Abstract
Introduction
Despite consensus guidelines on best practice in the care of older patients with cancer, geriatric assessment (GA) has yet to be optimally integrated into the field of oncology in most countries. There is a relative lack of consensus in the published literature as to the best approach to take, and there is a degree of uncertainty as to how integration of geriatric medicine principles might optimally predict patient outcomes.
The aim of the current study was to obtain consensus on GA in oncology to inform the implementation of a geriatric oncology programme.
Methods
A four round Delphi process was employed. The Delphi method is a structured group facilitation process, using multiple iterations in order to gain consensus on a given topic
Results
Consensus was reached on the optimal assessment method and interventions required for the commonly employed domains of GA. Other aspects of GA, such as screening methods and age cutoff for assessment represented a higher degree of disagreement.
Discussion
The expert panel employed in this study clearly identified the criteria that should be included in a clinical geriatric oncology programme. In the absence of evidence-based guidelines, this may prove useful in the care of older cancer patients.
Introduction
It is widely reported that older patients with cancer are undertreated compared to their younger counterparts (Beckett et al., 2012, Peake et al., 2003, Hubbard and Jatoi, 2011). Survival data from national cancer registries and institutions such as EUROCARE (De Angelis et al., 2014), have highlighted significantly poorer outcomes for older patients. There is a lack of empirical data related to tolerability of cancer-directed treatment in older patients, due to the traditional exclusion of older patients from cancer clinical trials (Hutchins et al., 1999, Lewis et al., 2003, Murthy et al., 2004, Zulman et al., 2011, Talarico et al., 2004). Existing level 1 evidence and treatment guidelines tend to favour fitter older patients, and it remains uncertain what approach to take towards more vulnerable patients (Jatoi et al., 2005, Lugtenberg et al., 2011, Clough-Gorr and Silliman, 2008). Also, ageism may exist in cancer care (DOH, 2012), and indeed patients themselves may choose not to undergo aggressive treatment, especially if treatment could affect their quality of life (Fried et al., 2002, Yellen et al., 1994).
The Institute of Medicine's most recent report on cancer care (Levit et al., 2013) highlights the urgent need to gain more evidence regarding safe and effective treatments for undertreatment of older patients with cancer. Guidelines have advocated for a more objective pre-treatment assessment of older cancer patients (Wildiers et al., 2014). The ability to stratify patients according to their physiological age to help guide cancer treatment decisions for older patients is of paramount importance. It is thought that integration of geriatric medicine principles into oncology might better assist clinicians in making complex treatment decisions.
A geriatric assessment (GA) is defined as a “multidimensional interdisciplinary diagnostic process focussed on determining an older person's medical, psychological and functional capability in order to develop a coordinated and integrated plan for treatment and long term follow up”(Rubenstein et al., 1989). GA has been shown to improve outcomes in older adults in the geriatric medicine setting, with regard to reduced hospital admissions, improved functional status and better survival (Ellis et al., 2011, Stuck et al., 1993). The evidence for the benefits of GA in oncology include prediction of treatment related toxicity (Hurria et al., 2011, Shin et al., 2012, Aparicio et al., 2013, Extermann et al., 2012), treatment adherence (Puts et al., 2014, Spyropoulou et al., 2014, Kim et al., 2014), quality of life (Pottel et al., 2014, Ward et al., 2014), ability to inform oncologist's treatment decisions (Kenis et al., 2013, Caillet et al., 2011, Horgan et al., 2012, Aliamus et al., 2011, Aparicio et al., 2011, Decoster et al., 2013) and overall survival (Hamaker et al., 2011, Girones et al., 2011, Kanesvaran et al., 2011, Soubeyran et al., 2012). However, much of the current knowledge base for the effectiveness of GA in oncology is based on smaller retrospective studies of heterogeneous cancer patients, and better prognostic models are needed (Wildiers et al., 2014).
Despite consensus guidelines from The International Society of Geriatric Oncology (SIOG) (Extermann et al., 2005, Wildiers et al., 2014) , the National Comprehensive Cancer Network (NCCN) (Hurria et al., 2014) and European Organisation for Research and Treatment of Cancer (EORTC) (Pallis et al., 2011, Pallis et al., 2010), who have recommended GA be performed in all cancer patients, it has yet to be optimally integrated into the field of oncology in most countries. One difficulty in the published literature in relation to GA, lies with the lack of standardisation of assessment approaches to date (Puts et al., 2012). In the absence of evidence-based guidance, the Delphi method is frequently employed in healthcare to formulate expert consensus guidelines in a particular field (Simon et al., 2014, Uphoff et al., 2012, Yeung et al., 2012).
The aim of the current study was to obtain consensus on aspects of GA in oncology to inform the implementation of an Irish geriatric oncology programme. This is transferable to other countries and healthcare systems.
Methods
A four round online Delphi process was employed. The Delphi method is a structured group facilitation process, using multiple iterations in order to gain consensus on a given topic, and is widely used in medical research (Simon et al., 2014, Fearon et al., 2011, Diviani and Schulz, 2011).
The first Delphi round period began on September 2012 and the four round process was completed by July 2013, as follows:
Selection of an expert panel
The expert panel was purposively sampled upon individual expertise and knowledge, as follows: 1) recognized scientific expertise in geriatric oncology research or clinical experience, demonstrated by publication or clinical activities and participation in guideline development; 2) multidisciplinarity to facilitate diversity of views and expertise from a geriatric medicine and oncology perspective; and 3) both a national and international context to facilitate a global representation and exchange of state-of-the-art knowledge, with the aim of implementing an Irish geriatric oncology programme. The international expert panel was identified through active International Society of Geriatric Oncology (SIOG) affiliation. A follow-up search of Pubmed was then used to verify clinical and research activity. For the current study, whose focus is the implementation of a geriatric oncology programme in Ireland, it was deemed important to include a national panel of stakeholders also. The SIOG affiliated panel was mainly European-based, as a comparison study, which was designed to evaluate geriatric assessment interventions, was run concurrently in the US to gain the US perspective (Mohile et al., 2013). All Irish (Consultant) Radiation and Medical Oncologists and Geriatricians were identified through the relevant professional body, and invited to participate in this study. Surgeons were excluded from this study, as although it is appreciated that there are some commonalities, the pre-operative assessment of patients is necessarily different to the pre-treatment assessment of patients undergoing chemo-radiotherapy. It would therefore constitute a separate panel of expertise with different aims and objectives.
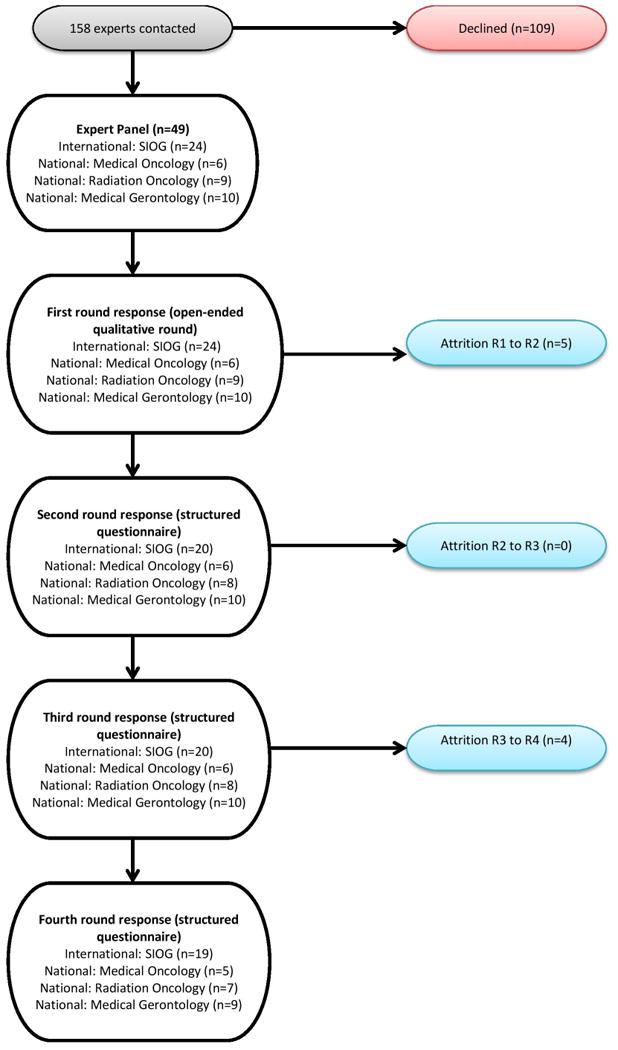
One hundred and fifty eight experts, in total, were contacted via email and provided with information regarding the study. Response rate varied per professional group, as follows: SIOG affiliated 55% (n=24/44), Radiation Oncology 31% (n=9/29), Medical Oncology 23% (n=6/26) and Geriatric Medicine 17% (n=10/59).
