Abstract
Background
Enteral access placement is performed among a variety of providers and specialties, yet there is a dearth of literature on trends and factors related to enteral access placement in the United States.
Objective
To examine trends in the incidence of enteral access procedures performed by gastroenterologists in the United States.
Design
Retrospective review of upper endoscopic procedures that involved percutaneous endoscopic gastrostomy (PEG) tube placement between 2000 and 2010 was performed.
Setting
Endoscopy sites participating in the Clinical Outcomes Research Initiative (CORI).
Patients
Patients undergoing an upper endoscopy.
Intervention
PEG tube placement.
Main Outcome Measurements
Number of PEG tubes placed.
Results
Overall PEG tube placement by a provider from 2000-2010 was 1.7% (number of PEG tubes performed/number of upper endoscopies performed) with the majority of them being performed by gastroenterologists. Very young and very old, non-white racial background (Hispanic OR 1.21; 95% CI, 1.13-1.28; blacks OR 2.24; 95% CI, 2.12-2.36) and males (OR 1.44; 95% CI, 1.39-1.50) were patient characteristics associated with greater PEG tube placement. In terms of practice setting, PEG tube placement occurred more frequently in a community/HMO environment and the East Coast. With respect to provider characteristics, male providers were less likely than females to perform a PEG tube (OR, 0.67; 95% CI, 0.64-0.71) and there was a trend that as providers were further out of medical school they were less likely to perform a PEG tube procedure. Interestingly, surgeons (OR 6.69; 95% CI, 6.18-7.24) and other providers (non-pediatric/non-general practitioner) (OR 3.22; 95% CI, 2.63-3.94) were more likely to perform PEG tubes than gastroenterologists.
Limitations
Participation in CORI is voluntary and may not capture data on non-GI providers.
Conclusions
Significant practice variation was noted in PEG tube placement in the United States with respect to patient and provider characteristics, geographic region and endoscopy settings.
Keywords: percutaneous endoscopic gastrostomy (PEG), endoscopy, health outcomes, quality, practice variation, enteral feeding
Introduction
Enteral access procedures (typically in the form of percutaneous endoscopic gastrostomy, PEG) allows the short and long term option of providing nutrients and medications into the gastrointestinal (GI) tract for patients who cannot maintain adequate oral intake1. Such procedures are performed most frequently by gastroenterologists2, but can also be performed by other providers such as surgeons and interventional radiologists3, and training in enteral access placement is integral to the education of residents and fellows in all of these specialties4.
At the moment, there is a lack of current data on enteral access placement in the United States with significant gaps in the literature. To date only one study has examined trends with respect to enteral access placement. The National Trends in Gastrointestinal Access Procedures Database reported that enteral access placement increased by 1.4% from 1997 to 2000 for Medicare beneficiaries. In this study, gastroenterologists most often performed these procedures (48.6%), followed by surgeons (25.1%) and radiologists (7.4%), but several interesting temporal trends were noted. Over the study time period, enteral access procedures performed by radiologists dramatically increased by 29.6% whereas enteral access procedures performed by gastroenterologists increased minimally by 6.9% with a decrease in surgeons performing these procedures by 4.9%2. To date, this limited decades-old study is the only one focusing on the topic of enteral access placement in the United States with no subsequent interval follow-up conducted. Given that the U.S. population is living longer, with more chronic medical problems that may necessitate the need for enteral access procedures, more current data on the incidence and associated predictors of this procedure are clearly needed.
Consequently, our primary study aim was to examine trends in the incidence of enteral access procedures (specifically PEG tubes) performed by gastroenterologists in the United States over the past 11 years (2000-2010). Our secondary aim was to assess provider, endoscopy setting and patient related factors associated with PEG tube placement in the United States.
Methods
Study Design
We conducted a retrospective electronic medical record review of upper endoscopic procedures that involved percutaneous endoscopic gastrostomy (PEG) tube placement between 2000 and 2010 in the United States using the National Endoscopic Database (NED).
Data Source
Data for the study were abstracted from the Clinical Outcomes Research Initiative (CORI) using NED. CORI was developed to study the outcomes of GI endoscopic procedures across the United States.5 The CORI project began in 1995 under the auspices of the American Society for Gastrointestinal Endoscopy. Physicians participating in the CORI consortium produce GI endoscopy reports using a specialized electronic health record. Data from the reports are automatically sent electronically to a central data repository where they are pooled with data from other consortium participants in the NED. The data transmitted from the local site to the NED do not contain most patient or provider identifiers, and qualify as a Limited Data Set under 45 C.F.R. Section 164.514(e)(2). The data are then tested for completeness and accuracy and merged with data from all the other participating practices and stored in the NED. Practice sites include hospitals, private practices, ambulatory care centers, universities, and Veteran Affairs (VA) hospitals. The NED contains close to 2 million reports. Captured data includes procedure type, patient and endoscopist demographics, procedure indication, sedation used during the procedure, pathology and adverse events. In 2007, the NED received over 250,000 reports from 70 practice sites in 24 states with approximately 400 participating endoscopists.
