Abstract
There are no studies on the acute effect of ethanol on peripheral N-methyl-D-aspartate receptor (NMDAR)-mediated increases in reactive oxygen species (ROS) and blood pressure (BP). We tested the hypothesis that ethanol antagonism of peripheral NMDAR dampens systemic NMDA-evoked increases in vascular ROS and BP. We investigated the effect of ethanol (1 g/kg) on BP and heart rate (HR) responses elicited by systemic bolus (125–1000 μg/kg, intra-venous [i.v.]) or infused (180 μg/kg/min) NMDA in conscious male Sprague-Dawley rats. We also hypothesized that peripheral NMDAR blockade with DL-2-Amino-5-phosphonopentanoic acid (AP-5; 5 mg/kg, i.v.) uncovers an ethanol- (1 or 1.5 g/kg) evoked hypotensive response. Ethanol attenuated the peripheral NMDAR-mediated pressor and bradycardic responses caused by NMDA infusion, and ex vivo studies revealed parallel ethanol attenuation of peripheral NMDAR-mediated increases in vascular ROS. While ethanol (1 or 1.5 g/kg) alone had no effect on BP, the higher dose caused a hypotensive response in the presence of NMDAR blockade (AP-5). Blood ethanol concentrations were not statistically different in the groups that received ethanol alone or along with NMDA or AP-5. These findings are the first to demonstrate ethanol attenuation of peripheral NMDAR-mediated pressor response, and the uncovering of ethanol-evoked hypotension in the presence of peripheral NMDAR blockade.
Keywords: ethanol, peripheral NMDAR, blood pressure, oxidative stress, AP-5, NO
Introduction
Previous studies have focused on central N-methyl-D-aspartate receptor (NMDAR) involvement in behavioral as well as cardiovascular function (Vyklicky et al., 2014). Further, numerous studies have reported on the ethanol-NMDAR interaction within the central nervous system (Carpenter-Hyland & Chandler, 2007; Möykkynen & Korpi, 2012; Toropainen et al., 1997). However, the NMDAR is also expressed within peripheral tissues including the kidney, sex organs, keratinocytes, and the cardiovascular system (Deng, Valdivielso, Munger, Blantz, & Thomson, 2002; Gill & Pulido, 2001; Morhenn, Murakami, O'Grady, Nordberg, & Gallo, 2004). Specifically, the NMDAR is localized within the heart and vascular (aorta and mesenteric arteries) tissues (Carlton, Chung, Ding, & Coggeshall, 1998; Gill & Pulido, 2001; Leung et al., 2002; McGee & Abdel-Rahman, 2012), and its activation causes dose-related pressor responses in conscious rats (McGee & Abdel-Rahman, 2012). Similar to its neuronal action (Gunasekar, Kanthasamy, Borowitz, & Isom, 1995; Reyes, Brennan, Shen, Baldwin, & Swanson, 2012), peripheral NMDAR mediates vascular oxidative stress (McGee & Abdel-Rahman, 2010).
Reported findings highlight the NMDAR as a prominent molecular target for ethanol (Lovinger, White, & Weight, 1989; Xu, Smothers, Trudell, & Woodward, 2012). While acute ethanol attenuates central NMDA-evoked behavioral and cardiovascular responses (Allgaier, 2002; El-Mas & Abdel-Rahman, 1993; Kumari, 2001; Lin, Hsieh, Shiu, Chiu, & Lai, 2003; Mao & Abdel-Rahman, 1995), NMDA or ethanol produces oxidative stress (Augustyniak, Michalak, & Skrzydlewska, 2005; Collins & Neafsey, 2012). It has not been investigated whether acute ethanol interaction with the peripheral NMDAR influences the NMDA-evoked cardiovascular responses and vascular oxidative stress. It is also not known if prior blockade of peripheral NMDAR influences the BP and HR responses elicited by moderate doses of ethanol. Importantly, in male conscious rats (El-Mas & Abdel-Rahman, 1999) and normotensive humans (Abdel-Rahman, Merrill, & Wooles, 1987), moderate doses of ethanol do not change BP because the sympathoexcitatory action of ethanol (Abdel-Rahman, Carroll, & El-Mas, 1992) is counterbalanced by its peripheral vasodilating effects. Further, excitatory NMDA receptors are expressed in sympathetic innervation of resistance vessels, the heart, and in the adrenal medulla (Carlton et al., 1998; Schwendt & Jezová, 2001).
Here, we tested the hypothesis that peripheral NMDAR blockade by acute ethanol dampens the peripheral NMDA-evoked increases in vascular ROS and BP. We adopted two approaches to investigate the effect of ethanol (1 g/kg by gavage) in conscious unrestrained rats to circumvent the potential confounding effects of anesthetics on the cardiovascular responses. First, we investigated the effect of ethanol on the dose-pressor response curves constructed with bolus i.v. injections of NMDA (125–1000 μg/kg). Second, we investigated the effect of ethanol on the pressor response elicited by sustained activation of peripheral NMDAR with NMDA infusion (180 μg/kg/min). Further, we tested the hypothesis that prior peripheral NMDAR blockade might uncover a dose-dependent hypotensive effect of ethanol (1 or 1.5 g/kg); the selective NMDAR blocker AP-5, which does not cross the blood-brain barrier (Itoh, Sakata, Watanabe, Aikawa, & Fujii, 2008), was used in this experiment. In addition, we conducted ex vivo studies on vascular tissues to elucidate the effects of acute ethanol-NMDAR interaction on vascular NO and oxidative stress.