Initial survey items were based on a review of the literature. The first round (R1) was an open round. R1 comprised 49 members, encompassing four disciplines: Radiation Oncology (n=9), Medical Oncology (n=6), Geriatric Medicine (n=10) and SIOG-affiliated (n=24).
Attrition between rounds was minimal, with five panel members choosing not to participate further after R1 (one from Radiation Oncology, four from the SIOG affiliated group). There was no further attrition between rounds two (R2) and three (R3), while four participants did not proceed to the final round (R4), one member from each of the respective professional subgroups. A nonrespondent bias check was conducted after the R1 Delphi to verify that nonresponders did not differ demographically from responders.
Defining consensus and stability
A predetermined threshold for consensus was chosen, as per best practice in Delphi studies. Consensus in Delphi studies is often calculated by using the interquartile range(IQR) (Jones and Hunter, 1995). It is widely accepted as an objective and rigorous method of defining consensus in Delphi studies (von der Gracht, 2012, De Vet et al., 2005). An IQR ≤1 can be considered as good consensus on a five-point Likert scale, IQR ≤2 for a ten-point scale (Linstone and Turoff, 1975).
Stability, or the degree of permanence of participants' vote distribution over successive rounds, reflects consensus. Changes of less than 15% offer a working definition of stability in the literature, when the responses obtained in two successive rounds are shown to not be statistically significantly different from each other (Dajani et al., 1979). Group stability, rather than individual stability was assessed in the current study.
For nominal data, consensus was defined as 67% i.e. two-thirds majority. For likert scales, items reaching consensus, based on the a priori IQR definitions, were re-presented in the following round to ensure stability of responses in two successive rounds.
First Delphi Round
A survey (http://www.surveymonkey.com) was designed consisting of open-ended questions related to the agenda above. Questionnaires were accessed via a secure URL link, generated by survey monkey, and sent via email. Each participant used a study ID when completing each round, whose identity was known only to a designated gatekeeper who secured the study code. Reminder emails were sent before the pre-defined deadline for completion in all cases, in order to enhance participation.
The R1 survey consisted of demographic information and qualitative development of guidelines relating to use of GA, organisation of geriatric oncology activity, use of GA tools and interventions, stratification of patients for full GA and perceived importance of GA in the decision making process. Sample questions used included “What staff members participate in the interpretation of geriatric assessment?”, “Who is offered geriatric assessment (i.e. characteristics of patients)?” and “Please list any geriatric assessment tool(s) that you currently use in your clinical practice”. As per the classical Delphi approach, the rationale for the use of open-ended questions in R1 is to reduce bias, allowing participants relative freedom in their responses (Hasson et al., 2000).
Surveys were piloted in advance to ensure comprehension and promote clarity.
Second Delphi Round
In R2, the goal was to design a questionnaire, with quantifiable ranking/rating scales, using information put forward by participants in the first round. This formed the basis for subsequent rounds, whereby items were eliminated only through consensus and stability. Summary statements were amalgamated, grouping related content together and then distributed to participants in a full report before each successive round. Descriptive statistics were reported, and open responses, from additional comment text boxes, were included in appendices (ensuring anonymity), with broad summaries and synopses in the main text. Box and whisker plots were also displayed in the feedback report for certain aspects, to display the distribution of responses and to highlight outliers, who were identified by study ID. It was anticipated that this would aid convergence towards group consensus. Participants were asked to rate or rank certain aspects of the geriatric oncology process, under the aforementioned broad headings i.e. selection of patients for GA, appropriate assessments and interventions for older oncology patients, implementation strategies as well as education and training requirements. Participants “voted” using a 10-point scale to indicate the level of importance attributed to a particular statement, or to rank order items presented to them, or by simply answering yes or no to a given question. The method of voting chosen depended on the type of information sought. Some statements required a simple yes or no answer, while others were ranked in order of preference, from a selection of choices e.g. assessment tools/interventions. A 10 point likert scale was used to measure level of agreement with a statement, or the level of importance attributable to it. The process of feedback and re-presentation of statements not reaching consensus was repeated for each round. Experts were asked to consider their responses in the context of the group response, along with the summarised report and then re-rate the statements. Where items reached consensus they were re-introduced once more to ensure stability, as per the pre-defined methodological approach.
Third Delphi Round
For R3, survey items remained unchanged. In order to ensure stability, it was necessary to produce a duplicate round of all items used in R2. Each member of the panel was provided with an anonymous summary of the expert's opinion from the previous round in order to aid decision making.
Final Delphi Round
Only items that had not achieved consensus and stability in the previous rounds were presented in R4. Only the top three options (identified by mean rank/rating) were presented in the final round where consensus had yet to be achieved, in a final effort to “force” consensus.
Open comments were encouraged throughout in a combined qualitative and quantitative approach. The Delphi process is summarised in Fig. 1.
Fig 1. Overview of Delphi process.
Data analysis
Data were analysed anonymously by encoding panel members with their survey ID numbers. Data were exported from Survey Monkey and analysed using SPSS v20.0. Demographic characteristics were analysed using descriptive statistics. The stratification of patients for GA, ranking of GA domains and assessments/interventions was reported as ordinal data. The median was used to measure the group aggregate rating. The median rating was interpreted along with the IQR to determine consensus of the statements, as outlined previously. The median and interquartile range were calculated based on all participating respondents. Missing answers were regarded as nonparticipation, and the panel was directed not to provide guidance on items it was unsure about. This was considered important due to the heterogeneous nature of the expert panel.
Kendall's coefficient of concordance (W) was performed to measure the degree of consensus among experts (Schmidt, 1997). Kendall's W ranges from 0 (no agreement) to 1 (complete agreement). The Kruskal Wallis test was used to analyse differences in independent variables among the different subgroups of experts.
The significance level for determining statistical difference was defined at P≤ 0.05.
Ethical Considerations
Ethical approval was granted by the Research Ethics Committee of the Faculty of Health Sciences in Trinity College Dublin. All participants gave informed consent to participate in the study.
Results
Demographics
Demographics of study participants are presented in Table 1, by professional group i.e. SIOG affiliated panel, Irish radiation oncologists, medical oncologists and geriatricians. Participants were also asked to rate the current evidence base in geriatric oncology, on a ten point likert scale, as part of the initial demographics round. Overall, this was rated at an overall mean value of 4.3 ± 1.8. For the SIOG affiliated panel, this increased marginally to 4.6± 2.1.
Table 1. Demographic characteristics of study participants by professional group i.e. SIOG affiliated panel, Irish radiation oncologists, medical oncologists and geriatricians.
| Demographic and clinical characteristics of the Expert Panel on GA in Oncology | |||||
|---|---|---|---|---|---|
|
| |||||
| SIOG Group (n=24) | |||||
|
| |||||
| Characteristics | n | % Entire Expert Panel | Mean±SD | Median | Range |
|
| |||||
| Age (y) | 24 | 49 | 44.8+9.4 | 43 | 34-73 |
|
| |||||
| Female gender | 11 | 22 | |||
|
| |||||
| Male gender | 13 | 27 | |||
|
| |||||
| Years in clinical practice | 24 | 49 | 12.8+11.0 | 11 | 1-48 |
|
| |||||
| Current evidence base in GO (scale 1-10) | 24 | 49 | 4.6+2.1 | 4.5 | 1-9 |
|
| |||||
| In receipt of funding for GO research | 8 | 16 | |||
|
| |||||
| Caseload Older Patients with Cancer Seen/Week | 22 | 45 | 24+30.5 | 18 | 1-150 |
| Additional Expertise of the SIOG group | |||||
|---|---|---|---|---|---|
|
| |||||
| Yes n(%) | No n(%) | ||||
| I do more clinical work than research | 18 (82%) | 4 (18%) | |||
| I mentor others in GO | 18 (86%) | 3 (14%) | |||
| I describe myself as a geriatric oncologist | 10 (48%) | 11 (52%) | |||
| Medical Gerontology Group (n=10*) | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | n | % Entire Expert Panel | Mean±SD | Median | Range |
|
| |||||
| Age (y) | 9 | 18 | 43.4+3.5 | 44 | 39-48 |
|
| |||||
| Female gender | 5 | 10 | |||
|
| |||||
| Male gender | 5 | 10 | |||
|
| |||||
| Years in clinical practice | 9 | 18 | 7.9+3.2 | 8 | 2-12 |
|
| |||||
| Current evidence base in GO (scale 1-10) | 9 | 18 | 3.8+1.4 | 1-5 | |
|
| |||||
| In receipt of funding for GO research | 2 | 4 | |||
|
| |||||
| Caseload Older Patients with Cancer Seen/Week | 9 | 18 | 2.4+1.4 | 2 | 1-5 |
| Radiation Oncology Group (n=9) | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | n | % Entire Expert Panel | Mean±SD | Median | Range |
|
| |||||
| Age (y) | 9 | 18 | 45.4+8.1 | 43 | 35-62 |
|
| |||||
| Female gender | 4 | 17 | |||
|
| |||||
| Male gender | 5 | 21 | |||
|
| |||||
| Years in clinical practice | 9 | 18 | 12+8.7 | 9 | 3-29 |
|
| |||||
| Current evidence base in GO (scale 1-10) | 9 | 18 | 4.1+1.8 | 4 | 2-7 |
|
| |||||
| In receipt of funding for GO research | 0 | 0 | |||
|
| |||||
| Caseload Older Patients with Cancer Seen/Week | 9 | 18 | 8.7+12.4 | 4 | two to forty |
| Medical Oncology Group (n=6) | |||||
|---|---|---|---|---|---|
|
| |||||
| Characteristics | n | % Entire Expert Panel | Mean±SD | Median | Range |
|
| |||||
| Age (y) | 6 | 12 | 43+8.0 | 40 | 20-56 |
|
| |||||
| Female gender | 1 | 4 | |||
|
| |||||
| Male gender | 5 | 21 | |||
|
| |||||
| Years in clinical practice | 6 | 12 | 10+8.4 | 8 | 2-12 |
|
| |||||
| Current evidence base in GO (scale 1-10) | 6 | 12 | 4.2+10 | 4 | 1-5 |
|
| |||||
| In receipt of funding for GO research | 0 | 0 | |||
|
| |||||
| Caseload Older Patients with Cancer Seen/Week | 6 | 12 | 16.2+6.3 | 16 | 10-25 |
One participant declined to complete the demographic summary
Selection of patients for GA
R1 sought the opinion of the contributing professional groups regarding which patients should be routinely referred for GA. There was no consensus in the first three rounds regarding this aspect of GA. Consensus was finally reached in R4 that all patients aged 70 and over, and those who are younger with age-related issues or concerns, should be referred for GA. See Table 2 for a summary of descriptive statistics per round, and supplementary data for a box and whisker plot with all categories included, showing additional options presented in previous rounds.