Data Abstraction
Clinical and demographic data for patients and providers as well as procedure/endoscopy site data was abstracted from the electronic medical records in the NED for all patients who underwent an upper endoscopy from 2000 to 2010. For the study, four key data groups were abstracted. First, patient data abstracted included age, sex and race. Second, procedure data was recorded and included procedure indication and trainee involvement in procedures. Third, endoscopy site data included geographic location, procedure setting (inpatient, outpatient, emergency), and endoscopy site type (academic, community/health maintenance organization, VA/military). Last, provider's sex, race, specialty (gastroenterology, gastroenterology pediatrics, surgery, general practitioner/pediatrician, other), and years since graduating from medical school were also documented. Upper endoscopic procedures were stratified into 2 groups based on whether a patient had received a PEG tube or not.
Statistical Analysis
Baseline demographics on patients, providers, procedure, and endoscopy sites were calculated as means for categorical data and proportions for nominal data. The primary outcome variable for the study was PEG tube placement. For analyses involving the incidence calculation and regression analysis for PEG tube placement only data from stable endoscopy sites were used (endoscopy sites that contributed upper endoscopy data to the study cohort for both years 2000 and 2010). Incidence calculations represented the number of PEG tubes performed/number of upper endoscopies performed during a specified time period. The incidence rate and annual percentage change of PEG tube procedures performed in the United States from 2000 to 2010 was calculated. These results were then stratified by provider type, endoscopy site, geographic region, trainee participation and years since provider graduated medical school.
We then attempted to identify predictors that may be associated with PEG tube placement in the United States. First, a univariate analysis was performed to determine predictors that influenced the placement of a PEG tube during 2000 to 2010. Individual predictors assessed included patient, procedure, endoscopy site and provider characteristics as well as fellow participation. Patient characteristics that were analyzed included age, sex, and race. Procedure indications of anorexia, early satiety, feeding refusal, malabsorption, nausea/vomiting and weight loss were examined in the analysis. For endoscopy site, geographic region and site type were included. Last, provider characteristics such as sex, race, specialty and years since medical school graduation were analyzed. The Student t-test and chi- square tests were used as appropriate to determine which individual predictors were associated with PEG tube placement. Statistically significant results are noted with a p value < 0.05. Second, 2 multivariate logistic regression analyses were performed. The first regression analysis included all individual predictors that were statistically significant from the univariate analysis. In the second regression analysis procedure indication and geographic region were excluded as predictors from the model. Procedure indication was not included in the final model because in the vast majority of cases providers entered one indication (eg, PEG tube placement) rather than symptom based indications. Also, because an exhaustive list of indications was not included in the analysis this predictor was excluded from the second model. Additionally, geographic region was excluded as it was believed that this factor only described regional variations in CORI-participating practices as opposed to such variations in actual GI endoscopy practice as CORI is not set up to be geographically diverse (e.g. some regions have greater participation by academic centers whereas others may have more VA/military participation). Also, the distribution of endoscopy sites was not necessarily matched with the population of various regions. Of note, given that the distribution of patient ages was U-shaped (e.g. high incidence in young and older patients, much lower in young adults and middle-aged patients), all analyses were performed with age as a categorical variable. For both logistic regression analyses the area under the receiver operating characteristic (ROC) curve and the Hosmer and Lemeshow test were used to assess for the multivariate model's sensitivity and goodness of fit, respectively.
Ethical Considerations
This study was approved by the University of California, San Francisco Human Committee on Human Research. The NED was given approval by the IRB of the Oregon Health & Science University (eIRB #7331) in October 2011. This specific study used a limited dataset and was therefore exempted from further IRB review.
Results
Baseline Characteristics
Table 1 highlights the demographic characteristics captured on patients, procedures, endoscopy sites and providers who were included in the study cohort. Mean patient age was 54.5 ± 20.2 years with 71.6% of patients falling in the age range of 40-79 years. The racial background of patients was overwhelmingly white with nearly an equal number of female and males undergoing upper endoscopic procedures. Among upper endoscopies recorded in CORI during the study time period the majority were performed in the outpatient setting (75.4%) and in a community/healthcare maintenance organization (HMO) (70.5%). Of providers who documented their procedures in CORI the vast majority were white male gastroenterologists. Over 68.0% of providers in the study cohort had graduated from medical school in the last 10 to 29 years with a mean of 21.0 + 9.0 years in practice.
Table 1.
Demographic characteristics of patients, procedures, endoscopy sites and providers included in the study.