Materials & methods
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) weighing 275–325 grams (10–11 weeks old) were used in this study. Rats were housed individually in separate cages and allowed free access to Purina chow and water. The temperature was maintained at 22 ± 1 °C, and a 12-12 hour light-dark cycle was maintained with the lights automatically turned off at 7:00 PM. Surgical procedures and animal experiments were conducted in accordance with the institutional animal use and care guidelines and the Institute of Laboratory Animal Resources.
Intravascular catheterization
Femoral artery and vein catheterization was performed as previously done in our laboratory (Abdel-Rahman, 1994). Animals received buprenorphine (0.03 mg/kg) 30 min prior to surgery and were anesthetized with an intra-peritoneal injection of ketamine (9 mg/100 g) and xylazine (1 mg/100 g). Catheters consisting of 5-cm PE-10 tubing bonded to 15-cm PE-50 tubing were placed into the abdominal aorta and vena cava via the left femoral vessels for measurement of arterial pressure and intravenous injections, respectively. Two venous catheters were inserted into the femoral vein to permit i.v. bolus administration and/or infusion of drugs. Catheters were tunneled subcutaneously and exteriorized at the back of the neck between the scapulae. Vascular catheters were flushed with heparinized saline and plugged by stainless-steel pins. Incisions were closed with surgical clips and swabbed with povidine-iodine solution. Postoperative care included buprenorphine (0.03 mg/kg) and penicillin G procaine (100,000 U/kg). The animals were allowed 2 days following surgery before conducting experiments.
On the day of the experiment, the arterial catheter was connected to a pressure transducer for measurement of blood pressure in conscious freely moving rats. At least 30 min were allowed for stabilization of blood pressure and heart rate at the beginning of an experiment. Blood pressure (BP) was recorded by ML870 (PowerLab 8/30) and analyzed by LabChart (v.6) pro software (AD Instruments, Colorado Springs, CO). Heart rate was extracted from the BP recording by the LabChart (v.6) blood pressure analysis module, and both variables were continuously recorded and stored for offline analysis.
Quantification of aortic reactive oxygen species
The 2′, 7′-dichlorofluorescein (DCF) biochemical assay was utilized for quantification of ROS as reported (Zou, Jung, Kim, Yu, & Chung, 2004) with the following modifications. Homogenization was performed using Radnoti tissue grinders (Radnoti Glass Technology, Monrovia, CA) to increase protein yield, and kinetic readings were taken at 5-min intervals for 30 min at 37 °C. ROS levels were calculated by relative DCF fluorescence per μg protein.
Measurement of nitrite/nitrate (NOx) level
The NOx (nitrite/nitrate) content was measured using a colorimetric assay kit according to manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI) and as detailed (Misko, Schilling, Salvemini, Moore, & Currie, 1993).
Blood alcohol concentration
Blood alcohol concentrations were determined in blood samples (0.2 mL/sample), which were drawn from each rat 30 and 60 min after ethanol administration. Blood samples were centrifuged at 5000 rpm for 10 min. The supernatant was aspirated and stored at °80 °C until analyzed. The plasma alcohol content was measured by the enzymatic method based on reported studies (including ours) utilizing a 7-point standard curve (Bender & Abdel-Rahman, 2010; Bernt & Guttman, 1974). Absorbance was measured at 340 nm using the Biotek Synergy HT microplate reader.
Experimental groups and protocols
Experiment 1: Effect of ethanol on peripheral NMDA-mediated pressor response
Two groups (n = 5 each) of conscious male rats were used to investigate the effect of ethanol (1 g/kg by gavage) on blood pressure and heart rate. Following MAP and HR stabilization, saline (vehicle for AP-5 in Experiment 2) was administered 30 min before ethanol (1 g/kg; 10 mL/kg of 13% ethanol diluted in water by gavage) or its vehicle (water). In two additional groups of rats (n = 5–6 each), we investigated the effect of oral ethanol (1 g/kg) or its vehicle (water), administered 30 min earlier, on the dose-related pressor response elicited by systemic NMDA (125, 250, 500, or 1000 μg/kg; i.v.). The pre-NMDA MAP and HR constituted the average values over the 30-min period that followed ethanol or water administration. NMDA was administered at 15-min intervals to allow full recovery from the preceding dose except for the final (1000 μg/kg) dose. The dose of ethanol is based on a previous study (El-Mas & Abdel-Rahman, 1999). The vascular tissues were collected at the conclusion of the integrative studies and stored for subsequent molecular studies; in NMDA-treated rats, the aortas were collected at the peak of the pressor response caused by the highest NMDA dose. Blood samples (0.2 mL) were collected at baseline, following ethanol in the absence or presence of NMDA or AP-5 for blood ethanol concentration measurements.
Experiment 2: Effect of ethanol on the cardiovascular responses elicited by sustained NMDAR activation
Four groups (n = 5 each) of conscious male rats were used to investigate the effect of ethanol (1 g/kg by gavage) on the cardiovascular responses elicited by sustained activation of peripheral NMDAR. The optimal NMDA dose/infusion rate that produced an approximately 25-mmHg increase in BP was established in our recent study (McGee & Abdel-Rahman, 2012). The rats were pretreated with water (vehicle) or ethanol (1 g/kg; 10 mL/kg of 13% ethanol diluted in water) 30 min prior to NMDA (180 μg/kg/min) or saline infusion at a rate of 9 μL/min (Harvard compact pump, Model 975) for 30 min. As in the previous experiment, the pre-NMDA MAP and HR constituted the average values over the 30-min period that followed ethanol or water administration. Blood pressure and heart rate were measured for an additional 30 min during the NMDA infusion.