Table 2.
Results for Patient Stratification for GA, in order of mean rank per individual round. An IQR ≤2 was applicable for consensus in rounds 2 and 3 (8-10 options), with an IQR of ≤1 for the final round (<5 options).
| Rank | Median | Mode | Interquartile Range | Consensus {R4 W=0.452, 2 df, p<0.001) |
|---|---|---|---|---|
| 1. All patients aged 70 and over, and those who are younger with age-related issues or concerns | R2: 3.00 | 1.00 | {1.00, 5.00} | No |
| R3: 2.00 | 1.00 | {1.00, 3.50} | No | |
| R4: 1.00 | 1.00 | {1.00, 2.00} | Yes | |
|
| ||||
| 2. All patients aged 75 and over, and those who are younger with age-related issues or concerns | R2: 3.00 | 2.00 | {2.00, 5.00} | No |
| R3: 3.00 | 2.00 | {2.00, 5.00} | No | |
| R4: 2.00 | 2.00 | {1.00, 3.00} | No | |
|
| ||||
| 3. All patients aged 70 and over | R2: 6.00 | 8.00 | {3.00, 8.00} | No |
| R3: 4.00 | 1.00 | {2.00, 7.00} | No | |
| R4: 3.00 | 3.00 | {2.00, 3.00} | No | |
For the final round age cutoff variable, W was calculated (W = 0.452) and found to be statistically significant (at p < 0.001). This indicates moderate agreement with the final ranking. The Kruskal Wallis test demonstrated that there was no significant difference in relation to how ranks were applied among the four subgroups.
Appropriate assessments and interventions for Oncology
Screening Tools
Consensus was not reached on the use of a shorter screening tool that would identify those patients who could potentially benefit from GA, versus those who would not.
Only the top three screening options were presented in the final round in an effort to force consensus. The abbreviated CGA (aCGA) was ranked highest overall, however it did not achieve consensus. As the degree of familiarity with the screening tools under consideration was specific to the field of geriatric oncology, and many of the tools were relatively new by comparison to other GA domains, subgroup analysis of the SIOG affiliated group was also carried out. This analysis of the SIOG affiliated group indicated an overall preference for the G8 screening tool, but this did not reach consensus. However, there was consensus among the SIOG group in R3 and R4 regarding the lower ranked VES-13, with a mean rank of 2.13 and IQR of 1. See Table 3 for further details.
Table 3.
Best Choice of Screening Tool in Oncology (in order of preference: 1=1st place etc.) Subgroup analysis of the SIOG affiliated group is presented in italics. Please note that consensus was defined as an IQR of 2 for rounds 2 and 3, and 1 for Round 4 (as only 3 items presented to participants in final round).
| Screening Tool | Median | Mode | Interquartile Range | Consensus (R4 All:W=0.002, 2df, p=0.957;R4 SIOG:W=0.016, 2df, p=0.779) | |
|---|---|---|---|---|---|
| 1. aCGA | R2 (All): | 3.00 | 1.00 | {1.00, 7.00} | No |
| R2 (SIOG): | 2.50 | 1.00 | {1.00, 4.50} | No | |
| R3 (All): | 3.00 | 1.00 | {1.00, 4.50} | No | |
| R3 (SIOG): | 3.00 | 1.00 | {1.50, 6.00} | No | |
| R4 (All): | 2.00 | 1.00 | {1.00, 3.00} | No | |
| R4 (SIOG): | 2.00 | 2.00 | {1.00, 3.00} | No | |
|
| |||||
| 2. G8 | R2 (All): | 3.00 | 1.00 | {1.00, 5.00} | No |
| R2 (SIOG): | 2.00 | 1.00 | {1.00, 3.00} | Yes | |
| R3 (All): | 2.00 | 1.00 | {1.00, 4.00} | No | |
| R3 (SIOG): | 2.00 | 1.00 | {1.00, 3.00} | Yes | |
| R4 (All): | 2.00 | 1.00 | {1.00, 3.00} | No | |
| R4 (SIOG): | 1.50 | 1.00 | {1.00, 3.00} | No | |
|
| |||||
| 3. VES-13 | R2 (All): | 3.00 | 2.00 | {2.00, 4.00} | No |
| R2 (SIOG): | 2.50 | 2.00 | {2.00, 4.00} | No | |
| R3 (All): | 2.00 | 2.00 | {1.75, 3.25} | No | |
| R3 (SIOG): | 2.00 | 2.00 | {2.00, 3.00} | Yes | |
| R4 (All): | 2.00 | 2.00 | {1.00, 3.00} | No | |
| R4 (SIOG): | 2.00 | 2.00 | {2.00, 3.00} | Yes | |
|
| |||||
| Others under consideration | Groningen Frailty Indicator (GFI), functional status, objective physical performance (OPP), self-rated health, ECOG performance status, Karnofsky performance status, Cancer and Aging Research Group (CARG), Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH), self-rated health | ||||
Statistical tests for concordance and intergroup variability proved insignificant for selection of screening tools.
Geriatric Assessment and Interventions
A recent systematic review (Puts et al., 2012) was used as the basis for selection of relevant GA domains in Oncology, which were used for this Delphi study. Panellists were also invited to contribute other domains and assessments. The importance of each domain was ranked in each round, as can be seen in Table 4. For the final round, W was calculated (W = 0.427) and found to be statistically significant (p < 0.001), indicating moderate agreement among the expert panel in relation to the importance of each domain.
Table 4. Importance of Each Domain in Oncology in Rank Order (1=1st place etc.).