| Variable | Total (%) (N=824,453) | No PEG placement (%) N=812,226 | PEG placement (%) N=12,227 | p-value |
|---|---|---|---|---|
| Patient | ||||
| Age (years) | 54.5 ± 20.2 | 54.4 ± 20.1 | 62.7 ± 24.3 | <0.001 |
| Infant (<1) | 3,437 (0.4) | 3,141 (0.4) | 296 (2.4) | <0.001 |
| 1-9 | 27,093 (3.3) | 26,435 (3.3) | 658 (5.4) | |
| 10-19 | 38,850 (4.7) | 38,604 (4.8) | 246 (2.0) | |
| 20-29 | 33,515 (4.1) | 33,242 (4.1) | 273 (2.2) | |
| 30-39 | 64,338 (7.8) | 64,020 (7.9) | 318 (2.6) | |
| 40-49 | 122,414 (14.8) | 121,681 (15.0) | 733 (6.0) | |
| 50-59 | 176,514 (21.4) | 174,816 (21.5) | 1,698 (13.9) | |
| 60-69 | 160,836 (19.5) | 158,770 (19.5) | 2,066 (16.9) | |
| 70-79 | 130,991 (15.9) | 128,169 (15.8) | 2,822 (23.1) | |
| 80-89 | 60,590 (7.3) | 58,071 (7.1) | 2,519 (20.6) | |
| ≥90 | 5,875 (0.7) | 5,277 (0.6) | 598 (4.9) | |
| Race/Ethnicity | ||||
| White | 649,601 (78.8) | 64,1645 (79.0) | 7,956 (65.1) | <0.001 |
| Hispanic | 79,510 (9.6) | 78,132 (9.6) | 1,378 (11.3) | |
| Black | 59,409 (7.2) | 57,122 (7.0) | 2,287 (18.7) | |
| Asian/Pacific Islander | 15,528 (1.9) | 15,259 (1.9) | 269 (2.2) | |
| American Indian | 9,382 (1.1) | 9,259 (1.1) | 123 (1.0) | |
| Multiracial | 1,760 (0.2) | 1,718 (0.2) | 42 (0.3) | |
| Other | 654 (0.1) | 645 (0.1) | 9 (0.1) | |
| Unknown | 8,609 (1.0) | 8,446 (1.0) | 163 (1.3) | |
| Sex | ||||
| Female | 411,127 (49.9) | 406,258 (50.0) | 4,869 (39.8) | <0.001 |
| Male | 413,326 (50.1) | 405,968 (50.0) | 7,358 (60.2) | |
| Procedure | ||||
| Indication | ||||
| Nausea/Vomiting | 72,668 (8.8) | 72,527 (8.9) | 141 (1.2) | <0.001 |
| Weight loss | 30,193 (3.7) | 29,465 (3.6) | 728 (6.0) | <0.001 |
| Anorexia | 5,529 (2.0) | 5,285 (0.7) | 244 (2.0) | <0.001 |
| Early satiety | 8,369 (1.0) | 8,338 (1.0) | 31 (0.3) | <0.001 |
| Malabsorption | 1,661 (0.2) | 1,659 (0.2) | 2 (0) | <0.001 |
| Feeding refusal | 1,210 (0.1) | 512 (0.1) | 698 (5.7) | <0.001 |
| Setting | ||||
| Inpatient | 69,053 (8.4) | 63,545 (7.8) | 5,508 (45.0) | <0.001 |
| Outpatient | 621,330 (75.4) | 617,311 (76.0) | 4,019 (32.9) | |
| Emergency | 218 (0) | 217 (0) | 1 (0) | |
| Unknown | 133,852 (16.2) | 131,153 (16.1) | 2,699 (22.1) | |
| Fellow Involvement | 138,197 (16.8) | 133,741 (16.5) | 4,456 (36.4) | <0.001 |
| Endoscopy Site | ||||
| Geographic Region | ||||
| North East | 153,861 (18.7) | 149,851 (18.4) | 4,010 (32.8) | <0.001 |
| South East | 130,303 (15.8) | 127,426 (15.7) | 1,995 (16.3) | |
| North Central | 102,316 (12.4) | 101,711 (12.5) | 605 (4.9) | |
| South Central | 105,228 (12.8) | 103,233 (12.7) | 1,995 (16.3) | |
| North West | 88,104 (10.7) | 87,685 (10.8) | 419 (3.4) | |
| South West | 244,641 (29.7) | 242,320 (29.8) | 2,321 (19.0) | |
| Type | ||||
| Academic | 135,313 (16.4) | 132,735 (16.3) | 2,578 (21.1) | <0.001 |
| Community/HMO | 581,239 (70.5) | 573,246 (70.6) | 7,993 (65.4) | |
| VA/Military | 107,901 (13.1) | 106,245 (13.1) | 1,656 (13.5) | |
| Provider | ||||
| Sex | ||||
| Female | 99,836 (12.1) | 97,635 (11.8) | 2,201 (18.) | <0.001 |
| Male | 695,882 (84.4) | 686,578 (84.5) | 9,304 (76.1) | |
| Unknown | 28,735 (3.5) | 28,013 (3.5) | 722 (5.9) | |
| Race/Ethnicity | ||||
| White | 424,201 (51.5) | 419,348 (51.6) | 4,853 (39.7) | <0.001 |
| Hispanic | 17,137 (2.1) | 16,919 (2.1) | 218 (1.8) | |
| Black | 3,173 (0.4) | 3,132 (0.4) | 41 (0.3) | |
| Asian/Pacific Islander | 112,538 (13.7) | 109,639 (13.5) | 2,899 (23.7) | |
| American Indian | 440 (0.1) | 439 (0.1) | 1 (0) | |
| Other | 26,300 (3.2) | 25,836 (3.2) | 464 (3.8) | |
| Unknown | 240,664 (29.2) | 236,913 (29.2) | 3,751 (30.7) | |
| Specialty | ||||
| Gastroenterology | 700,465 (85.0) | 691,209 (85.1) | 9,256 (75.7) | <0.001 |
| Pediatric gastroenterology | 53,720 (6.5) | 52,794 (6.5) | 926 (7.6) | |
| General practitioner/pediatrician | 10,735 (1.3) | 10,587 (1.3) | 148 (1.2) | |
| Surgery | 17,193 (2.1) | 16,323 (2.0) | 870 (7.1) | |
| Other | 3,431 (0.4) | 3,314 (0.4) | 117 (1.0) | |
| Unknown | 38,909 (4.7) | 37,999 (4.7) | 910 (7.4) | |
| Years since medical school graduation | 21.0 ± 9.0 | 21.0 ± 9.0 | 20.1 ± 9.5 | <0.001 |
| 3-9 | 67,542 (8.2) | 66,272 (8.2) | 1,270 (10.4) | <0.001 |
| 10-19 | 298,406 (36.2) | 293,608 (36.1) | 4,798 (39.2) | |
| 20-29 | 262,085 (31.8) | 258,777 (31.9) | 3,308 (27.1) | |
| 30-39 | 116,597 (14.1) | 115,449 (14.2) | 1,148 (9.4) | |
| 40-49 | 26,790 (3.2) | 26,214 (3.2) | 576 (4.7) | |
| Unknown | 53,033 (6.4) | 51,906 (6.4) | 1,127 (2.1) | |
Percutaneous Endoscopic Gastrostomy Tube Placement Incidence, 2000-2010