Experiment 3: Effect of prior NMDAR blockade on the cardiovascular responses elicited by ethanol
Four groups (n = 4–6 each) of rats were used to investigate the effect of pretreatment with the selective NMDAR antagonist, DL-AP-5, on the cardiovascular effects of ethanol (1 or 1.5 g/kg). The selected DL-AP-5 dose (5 mg/kg, i.v.), which does not cross the blood-brain barrier, caused no change in BP or HR, but virtually abolished the pressor response elicited by NMDA infusion (McGee & Abdel-Rahman, 2012). Groups 1 and 2 received AP-5, 30 min before ethanol (1 g/kg) or water; notably, the effects of ethanol (1 g/kg), compared to water, on BP and HR were investigated under Experiment 1. Groups 3 and 4 received saline (vehicle for AP-5) or AP-5 30 min before ethanol (1.5 g/kg). The pre-ethanol or water MAP and HR constituted the average values over the 30-min period that followed AP-5 or saline administration. BP and HR were measured for 30 min following ethanol or water administration before euthanasia and tissue collection.
Ex vivo studies
Blood samples (0.2 mL) were collected at baseline and following ethanol administration in the absence or presence of DL-AP-5 or NMDA for measurements of blood ethanol concentration. The vascular tissues collected at the peak of the pressor response elicited by the 1000 μg/kg NMDA or at the end of the 15-min NMDA infusion were used for measurements of vascular NO and ROS, as detailed in our recent study (McGee & Abdel-Rahman, 2012).
Drugs
N-M ethyl-D-Aspartic Acid was purchased from Sigma Aldrich (St. Louis, MO). DL-2-Amino-5-phosphonopentanoic acid was purchased from Tocris Biosciences (Bristol, United Kingdom). Ethanol was purchased from Ultrapure (Darien, CT) and sterile saline was purchased from B.Braun Medical (Irvine, CA).
Data analysis & statistics
Values were expressed as means ± SEM. Mean Arterial Pressure (MAP) was computed as [1/3 × (Systolic ° Diastolic)] + Diastolic. Statistical analyses were conducted using a two-way repeated-measures ANOVA with Tukey’s post hoc test and Student’s t test. Graphpad Prism 5.0 software was used to perform statistical analyses. p < 0.05 was considered significant.
Results
Ethanol attenuates NMDA-evoked pressor response
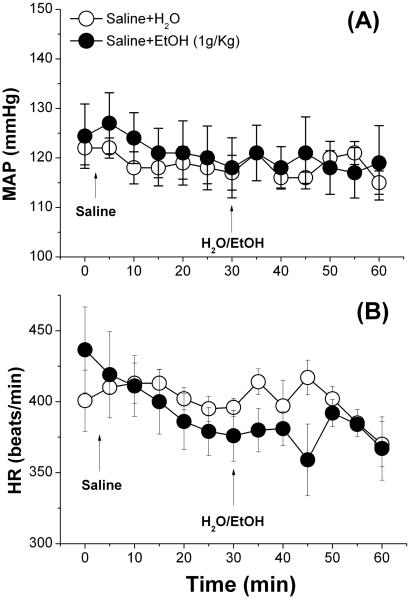
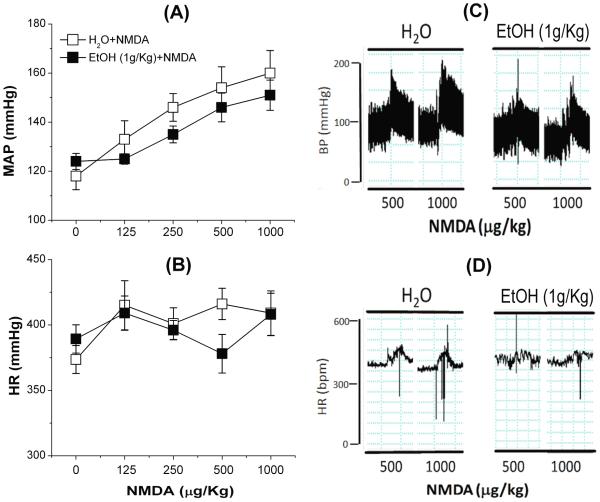
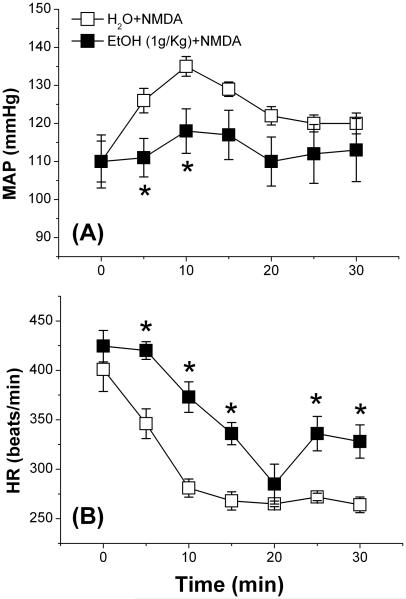
Ethanol (1 g/kg), compared to its vehicle (water), had no effect on BP or HR (Fig. 1), and had no effect on the dose-dependent pressor response or the dose-independent tachycardia elicited by bolus NMDA injections (125–1000 μg/kg, i.v.) (Fig. 2A, B). Representative tracings of BP and HR elicited by bolus NMDA in the absence or presence of ethanol are shown (Fig. 2C, D). NMDA infusion (180 μg/kg/min) caused increases in blood pressure that reached a significant (p < 0.05) peak (~25 mmHg) at 10 min before its gradual decline (Fig. 3A). The BP response was associated with a drastic bradycardic response (Fig. 3B). Ethanol (1 g/kg) significantly (p < 0.05) attenuated the peripheral NMDAR-mediated pressor (Fig. 3A) and bradycardic (Fig. 3B) responses caused by NMDA infusion.
