Abstract
Current histopathologic classification schemes for gastric adenocarcinoma have limited clinical utility and are difficult to apply due to tumor heterogeneity. Elucidation of molecular subtypes of gastric cancer may contribute to our understanding of gastric cancer biology and to the development of new molecular markers that may lead to improved diagnosis, therapy, or prognosis. We previously demonstrated that Epstein-Barr virus infected gastric cancers have a distinct human gene expression profile compared to uninfected cancers. We now examine the histopathologic features characterizing infected (n=14) and uninfected (n=89) cancers, the latter of which are now further divided into two major molecular subtypes based on expression patterns of 93 RNAs. One uninfected gastric cancer subtype was distinguished by upregulation of three genes with neuroendocrine function (CHGA, GAST, and REG4 encoding chromogranin, gastrin and the secreted peptide REG4 involved in epithelial cell regeneration), implicating hormonal factors in the pathogenesis of a major class of gastric adenocarcinomas. Evidence of neuroendocrine differentiation (molecular, immunohistochemical, or morphologic) was mutually exclusive of EBV infection. EBV infected tumors tended to have solid-type morphology with lymphoid stroma. This study reveals novel molecular subtypes of gastric cancer and their associated morphologies that demonstrate divergent neuroendocrine features.
Background
Gastric adenocarcinoma has remarkable morphologically heterogeneity, and many descriptive histologic classification schemes have been proposed over the past several decades1,2,3,4,5,6. Although some histologic types have prognostic and epidemiologic correlates, further progress is needed to classify tumors in a manner that impacts patient management. Variable clinical behavior and response to intervention attest to underlying biologic diversity. The lack of strong predictors of clinical outcome with any single histologic classification scheme highlights the complicated landscape of gastric cancer morphology which hampers comparative analysis across studies and underscores the need to devise novel methods of predicting response to current and future therapies.
The widely used Lauren classification divides the majority of gastric cancers into intestinal and diffuse types, and this dichotomous scheme has some clinicopathologic and epidemiologic relevance. However, there are other histologic patterns of prognostic significance, such as mixed7,8,9,10, solid7,8,11,12, and hepatoid13,14, that are not distinguished under the Lauren scheme. Intratumoral heterogeneity is common, and different histologic features often coexist within a single tumor, thus confounding the morphologic classification attempts and raising concerns about their accuracy and reproducibility7,15,16,17.
Gastric adenocarcinomas also have diverse pathogenesis, with Helicobacter pylori and Epstein-Barr virus (EBV) both designated as associated class 1 oncogenic pathogens by the World Health Organization (WHO). Approximately 10% of gastric adenocarcinomas harbor EBV infection in the neoplastic epithelial cells18. The prevalence of EBV infection is significantly higher (>80%) in the specific histologic type variably termed “gastric carcinoma with lymphoid stroma” or “lymphoepithelioma-like carcinoma”19,20,21,22,23,24. This tumor type is characterized by undifferentiated epithelial cells and an abundant lymphocytic infiltrate distributed uniformly throughout the tumor, similar to EBV-associated nasopharyngeal carcinoma. Gastric carcinoma with lymphoid stroma has a survival advantage over other histologic types22,23, however the literature varies on whether EBV positivity alone or histopathologic appearance trumps in assigning prognosis 23,25,26,27,28. When EBV-associated gastric cancers are classified according to their histologic patterns, some studies report predominance of Lauren intestinal type26, tubular type26,29,30, Lauren diffuse type20,23,27, and medullary/solid type30,31. This variability may illustrate the interpretative bias inherent in applying morphologic criteria.
