distributed in somatic cells including ACs (Fig. S1A-A”, arrow); whereas, in slightly older gonads (i.e., ML3), dpERK staining was detected in differentiating TF cells as well as in non-differentiated ACs (Fig. S1B-B”; arrowhead and arrow, respectively). Previous studies have detected dpERK staining within the IC population, somatic support cells for PGCs present in the medial domain of developing ovaries, and demonstrated that this activation relates to intracellular signaling downstream of EGFR activation (Gilboa and Lehmann, 2006). As we detected dpERK in anterior regions (Fig. S1A’) including the domain of TF specification (Fig. S1B’), these results suggest that signaling downstream of other receptor tyrosine kinases, possibly FGFR, may also occur at this stage.
Collectively, these data show that FGF signaling is spatially and temporally positioned within domains able to impact differentiating TF cells and ACs.
Htl-receptor activation by the Pyr FGF ligand regulates differentiation of TF cells
To assay a role for FGF signaling in the developing ovary, we assayed phenotypes resulting from loss of htl. htl mutants could not be assayed directly because null mutants are zygotically lethal at the embryo/early larval stages (data not shown; Gisselbrecht et al., 1996). Therefore an RNAi approach was taken. Specifically, the pan-somatic driver c587.GAL4 was used to support expression of two UAS-RNAi transgenes (VDRC40627 and VDRC6692) targeted to distinct regions of the htl gene.
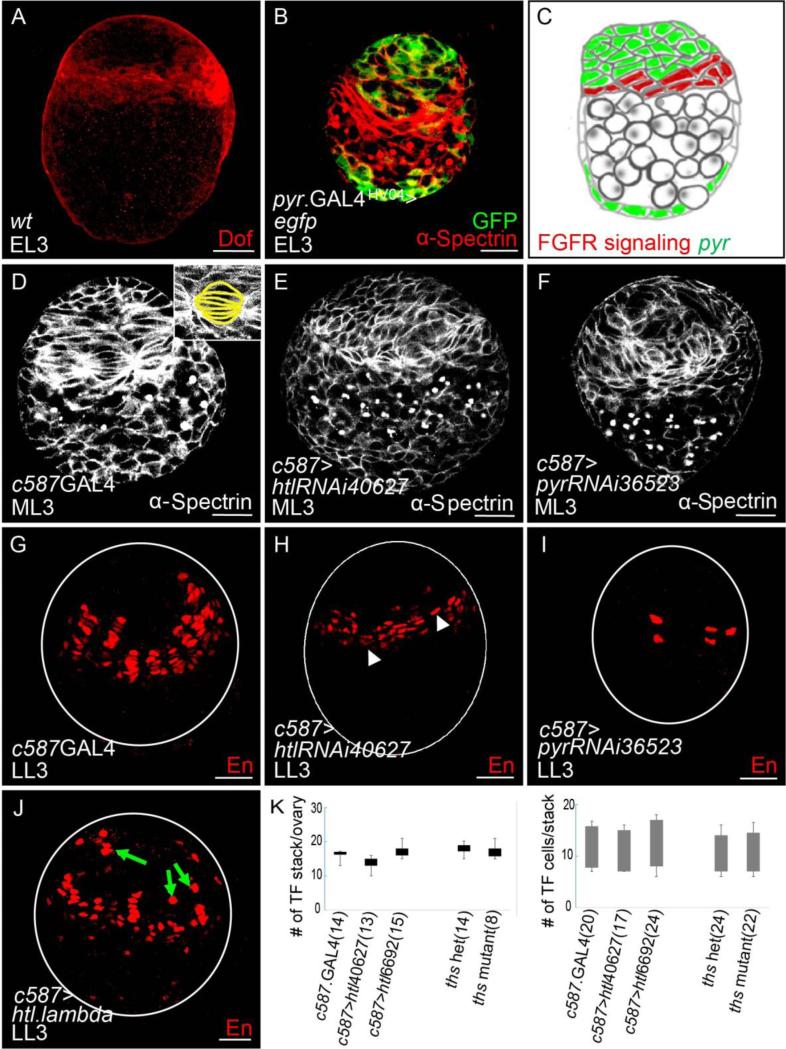
To start, the role of FGF signaling in supporting TF development was investigated. The earliest Dof expression was observed at the EL3 stage, in a domain in which TF cells are specified at the later larval stage (Fig. 3A), suggesting a role for FGF in TF development. TF cells start off lens-shaped at the ML3 stage (Fig. 3D), and they later transform into blunt-edged disc shaped cells as the differentiation process progresses resulting in complete separation between TF stacks (Godt and Laski, 1995; Sahut-Barnola et al., 1995). At the LL3 stage, TF cells resolve into a number of individual stacks that each contain approximately 7-9 cells exhibiting flattened nuclei. Differentiated TF cells can be identified by expression of the transcription factor Engrailed (En) within nuclei (Fig. 3G), which is maintained throughout gonadal development. Upon htl KD, the number of En+ TF cells was reduced at LL3 stage (Fig. 3H, white arrowhead). In contrast, when a constitutively active form of the Htl receptor (“htl lambda”) was expressed within somatic tissues, ectopic expression of En was observed (Fig. 3J, green arrow). When a germline specific GAL4 driver (nos.Gal4) was used to support either htl.RNAi or expression of constitutively active Htl receptor, no effect on En expression within TFs was observed (data not shown). These results suggest the Htl FGF receptor acts in somatic cells to regulate TF specification, possibly, by controlling gene activation.