Fig. 1.
Effect of ethanol (EtOH, 1 g/kg by gavage) or equal volume of water (H2O) on A) mean arterial pressure (MAP) and B) heart rate (HR) in conscious male Sprague-Dawley rats. Values are means ± SEM of 4–6 observations.
Fig. 2.
Effect of ethanol (EtOH, 1 g/kg by gavage) or equal volume of water (H2O) on bolus injections NMDA-evoked (125, 250, 500, or 1000 μg/kg; i.v.) changes in mean arterial pressure (MAP; A) and heart rate (HR; B) in conscious male Sprague-Dawley rats. Representative tracings show the effect of NMDA on mean arterial pressure (MAP; C) and heart rate (BPM; D) in the absence or presence of ethanol. Values are means ± SEM of 4–6 observations.
Fig. 3.
Effect of ethanol (EtOH) on mean arterial pressure (MAP; A) and heart rate (BPM; B) elicited by 30-min NMDA infusion (180 μg/kg/min; 9 μL/min) in conscious male Sprague-Dawley rats. Values are means ± SEM of 4–6 observations. *p < 0.05 vs. water (H2O) + NMDA.
Prior peripheral NMDAR blockade uncovers dose-related hypotensive effect of ethanol
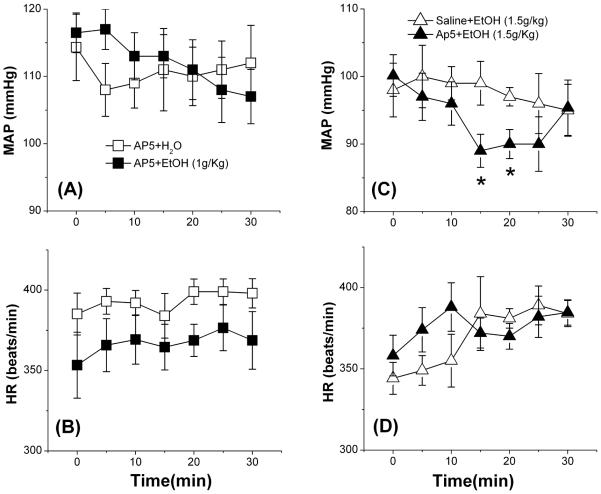
Consistent with our previous findings (McGee & Abdel-Rahman, 2012), AP-5 had no effect on MAP or HR during the 30-min pre-treatment period (data not shown). Further, the lower (1 g/kg) and higher (1.5 g/kg) doses of ethanol had no effect on MAP (Figs. 1A and 4C) or HR (Figs. 1B and 4D). However, while prior peripheral NMDAR blockade (AP-5) did not influence BP or HR in the presence of the lower (1 g/kg) dose of ethanol (Fig. 4A, B), it uncovered a significant (p < 0.05) hypotensive response when combined with the higher (1.5 g/kg) dose of ethanol (Fig. 4C).
Fig. 4.
Effect of NMDAR antagonist DL-AP-5 (5 mg/kg, i.v.) in the absence or presence of ethanol (EtOH 1 or 1.5 g/kg by gavage), or equal volume of water (H2O), on mean arterial pressure (MAP; A, C) and heart rate (BPM; B, D) in conscious male Sprague-Dawley rats. Values are means ± SEM of 4–6 observations.*p < 0.05 vs. saline (Sal) + ethanol.