| Domain and Rank | Round | Median | Mode | Interquartile Range | Consensus {R4:W=0.427, 8df, p<0.001} |
|---|---|---|---|---|---|
| 1. Functional status | R2: | 10.00 | 10.00 | {8.00, 10.00} | Yes |
| R3: | 10.00 | 10.00 | {8.25, 10.00} | Yes | |
| R4: | 10.00 | 10.00 | {9.00, 10.00} | Yes | |
|
| |||||
| 2. Objective physical performance status | R2: | 9.00 | 10.00 | {7.25, 10.00} | No |
| R3: | 9.00 | 10.00 | {8.00, 10.00} | Yes | |
| R4: | 9.00 | 10.00 | {8.00, 10.00} | Yes | |
|
| |||||
| 3.Comorbidities | R2: | 9.00 | 10.00 | {6.25, 10.00} | No |
| R3: | 9.00 | 10.00 | {7.25, 10.00} | No | |
| R4: | 9.00 | 10.00 | {8.00, 10.00} | Yes | |
|
| |||||
| 4. Cognitive status | R2: | 9.00 | 10.00 | {8.00, 10.00} | Yes |
| R3: | 9.50 | 10.00 | {8.00, 10.00} | Yes | |
| R4: | 9.00 | 10.00 | {8.00, 10.00} | Yes | |
|
| |||||
| 5.Nutritional status | R2: | 8.00 | 8.00 | {7.00, 9.00} | Yes |
| R3: | 8.00 | 6.00 | {6.00, 9.00} | No | |
| R4: | 8.00 | 8.00 | {6.00, 9.00} | No | |
|
| |||||
| 6.Social support status | R2: | 7.00 | 7.00 | {6.00, 9.00} | No |
| R3: | 8.00 | 8.00 | {6.00, 9.00} | No | |
| R4: | 8.00 | 8.00 | {6.00, 9.00} | No | |
|
| |||||
| 7.Polypharmacy | R2: | 7.00 | 9.00 | {5.00, 9.00} | No |
| R3: | 7.50 | 8.00 | {5.00, 9.00} | No | |
| R4: | 7.00 | 5.00 | {5.00, 9.00} | No | |
|
| |||||
| 8.Psychological status - depression | R2: | 6.00 | 6.00 | {5.00, 8.00} | No |
| R3: | 7.00 | 67.00 | {5.00, 8.00} | No | |
| R4: | 7.00 | 9.00 | {5.00, 9.00} | No | |
|
| |||||
| 9.Psychological status - anxiety | R2: | 6.00 | 6.00 | {4.25, 8.00} | No |
| R3: | 7.00 | 8.00 | {4.00, 8.00} | No | |
| R4: | 6.00 | 7.00 | {5.00, 8.00} | No | |
Kruskal Wallis tests found a statistically significant difference between the four subgroups, only in relation to social support status (H=11.35, 3 df, p=0.01). Significant difference in mean rank was found between the SIOG group and Radiation Oncology (H=9.053, 1 df, p=0.003). Radiation Oncology ranked this aspect of GA much lower (mean=5.08) than their SIOG colleagues (mean=14.97).
Overall, panellists rated functional status (subjective and objective measures) as the most important domain in influencing oncology decisions, followed by comorbidities and cognition. Other domains did not reach consensus in relation to overall importance.
Consensus was reached on the optimal assessment method and interventions required for the commonly employed domains of GA, apart from polypharmacy assessment.
Table 5 outlines the consensus achieved for selected domains of GA in Oncology in R4. There was significant agreement among the expert panel with respect as to how they ranked the relative importance of each assessment and intervention. There was no consensus regarding polypharmacy assessment, but the expert panel agreed that geriatricians should be consulted regarding management of medications. The strength of agreement varied from weak agreement (functional status, nutritional status and depression assessments), to moderate (interventions for comorbidities, social support and anxiety/depression) to strong (cognition, comorbidities and nutritional status assessments). See table 5 for W values and further details. Information on assessments and interventions in previous rounds, including other items considered, may be found in supplementary data.
Table 5.
Top 3 assessments and interventions for older patients with cancer (in order of preference: 1=1st place etc.). Kendall's W is also indicated for each domain.
| Functional Status Assessment {R3 W=0.266, 8df, p<0.001} | |||||
|---|---|---|---|---|---|
| Item | Mean Rank | Median | Mode | Interquartile Range | Consensus |
| 1. Activities of Daily Living (ADL)/Instrumental Activities of Daily Living (IADL) in combination | R3:1.86 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Functional Status Interventions {R3 W=0.189, 2df, p=0.001} | |||||
| 1. Physiotherapy referral | R4: 1.59 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Physical Performance Impairment: Assessment (R4 W=0.267, 2df, p<0.001) | |||||
| 1. Timed Up and Go (TUG) | R4:1.67 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Physical Performance Impairment: Interventions (R3 W=0.266, 8df, p<0.001) | |||||
| 1. Physiotherapy Referral | R3:1.24 | 1.00 | 1.00 | {1.00, 1.00} | Yes |
| Cognitive Status: Assessment (R3 W=0.667, 14df, p<0.001) | |||||
| 1. Mini Mental State. Examination (MMSE) | R3:1.55 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Cognitive Status: Interventions (R4 W=0.222, 2df, p=0.001) | |||||
| 1. Geriatrician referral | R4:1.46 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Co-morbidities: Assessment (R3 W=0.662, 4df, p<0.001) | |||||
| 1. Charlson Comorbidity Index | R3:1.53 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Co-morbidities: Interventions (R3 W=0.356, 2df, p<0.001) | |||||
| 1. Geriatrician Referral | R3:1.43 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Polypharmacy: Assessment (R4 W=0.003, 2df, p=0.90) | |||||
| 1. List of Medications | R4:1.95 | 2.00 | 1.00 | {1.00, 3.00} | No |
| Polypharmacy: Interventions (R4 W=0.186, 2df, p=0.001) | |||||
| 1. Geriatrician Referral | R3:1.54 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Nutritional Status: Assessment (R4 W=0.203, 2df, p=0.002) | |||||
| 1. Mini Nutritional Assessment (MNA) Short form | R4:1.50 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Nutritional Status: Interventions (R3 W=0.605, 1df, p<0.001) | |||||
| 1. Dietician Referral | R3:1.11 | 1.00 | 1.00 | {1.00,1.00} | Yes |
| Social Support Status: Assessment (R3 W=0.732, 3df, p<0.001) | |||||
| 1. Patient History/caregiver interview | R3:1.27 | 1.00 | 1.00 | {1.00, 1.00} | Yes |
| Social Support: Interventions (R3 W=0.309, 4df, p<0.001) | |||||
| 1. Social work referral | R3:1.57 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Anxiety: Assessment (R3 W=0.345, 2df, p<0.001) | |||||
| 1. Patient history/Interview | R3:1.62 | 2.00 | 1.00 | {1.00, 2.00} | Yes |
| Anxiety: Interventions (R3 W=0.492, 5df, p<0.001) | |||||
| 1. Referral to a Psychiatrist/Psychologist/Cogntive Behavioural Therapy | R3:1.63 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
| Depression: Assessment (R3 W=0.117, 3df, p=0.006) | |||||
| 1. Geriatric Depression Scale (GDS) Short form | R3:1.86 | 2.00 | 1.00 | {1.00, 2.00} | Yes |
| Depression: Interventions (R3 W=0.451, 5df, p<0.001) | |||||
| 1. Referral to a Psychiatrist/Psychologist/Cognitive Behavioural Therapy | R3:1.50 | 1.00 | 1.00 | {1.00, 2.00} | Yes |
There were no significant differences in the Kruskal-Wallis H-test results for items reaching consensus, thereby indicating expert agreement in variable ranking among the four professional subgroups.
Discussion
Currently, formal geriatric assessment tools are rarely employed by oncologists, not only in Ireland, but internationally. Underutilisation of GA may be due to the lack of consensus in relation to the application of geriatric assessments and interventions in oncology, as well as the lack of level 1 evidence for the efficacy of this approach. The current Delphi study aimed to gain consensus from an expert panel of national and international stakeholders regarding the optimal assessment methods in oncology.
The panellists in this study clearly identified the criteria that should be included in a clinical geriatric oncology programme. Patient stratification and essential assessments and interventions to be included were identified through expert consensus. As the panellists in this study vocalised, the current evidence base in geriatric oncology (rated 4/10) is insufficient to advise on the optimal assessment of older oncology patients, and guidance of an expert panel with related expertise is an appropriate alternative.
Content validity is ensured in Delphi studies when the expert panel has appropriate expertise and clinical experience (Goodman, 1987). As geriatric oncology is considered to be a specialised area, selection of this expert panel was well considered, and includes contributions from a wide range of experts. Overall, during the consultation process, attrition rates were low, ensuring the validity of the final results (Hasson et al., 2000, Lopez, 2003).
The first task of the expert panel was definition of an age cutoff for routine referral for GA. An age cutoff for older adults with cancer is difficult to define due to the considerable heterogeneity in the ageing process. Some organisations, such as SIOG (Wildiers et al., 2014) and the EORTC (Pallis et al., 2010) use an age cut-off of 70, others use 65. The European Medicines Agency (Great Britain. Medicines Control, 1993) considers 65 years of age as a cut-off for the definition of “old”, from a regulatory perspective. In the current study, consensus was finally reached in round 4 that all patients over the age of 70, and those who are younger with age related issues or concerns, should be referred for geriatric assessment. In the final round the overall level of agreement was good (W=0.452, p<0.001).The expert panel may have been reluctant to provide an age cut-off in previous rounds, as it contradicts the basic principle on which geriatric medicine is founded i.e. definition of physiological age, rather than chronological age. In the words of one participant, “it is pragmatic to choose an age above which the incidence of issues is high enough for a routine policy, but this should not preclude the younger patients being assessed. To some degree the choice of age should reflect local patterns of age related problems.” This comment is in line with current SIOG recommendations (Wildiers et al., 2014), which may have biased the results, given the relatively large proportion of SIOG affiliated members.