Figure 3. FGF signaling activated by Htl FGF receptor supports TF cell differentiation at the larval stage.
(A) Immunostaining of wildtype ovary of early-larval third instar (EL3) stage using anti-Dof antibody (red).
(B) Immunostaining of an ovary of EL3 stage using anti-GFP (green) and anti-α-Spectrin (red) antibodies to examine expression of UAS.GFP supported by pyr.GAL4HA04.
(C) Schematic showing inferred activation domain of FGF signaling (red) relative to pyr expression domain (green) at EL3.
(D-F) Immunostainings of ovaries at ML3 stage using anti-α-Spectrin antibody (white) to examine TF cell morphogenesis. A role for htl and pyr in somatic cells was examined using the c587.GAL4 driver: control, driver alone (D) compared with htl KD (using UAS.htl.RNAi40627, E) and pyr KD (using UAS.pyr.RNAi36523, F). Within the magnified inset of (D), TF cells are outlined in yellow to demonstrate normal morphology.
(G-J) Immunostainings of ovaries from late-larval third instar (LL3) stage using anti-En antibody (red) to detect differentiated TF cells. Effects on somatic cells were examined using the c587.GAL4 driver: control, driver alone (G) compared with htl KD (using UAS.htl.RNAi40627, H), pyr KD (using UAS.pyr.RNAi36523, I), and constitutively-active-Htl (using UAS.htl.lambda, J). Representative single confocal sections are displayed; and ovary outlines are marked by white circles. In (H), TF stalks that contain fewer En+ TF cells are marked by white arrowhead. In (J), ectopically expressed En+ cells are marked by green arrows.
(K) Graphs showing number of TF stalks per ovary (left) and TF cells per stack (right) in c587.GAL4 control, two htl KDs (using UAS.htl.RNAi40627 and UAS.htl.RNAi6692), ths heterozygote control, and ths mutant.
We further examined a role for each of the two ligands for the Htl receptor, Pyr or Ths, in supporting FGF signaling in this function. Neither ths mutant nor RNAi exhibited any change in TF morphology (Fig. S2E, compare with Fig. S2C; data not shown) or En expression (Fig. S2G, compare with Fig. S2F; data now shown) and suggested, instead, that Pyr is fulfilling Htl receptor activation in this role. The expression domain of Pyr FGF ligand was examined through assay of associated cis-regulatory domains. pyr.GAL4HA04 supports expression in somatic cells (Fig. 3B, Fig. S2A), while another cis-regulatory sequence from the vicinity of the pyr gene was found to support expression in PGCs (pyr.GAL4HV03; Figs. S2A and S2B).
To assay a role for Pyr, pyr KD by RNAi, through GAL4-mediated expression of a UAS-driven hairpin construct, was chosen due to zygotic lethality of all assayed pyr mutants at the embryonic/early larval stage. However, the only available RNAi construct directed to pyr (VDRC36523) has a number of off-targets. While pyr KD resulted in a decreased number of En+ cells at the LL3 stage (Fig. 3I), a phenotype shared with htl KD, earlier phenotypes were observed as well that were not shared with htl KD. Upon pyr KD, TF morphology was affected; few lens-shaped cells were observed at stage ML3 (Fig. 3F), which is a more severe effect than htl KD (Fig. 3E, compare with Fig. 3D), suggesting either that off-targets of pyr.RNAi36523 are responsible and/or that the htl RNAi presents only a partial loss-of-function phenotype. In contrast, when pyr was knocked-down in the germline cells, TF morphology appeared normal (Fig. S2D, compare with Fig. S2C). Taken together, FGF signaling, likely mediated by somatically expressed Pyr at the larval stage, contributes to ovarian morphogenesis by directly promoting TF cell differentiation.
Next, we investigated whether these observed differentiation defects, changes in En+ cell numbers within TF precursor cells, affected TF number at later stages. The number of TF stack per ovary and the number of TF cells per TF stack were counted at the LL3 stage based on staining with rhodamine phalloidin and TO-PRO3 (marking TF cells and nuclei, respectively; Fig. S3). No effects on TF cell or stack numbers were observed in htl KD or ths mutant ovary at these earlier stages (Fig. 3K); neither were any effects on the adult stem cell niche identified at the adult stage (data not shown). These results suggested that the identified role for FGF in regulating gene expression within TF precursor cells is unlikely to relate to the fertility defects observed in FGF mutants. We focused instead on whether FGF signaling has additional roles in the developing gonad at later stages that relate to fertility.