Ethanol attenuates NMDA-evoked increases in vascular NOx and ROS
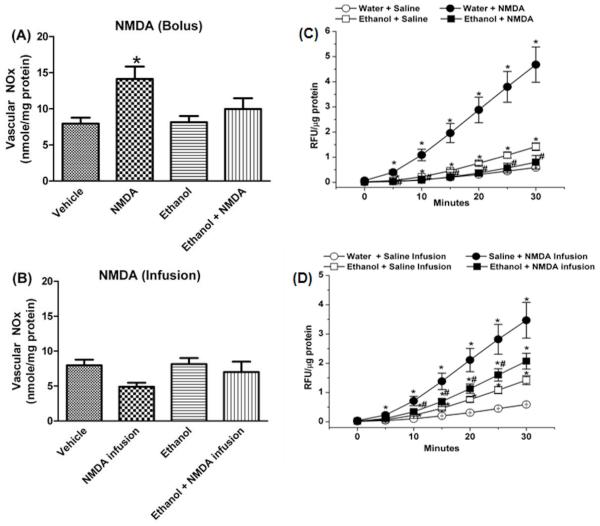
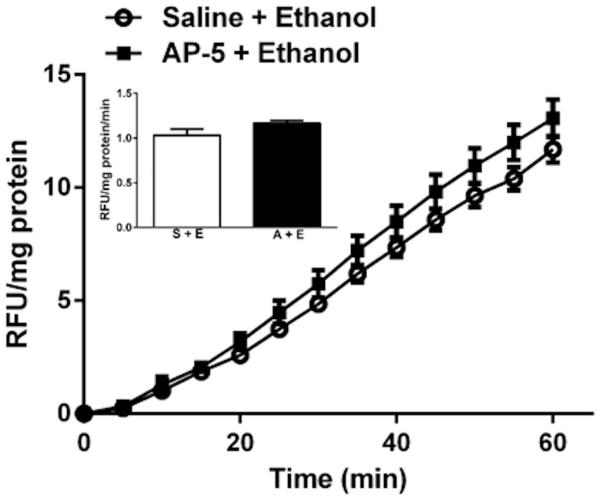
Ethanol (1 g/kg) alone had no effect on vascular NOx (Fig. 5A), but significantly (p < 0.05) increased vascular ROS (Fig. 5C). Vascular (aortic) tissues collected at the peak of the pressor response caused by a bolus of NMDA (1000 μg/kg) exhibited significant (p < 0.05) increases in vascular NOx (Fig. 5A) and ROS (Fig. 5C) levels. Similarly, NMDA infusion caused significant (p < 0.05) increases in vascular ROS (Fig. 5D), but caused a reduction in vascular NOx (Fig. 5B). Pretreatment with ethanol (1 g/kg) attenuated the increases in vascular NOx (Fig. 5A) and ROS (Fig. 5C) caused by bolus NMDA injection and resulted in NOx and ROS values that were not significantly (p > 0.05) different from the corresponding control values (Fig. 5). Further, ethanol (1 g/kg) significantly (p < 0.05) attenuated the increase in vascular ROS caused by NMDA infusion (Fig. 5D). Compared to saline pretreatment, AP-5 did not alter the increase in vascular ROS caused by the higher (1.5 g/kg) dose of ethanol (Fig. 6). Blood ethanol concentrations were not statistically different in the groups that received ethanol alone or along with NMDA or AP-5 (Table 1).
Fig. 5.
Effect of i.v. bolus NMDA (1000 μg/kg; A) or infusion (180 μg/kg/min; 9 μL/min; B) on aortic nitrite/nitrate (NOx) content in the absence or presence of ethanol (1 g/kg; 10 mL/kg of 13% ethanol in water). ROS generation, measured by DCFH-DA, in aortas obtained from rats treated with: water (vehicle), NMDA, ethanol, ethanol + NMDA using the bolus C) or infusion D) paradigm for activating peripheral NMDAR. Aorta homogenates were incubated at 37 °C with DCFH for 30 min. Kinetic readings were recorded at 5-min intervals; relative fluorescence units were normalized to protein. Values are means ± SEM of 4–6 observations. *p < 0.05 vs. saline (control); #p < 0.05 vs. NMDA.
Fig. 6.
ROS generation, measured by DCFH-DA, in aortas obtained from rats treated with ethanol (1.5 g/kg) 30 min following AP-5 (5 mg/kg, i.v.) or its vehicle (saline). Aorta homogenates were incubated at 37 °C with DCFH for 30 min. Kinetic readings were recorded at 5-min intervals; relative fluorescence units were normalized to protein. Values are means ± SEM of 4–6 observations.
Table 1.
Blood ethanol concentrations (mg/dL) following ethanol (1 g/kg; 10 mL/kg of 13% ethanol in water) in conscious male Sprague-Dawley rats
| Time (min) | ||
|---|---|---|
| Group | 30 | 60 |
| Ethanol + Saline | 42 ± 6.0 | 42 ± 9.7 |
| Ethanol + NMDA | 26 ± 1.3* | 24 ± 6 |
| Ethanol + NMDA infusion | 36 ± 2.6 | 29 ± 1.7 |
| Saline + Ethanol (1 g/kg) | 39 ± 8.1 | 27 ± 2.9 |
| AP-5 + Ethanol (1 g/kg) | 28 ± 6.7 | 21 ± 5.4 |
| Saline + Ethanol (1.5 g/kg) | 83 ± 5.1 | |
| AP-5 + Ethanol (1.5 g/kg) | 91 ± 5.3 | |
Values are means ± standard error of the mean of 4–6 observations. AP-5, DL-2-Amino-5-phosphonopentanoic acid; NMDA, N-Methyl-D-Aspartate
p < 0.05 versus ethanol + saline treatment group (Student’s t test)
Discussion
The following are the most important findings of the present study, which is the first to deal with acute ethanol interaction with peripheral (vascular) NMDAR: i) ethanol attenuated the pressor responses caused by infused NMDA, ii) prior NMDAR antagonism (AP-5) uncovered a modest, but significant, hypotensive response caused by ethanol (1.5 g/kg), iii) ethanol had no effect on vascular NOx (index of NO), but significantly increased vascular ROS, and iv) ethanol attenuated the increase in vascular ROS elicited by NMDA infusion. Collectively, abrogation of vascular oxidative stress underlies ethanol attenuation of the peripheral NMDAR-mediated pressor response.