Consensus was not reached on the use of a shorter screening tool that would identify those patients from an oncology clinical practice who could potentially benefit from GA, versus those who would not. However, there was consensus among the SIOG panel in relation to the VES-13, although this was ranked the lowest of the three options presented in the final round. It may suggest suitability in the absence of suitable alternatives, and reflects the literature in this area which has yet to reveal a tool sufficiently sensitive and specific enough for use in oncology (Hamaker et al., 2012). A GA is time-consuming and resource intensive, which is one of the recognised barriers in the more widespread implementation of geriatric oncology. To mitigate this, a number of studies have been conducted, focussing on screening tools that may be used to distinguish fit older patients who are able to tolerate standard treatment versus those who may be considered more vulnerable or frail (Rodin and Mohile, 2007, Luce et al., 2012, Bellera et al., 2012, Huisman et al., 2014) The majority of the expert panel felt that screening should be implemented, but were divided approximately 50:50 between those who would recommend a particular screening tool, versus those who could not identify an appropriate choice. In a recent systematic review (Hamaker et al., 2012), Hamaker and colleagues concluded that none of the currently available frailty screening methods have sufficient sensitivity or specificity for predicting outcome on GA. Many of the screening tools included in the Hamaker review were rated by the expert panel, who failed to reach consensus. While the pursuit of a shorter screening tool is worthwhile, especially for centres lacking dedicated geriatric oncology services, its investigation may be premature in some respects. Many of the current screening tools are broadly based on one or more domains of GA e.g. the G8 is mainly based on nutritional status, while the VES-13 is based on functional status. Greater knowledge of the impact of these individual domains on patient outcomes in oncology is needed for various patient groups and endpoints of interest.
The lack of consensus regarding which domains to be included in a GA, and what assessments and interventions should be used, was identified as one of the main barriers to advancing the field of geriatric oncology at the current time (Puts et al., 2012). This Delphi study aimed to address that with the rating of all domains identified by the expert panel as relevant, and selection of appropriate assessment tools. Consensus was reached on all GA assessments and interventions considered to be important, apart from polypharmacy assessment, with significant agreement achieved, and no individual differences between the professional subgroups. It could be argued that continuation of the study to a fifth round may have secured consensus for items such as polypharmacy, or use of a screening tool. There are no guidelines in relation to the optimal number of Delphi rounds that should be employed in a study of this kind, but generally four is a maximum (Boulkedid et al., 2011). It is advised to exercise caution with excessive rounds, at the expense of expert panel attrition (Hasson et al., 2000, Linstone and Turoff, 1975). Due to the repetitive nature of this study, and the substantial time demands required, it was deemed appropriate to only use four rounds, in order to minimise respondent fatigue. Other studies have used a modified Delphi approach, with the integration of a face-to-face meeting, with subsequent ranked rounds. As a multinational expert panel was employed in this study, this was not feasible. However, there are also recognisiable limitations to face-to-face meetings, due to the dominance of certain individuals (Murphy et al., 1998), different personalities (Jairath and Weinstein, 1994), as well as time limitations. The Delphi method affords other advantages such as anonymity (Rowe et al., 1991), democracy (Butterworth and Bishop, 1995) and structured conformity (Goodman, 1987). A comparison of both the Delphi method and the nominal group technique highlighted greater consensus and depth of understanding for the latter, but much higher reliability for the Delphi method (Hutchings et al., 2006). This reliability can be further enhanced by the use of appropriate, standard feedback (Campbell et al., 1999) as well as multiple professional groups, both illustrated in this study, where subset analysis was used as appropriate.
A number of “voting” methods were used in the current study, depending on the type of information sought e.g. yes/no responses, versus ordinal scales, with different definitions of consensus applied. This may also have affected our inability to reach agreement on some items, however it must be acknowledged that dissensus is equally meaningful(von der Gracht, 2012). Defining consensus is one of the most contentious aspects of the Delphi method, and its measurement varies greatly in the literature (Rayens and Hahn, 2000, Crisp et al., 1997). The more stringent the criteria, the more difficult it is to achieve consensus among the expert panel, while less stringent criteria can also limit the meaningfulness of the consultation process. In addition to measuring consensus, it is also important to measure the relative strength and stability of that agreement, for which Kendall's W(Schmidt, 1997) may be used, as calculated in this study.
The final assessment and intervention algorithm may be considered a minimum dataset, but importantly, it is not all-inclusive. There are additional domains that would greatly benefit patients from a holistic care perspective, if time and resources permitted e.g. spiritual care, sexuality issues, quality of life, amongst others. The EORTC Elderly Task Force (ETF) has previously established an Elderly Minimal Dataset (MinDS) with the proposed aim of harmonisation of data collection with regard to geriatric oncology studies. This included four elements, the Instrumental Activities of Daily Living (IADL), Charlson Comorbidity Index (CCI), G8 Geriatric Assessment Screening Tool (which includes a set of questions from the MNA) and social status. Apart from the G8, all of these have been selected by the expert panel, in addition to the following: Activities of Daily Living (ADLs), Mini Mental State Examination (MMSE), Timed Up and Go test (TUG), Mini Nutritional Assessment (MNA) and psychological assessment using patient interview and the Geriatric Depression Scale (GDS). The scope of a GA will therefore be broader than the EORTC's MinDs.
In relation to the relative importance of each domain, functional status was rated as the most important, followed by comorbidities and cognition, which is in agreement with the current literature. This is reflected by the literature to date (Ward et al., 2014, Soubeyran et al., 2012, Peel et al., 2013, Hermosillo-Rodriguez et al., 2013). However, lower ranked domains, such as psychological status are also important. Studies suggest that older age may not predispose to increased anxiety levels in cancer patients, but may be associated with higher rates of depression (Nelson et al., 2009). Depressive symptoms have been associated with poorer outcomes (Roth and Modi, 2003, Katon et al., 2007), and even a higher suicide risk (Llorente et al., 2005) Further studies are needed to examine the impact of psychological distress and outcomes of older cancer patients. Polypharmacy was also rated lower than other domains, even though it has been identified as a significant cause of adverse drug events, greater hospital admission rates, reduced quality of life and increased falls risk in older patients in the acute care setting (Leipzig et al., 1999b, Leipzig et al., 1999a, McMahon et al., 2013). However, there is little data to date regarding polypharmacy and its potential effects in cancer patients. Shedding light on this little known area, Maggiore et al (Maggiore et al., 2014), in a recent study of 500 patients, found that polypharmacy and potentially inappropriate medication use were common in older adults with cancer, but not associated with additional morbidity or hospitalisation.
Interventions that were identified for deficits in each domain underline the importance of multidisciplinary team collaboration, particularly close collaborative links with the geriatric medicine team. U.S. based geriatric oncologists similarly reached consensus on multidisciplinary input to design interventions for older adults (Mohile et al., 2013) and future research efforts will compare and contrast international perspectives. While employment of a geriatrician dedicated to oncology patients is highly desirable, this isn't always feasible. However, the results of this Delphi study highlight the importance of having a geriatrician participate in the care of older patients with cancer and thus incorporating geriatricians into multidisciplinary oncology care should be the ultimate aim of every organisation.
Limitations
Given the aforementioned absence of high quality studies to date (Puts et al., 2012), the use of a Delphi panel is justified. However, bias is an inherent risk in such an approach (Hasson et al., 2000). This may be overcome to a certain extent by the adoption of a heterogeneous panel (Duffield, 1993, Murphy et al., 1998), such as the four groups consulted here. Each group added a valuable perspective for clinical practice, while simultaneously benefitting from the opinions of others. Another important limitation of this process is that new relevant data may have been published subsequent to the Delphi study. In addition, response rate was low, which may also influence the results.
The panel was mainly European-based, as a similar study was conducted in the US concurrently (Mohile et al., 2013). The results may therefore represent a bias towards European practice.
This study did not include a face-to-face meeting, as per the modified Delphi approach often employed in guideline development. However, our approach avoids the disadvantages inherent in group processes, where one panellist might dominate discussions and may unduly influence consensus (Boulkedid et al., 2011), as previously discussed.
While panellists agreed on the assessments and interventions that are important in oncology, the subsequent usefulness of the information provided depends on the individual organisation. Resources are a key concern, hence the desire to find a shorter screening tool to avoid lengthy consultations. Collaboration with geriatric medicine colleagues is essential, and employment of at least one dedicated geriatrician for oncology should be a primary aim for every oncology department. However, several members of the expert panel have alluded to the shortage of geriatricians for this purpose, and there is a known shortage of geriatricians worldwide. It must also be acknowledged that decision making in oncology is inherently complex, and that complexity could not be captured in a study of this kind. A more detailed analysis of decision making in older adults warrants further investigation under more controlled, site-specific conditions.
Conclusion
In the absence of evidence-based guidelines, this Delphi expert consensus on geriatric oncology design and implementation provides a useful template for clinicians regarding multidimensional assessment of older patients with cancer. This Delphi consensus study is part of a broader programme of research. More data is needed to clarify the clinical efficacy of this approach. GA as a model of care for patients with cancer is currently under investigation, and will contribute to the development of existing guidelines and practices. In addition, as highlighted previously (Puts et al., 2012), the instruments that have been selected as part of this Delphi process were validated in the geriatric medicine setting, although their psychometric properties have yet to be established in oncology
In the absence of level 1evidence for the benefits of geriatric assessment in oncology, one should still endeavor to incorporate its principle components into clinical practice. There is a wealth of evidence for its benefits in the non-oncologic setting. These outcomes and the provision of a more holistic approach to the care of older patients should be a key pursuit in cancer care.