Htl-receptor activation by the Ths FGF ligand regulates apical cell proliferation
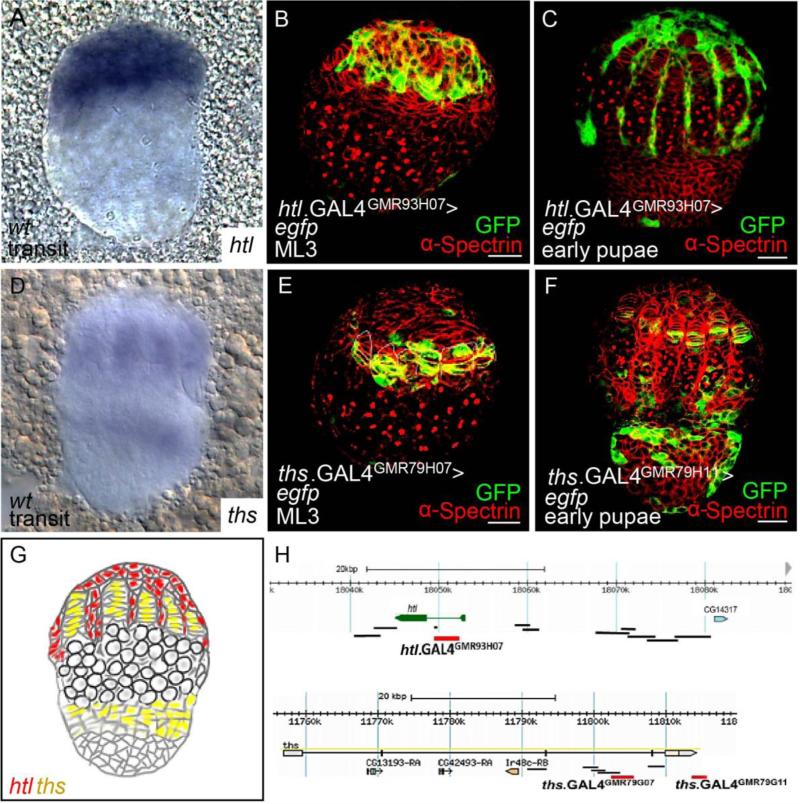
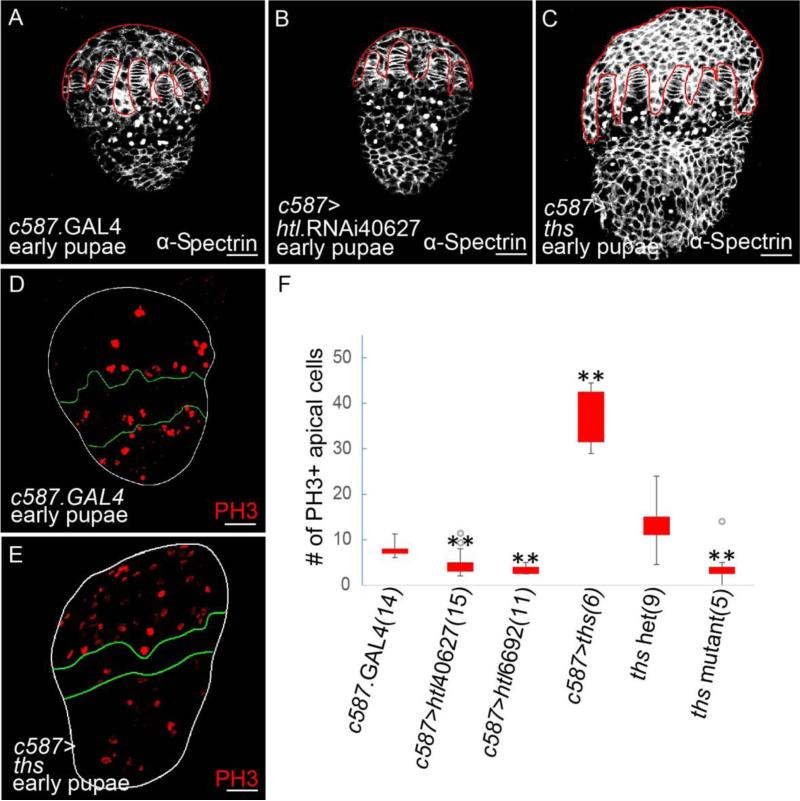
At the transition stage from larva to pupa, htl transcripts were detected in the ACs by in situhybridization (Fig. 4A). The domain of FGF signaling in developing gonads also was investigated by assaying expression supported by the various htl.GAL4 and ths.GAL4 drivers (Fig. 4H), which were found to support expression in different somatic cell types. Once again, only one driver htl.GAL4GMR93H07 supported expression in the developing gonad within ACs at the ML3 and early pupae stage (Fig. 4B and 4C, respectively); the same construct that was found to support expression in the epithelial sheath of adult ovaries (Fig. 1I).
Figure 4. At the early pupal stage, htl is expressed in the ACs, whereas ths is expressed in the TF and presumptive basal stalk cells.
(A,D) In situ hybridization using antisense riboprobes to detect htl (A) and ths (D) transcripts in wildtype ovaries at the stage of transition from larvae to pupae.
(B,C,E,F) Immunostainings of ovaries expressing UAS.GFP driven by htl.GAL4GMR93H07 (B,C), ths.GAL4GMR79H07(E), and ths.GAL4GMR79H11(F) detected using anti-GFP (green) and anti-α-Spectrin (red) antibodies. Two different stages are shown: ML3 (B,E) and early pupal stage (C,F).
(G) Schematic showing expression domains of htl (red) and ths (yellow) at the transition stage.
(H) Location of non-coding DNA regions used to make GAL4 drivers, which were assayed for expression in the developing ovary. Those that did or did not support expression are labeled red or black, respectively.
ths transcripts were detected in TF and presumptive basal stalk cells by in situ hybridization (Fig. 4D). Moreover, we identified cis-regulatory sequences flanking the ths gene able to drive expression in these regions. ths.GAL4GMR79H07 and ths.GAL4GMR79G11 drivers both support expression in TF cells (Fig. 4E and 4F, respectively); whereas the ths.GAL4GMR79G11 driver alone supports expression, additionally, within the basal/ basal stalk cells (Fig. 4F). Based on (i) the specific expression of Htl within ACs; (ii) the abutting domains of expression of the htl and ths genes (Fig. 4G); as well as (iii) the finding that the ovarian muscle tissue defects are present in ths mutants, we investigated whether the Ths FGF ligand activates Htl to support ovarian muscle sheath formation through regulation of ACs.