The present study dealt with the cardiovascular consequences of ethanol interaction with the peripheral NMDAR in conscious male rats. It is imperative to note that the selected doses of ethanol (1 or 1.5 g/kg) had no significant effect on BP, which agrees with our reported findings in conscious normotensive male rats (El-Mas & Abdel-Rahman, 1999) and humans (Abdel-Rahman et al., 1987). Therefore, the use of the same model system in the present study circumvented potential confounding effects of ethanol-evoked changes in baseline BP, and its potential impact on the pressor response elicited by peripheral NMDAR. The NMDAR is a prominent molecular target for ethanol within the brain (Möykkynen & Korpi, 2012). Notably, our recent study is the first to suggest a mechanistic role for vascular oxidative stress in the peripheral NMDAR-mediated pressor response (McGee & Abdel-Rahman, 2012). Given the ability of acute ethanol to attenuate a number of central NMDAR-mediated neurobiological effects (Lovinger et al., 1989; Woodward, 1999), we hypothesized that ethanol would attenuate the peripheral NMDAR-mediated pressor response. While consistent with this view, ethanol attenuation of the NMDA dose-dependent pressor responses did not achieve statistical significance. The latter most likely resulted from the variability in BP responses when presented as an absolute value (Fig. 2). However, our hypothesis is unequivocally supported by the drastic ethanol-evoked attenuation of the sustained pressor response elicited by infused NMDA (Fig. 3). These new findings agree with reported findings (including our own), which demonstrated ethanol’s ability to attenuate BP responses elicited by NMDAR activation in cardiovascular-controlling nuclei in the brainstem (El-Mas & Abdel-Rahman, 1993; Lai, Chang, & Lin, 2004).
A second objective of the current study was the elucidation of the role of the peripheral NMDAR in the acute cardiovascular effects of ethanol. We hypothesized that peripheral NMDAR blockade might uncover a hypotensive effect of moderate doses of ethanol that have little effect on BP when administered alone. Therefore, we selected a dose of the selective NMDAR blocker AP-5 (5 mg/kg, i.v.), which had no effect on BP, but attenuated the NMDAR-evoked pressor response in our recent study (McGee & Abdel-Rahman, 2012). Contrary to our hypothesis, prior peripheral NMDAR blockade (AP-5) did not uncover BP reduction by subsequently administered ethanol (1 g/kg) (Fig. 4A). We then considered the possibility that a higher ethanol dose is needed to uncover its hypotensive effect in AP-5 pretreated rats. This possibility was confirmed when a higher (1.5 g/kg) dose of ethanol was administered in the presence of peripheral NMDAR (Fig. 4C). Importantly, in male conscious rats (El-Mas & Abdel-Rahman, 1999) as well as in humans (Abdel-Rahman et al., 1987), similar doses (0.5–1.5 g/kg) of ethanol had no effect on BP but elicited a tachycardic response. In the present study, ethanol (1 g/kg) had no effect on HR (Fig. 1B), but attenuated the bradycardic response caused by NMDA infusion (Fig. 3B), along with the virtual abolition of the NMDA-evoked pressor response (Fig. 3). Future studies are warranted to elucidate the molecular mechanisms of the bradycardia caused by NMDA infusion, particularly in the presence of ethanol.
Recently, we provided the first evidence that neuronal NOS-derived NO contributed to peripheral (vascular) NMDAR-mediated pressor response (McGee & Abdel-Rahman, 2012). However, since ethanol modulates the NMDAR-NOS signaling pathway (Deng & Deitrich, 2007), we hypothesized that ethanol might influence the NMDAR-mediated NO (vascular nitrate/nitrite; NOx) release in a manner that might explain ethanol attenuation of the peripheral NMDAR-mediated pressor response. In agreement with our recent findings (McGee & Abdel-Rahman, 2012), NOx was significantly increased in vascular tissue collected at the peak of the pressor response caused by the highest NMDA bolus dose (Fig. 5A), and this response was attenuated by ethanol (Fig. 5A). The current biochemical findings are consistent with acute ethanol’s ability to attenuate NMDAR-mediated nNOS activation in neuronal tissues in vitro (Chandler, Guzman, Sumners, & Crews, 1994; Czapski, Sun, & Strosznajder, 2002). However, this relevance of this effect may be tissue-specific because ethanol had no impact on the pressor response caused by bolus NMDA injections. Further, NOx level was not altered in the vasculature of rats during the pressor response caused by NMDA infusion in the absence or presence of ethanol (Fig. 5B). These findings argue against a role for vascular NO in the sustained pressor response caused by NMDAR infusion or in ethanol attenuation of such a response. It was important, therefore, to elucidate the role of vascular ROS in ethanol-peripheral NMDAR interaction.
Systemic NMDA (bolus injection or infusion) generated substantial increases in vascular ROS (Fig. 5), consistent with our recent findings in the same tissue (McGee & Abdel-Rahman, 2012, 2014) and in other tissues (Gunasekar et al., 1995). In agreement with reported findings (Krenz & Korthuis, 2012; Yogi et al., 2010), ethanol produced modest, but significant increases in ROS, at least partly, via its oxidative metabolites, acetaldehyde and acetate (Deng & Deitrich, 2007; Seitz & Stickel, 2007). Interestingly, despite ethanol’s or NMDA’s ability to increase vascular ROS, ethanol pretreatment significantly attenuated the peripheral NMDAR-mediated increases in ROS. Together, the present findings suggest that attenuation of vascular oxidative stress contributes, at least partly, to ethanol attenuation of the pressor effect caused by systemic NMDA infusion. Finally, vascular ROS generation caused by the higher (1.5 g/kg) dose of ethanol was NMDAR-independent because it persisted in the presence of AP-5 (Fig. 6). Future studies are needed to elucidate the mechanisms of these intriguing biochemical findings.