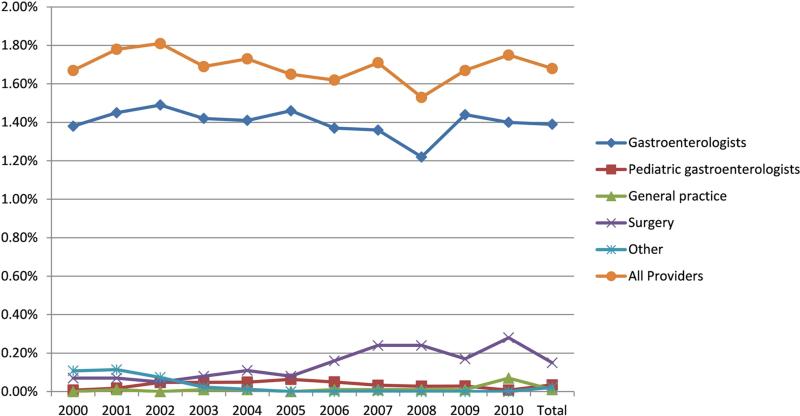
Only a small fraction of PEG tubes were placed by providers who performed upper endoscopies in CORI practices from 2000 to 2010. During these 11 years the incidence of PEG tube placement in stable CORI endoscopy sites was 1.7% (e.g. number of PEG tubes performed/number of upper endoscopies performed) with the majority of PEG tube placements being performed by gastroenterologists (incidence of 1.6% among gastroenterologists who performed upper endoscopies) (Figure 1). Although the overall incidence of PEG tube placement by all providers from 2000 to 2010 remained constant there was a statistically significant decrease in the incidence of PEG tube placement among gastroenterologists between 2000 and 2010 in stable CORI endoscopy sites (decreased by 4.9%). Of note, the greatest decline in the incidence of overall PEG tube placement occurred from 2007 to 2008 with a decline of 10.5% (p=0.02) and was driven by a significant decline in PEG tube placement by gastroenterologists. Interestingly, the incidence of PEG tube placement recovered in the subsequent year (2009) increasing by 9.2% (p=0.06).
Figure 1.
Incidence of PEG tube placement among stable endoscopy sites that participated in CORI stratified by provider type (e.g. number of PEG tubes performed/number of upper endoscopies performed). General practice includes Family Medicine, Internal Medicine, and non-GI Pediatricians.
Further stratification of PEG tube placement incidence revealed several interesting trends. For example, there was a statistically significant increase in the incidence of PEG tube placement by gastroenterologists at academic centers and community/HMO practices between 2000 and 2010 (increase of 28.1% and 17.9%, respectively). At the same time, PEG tube placement incidence significantly declined at VA/military centers (45.0%). Also there was variation in the incidence of PEG tube placement based on the number of years that a provider had been practicing. The incidence of PEG tube placement increased during the study timeframe for providers who had been out of medical school for 10 to 19 and 20 to 29 years; conversely for providers who had graduated from medical school 30 to 39 years ago there was a statistically significant decline in the incidence of PEG tube placement.
Variation and Predictors of PEG Tube Placement Among CORI Users in the United States
A number of patient, procedure, endoscopy site and provider characteristics were associated with PEG tube placement (Tables 1 and 2). In multivariate logistic regression, both very young and very old age were important predictors for PEG tube placement with most age groups less likely to have a PEG tube placed when compared with the infant age group (< 1 year). As a proportion of all EGDs, blacks, Hispanics, and Asians were more likely to have received a PEG tube compared with whites whereas male patients had more PEG tube placements than female patients. Inpatients were almost 8 times more likely to receive a PEG tube compared with outpatients (OR 7.91; 95% CI, 7.56-8.28) and fellows were nearly twice as likely to participate in the procedure (OR 2.09; 95% CI, 1.98-2.20). In terms of practice setting, PEG tube placement occurred more frequently in a community/HMO environment; PEG tubes were 8% less likely to occur in an academic center and 34% less likely to be performed in a VA/military hospital compared with a community/HMO setting. Furthermore, with respect to provider characteristics, male providers were 33% less likely to perform a PEG tube than females and there was a statistically significant trend that as providers were further out of medical school they were less likely to perform a PEG tube procedure. Interestingly, surgeons (OR 6.69; 95% CI, 6.18-7.24) and other providers (non-pediatric, non-general practitioner) (OR 3.22; 95% CI, 2.63-3.94) were more likely to perform PEG tubes than gastroenterologists. Although geographic region was not initially included in the model, when it was incorporated into it all of the previous predictors remained statistically significant and the East Coast (North East and South East) were the areas most strongly associated with the placement of PEG tubes when compared with the North West (OR 4.03; 95% CI, 3.61- 4.49 and OR 4.20; 95% CI, 3.74-4.70, respectively). The area under the receiver operating characteristic curve was c=0.84 and the Hosmer and Lemeshow Goodness of Fit was χ^2 = 43.1 (p=<0.0001) for the logistic regression model.