At the middle pupal stage, AC migration from the anterior to posterior region is apparent (Fig. 2D). Primordial ovarioles lose direct contact with each other, as the space between them is filled with ACs that migrate in between them; first at anterior regions (Fig. 5C) and, subsequently, at posterior regions as well (Fig. 5E). In contrast, many fewer ACs were observed between the primordial ovarioles of ths mutant ovaries (Fig. 5B, compare with Fig. 5A; Fig. 5D, compare with Fig. 5C). This finding suggested that FGF signaling through Htl and Ths controls apical cell migration and/or proliferation.
Figure 5. FGF signaling is not necessary for AC migration during ovarian development.
Stainings of ovaries using anti-α-Spectrin (A-D, white), anti-FasIII (E-I, red), and anti-RFP (G, green) antibodies or rhodamine phalloidin (E,F,H,I, green).
(A-F) Ovaries at the middle pupa stage obtained from wildtype (A,C,E) and ths mutants (B,D,F). Migrating ACs located between presumptive ovarioles are marked with yellow arrows (C-F).
(G) Htl.mCherry reporter introduced into ths mutant background stained with anti-RFP (green) and anti-FasIII (red) to detect ACs and basal stalks, respectively.
(H,I) Staining of wildtype (H) or ths mutant (I) at the late pupal stage showing gaps in the muscle sheath.
Even in ths mutants, however, somatic cells were identified near basal stalks at the posterior suggesting AC migration was occurring, though the number of cells present in this domain was reduced compared with wildtype (Fig. 5F, compare with Fig. 5E). AC number and organization phenotypes in ths mutants were examined more closely by introducing the htl-mcherry transgene, which marks ACs, into this background. At the middle pupal stage when AC migration completes, we found that mCherry-expressing ACs do reach their final destination (the posterior of the developing gonad) in ths mutants (Fig. 5G, white arrows). However, ths mutant exhibited aberrant muscle tissues formation at later stages. The muscle tissues failed to completely encompass the whole ovary and appeared torn (Fig. 5I, compare with Fig. 5H). In htl KD ovaries, size reduction of the apical cell population was apparent (Fig. 6B, compare with Fig. 6A). In contrast, when Ths was ectopically expressed in somatic cells, ACs over-proliferated (Fig. 6C, compare with Fig. 6A). These results demonstrated that FGF signaling is not required for AC migration but suggested instead that FGF signaling regulates AC number.
Figure 6. FGF signaling regulates the AC population size by controlling cells’ proliferation at the early pupal stage.
(A-E) Immunostainings of ovaries at the early pupae stage using anti-α-Spectrin (A-C, white) and anti-Phospho-Histone H3 (PH3, D,E, red) antibodies. A role for htl and ths in somatic cells was examined using the c587.GAL4 driver: control, driver alone (A,D) compared with htl KD (using UAS.htl.RNAi40627, B) and ths-ectopic expression (C,E). AC domain in (A-C) is outlined in red. In (D,E), ovaries are outlined in white, while the location of germ cell is bounded by green lines.
(F) A graph showing number of PH3+ ACs in c587.GAL4 control, two htl KDs (using UAS.htl.RNAi40627 and UAS.htl.RNAi6692), ths heterozygote control, and ths mutant. The sample size is indicated within brackets. Gray circles represent outlier datapoints. For statistical analysis, two-tailed Student's t-test was used, and ρ<0.05 was considered significant (**).
To test this idea directly, we examined the number of mitotic ACs by counting Phospho-Histone-H3 (PH3) positive ACs (e.g. Deng et al., 2001). In the htl KD ovaries upon expression of htl-RNAi40627 or htl-RNAi6692 in somatic cells, significant decreases in PH3+ ACs were observed (Fig. 6F). Similarly, ths mutant ovaries also exhibited a reduction in the number of PH3+ ACs compared with ovaries from ths heterozygotes, which served as control (Fig. 6F). In contrast, when Ths was ectopically expressed in somatic cells, the number of PH3+ ACs was increased significantly (Fig. 6E,F, compared with Fig. 6D). Collectively, these results suggest FGF signaling mediated by interaction between Htl FGFR and Ths FGF controls the AC population size by promoting cells’ proliferation, to support AC migration and proper ovarian muscle generation.