High grade neuroendocrine carcinoma is another heterogeneous group of gastric malignancies classified and staged separately from gastric adenocarcinomas by the WHO32. It is frequently under-recognized because of the broad morphologic spectrum (from classic small cell carcinoma to poorly defined large cell carcinoma) and overlap with poorly differentiated adenocarcinoma33,34,35,36. Admixed adenocarcinoma components can be found in tumors of any of the neuroendocrine carcinoma subtypes, presenting a challenge in terms of the overall tumor classification. In fact, evidence of focal neuroendocrine differentiation has been found in over 50% of tumors otherwise classified as adenocarcinoma37,38,39,40. It is particularly common in signet ring cell carcinomas, leading some to propose that signet ring cells derive from a pluripotent neuroendocrine stem cell 41,42,43,44. Molecular and infectious correlates of neuroendocrine differentiation have not been systematically investigated.
Molecular profiling holds promise for adding value in categorizing disease in a manner that is clinically meaningful. We previously demonstrated that EBV-infected gastric cancers have a distinct human gene expression profile compared to uninfected gastric cancers45. In the current study, we examine RNA expression patterns to further divide uninfected cancers into two principal molecular subtypes, and we evaluate associations with tumor histology and neuroendocrine gene expression. This study is the first, to our knowledge, to investigate the relationship between EBV infection and neuroendocrine differentiation in gastric cancer.
Materials and Methods
Tissue and molecular profiling
Consecutive gastric adenocarcinoma cases on which sufficient residual formalin-fixed paraffin-embedded tissue was available were assembled from pathology archives of three hospitals in disparate parts of the world, 30 from the University of North Carolina Hospitals in Chapel Hill, USA, 133 from Western Regional Hospital in Santa de Rosa, Honduras, and 24 from Wakayama Medical University, Wakayama, Japan. RNA profile data on this case cohort were previously published, and histopathology correlates are the subject of the current study. Studies were performed with approval of the University of North Carolina Biomedical Institutional Review board45.
Paraffin-embedded tissue sections were stained with hematoxylin and eosin (H&E) and reviewed to identify tumor for subsequent macrodissection from unstained adjacent sections. Nucleic acid was extracted and RNA expression analysis was performed on the nCounter system (Nanostring) as previously described45. A custom panel of 96 RNAs (Gastrogenus v1™ panel) was measured which includes 73 human mRNAs of which 4 were housekeeper transcripts, 20 viral RNAs (7 latent and 9 lytic EBV mRNA transcripts, EBER1 and EBER2 non-coding RNAs, and two cytomegalovirus mRNAs), and 3 spiked ERCC RNA controls. The human RNAs were selected based on their proposed roles in gastric cancer pathogenesis, inflammatory response, and/or gastric cancer therapeutic targeting and monitoring. The viral RNAs were selected to detect EBV and to measure expression of latent and lytic viral transcripts. After excluding samples with insufficient RNA or insufficient histopathologic material for morphologic assessment, a total 103 gastric carcinomas were included in the study. Of note, biopsies tended to yield better quality RNA than did resection specimens, possibly because biopsies tend to be immersed in formalin preservative much more quickly than are resected tumors.
Histologic analysis
All tumors were classified by both the Lauren system1 and by the Carneiro system7,46. In the Carneiro classification of gastric cancer, mixed carcinomas consisting of both glands and infiltrating single cells, as well as solid carcinomas, are classified separately from purely gland-forming tumors and isolated-cell tumors. The latter two tumor categories correspond to Lauren intestinal and diffuse types, respectively. Solid carcinomas are composed of tumor cell sheets with little or no gland formation and these tend to form expansile rather than infiltrative masses11. Solid tumors typically lack a significant stromal component and correspond to the medullary type distinguished by Kubo4. Others have used yet another term, “cohesive carcinoma”8, when individual cells or small groups of tumor cells in solid/medullary/cohesive formations lack investment by stroma in contrast to isolated-cell type. Thus, in the Carneiro classification scheme histologic group 1 is isolated-cell, group 2 is gland-forming, group 3 has mixed gland-forming and single cell components, and group 4 is solid. A number of cases remained unclassified by this four-group system due to the scant nature and quality of biopsy material. Tumors with neuroendocrine morphology by H&E were also placed in the unclassified category as well as being considered separately for statistical analysis (see below).