Table 2.
Multivariate logistic regression model of PEG tube placement among CORI users in the United States. Note: Procedure indication and geographic region were not included in the model.
| Variable | Odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Patient | ||
| Age (years) | ||
| Infant (< 1 year) | Reference | |
| 1-9 | 0.37 (0.32-0.43) | <0.001 |
| 10-19 | 0.09 (0.07-0.10) | <0.001 |
| 20-29 | 0.09 (0.07-0.11) | <0.001 |
| 30-39 | 0.05 (0.04-0.06) | <0.001 |
| 40-49 | 0.06 (0.05-0.08) | <0.001 |
| 50-59 | 0.11 (0.09-0.13) | <0.001 |
| 60-69 | 0.15 (0.12-0.18) | <0.001 |
| 70-79 | 0.24 (0.20-0.29) | <0.001 |
| 80-89 | 0.40 (0.33-0.48) | <0.001 |
| ≥90 | 0.83 (0.68-1.02) | 0.08 |
| Race/Ethnicity | ||
| White | Reference | |
| Hispanic | 1.21 (1.13-1.28) | <0.001 |
| Black | 2.24 (2.12-2.36) | <0.001 |
| Asian/Pacific Islander | 1.16 (1.02-1.32) | 0.03 |
| American Indian | 1.00 (0.83-1.20) | 1.0 |
| Multiracial | 1.78 (1.29-2.45) | <0.001 |
| Unknown | 1.35 (1.14-1.59) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.44 (1.39-1.50) | <0.001 |
| Procedure | ||
| Setting | ||
| Outpatient | Reference | |
| Inpatient | 7.91 (7.56-8.28) | <0.001 |
| Emergency | 0.47 (0.07-3.36) | 0.5 |
| Unknown | 3.07 (2.92-3.24) | <0.001 |
| Fellow involvement | 2.09 (1.98-2.20) | <0.001 |
| Endoscopy Site | ||
| Type | ||
| Community/HMO1 | Reference | |
| Academic | 0.92 (0.87-0.97) | 0.004 |
| VA2/Military | 0.66 (0.62-0.71) | <0.001 |
| Provider | ||
| Sex | ||
| Female | Reference | |
| Male | 0.67 (0.64-0.71) | <0.001 |
| Unknown | 0.95 (0.81-1.12) | 0.5 |
| Race/Ethnicity | ||
| White | Reference | |
| Asian/Pacific Islander | 1.57 (1.49-1.65) | <0.001 |
| Hispanic | 0.81 (0.70-0.93) | 0.003 |
| Black | 1.03 (0.75-1.41) | 0.9 |
| American Indian | 0.35 (0.05-2.50) | 0.3 |
| Unknown | 0.90 (0.85-0.94) | <0.001 |
| Specialty | ||
| Gastroenterology | Reference | |
| Pediatric gastroenterology | 0.90 (0.77-1.04) | 0.1 |
| General practitioner/pediatrician | 1.05 (0.88-1.25) | 0.6 |
| Surgery | 6.69 (6.18-7.24) | <0.001 |
| Other | 3.22 (2.63-3.94) | <0.001 |
| Unknown | 1.41 (1.18-1.69) | <0.001 |
| Years since medical school graduation | ||
| 3-9 | Reference | |
| 10-19 | 0.98 (0.92-1.05) | 0.5 |
| 20-29 | 0.79 (0.73-0.84) | <0.001 |
| 30-39 | 0.67 (0.62-0.73) | <0.001 |
| 40-49 | 0.73 (0.66-0.82) | <0.001 |
| Unknown | 0.76 (0.66-0.88) | <0.001 |
HMO: Health maintenance organization.
VA: Veterans Administration.
Discussion
PEG tube placement remains a controversial issue in medicine with a variety of indications for its placement and a number of providers who can perform the procedure1, 3. Historically, gastroenterologists have predominantly placed PEG tubes, but although there is data on the safety6-8 and outcomes9-12 of this procedure, there is scant literature on the incidence of and predictors for PEG tube placement in the United States.2 In a large cohort of stable endoscopy sites across the United States that used the CORI database, we determined the overall incidence of PEG tube placement by gastroenterologists was 1.6% and that there was a significant decrease in the placement of PEG tubes by gastroenterologists over an 11-year period. Very young and very old, non-white ethnicity and inpatient status were important patient predictors of PEG tube placement whereas academic centers and veterans administration/military hospitals were inversely associated with PEG tube placements. Interestingly, non-gastroenterologists and younger providers were more likely to place PEG tubes in our study. These data shed light on important patient, endoscopy setting and provider factors related to PEG tube placement and illustrate the need for further studies to address many of the important observations we noted.