FGF signaling is required for ovarian muscle tissue morphogenesis during ovarian development
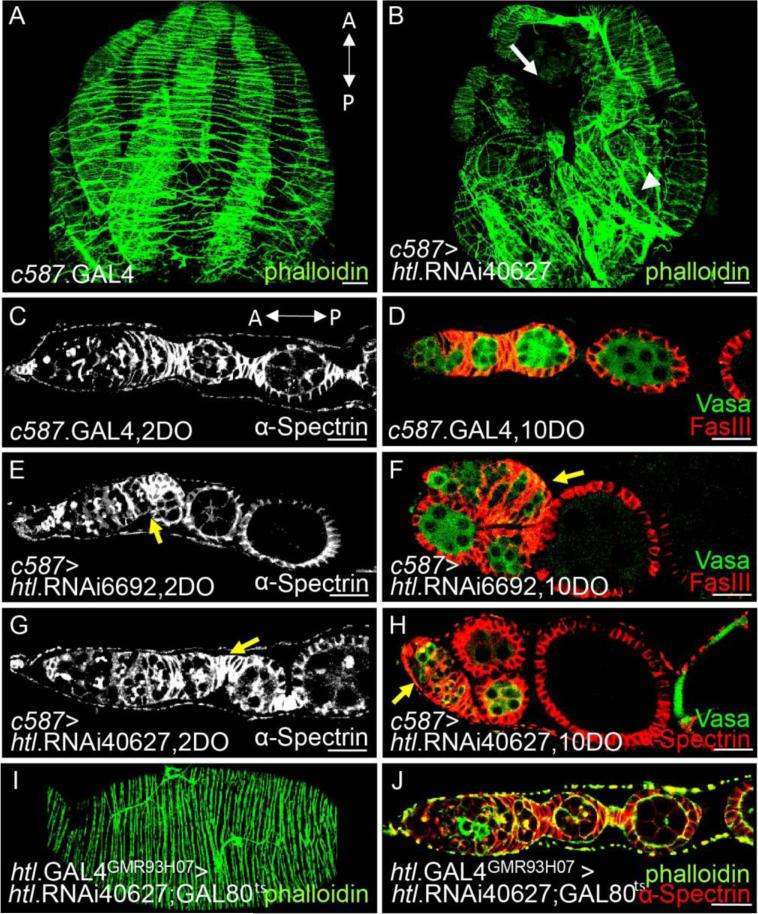
We further investigated whether these larval and pupal FGF signaling functions relate to phenotypes observed in the adult ovaries for ths mutants (e.g., lack of epithelial sheath; Fig. 1B). In the wildtype adult ovary, the peritoneal sheath is composed of thick muscle bundles encompassing each ovary that are aligned perpendicular to the anterior-posterior (AP) axis of the ovary. Additionally, thin muscle fibers are also present on the peritoneal sheath, which is organized in a parallel manner to its AP axis (Hudson et al., 2008). In htl KD ovaries, the muscle fibers on the peritoneal sheath appeared disorganized as they no longer aligned perpendicular to the AP axis (Fig. 7B, arrowhead, compare with 7A) and failed to encompass the entire ovary (Fig. 7B, white arrow).
Figure 7. Ovarian muscle sheaths provide structural support during oogenesis, which affects female fertility.
Stainings of adult ovaries by rhodamine phalloidin (A,B,I,J green) or anti-α-Spectrin (C,E,G, white; H,J red), anti-FasIII (D,F, red), and anti-Vasa (D,F,H, green) antibodies.
(A,B) The role of htl in morphogenesis of ovarian muscles was examined using c587.GAL4 somatic cell driver: control, driver alone (A) compared with htl KD (using UAS.htl.RNAi40627, B). A white arrow and arrowhead in (B) mark absence and aberrant organization of ovarian muscle sheaths, respectively.
(C-H) The role of htl in structural organization of adult ovarioles was investigated using the somatic cell c587.GAL4 driver: control, driver alone (C,D) compared with htl KDs [using UAS.htl.RNAi6692 (E,F) and using UAS.htl.RNAi40627 (G,H)]. Yellow arrows in (E-H) mark “flop-down” egg chamber phenotypes. Phenotypes for two time points are shown: two days old (2OD; C,E,G) and ten days old (10OD: D,F,H).
(I,J) Timing of htl action was examined by using GAL80ts to limit htl KD to the adult stage. htl.GAL4GMR93H07 driver together with GAL80ts were used to support expression of UAS.htl.RNAi40627 only in adults, by switch to growth at 29°C. In panels (A) and (C), “A” denotes the anterior tip and “P” denotes the posterior tip.
htl KD ovarioles exhibited additional structural abnormalities relating to organization of egg chambers. In wildtype, regardless of age, germaria and egg chambers were aligned linearly (Fig. 7C,D). In contrast, when htl was knocked down by expressing htl.RNAi40627 or htl.RNAi6692 in somatic cells, the linear alignment of ovarioles was disrupted. Young egg chambers, isolated from two day-old females, appeared clumped beside the germarium (Fig. 7E,G, yellow arrow, compare with Fig. 7C); a phenotype described previously as a “flop-down” egg chamber (Cohen et al., 2002). In ovaries isolated from older flies, ten days in age, this phenotype was exacerbated (Fig 7F,H, yellow arrow, compare with Fig. 7D).
This result suggested that FGF coordinates movement of egg chambers within ovarioles through regulation of ovarian muscles. To investigate, live imaging was used to observe contraction of ovaries when cultured in vitro. The wildtype ovary exhibited smooth and constant rhythmic contraction (Movie S1), as described previously (Middleton et al., 2006). However, in htl KD ovaries, in which htl.RNAi40627 or htl.RNAi6692 was induced in somatic cells by c587.GAL4 at earlier stages, muscle contractions were present but appeared uncoordinated. In some htl KD ovaries, though contractions appeared to extend throughout the length of the ovary, erratic movements resulted (Movie S2, compare with Movie S1). In other cases, muscle contractions limited to the anterior region of the ovary and absent from the posterior (Movies S3, S4 compare with Movie S1). As htl RNAi also resulted in a decrease in egg-laying, collectively, these data suggest that proper organization of ovarian muscle tissues is crucial to maintain coordinate muscle contractions required to support fertility.