Acknowledgments
The authors would like to thank all experts who participated in this study for their time, dedication and considered opinions.
Geriatric Medicine (Ireland)
Dr. Josie Clare, Consultant Geriatrician, Cork University Hospital, Cork
Dr. Suzanne Timmons, Geriatrician and Senior Lecturer Centre for Gerontology and Rehabilitation, School of Medicine, University College Cork
Prof William Molloy, Centre for Gerontology and Rehabilitation, School of Medicine, University College Cork
Dr. Eithne Harkin, Consultant Geriatrician, Wexford General hospital
Dr. David Robinson, Consultant Physician in Geriatric & General (Internal) Medicine and Clinical Lecturer in Medical Gerontology, Trinity College Dublin
Dr. John Gerard Doherty, Consultant Geriatrician, Sligo General hospital
Dr. Conal Cunningham, Clinical Director, Medicine for the Elderly Directorate (MedEL), Consultant Geriatrician, Medicine for the Elderly, Clinical Senior Lecturer, Department of Medical Gerontology, Trinity College Dublin
Dr. Paula Hickey, Consultant Geriatrician, Sligo General Hospital
Dr. Emer Ahern, Consultant Geriatrician, St Luke's General Hospital, Kilkenny
Dr. Graham Hughes, Consultant Geriatrician, St Vincent's University Hospital, Dublin
Medical Oncology (Ireland)
Dr. Brian Bird, Consultant Medical Oncologist, Bon Secours Hospital, Cork
Dr. Conleth Murphy, Consultant Medical Oncologist, Bon Secours Hospital, Cork
Dr. Dearbhaile O Donnell, Consultant Medical Oncologist, St. James's Hospital, Dublin
Dr. Gregory Leonard, Consultant Medical Oncologist, University College Hospital Galway (UCHG), Galway
Dr. Ray McDermott, Consultant Medical Oncologist, St Vincent's University Hospital, Dublin
Dr. Eugene Moylan, Consultant Medical Oncologist, formerly Cork University Hospital, Cork *
Radiation Oncology (Ireland)
Dr. Moya Cunningham, Consultant Radiation Oncologist, Saint Luke's Radiation Oncology Network, Dublin
Dr. Aileen Flavin, Consultant Radiation Oncologist, Cork University Hospital, Cork
Dr. David Fitzpatrick, Consultant Radiation Oncologist, Saint Luke's Radiation Oncology Network, Dublin
Dr. Sinead Brennan, Consultant Radiation Oncologist, Saint Luke's Radiation Oncology Network, Dublin
Dr. Brian O'Neill, Consultant Radiation Oncologist, Saint Luke's Radiation Oncology Network, Dublin
Dr. Charles Gillham, Consultant Radiation Oncologist, Saint Luke's Radiation Oncology Network, Dublin
Dr. Goran Bjelkengren, Consultant Radiation Oncologist, Cork University Hospital, Cork
Dr. Dayle Hacking, Consultant Radiation Oncologist, UPMC Whitfield Cancer Centre, Waterford
SIOG Panel
Prof Kerri Clough-Gorr, Institute of Social & Preventive Medicine University of Bern, Switzerland
Professor Hans Wildiers, Belgium, Medical Oncologist and Chairman of the elderly task force of the European Organization of Research and Treatment of Cancer (EORTC)
Dr. M.E.Hamaker, Geriatrician, Diakonessenhuis, Utrecht, Netherlands
Dr. Eduard Maartense, Medical Oncologist, Reinier de Graaf , Netherlands
Dr. MJ Molina-Garrido, Head of the Unit of Cancer in the Elderly. Medical Oncology Department, Hospital General Virgen de la Luz, Cuenca, Spain
Dr. Alistair E. Ring, Consultant Medical Oncologist, Royal Marsden NHS Foundation Trust, UK
Dr. Regina Gironés Sarrió, Medical Oncology Unit, Hospital Lluís Alcanyís, Xàtiva, Valencia, Spain.
Dr. Ravindran Kanesvaran, Medical Oncologist and President Singapore Society of Oncology, Singapore
Dr. Siri Rostoft, MD, PhD, Geriatrician/Senior Physician, Department of Geriatric Medicine, Oslo University Hospital, Norway
Dr. Etienne Brain, Consultant Medical Oncologist and Incoming President International Society of Geriatric Oncology, France
Dr. Laura Biganzoli, MD, Medical Oncologist, Medical Oncology Dept., Nuovo Ospedale di Prato, Istituto Toscano Tumori, Prato, Italy
Dr. Catherine Terret, Medical oncologist; Head of the Coordination Unit of Geriatric Oncology of the Rhone Department of Medical Oncology, Lyon, France
Dr. Christopher Steer, Medical Oncologist and chair of the geriatric oncology interest group of the Clinical Oncological Society of Australia (COSA), Australia
Dr. Ulrich Wedding, Medical Oncologist and Treasurer of the EORTC Cancer in the Elderly Task Force, Germany
Dr. Bernard Marty Chantal, Medical Oncologist, ONCORAD Clinique Pasteur, Toulouse, France
Dr. Athanasios Karampeazis, Medical Oncologist, Athens, Greece
Dr. Emmanuel Mitry, Institut Curie Hopital Rene Huguenin, Saint-cloud, France
Professor Margot Ann Gosney, Director, Professor of Elderly Care Medicine, University of Reading/Royal Berkshire NHS Foundation Trust, UK
Dr. Theodora Karnakis, Department of Geriatrics and Oncology, Sao Paolo, Brazil
Dr. Anne Horgan, Consultant Medical Oncologist, Waterford Regional Hospital, Ireland
Footnotes
We have no disclosures of interest to make.
Now resident in Australia
Contributor Information
A. O'Donovan, Email: anita.odonovan@tcd.ie.
S.G. Mohile, Email: Supriya_Mohile@URMC.Rochester.edu.
M. Leech, Email: leechm@tcd.ie.
References
- Aliamus V, Adam C, Druet-Cabanac M, Dantoine T, Vergnenegre A. Impact de l'évaluation gériatrique sur la décision de traitement en oncologie thoracique. Revue des Maladies Respiratoires. 2011;28:1124–1130. doi: 10.1016/j.rmr.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Aparicio T, Girard L, Bouarioua N, Patry C, Legrain S, Soule JC. A mini geriatric assessment helps treatment decision in elderly patients with digestive cancer. A pilot study. Crit Rev Oncol Hematol. 2011;77:63–9. doi: 10.1016/j.critrevonc.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Aparicio T, Jouve JL, Teillet L, Gargot D, Subtil F, Le Brun-Ly V, Cretin J, Locher C, Bouché O, Breysacher G, Charneau J, Seitz JF, Gasmi M, Stefani L, Ramdani M, Lecomte T, Mitry E. Geriatric Factors Predict Chemotherapy Feasibility: Ancillary Results of FFCD 2001-02 Phase III Study in First-Line Chemotherapy for Metastatic Colorectal Cancer in Elderly Patients. Journal of Clinical Oncology. 2013;31:1464–1470. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- Beckett P, Callister M, Tata LJ, Harrison R, Peake MD, Stanley R, Woolhouse I, Slade M, Hubbard RB. Clinical management of older people with non-small cell lung cancer in England. Thorax. 2012;67:836–9. doi: 10.1136/thoraxjnl-2011-200994. [DOI] [PubMed] [Google Scholar]
- Bellera CA, Rainfray M, Mathoulin-Pélissier S, Mertens C, Delva F, Fonck M, Soubeyran PL. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Annals of Oncology. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6:e20476. doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth T, Bishop V. Identifying the characteristics of optimum practice: findings from a survey of practice experts in nursing, midwifery and health visiting. J Adv Nurs. 1995;22:24–32. doi: 10.1046/j.1365-2648.1995.22010024.x. [DOI] [PubMed] [Google Scholar]
- Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, Bastuji-Garin S, Culine S, Paillaud E. Comprehensive Geriatric Assessment in the Decision-Making Process in Elderly Patients With Cancer: ELCAPA Study. Journal of Clinical Oncology. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- Campbell SM, Hann M, Roland MO, Quayle JA, Shekelle PG. The effect of panel membership and feedback on ratings in a two-round Delphi survey: results of a randomized controlled trial. Med Care. 1999;37:964–8. doi: 10.1097/00005650-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Clough-Gorr KM, Silliman RA. Translation Requires Evidence: Does Cancer-Specific CGA Lead to Better Care and Outcomes? Oncology (Williston Park) 2008;22:925–928. [PMC free article] [PubMed] [Google Scholar]
- Crisp J, Pelletier D, Duffield C, Adams A, Nagy S. The Delphi method? Nurs Res. 1997;46:116–8. doi: 10.1097/00006199-199703000-00010. [DOI] [PubMed] [Google Scholar]
- Dajani JS, Sincoff MZ, Talley WK. Stability and agreement criteria for the termination of Delphi studies. Technological Forecasting and Social Change. 1979;13:83–90. [Google Scholar]
- De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, Bielska-Lasota M, Engholm G, Nennecke A, Siesling S, Berrino F, Capocaccia R, Group, E. W. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncology. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- De Vet E, Brug J, De Nooijer J, Dijkstra A, De Vries NK. Determinants of forward stage transitions: a Delphi study. Health Education Research. 2005;20:195–205. doi: 10.1093/her/cyg111. [DOI] [PubMed] [Google Scholar]