Our study raises several interesting points on the topic of PEG tube placement. The first point is the apparent shift of PEG tube placement away from gastroenterologists to other specialty services. In contrast to previous research2 we discovered the overall incidence of PEG tube placement by gastroenterologists has declined over the years and that surgery and other specialties (likely representing interventional radiology (IR) services) were more likely to place PEG tubes. Reasons for this observed shift are many. First, IR services have become more readily available and accessible, scheduling an IR procedure is relatively easier, placement of PEG tubes by IR only requires local anesthesia in many cases, is less expensive13 and in most circumstances a formal consultation is not required by IR. Second, the increase in PEG tube placement by non-gastroenterologists such as surgeons and IR may also be a reflection of easier access to anesthesia and operating room services by these specialties thereby allowing the procedure to be performed more expeditiously. Similarly, a third reason for this change may be that the complexity of patients requiring enteral nutrition has increased over the years. For example, patients considered for enteral access are frequently severely malnourished or have multiple existing comorbid medical conditions. In these instances a patient's risk of having an adverse event during the procedure, especially with sedation, is increased and thus referring providers may favor services that only use local anesthesia for the procedure or have better access to anesthesia services. Finally, patients needing enteral access may have altered anatomy or an obstruction of the oropharynx/esophagus due to an underlying malignancy whereby placing a PEG tube may not be feasible. Such situations require other services to provide enteral access using a non-endoscopic approach. Regardless, it is quite difficult to determine the exact reason(s) for this observed shift away from gastroenterologists placing PEG tubes and additional studies are required to further verify this trend as well as explore reasons for such a shift.
The second point to highlight from our study is the practice variation we noted in PEG tube placement. In recent years there has been substantial debate on unwarranted variations in U.S. healthcare14, 15. Yet, of the minimal data that has been presented on healthcare variations with regards to GI delivery of care most have focused on colonoscopy16-22 and upper endoscopy16 with none examining enteral access placement. Given the invasiveness and risk of the procedure, debate and controversy surrounding its placement23 and high costs associated with enteral access placement, understanding its practice variation is essential. Evident in our study was tremendous practice variation based on a number of factors such as provider characteristics, geographic region, and endoscopy setting. For instance, both older and male providers were less likely to place PEG tubes in our study. A number of potential reasons may explain these findings. As providers age they themselves become more personally engaged with the healthcare system either as an individual or by caring for a family member and thus older providers might be less likely to recommend more aggressive/invasive treatment for their patients24, 25. Additionally, older providers may be less willing to perform therapeutic procedures (e.g. PEG) later in their career as they may be more “risk adverse” with such procedures given the greater medico-legal risk and adverse events associated with performing this procedure. Furthermore, we noted that there were provider gender differences in the placement of PEG tubes which was a surprising finding. Limited literature has illustrated that female providers engage in more active partnership behaviors, and positive and emotionally focused talk as well as have longer patient visits than their male counterparts26. Spending more time with their patients and possibly possessing a more positive outlook on a medical situation/quality of life may explain why female providers would recommend and potentially place more PEG tubes for their patients. However, this research is limited to the primary care setting and has yet to be replicated in the GI arena. Consequently, further research is needed to better understand these interactions between patients, provider gender and communication in relation to the placement of PEG tubes.
Additionally, we noted further practice variation with respect to endoscopy setting and geography. For example, patients in a community setting were more likely to undergo PEG tube placement than academic or VA medical centers. These differences may reflect more of a willingness by community providers to place PEG tubes based on an overall healthier patient population27, improved reimbursement rates and enhanced operational efficiencies28, 29 built into community based systems. Last, patients residing in the East coast were more likely to have a PEG tube placed when compared with other regions in the country. This observation may reflect an older patient population concentrated in this region30 or reflect regional attitudes, perceptions, and practice patterns by providers in this part of the country. All of these observed variations in our study have significant implications with respect to the quality, efficiency and equity in health service delivery for enteral access placement in the United States, but clearly more robust and well-designed studies are needed to further understand and address these important observations.
One last notable point from our study was the racial variation observed in PEG tube placement among patients. Non-whites, and in particular blacks and Hispanics, were significantly more likely to have a PEG tube placed as a proportion of all EGDs. Distrust of the medical system and perceptions of providers potentially withholding care31, 32 have been well documented among non-whites in the U.S. healthcare system and may partially explain our findings. Moreover, these beliefs coupled with many individual's ideas that nutrition is a basic element of human life may influence particular groups to more strongly favor PEG tubes even in situations where its benefits may be minimal. Such a finding illustrates the potential cultural, historical and religious beliefs among patients and how it impacts their healthcare decisions. Finally, patient's level of education, income level/socioeconomic status and insurance status/type may also play a role in this practice variation we noted. Unfortunately, this information was not captured in the CORI database and future studies that incorporate this data will need to be conducted to further answer this question. Overall, this is an important area that merits further research, specifically through patient interviews and surveys, in order to further investigate this pattern and to understand patient perceptions about PEG tube placement.