To determine whether these adult phenotypes relate to secondary roles for FGF at this adult ovarian stage or instead stem from earlier functions (e.g. proliferation of ACs at the larval/pupal stages), we examined mutant phenotypes associated with htl KD, specifically, at the adult stage. A htl.RNAi40627 transgene was expressed via htl.GAL4GMR93H07 together with GAL80ts to support temporal control of the KD within the epithelial sheath of adult ovaries (see Materials & Methods). In htl KD at the adult stage, epithelial and peritoneal sheath muscle tissues were present (Fig. 7J) and the muscle tissues appeared morphologically normal (Fig. 7I). Ovarioles were aligned linearly regardless of age (Fig. 7J). Furthermore, muscle contractions (Movie S5) and egg-laying frequency (data not shown) were normal as well. Taken together, these data demonstrate that Htl function at earlier stages in the larval and pupal stages is required for proper ovarian muscle tissue morphogenesis to support fertility.
DISCUSSION
The data presented demonstrate that FGF signaling acts in the gonadal somatic cells throughout larval and pupal development of the Drosophila ovary. Early, at the larval stage, FGF signaling also promotes TF cell differentiation through the Htl FGFR and its FGF ligand Pyr. An additional role for FGF signaling in supporting AC migration, at a slightly later stage, was uncovered that is required for normal ovary function. Rather than providing a directional cue to support AC migration, our results are consistent with the view that FGF acts to regulate AC number. FGF signaling regulates AC number through influence on cell proliferation. In this manner, FGF ensures sufficient numbers of cells are present along the length of the developing gonad so that the epithelial and peritoneal sheaths can encompass the entire ovary. The sterility phenotype of ths Drosophila females is likely explained by this earlier role for FGF, in supporting development of this muscle tissue, during gonadal development. Without proper muscle development, oogenesis is not supported.
As a function of FGF signaling pathway during ovarian development, we identified defects in gene expression within TF cells upon loss of FGF signaling. Most of htl KD mutants did not develop into adults. However, a few escapers were observed, possibly due to incomplete KD of htl levels by the RNAi approach. When these escapers were examined at the adult stage in terms of TF cell development and functionality, no apparent phenotypes were observed. Thus, it is unclear how the FGF-specific differentiation defects identified here at the larval stage affect ovary function. Furthermore, in the adult ovary, TF cells are contiguous with the epithelial sheath (Cohen et al., 2002). However, we show here that FGF signaling has distinct functions in the TF cells versus ACs (epithelial sheath), suggesting regulation of these cell types is separable.
During ovarian development and oogenesis, expression of genes ths, pyr, and htl is dynamic. For instance, the expression of the htl gene, encoding the FGFR, is not limited to TF cells and ACs. Htl expression through the Htl.mCherry reporter is also detected within the swarm cells at ML3, basal cells at the early pupal stage, and within the epithelial/peritoneal sheaths at the adult stage, suggesting additional roles during gonadal development and oogenesis. It is likely that FGF signaling, which is expressed in multiple domains of the developing ovary, supports various roles and possibly promotes differentiation or proliferation of additional cell types not examined here. Furthermore, why multiple FGF ligands are necessary to support FGF receptor activation is unclear, but the prevailing view is that ligands may influence different FGF response outputs. In the ovary, Pyr and Ths appear to encompass distinct functions as Pyr regulates TF cell differentiation at the larval stage, while Ths controls AC number at the early pupal stage. Thus, with FGF signaling components and primary function defined, the Drosophila ovary is an excellent system to study molecular mechanisms regulating FGF signaling activity.
We show here that control of FGF signaling is critical for the regulation of ovary growth as it acts as a mitogen, a role demonstrated previously in Drosophila only in the formation of air sacs of the tracheal system (Sato and Kornberg, 2002). In ovaries ectopically expressing Ths FGF8-like ligand, vast over-proliferation was observed, which is one of the hallmarks of cancer. Many cell-based studies and mouse models have demonstrated that FGF signaling promotes tumor cell proliferation. Frequent amplification of the FGF1 gene in humans, resulting in increased gene expression, has also been reported in ovarian cancer (Birrer et al., 2007). In ovarian cancer states exhibiting increased FGF1 ligand expression, this factor functions to promote angiogenesis in a paracrine fashion but also may function in an autocrine manner to promote tumor cell proliferation (Birrer et al., 2007; Turner and Grose, 2010). Taken together, study of the development of this organ may serve as excellent system to study the interplay between FGF signaling and cell proliferation with the possibility of far-reaching implications to the cancer biology field.
For example, as multiple signaling pathways have been implicated in the control of cell proliferation during ovary development in Drosophila, it is likely that mechanisms exist to co-regulate the activation of multiple signaling pathways to integrate growth of this tissue in a balanced manner. The Hippo pathway cooperates with EGFR signaling to maintain homeostasis of ICs and PGCs, and it also interacts with JAK/STAT pathways to control number of TF cells at the larval stage (rev. in Gilboa, 2015; Sarikaya and Extavour, 2015). As we found that the Ths FGF ligand is produced by TF cells and provides the cue that stimulates AC proliferation via FGF signaling pathway activation, it is possible that Hippo, which specifies number of TF cells, plays a role in controlling FGF signaling to coordinate the number of muscle precursors produced relative to size of the ovary. Each ovarian cells type appears to utilize a different signaling pathway to regulate proliferation of cells to support balanced growth of the gonad.