It is important to acknowledge a limitation of the present study and to comment on the biological relevance of the present findings. First, the ex vivo biochemical studies were not conducted on resistance vessels. We used the aorta because it provided the needed amount of tissue (protein) to conduct the biochemical studies. Notably, similar to the resistance vessels (Carlton et al., 1998), the aorta also expresses NMDAR and exhibits NMDA-evoked contraction in vitro (McGee & Abdel-Rahman, 2012). Second, while the endogenous ligand for the peripheral NMDAR remains elusive, the location of NMDAR in sympathetic innervation of resistance vessels (Carlton et al., 1998) seems to implicate the NMDAR in the regulation of vascular tone. Based on this premise, future studies are warranted on the effect of ethanol on NMDAR signaling in resistance vessels in animal models that exhibit tonically enhanced peripheral NMDAR signaling.
In summary, the present study yields new insight into ethanol’s interaction with peripheral (vascular) NMDAR and its impact on cardiovascular responses elicited by NMDA and ethanol in conscious rats. We demonstrated ethanol’s ability to attenuate the peripheral NMDAR-mediated pressor response caused by NMDA infusion, at least partly, via attenuation of ROS generation. Further, prior peripheral NMDAR blockade (AP-5) uncovered a hypotensive response caused by a relatively high ethanol dose. Collectively, these findings support a protective role for ethanol against NMDA-mediated oxidative stress. More studies are warranted to investigate the molecular mechanisms that underlie the reciprocal interaction between ethanol and peripheral NMDAR.
Highlights.
Ethanol attenuated systemic NMDA-induced pressor responses.
Prior NMDAR blockade uncovered ethanol-evoked hypotensive response.
AP-5 had no effect on ethanol induction of vascular ROS levels.
Acknowledgment
This work was supported by the National Institute of Alcohol Abuse and Alcoholism [2R01 AA07839-19 and AA07839-18S].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A. Acute effects of ethanol on cardiac output and its derivatives in spontaneously hypertensive and normotensive rats. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:1150–1158. [PubMed] [Google Scholar]
- Abdel-Rahman AA, Carroll RG, el-Mas MM. Role of the sympathetic nervous system in the alcohol-guanabenz hemodynamic interaction. Canadian Journal of Physiology and Pharmacology. 1992;70:1217–1224. doi: 10.1139/y92-169. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman AR, Merrill RH, Wooles WR. Effect of acute ethanol administration on the baroreceptor reflex control of heart rate in normotensive human volunteers. Clinical Science (Lond) 1987;72:113–122. doi: 10.1042/cs0720113. [DOI] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochemistry International. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Augustyniak A, Michalak K, Skrzydlewska E. The action of oxidative stress induced by ethanol on the central nervous system (CNS) Postępy Higieny i Medycyny Doświadczalnej. 2005;59:464–471. [PubMed] [Google Scholar]
- Bender TS, Abdel-Rahman AA. Differential central NOS-NO signaling underlies clonidine exacerbation of ethanol-evoked behavioral impairment. Alcoholism: Clinical and Experimental Research. 2010;34:555–566. doi: 10.1111/j.1530-0277.2009.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernt E, Guttman I. Ethanol: Determination with alcohol dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd Vol. 3. Academic Press; 1974. pp. 1499–1502. [Google Scholar]
- Carlton SM, Chung K, Ding Z, Coggeshall RE. Glutamate receptors on postganglionic sympathetic axons. Neuroscience. 1998;83:601–605. doi: 10.1016/s0306-4522(97)00406-5. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Adaptive plasticity of NMDA receptors and dendritic spines: implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacology, Biochemistry, and Behavior. 2007;86:200–208. doi: 10.1016/j.pbb.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Guzman NJ, Sumners C, Crews FT. Magnesium and zinc potentiate ethanol inhibition of N-methyl-D-aspartate-stimulated nitric oxide synthase in cortical neurons. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:67–75. [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ. Ethanol and adult CNS neurodamage: oxidative stress, but possibly not excitotoxicity. Frontiers in Bioscience (Elite edition) 2012;4:1358–1367. doi: 10.2741/465. [DOI] [PubMed] [Google Scholar]
- Czapski G, Sun G, Strosznajder J. Inhibition of N-methyl-D-aspartic acid-nitric oxide synthase in rat hippocampal slices by ethanol. Evidence for the involvement of tetrahydrobiopterin but not lipid peroxidation. Journal of Biomedical Science. 2002;9:3–9. doi: 10.1007/BF02256572. [DOI] [PubMed] [Google Scholar]
- Deng A, Valdivielso JM, Munger KA, Blantz RC, Thomson SC. Vasodilatory N-methyl-D-aspartate receptors are constitutively expressed in rat kidney. Journal of the American Society of Nephrology. 2002;13:1381–1384. doi: 10.1097/01.asn.0000013293.11876.4e. [DOI] [PubMed] [Google Scholar]