There are several limitations to our study. First, participation in the CORI database is voluntary and thus introduces participation bias; consequently our results may not be generalizable to all endoscopy center populations across the United States. However, a diverse range of groups including community based practices, academic centers and VA medical centers across the United States are represented in CORI and we examined over 800,000 upper endoscopies that were performed during the study period. Second, CORI does not fully capture data on other non-GI providers who may perform PEG tubes given that it is mostly used in GI practices. As a result, we may be under-reporting the true incidence of PEG placement in the United States and specifically the number of PEG tubes performed by non-GI providers may be even higher than we observed. Similarly, underreporting of trainee involvement may be present as only the attending physician has to be populated in the CORI database and including trainee participation is voluntary. Along these lines, the low number of trainees observed in PEG tube placement may be a reflection of endoscopy sites included in the study. The majority of endoscopy sites participating in CORI were outpatient practices and not academic sites. Thirdly, we were unable to account for the precipitous decline and then rise in PEG tube placement from 2007-2009. A number of potential factors could have explained this finding such as news reports altering provider or patient perception, published papers on the topic, or possible guideline changes with respect to PEG tubes. Although none of these factors could be confirmed or were reported, in 2007 one manufacturer of PEG tube kits made significant changes to their placement kit yet it was not available for some time. Such a change could have impacted PEG tube placement and could imply that manufacturers of endoscopic equipment affect providers’ procedure-performing habits. However, the type of PEG kit used for the procedure is not documented in CORI and such a hypothesis could not be verified. Another possibility is that the decline starting in 2007,with a return to baseline in 2009,could also reflect variance within the dataset. Last, we were unable to assess both provider and patient attitudes and beliefs on PEG tube placement to further understand a number of the variations we observed. Nevertheless, our study has much strength compared to previous literature in that we covered a much broader time period, did not rely solely on administrative claims data, did not focus solely on one particular age group and are the first to examine predictors and practice patterns of PEG tube placement.
In summary, among a large cohort of providers who provide endoscopic services, the incidence of PEG tube placement among patients undergoing upper endoscopy in the United States, as estimated by the NED, was 1.7% during 2000 to 2010. During the same time period, the overall incidence of PEG tube placement by gastroenterologists decreased with other non-GI providers more likely to perform them. Furthermore, significant practice variation was noted in PEG tube placement with respect to patient and provider characteristics, geographic region and endoscopy settings. These results highlight the need for more research in this area in order to improve the quality, cost, and utilization of resources dedicated to this procedure. Furthermore, now that the demographics of patients receiving PEG tubes have been characterized, further research is needed to determine if PEG tubes benefit in terms of nutrition, survival and quality of life.
Acknowledgments
Funding: Cook, Inc; CORI is supported with funding from NIDDK U01DK57132, R33-DK61778-01, and R21-CA131626. In addition, the practice network (Clinical Outcomes Research Initiative) has received support from the following entities to support the infrastructure of the practice-based network: AstraZeneca, Novartis, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research.
Abbreviations
- IR
interventional radiology
- CI
Confidence interval
- OR
Odds ratio
- GI
gastroenterology
- PEG
percutaneous endoscopic gastrostomy
- CORI
Clinical Outcomes Research Initiative
- NED
National Endoscopic Database
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Lukejohn Day (conception and design; analysis and interpretation of data of the article; critical revision of the article for important intellectual content; final approval of the article)
Michelle Nazareth (conception and design; critical revision of the article for important intellectual content; final approval of the article)
Justin Sewell (critical revision of the article for important intellectual content; final approval of the article)
Lucas Williams (conception and design; analysis and interpretation of data of the article; critical revision of the article for important intellectual content; final approval of the article)
David Lieberman (conception and design; critical revision of the article for important intellectual content; final approval of the article)
Conflicts of interest: Dr. Lieberman is the executive director of CORI, a non-profit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the OHSU and Portland VA Conflict of Interest in Research Committees.
References
- 1.Jain R, Maple JT, Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Fanelli RD, Fisher L, Fukami N, Ikenberry SO, Jue T, Khan K, Krinsky ML, Malpas P, Sharaf RN, Dominitz JA. The role of endoscopy in enteral feeding. Gastrointest Endosc. 2011;74:7–12. doi: 10.1016/j.gie.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Duszak R, Jr., Mabry MR. National trends in gastrointestinal access procedures: an analysis of Medicare services provided by radiologists and other specialists. J Vasc Interv Radiol. 2003;14:1031–6. doi: 10.1097/01.rvi.0000082983.48544.2c. [DOI] [PubMed] [Google Scholar]