Our data also suggest that ovarian muscle contractions provide mechanical supports to promote fertility. In htl KD mutants, the ovaries exhibited incomplete generation of ovarian muscle sheaths, and the organization of muscle sheaths was aberrant. These ovaries had uncoordinated muscle contraction throughout the ovaries and exhibited “flop-down” egg chambers. Furthermore, their egg deposit frequencies were significantly decreased. In contrast, when htl was knocked down only at the adult stage, none of these phenotypes were observed, suggesting that phenotypes observed in htl KD adult ovaries, when htl.RNAi was activated early, are consequences of abnormal muscle sheaths generation at the larval/pupal stage. This viewpoint is also further supported by study of a Drosophila Wnt ligand, DWnt4, during ovarian development. During this stage, DWnt4 controls AC migration in a focal adhesion kinase dependent manner by activating the Drosophila Frizzled 2 receptor (DFz2) (Cohen et al., 2002). DWnt4 mutants fail to generate full-length epithelial sheaths (Cohen et al., 2002). Also similar to the ths mutant phenotype, DFz2 mutants are viable and sterile (Chen and Struhl, 1999). Furthermore, like htl KD ovarioles, Dfz2 mutants and Dwnt4 mutants exhibit a “flop-down” ovariole phenotype (Cohen et al., 2002). Taken together, we propose that dysfunctional epithelial sheath formation, due to reduced FGF signaling (this study) and possibly also Wnt signaling, results in sterility.
CONCLUSION
Here we provide the first evidence that FGF signaling is an important regulator of ovarian muscle development in Drosophila and that this role in ovary morphogenesis is required to support fertility. This study also establishes the Drosophila ovary as a model system to uncover how FGF influences proliferation relating to tissue growth.
Supplementary Material
Highlights.
Mutants for FGF ligand thisbe are viable but sterile
Investigated role for FGF in ovary morphogenesis
Heartless FGF receptor and its ligands are expressed in the developing gonad
FGF influences apical cell proliferation to support cells’ migration and form muscles
Sterility of FGF mutants stems from abnormal development of muscles in gonad
ACKNOWLEDGEMENTS
We thank Leslie Dunipace and Ha Vu for generating fly stocks used in this study including htl.mCherry and pyr.GAL4s and Va Si for initiating study of the FGF mutant sterility phenotype. We also are grateful to D. Godt and M. Leptin for sending antibodies and many Drosophila researchers for sharing published fly stocks. This study was supported by a grant to A.S. from the NIH/NIGMS R01GM104838 as well as support of J.I. from CIRM Bridges to Stem Cell Research Program grant (Cal Poly and Cal State Los Angeles) TB1-01176 and NIH/NRSA training grant (Caltech) 5T32GM07616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bae YK, Trisnadi N, Kadam S, Stathopoulos A. The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator? Cell adhesion & migration. 2012;6:397–403. doi: 10.4161/cam.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu M, Mukhopadhyay S, Chatterjee U, Roy SS. FGF16 promotes invasive behavior of SKOV-3 ovarian cancer cells through activation of mitogen-activated protein kinase (MAPK) signaling pathway. J Biol Chem. 2014;289:1415–1428. doi: 10.1074/jbc.M113.535427. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Berisha B, Steffl M, Amselgruber W, Schams D. Changes in fibroblast growth factor 2 and its receptors in bovine follicles before and after GnRH application and after ovulation. Reproduction. 2006;131:319–329. doi: 10.1530/rep.1.00798. [DOI] [PubMed] [Google Scholar]
- Birrer MJ, Johnson ME, Hao K, Wong KK, Park DC, Bell A, Welch WR, Berkowitz RS, Mok SC. Whole genome oligonucleotide-based array comparative genomic hybridization analysis identified fibroblast growth factor 1 as a prognostic marker for advanced-stage serous ovarian adenocarcinomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:2281–2287. doi: 10.1200/JCO.2006.09.0795. [DOI] [PubMed] [Google Scholar]
- Buratini J, Jr., Pinto MG, Castilho AC, Amorim RL, Giometti IC, Portela VM, Nicola ES, Price CA. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 2B, in bovine follicles. Biology of reproduction. 2007;77:743–750. doi: 10.1095/biolreprod.107.062273. [DOI] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Mariol MC, Wallace RM, Weyers J, Kamberov YG, Pradel J, Wilder EL. DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Developmental cell. 2002;2:437–448. doi: 10.1016/s1534-5807(02)00142-9. [DOI] [PubMed] [Google Scholar]
- Couderc JL, Godt D, Zollman S, Chen J, Li M, Tiong S, Cramton SE, Sahut-Barnola I, Laski FA. The bric a brac locus consists of two paralogous genes encoding BTB/POZ domain proteins and acts as a homeotic and morphogenetic regulator of imaginal development in Drosophila. Development (Cambridge, England) 2002;129:2419–2433. doi: 10.1242/dev.129.10.2419. [DOI] [PubMed] [Google Scholar]
- Cummings MR. Ultrastructure of Ovarian Epithelial Sheath in Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol. 1974;3:137–145. [Google Scholar]
- Deng WM, Althauser C, Ruohola-Baker H. Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development. 2001;128:4737–4746. doi: 10.1242/dev.128.23.4737. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Buszczak M. Finding a niche: studies from the Drosophila ovary. Stem cell research & therapy. 2011;2:45. doi: 10.1186/scrt86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B, Poueymirou W, Papaioannou VE, DeChiara TM, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science (New York, N.Y.) 1995;267:246–249. doi: 10.1126/science.7809630. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development. 1997;124:3535–3541. doi: 10.1242/dev.124.18.3535. [DOI] [PubMed] [Google Scholar]