- Deng XS, Deitrich RA. Ethanol metabolism and effects: nitric oxide and its interaction. Current Clinical Pharmacology. 2007;2:145–153. doi: 10.2174/157488407780598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Role of NMDA and non-NMDA receptors in the nucleus tractus solitarius in the depressant effect of ethanol on baroreflexes. The Journal of Pharmacology and Experimental Therapeutics. 1993;266:602–610. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Sexually dimorphic hemodynamic effects of intragastric ethanol in conscious rats. Clinical and Experimental Hypertension. 1999;21:1429–1445. doi: 10.3109/10641969909070858. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicologic Pathology. 2001;29:208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. Journal of Neurochemistry. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- Itoh K, Sakata M, Watanabe M, Aikawa Y, Fujii H. The entry of manganese ions into the brain is accelerated by the activation of N-methyl- D-aspartate receptors. Neuroscience. 2008;154:732–740. doi: 10.1016/j.neuroscience.2008.03.080. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. Journal of Molecular and Cellular Cardiology. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M. Differential effects of chronic ethanol treatment on N-methyl- D-aspartate R1 splice variants in fetal cortical neurons. The Journal of Biological Chemistry. 2001;276:29764–29771. doi: 10.1074/jbc.M100317200. [DOI] [PubMed] [Google Scholar]
- Lai CC, Chang MC, Lin HH. Acute tolerance to ethanol inhibition of NMDA-induced responses in rat rostral ventrolateral medulla neurons. Journal of Biomedical Science. 2004;11:482–492. doi: 10.1007/BF02256097. [DOI] [PubMed] [Google Scholar]
- Leung JC, Travis BR, Verlander JW, Sandhu SK, Yang S-G, Zea AH, et al. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;283:R964–971. doi: 10.1152/ajpregu.00629.2001. [DOI] [PubMed] [Google Scholar]
- Lin HH, Hsieh W-K, Shiu J-Y, Chiu TH, Lai C-C. Inhibition by ethanol of NMDA-induced responses and acute tolerance to the inhibition in rat sympathetic preganglionic neurons in vitro and in vivo. British Journal of Pharmacology. 2003;140:955–963. doi: 10.1038/sj.bjp.0705512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Mao L, Abdel-Rahman AA. Blockade of L-glutamate receptors in the rostral ventrolateral medulla contributes to ethanol-evoked impairment of baroreflexes in conscious rats. Brain Research Bulletin. 1995;37:513–521. doi: 10.1016/0361-9230(95)00034-c. [DOI] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA. Mechanism of Peripheral NMDA Receptor-Mediated Pressor Response in Conscious Rats. The FASEB Journal. 2010;24:lb523. [Google Scholar]
- McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric- oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. The Journal of Pharmacology and Experimental Therapeutics. 2012;342:461–471. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA. Enhanced vascular PI3K/Akt-NOX signaling underlies the peripheral NMDAR-mediated pressor response in conscious rats. Journal of Cardiovascular Pharmacology. 2014;63:395–405. doi: 10.1097/FJC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Analytical Biochemistry. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Morhenn VB, Murakami M, O'Grady T, Nordberg J, Gallo RL. Characterization of the expression and function of N-methyl-D-aspartate receptor in keratinocytes. Experimental Dermatology. 2004;13:505–511. doi: 10.1111/j.0906-6705.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- Möykkynen T, Korpi ER. Acute effects of ethanol on glutamate receptors. Basic & Clinical Pharmacology & Toxicology. 2012;111:4–13. doi: 10.1111/j.1742-7843.2012.00879.x. [DOI] [PubMed] [Google Scholar]
- Reyes RC, Brennan AM, Shen Y, Baldwin Y, Swanson RA. Activation of neuronal NMDA receptors induces superoxide-mediated oxidative stress in neighboring neurons and astrocytes. The Journal of Neuroscience. 2012;32:12973–12978. doi: 10.1523/JNEUROSCI.1597-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Jezová D. Gene expression of NMDA receptor subunits in rat adrenals under basal and stress conditions. Journal of Physiology and Pharmacology. 2001;52:719–727. [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Reviews. Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- Toropainen M, Näkki R, Honkanen A, Rosenberg PH, Laurie DJ, Pelto- Huikko M, et al. Behavioral sensitivity and ethanol potentiation of the N-methyl-D-aspartate receptor antagonist MK-801 in a rat line selected for high ethanol sensitivity. Alcoholism: Clinical and Experimental Research. 1997;21:666–671. [PubMed] [Google Scholar]
- Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiological Research. 2014;63(Suppl 1):S191–203. doi: 10.33549/physiolres.932678. [DOI] [PubMed] [Google Scholar]
- Woodward J. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochemistry International. 1999;35:107–113. [PubMed] [Google Scholar]
- Xu M, Smothers CT, Trudell J, Woodward JJ. Ethanol inhibition of consecutively open N-methyl-D-aspartate receptors. The Journal of Pharmacology and Experimental Therapeutics. 2012;340:218–226. doi: 10.1124/jpet.111.187179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogi A, Callera GE, Hipólito UV, Silva CR, Touyz RM, Tirapelli CR. Ethanol-induced vasoconstriction is mediated via redox-sensitive cyclo-oxygenase-dependent mechanisms. Clinical Science (Lond) 2010;118:657–668. doi: 10.1042/CS20090352. [DOI] [PubMed] [Google Scholar]
- Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB Journal. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]