- Decoster L, Kenis C, Van Puyvelde K, Flamaing J, Conings G, De Grève J, Mets T, Milisen K, Lobelle JP, Wildiers H. The influence of clinical assessment (including age) and geriatric assessment on treatment decisions in older patients with cancer. Journal of Geriatric Oncology. 2013;4:235–241. doi: 10.1016/j.jgo.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Diviani N, Schulz PJ. What should laypersons know about cancer? Towards an operational definition of cancer literacy. Patient Educ Couns. 2011;85:487–92. doi: 10.1016/j.pec.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Department of Health (England) Department of Health; 2012. [Accessed 11/10/14]. The impact of patient age on decision making in oncology. Online. Available at: www.gov.uk/government/publications/the-impact-of-patient-age-on-clinical-decision-making-in-oncology. [Google Scholar]
- Duffield C. The Delphi technique: a comparison of results obtained using two expert panels. International Journal of Nursing Studies. 1993;30:227–237. doi: 10.1016/0020-7489(93)90033-q. [DOI] [PubMed] [Google Scholar]
- Ellis G, Whitehead MA, Robinson D, O'Neill D, Langhorne P. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. Bmj. 2011;343:d6553. doi: 10.1136/bmj.d6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, Lichtman S, Mor V, Monfardini S, Repetto L, Sorbye L, Topinkova E, Task Force on, C. G. A. O. T. I. S. O. G. O. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG) Crit Rev Oncol Hematol. 2005;55:241–52. doi: 10.1016/j.critrevonc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, Defelice J, Levine RM, Lubiner ET, Reyes P, Schreiber FJ, 3rd, Balducci L. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–86. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, Macdonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- Fried TR, Bradley EH, Towle VR, Allore H. Understanding the Treatment Preferences of Seriously Ill Patients. New England Journal of Medicine. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- Girones R, Torregrosa D, Gomez-Codina J, Maestu I, Tenias JM, Rosell R. Prognostic impact of comorbidity in elderly lung cancer patients: use and comparison of two scores. Lung Cancer. 2011;72:108–13. doi: 10.1016/j.lungcan.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Goodman CM. The Delphi technique: a critique. Journal of Advanced Nursing. 1987;12:729–734. doi: 10.1111/j.1365-2648.1987.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Great Britain. Medicines Control, A. Studies in support of special populations : geriatrics (ICH) Great Britain: Medicines Control Agency; 1993. [Google Scholar]
- Hamaker ME, Buurman BM, Van Munster BC, Kuper I, Smorenburg CH, De Rooij SE. The Value of a Comprehensive Geriatric Assessment for Patient Care in Acutely Hospitalized Older Patients with Cancer. Oncologist. 2011;16:1403–1412. doi: 10.1634/theoncologist.2010-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaker ME, Jonker JM, De Rooij SE, Vos AG, Smorenburg CH, Van Munster BC. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012;13:e437–e444. doi: 10.1016/S1470-2045(12)70259-0. [DOI] [PubMed] [Google Scholar]
- Hasson F, Keeney S, Mckenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- Hermosillo-Rodriguez J, Anaya DA, Sada Y, Walder A, Amspoker AB, Berger DH, Naik AD. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. Journal of Geriatric Oncology. 2013 doi: 10.1016/j.jgo.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Horgan AM, Leighl NB, Coate L, Liu G, Palepu P, Knox JJ, Perera N, Emami M, Alibhai SM. Impact and feasibility of a comprehensive geriatric assessment in the oncology setting: a pilot study. Am J Clin Oncol. 2012;35:322–8. doi: 10.1097/COC.0b013e318210f9ce. [DOI] [PubMed] [Google Scholar]
- Hubbard J, Jatoi A. Adjuvant chemotherapy in colon cancer: ageism or appropriate care? J Clin Oncol. 2011;29:3209–10. doi: 10.1200/JCO.2011.35.8630. [DOI] [PubMed] [Google Scholar]
- Huisman MG, Van Leeuwen BL, Ugolini G, Montroni I, Spiliotis J, Stabilini C, Carino ND, Farinella E, De Bock GH, Audisio RA. “Timed up & go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One. 2014;9:e86863. doi: 10.1371/journal.pone.0086863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Bhatia S, Katheria V, Klapper S, Hansen K, Ramani R, Lachs M, Wong FL, Tew WP. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of Clinical Oncology. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A, Wildes T, Blair SL, Browner IS, Cohen HJ, Deshazo M, Dotan E, Edil BH, Extermann M, Ganti AK, Holmes HM, Jagsi R, Karlekar MB, Keating NL, Korc-Grodzicki B, McKoy JM, Medeiros BC, Mrozek E, O'Connor T, Rugo HS, Rupper RW, Silliman RA, Stirewalt DL, Tew WP, Walter LC, Weir AB, 3rd, Bergman MA, Sundar H. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12:82–126. doi: 10.6004/jnccn.2014.0009. [DOI] [PubMed] [Google Scholar]
- Hutchings A, Raine R, Sanderson C, Black N. A comparison of formal consensus methods used for developing clinical guidelines. J Health Serv Res Policy. 2006;11:218–24. doi: 10.1258/135581906778476553. [DOI] [PubMed] [Google Scholar]
- Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- Jairath N, Weinstein J. The Delphi methodology (Part one): A useful administrative approach. Can J Nurs Adm. 1994;7:29–42. [PubMed] [Google Scholar]
- Jatoi A, Hillman S, Stella P, Green E, Adjei A, Nair S, Perez E, Amin B, Schild SE, Castillo R, Jett JR. Should elderly non-small-cell lung cancer patients be offered elderly-specific trials? Results of a pooled analysis from the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:9113–9. doi: 10.1200/JCO.2005.03.7465. [DOI] [PubMed] [Google Scholar]
- Jones J, Hunter D. Qualitative Research: Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–7. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- Katon W, Lin EHB, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. General Hospital Psychiatry. 2007;29:147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Kenis C, Bron D, Libert Y, Decoster L, Van Puyvelde K, Scalliet P, Cornette P, Pepersack T, Luce S, Langenaeken C, Rasschaert M, Allepaerts S, Van Rijswijk R, Milisen K, Flamaing J, Lobelle JP, Wildiers H. Relevance of a systematic geriatric screening and assessment in older patients with cancer: results of a prospective multicentric study. Annals of Oncology. 2013;24:1306–1312. doi: 10.1093/annonc/mds619. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim YJ, Lee KW, Chang H, Lee JO, Kim KI, Bang SM, Lee JS, Kim CH, Kim JH. The early discontinuation of palliative chemotherapy in older patients with cancer. Support Care Cancer. 2014;22:773–81. doi: 10.1007/s00520-013-2033-y. [DOI] [PubMed] [Google Scholar]
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999a;47:30–9. doi: 10.1111/j.1532-5415.1999.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999b;47:40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Levit L, Balogh E, Nass S, Pa G. National Academies Press; 2013. [Accessed 11/10/14]. Delivering high-quality cancer care: charting a new course for a system in crisis. Online. Available at: www.nap.edu/catalog.php?record_id=18359. [PubMed] [Google Scholar]
- Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, Housman MG, Escarce JJ. Participation of Patients 65 Years of Age or Older in Cancer Clinical Trials. Journal of Clinical Oncology. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Linstone HA, Turoff M. The Delphi method. Addison-Wesley; 1975. S.l. [Google Scholar]
- Llorente MD, Burke M, Gregory GR, Bosworth HB, Grambow SC, Horner RD, Golden A, Olsen EJ. Prostate cancer: a significant risk factor for late-life suicide. Am J Geriatr Psychiatry. 2005;13:195–201. doi: 10.1176/appi.ajgp.13.3.195. [DOI] [PubMed] [Google Scholar]
- Lopez V. Critical care nursing research priorities in Hong Kong. Journal of Advanced Nursing. 2003;43:578–587. doi: 10.1046/j.1365-2648.2003.02756.x. [DOI] [PubMed] [Google Scholar]
- Luce S, De Breucker S, Van Gossum A, Demols A, Mekinda Z, Ena G, Kentos A, Roumeguére T, Ghanooni R, Nouwynck C, Van Laethem JL, Sokolow Y, Simon P, Bailly B, Vervaet C, Marchand M, Pepersack T. How to identify older patients with cancer who should benefit from comprehensive geriatric assessment? Journal of Geriatric Oncology 2012 [Google Scholar]
- Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One. 2011;6:e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiore RJ, Dale W, Gross CP, Feng T, Tew WP, Mohile SG, Owusu C, Klepin HD, Lichtman SM, Gajra A, Ramani R, Katheria V, Zavala L, Hurria A, The, C. & Aging Research, G. Polypharmacy and Potentially Inappropriate Medication Use in Older Adults with Cancer Undergoing Chemotherapy: Effect on Chemotherapy-Related Toxicity and Hospitalization During Treatment. Journal of the American Geriatrics Society. 2014;62:1505–1512. doi: 10.1111/jgs.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon CG, Cahir CA, Kenny RA, Bennett K. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age and Ageing. 2013;43:44–50. doi: 10.1093/ageing/aft114. [DOI] [PubMed] [Google Scholar]
- Mohile S, Hurria A, Velarde C, Magnuson A, O'Donovan A, Dale W. Expert Consensus Panel on Geriatric Assessment Interventions in Oncology: the US Perspective. International Society of Geriatric Oncology Annual Conference; Copenhagen, Denmark. 2013. [Google Scholar]
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, Marteau T. Consensus development methods, and their use in clinical guideline development. Health Technol Assess. 1998;2:i–iv. 1–88. [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–6. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Weinberger MI, Balk E, Holland J, Breitbart W, Roth AJ. The Chronology of Distress, Anxiety, and Depression in Older Prostate Cancer Patients. Oncologist. 2009;14:891–899. doi: 10.1634/theoncologist.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, Lacombe D, Monfardini S, Scalliet P, Wildiers H. EORTC elderly task force position paper: Approach to the older cancer patient. European Journal of Cancer. 2010;46:1502–1513. doi: 10.1016/j.ejca.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Pallis AG, Ring A, Fortpied C, Penninckx B, Van Nes MC, Wedding U, Vonminckwitz G, Johnson CD, Wyld L, Timmer-Bonte A, Bonnetain F, Repetto L, Aapro M, Luciani A, Wildiers H. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol. 2011;22:1922–6. doi: 10.1093/annonc/mdq687. [DOI] [PubMed] [Google Scholar]
- Peake MD, Thompson S, Lowe D, Pearson MG, Centres, O. B. O. T. P. Ageism in the management of lung cancer. Age and Ageing. 2003;32:171–177. doi: 10.1093/ageing/32.2.171. [DOI] [PubMed] [Google Scholar]
- Peel NM, Kuys SS, Klein K. Gait Speed as a Measure in Geriatric Assessment in Clinical Settings: A Systematic Review. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2013;68:39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
- Pottel L, Lycke M, Boterberg T, Pottel H, Goethals L, Duprez F, Van Den Noortgate N, De Neve W, Rottey S, Geldhof K, Buyse V, Kargar-Samani K, Ghekiere V, Debruyne PR. Serial comprehensive geriatric assessment in elderly head and neck cancer patients undergoing curative radiotherapy identifies evolution of multidimensional health problems and is indicative of quality of life. Eur J Cancer Care (Engl) 2014;23:401–12. doi: 10.1111/ecc.12179. [DOI] [PubMed] [Google Scholar]
- Puts MT, Hardt J, Monette J, Girre V, Springall E, Alibhai SM. Use of geriatric assessment for older adults in the oncology setting: a systematic review. J Natl Cancer Inst. 2012;104:1133–63. doi: 10.1093/jnci/djs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts MTE, Tu HA, Tourangeau A, Howell D, Fitch M, Springall E, Alibhai SMH. Factors influencing adherence to cancer treatment in older adults with cancer: a systematic review. Annals of Oncology. 2014;25:564–577. doi: 10.1093/annonc/mdt433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayens MK, Hahn EJ. Building Consensus Using the Policy Delphi Method. Policy, Politics, & Nursing Practice. 2000;1:308–315. [Google Scholar]
- Rodin MB, Mohile SG. A Practical Approach to Geriatric Assessment in Oncology. Journal of Clinical Oncology. 2007;25:1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- Roth AJ, Modi R. Psychiatric issues in older cancer patients. Crit Rev Oncol Hematol. 2003;48:185–97. doi: 10.1016/j.critrevonc.2003.06.004. [DOI] [PubMed] [Google Scholar]
- Rowe G, Wright G, Bolger F. Delphi: A reevaluation of research and theory. Technological Forecasting and Social Change. 1991;39:235–251. [Google Scholar]
- Rubenstein LZ, Siu AL, Wieland D. Comprehensive geriatric assessment: toward understanding its efficacy. Aging (Milano) 1989;1:87–98. doi: 10.1007/BF03323881. [DOI] [PubMed] [Google Scholar]
- Schmidt RC. Managing Delphi Surveys Using Nonparametric Statistical Techniques. Decision Sciences. 1997;28:763–774. [Google Scholar]
- Shin DY, Lee JO, Kim YJ, Park MS, Lee KW, Kim KI, Bang SM, Lee JS, Kim CH, Kim JH. Toxicities and functional consequences of systemic chemotherapy in elderly Korean patients with cancer: A prospective cohort study using Comprehensive Geriatric Assessment. Journal of Geriatric Oncology. 2012;3:359–367. [Google Scholar]
- Simon ST, Weingärtner V, Higginson IJ, Voltz R, Bausewein C. Definition, Categorization, and Terminology of Episodic Breathlessness: Consensus by an International Delphi Survey. Journal of Pain and Symptom Management. 2014;47:828–838. doi: 10.1016/j.jpainsymman.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Soubeyran P, Fonck M, Blanc-Bisson C, Blanc JF, Ceccaldi J, Mertens C, Imbert Y, Cany L, Vogt L, Dauba J, Andriamampionona F, Houede N, Floquet A, Chomy F, Brouste V, Ravaud A, Bellera C, Rainfray M. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–34. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- Spyropoulou D, Pallis AG, Leotsinidis M, Kardamakis D. Completion of radiotherapy is associated with the Vulnerable Elders Survey-13 score in elderly patients with cancer. J Geriatr Oncol. 2014;5:20–5. doi: 10.1016/j.jgo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: A meta-analysis of controlled trials. The Lancet. 1993;342:1032–6. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- Talarico L, Chen G, Pazdur R. Enrollment of Elderly Patients in Clinical Trials for Cancer Drug Registration: A 7-Year Experience by the US Food and Drug Administration. Journal of Clinical Oncology. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- Uphoff EP, Wennekes L, Punt CJ, Grol RP, Wollersheim HC, Hermens RP, Ottevanger PB. Development of generic quality indicators for patient-centered cancer care by using a RAND modified Delphi method. Cancer Nurs. 2012;35:29–37. doi: 10.1097/NCC.0b013e318210e3a2. [DOI] [PubMed] [Google Scholar]
- Von Der Gracht HA. Consensus measurement in Delphi studies: Review and implications for future quality assurance. Technological Forecasting and Social Change. 2012;79:1525–1536. [Google Scholar]
- Ward P, Wang HJ, Dichmann R, Liem AK, Chan D, Patel R, Hu EH, Tchekmedyian NS, Wainberg ZA, Hecht JR, Naeim A. Physical function and quality of life in frail and/or elderly patients with metastatic colorectal cancer treated with capecitabine and bevacizumab: an exploratory analysis. J Geriatr Oncol. 2014 doi: 10.1016/j.jgo.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen MLG, Extermann M, Falandry C, Artz A, Brain E, Colloca G, Flamaing J, Karnakis T, Kenis C, Audisio RA, Mohile S, Repetto L, Van Leeuwen B, Milisen K, Hurria A. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. Journal of Clinical Oncology. 2014;32:2595–603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen SB, Cella DF, Leslie WT. Age and clinical decision making in oncology patients. Journal of the National Cancer Institute. 1994;86:1766–1770. doi: 10.1093/jnci/86.23.1766. [DOI] [PubMed] [Google Scholar]
- Yeung AR, Garg MK, Lawson J, McDonald MW, Quon H, Ridge JA, Saba N, Salama JK, Smith RV, Yom SS, Beitler JJ. ACR Appropriateness Criteria(R) ipsilateral radiation for squamous cell carcinoma of the tonsil. Head Neck. 2012;34:613–6. doi: 10.1002/hed.21993. [DOI] [PubMed] [Google Scholar]
- Zulman D, Sussman J, Chen X, Cigolle C, Blaum C, Hayward R. Examining the Evidence: A Systematic Review of the Inclusion and Analysis of Older Adults in Randomized Controlled Trials. Journal of General Internal Medicine. 2011;26:783–790. doi: 10.1007/s11606-010-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