- Gancz D, Gilboa L. Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila. Development (Cambridge, England) 2013;140:4145–4154. doi: 10.1242/dev.093773. [DOI] [PubMed] [Google Scholar]
- Gilboa L. Organizing stem cell units in the Drosophila ovary. Curr Opin Genet Dev. 2015;32C:31–36. doi: 10.1016/j.gde.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2006;443:97–100. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- Godt D, Laski FA. Mechanisms of cell rearrangement and cell recruitment in Drosophila ovary morphogenesis and the requirement of bric a brac. Development. 1995;121:173–187. doi: 10.1242/dev.121.1.173. [DOI] [PubMed] [Google Scholar]
- Huang P, Stern MJ. FGF signaling in flies and worms: more and more relevant to vertebrate biology. Cytokine Growth Factor Rev. 2005;16:151–158. doi: 10.1016/j.cytogfr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Hudson AM, Petrella LN, Tanaka AJ, Cooley L. Mononuclear muscle cells in Drosophila ovaries revealed by GFP protein traps. Developmental biology. 2008;314:329–340. doi: 10.1016/j.ydbio.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam F, Sutherland D, Huang W, Krasnow MA. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics. 1999;152:307–318. doi: 10.1093/genetics/152.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, Ghosh S, Stathopoulos A. Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development (Cambridge, England) 2012;139:699–708. doi: 10.1242/dev.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–747. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RC. The meiotic behavior of the Drosophila oocyte. Int Rev Cytol. 1970;28:125–168. doi: 10.1016/s0074-7696(08)62542-5. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell research. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Hiromi Y, Asaoka M. Egfr signaling controls the size of the stem cell precursor pool in the Drosophila ovary. Mech Dev. 2013;130:241–253. doi: 10.1016/j.mod.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Michelson AM, Gisselbrecht S, Buff E, Skeath JB. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development. 1998;125:4379–4389. doi: 10.1242/dev.125.22.4379. [DOI] [PubMed] [Google Scholar]
- Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CJ. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biol. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G, Goldfarb M. Receptor specificity of the fibroblast growth factor family. J Biol Chem. 1996;271:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, Mungall C, Svirskas R, Kadonaga JT, Doe CQ, Eisen MB, Celniker SE, Rubin GM. Tools for neuroanatomy and neurogenetics in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CB, Brand AH. Ectopic gene expression in Drosophila using GAL4 system. Methods. 1998;14:367–379. doi: 10.1006/meth.1998.0592. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocrine-related cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Prasad M, Jang AC, Starz-Gaiano M, Melani M, Montell DJ. A protocol for culturing Drosophila melanogaster stage 9 egg chambers for live imaging. Nat Protoc. 2007;2:2467–2473. doi: 10.1038/nprot.2007.363. [DOI] [PubMed] [Google Scholar]
- Ropiquet F, Giri D, Kwabi-Addo B, Mansukhani A, Ittmann M. Increased expression of fibroblast growth factor 6 in human prostatic intraepithelial neoplasia and prostate cancer. Cancer Res. 2000;60:4245–4250. [PubMed] [Google Scholar]
- Rottinger E, Saudemont A, Duboc V, Besnardeau L, McClay D, Lepage T. FGF signals guide migration of mesenchymal cells, control skeletal morphogenesis [corrected] and regulate gastrulation during sea urchin development. Development (Cambridge, England) 2008;135:353–365. doi: 10.1242/dev.014282. [DOI] [PubMed] [Google Scholar]
- Sahut-Barnola I, Godt D, Laski FA, Couderc JL. Drosophila ovary morphogenesis: analysis of terminal filament formation and identification of a gene required for this process. Developmental biology. 1995;170:127–135. doi: 10.1006/dbio.1995.1201. [DOI] [PubMed] [Google Scholar]
- Sarikaya DP, Extavour CG. The Hippo pathway regulates homeostatic growth of stem cell niche precursors in the Drosophila ovary. PLoS Genet. 2015;11:e1004962. doi: 10.1371/journal.pgen.1004962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Developmental cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- Sato T, Ogata J, Niki Y. BMP and Hh signaling affects primordial germ cell division in Drosophila. Zoological science. 2010;27:804–810. doi: 10.2108/zsj.27.804. [DOI] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development (Cambridge, England) 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–699. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulin S, Stathopoulos A. Analysis of Thisbe and Pyramus functional domains reveals evidence for cleavage of Drosophila FGFs. BMC developmental biology. 2010a;10:83. doi: 10.1186/1471-213X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulin S, Stathopoulos A. Extending the family table: Insights from beyond vertebrates into the regulation of embryonic development by FGFs. Birth Defects Res C Embryo Today. 2010b;90:214–227. doi: 10.1002/bdrc.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- Valve E, Penttila TL, Paranko J, Harkonen P. FGF-8 is expressed during specific phases of rodent oocyte and spermatogonium development. Biochemical and biophysical research communications. 1997;232:173–177. doi: 10.1006/bbrc.1997.6256. [DOI] [PubMed] [Google Scholar]
- Venken KJ, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- Vincent S, Wilson R, Coelho C, Affolter M, Leptin M. The Drosophila protein Dof is specifically required for FGF signaling. Mol Cell. 1998;2:515–525. doi: 10.1016/s1097-2765(00)80151-3. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic acids research. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.