- 3.Kwon RS, Banerjee S, Desilets D, Diehl DL, Farraye FA, Kaul V, Mamula P, Pedrosa MC, Rodriguez SA, Varadarajulu S, Song LM, Tierney WM. Enteral nutrition access devices. Gastrointest Endosc. 2010;72:236–48. doi: 10.1016/j.gie.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 4.The Gastroenterology Core Curriculum. Gastroenterology. (Third Edition) 2007;132:2012–8. doi: 10.1053/j.gastro.2007.03.079. [DOI] [PubMed] [Google Scholar]
- 5.Clinical Outcomes Research Initiative. [Accessed June 1 ahwco. [Google Scholar]
- 6.McClave SA, Chang WK. Complications of enteral access. Gastrointest Endosc. 2003;58:739–51. doi: 10.1016/s0016-5107(03)02147-3. [DOI] [PubMed] [Google Scholar]
- 7.Schrag SP, Sharma R, Jaik NP, Seamon MJ, Lukaszczyk JJ, Martin ND, Hoey BA, Stawicki SP. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis. 2007;16:407–18. [PubMed] [Google Scholar]
- 8.Wirth R, Voss C, Smoliner C, Sieber CC, Bauer JM, Volkert D. Complications and mortality after percutaneous endoscopic gastrostomy in geriatrics: a prospective multicenter observational trial. J Am Med Dir Assoc. 2012;13:228–33. doi: 10.1016/j.jamda.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764–72. doi: 10.1016/S0140-6736(05)17983-5. [DOI] [PubMed] [Google Scholar]
- 10.Stanga Z, Giger U, Marx A, DeLegge MH. Effect of jejunal long-term feeding in chronic pancreatitis. JPEN J Parenter Enteral Nutr. 2005;29:12–20. doi: 10.1177/014860710502900112. [DOI] [PubMed] [Google Scholar]
- 11.Grant MD, Rudberg MA, Brody JA. Gastrostomy placement and mortality among hospitalized Medicare beneficiaries. JAMA. 1998;279:1973–6. doi: 10.1001/jama.279.24.1973. [DOI] [PubMed] [Google Scholar]
- 12.Grant JP. Percutaneous endoscopic gastrostomy. Initial placement by single endoscopic technique and long-term follow-up. Ann Surg. 1993;217:168–74. doi: 10.1097/00000658-199302000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galaski A, Peng WW, Ellis M, Darling P, Common A, Tucker E. Gastrostomy tube placement by radiological versus endoscopic methods in an acute care setting: a retrospective review of frequency, indications, complications and outcomes. Can J Gastroenterol. 2009;23:109–14. doi: 10.1155/2009/801925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961–4. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasaitis L, Fisher ES, Skinner JS, Chandra A. Hospital quality and intensity of spending: is there an association? Health Aff (Millwood) 2009;28:w566–72. doi: 10.1377/hlthaff.28.4.w566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini SD, Eisen G, Mattek N, Schoenfeld P. Utilization of upper endoscopy for surveillance of gastric ulcers in the United States. Am J Gastroenterol. 2008;103:1920–5. doi: 10.1111/j.1572-0241.2008.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benchimol EI, Cook SF, Erichsen R, Long MD, Bernstein CN, Wong J, Carroll CF, Froslev T, Sampson T, Kappelman MD. International variation in medication prescription rates among elderly patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:878–89. doi: 10.1016/j.crohns.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Chaptini L, Chaaya A, Depalma F, Hunter K, Peikin S, Laine L. Variation in polyp size estimation among endoscopists and impact on surveillance intervals. Gastrointest Endosc. 2014 doi: 10.1016/j.gie.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 19.Cooper GS, Koroukian SM. Geographic variation among Medicare beneficiaries in the use of colorectal carcinoma screening procedures. Am J Gastroenterol. 2004;99:1544–50. doi: 10.1111/j.1572-0241.2004.30902.x. [DOI] [PubMed] [Google Scholar]
- 20.Gellad ZF, Voils CI, Lin L, Provenzale D. Clinical practice variation in the management of diminutive colorectal polyps: results of a national survey of gastroenterologists. Am J Gastroenterol. 2013;108:873–8. doi: 10.1038/ajg.2012.316. [DOI] [PubMed] [Google Scholar]
- 21.Radaelli F, Paggi S, Bortoli A, De Pretis G. Overutilization of post-polypectomy surveillance colonoscopy in clinical practice: a prospective, multicentre study. Dig Liver Dis. 2012;44:748–53. doi: 10.1016/j.dld.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Reding DJ, Hayes RB, Church T, Yurgalevich S, Doria-Rose VP, Hickey T, Riley T, Berg CD. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo B, Dornbrand L. Guiding the hand that feeds. Caring for the demented elderly. N Engl J Med. 1984;311:402–4. doi: 10.1056/NEJM198408093110611. [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra A, Reid RO, Adams JL, Friedberg MW, McGlynn EA, Hussey PS. Physicians with the least experience have higher cost profiles than do physicians with the most experience. Health Aff (Millwood) 2012;31:2453–63. doi: 10.1377/hlthaff.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pham HH, Landon BE, Reschovsky JD, Wu B, Schrag D. Rapidity and modality of imaging for acute low back pain in elderly patients. Arch Intern Med. 2009;169:972–81. doi: 10.1001/archinternmed.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roter DL, Hall JA, Aoki Y. Physician gender effects in medical communication: a meta-analytic review. JAMA. 2002;288:756–64. doi: 10.1001/jama.288.6.756. [DOI] [PubMed] [Google Scholar]
- 27.Hellinger FJ, Wong HS. Selection bias in HMOs: a review of the evidence. Med Care Res Rev. 2000;57:405–39. doi: 10.1177/107755870005700402. [DOI] [PubMed] [Google Scholar]
- 28.Rollins J, Lee K, Xu Y, Ozcan YA. Longitudinal study of health maintenance organization efficiency. Health Serv Manage Res. 2001;14:249–62. doi: 10.1177/095148480101400405. [DOI] [PubMed] [Google Scholar]
- 29.Baker LC, Bundorf MK, Kessler DP. HMO coverage reduces variations in the use of health care among patients under age sixty-five. Health Aff (Millwood) 2010;29:2068–74. doi: 10.1377/hlthaff.2009.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.TOP. 2010 [Google Scholar]
- 31.Boothe EJ. African American attitudes toward participation in health care. ABNF J. 1998;9:14–6. [PubMed] [Google Scholar]
- 32.Kennedy BR, Mathis CC, Woods AK. African Americans and their distrust of the health care system: healthcare for diverse populations. J Cult Divers. 2007;14:56–60. [PubMed] [Google Scholar]