Morphologic features suggesting neuroendocrine differentiation on H&E were evaluated as previously described for neuroendocrine carcinomas, namely, nested/organoid, trabecular, and rosette-forming architecture, relative cellular monotony, eosinophilic cytoplasm, indistinct cellular borders, and evenly dispersed chromatin3,33,34,47. Formal mitotic counts were not performed due to a preponderance of small biopsy specimens with limited numbers of high-power fields; however, mitoses were noted in the majority of tumors with neuroendocrine features. Tumors were characterized as signet ring cell carcinomas if >50% of malignant cells were signet ring cells. The degree of chronic inflammatory cell infiltrate within the tumor was graded semi-quantitatively as mild, moderate, or severe. Stromal fibrosis (desmoplasia) was also graded semi-quantitatively as mild, moderate or severe, reflecting a relative proportion of stromal spindle cells to tumor cells.
Histochemistry
EBV encoded RNA (EBER) in situ hybridization was performed on paraffin sections (Leica BOND Max system with EBER ASR probe and oligo dT control probe detected using the Bond Polymer AP Red Detection reagents, Leica Microsystems Inc., Buffalo Grove, IL)48,49 to determine if EBV was localized to malignant epithelial cells. EBV localization to scattered lymphocytes was seen in some EBV-positive and EBV-negative tumor tissues as previously reported50. Representative histologic tumor types from all three molecular groups were selected for immunohistochemistry using antibodies to chromogranin A (Leica, 1:200) and synaptophysin (Leica, 1:200). Avidin-biotin complex peroxidase techniques were used for signal detection on 4 μm sections of formalin-fixed, paraffin-embedded tissues. Antigen retrieval in hot citrate buffer at pH 6.0 was applied before immunostaining.
Statistics
Unsupervised hierarchical clustering revealed the EBV-infected molecular subtype for which EBV localization to malignant cells was proven by EBER in situ hybridization. Two additional uninfected molecular classes of gastric cancer were identified on heat maps created using Cluster 3.0 and JavaTreeView software algorithms applied to log2 transformed gene expression data collected on the nCounter system. Genes differentially expressed among the three molecular types of cancer were identified using non parametric Mann-Whitney tests, and p-values were adjusted using Bonferroni correction to account for multiple comparisons. A Bonferroni adjusted p value <0.05 connoted significant differential expression. Box plots show the median and middle two quartiles surrounded by whiskers depicting outliers which are far above or below the interquartile range (IQR) by >Q3+1.5*IQR or <Q1-1.5*IQR, respectively. Associations between discrete histologic parameters and the three molecular types of cancer were evaluated by Fisher's Exact Test or Pearson's Chi-squared test. Statistical analysis was repeated after limiting the cohort to the two uninfected gastric cancer molecular types.
Results
RNA expression profiling reveals three molecular subtypes of gastric adenocarcinoma
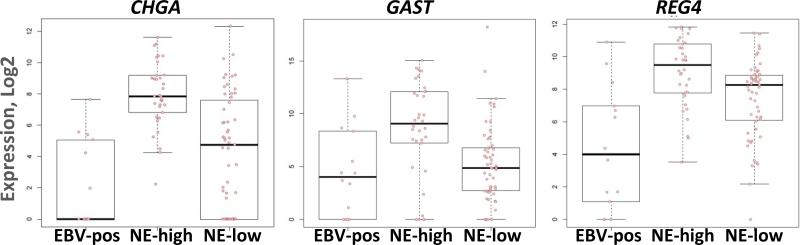
The 96-RNA probe test panel that included 73 human mRNAs, 20 viral RNAs, and three controls (Gastrogenus v1™ panel) was applied to 103 macrodissected gastric adenocarcinoma tissues. After data normalization, unsupervised clustering identified three major molecular subtypes, one with high expression of EBV RNAs, and two lacking substantial levels of EBV RNA. The cancers overexpressing EBV RNAs were called the EBV-positive molecular type (n=14), and all 14 were proven to have EBV localized to malignant cells by EBER in situ hybridization which is the gold standard assay for defining a tumor as infected 45,50. Three RNAs involved in the differentiation and signaling of neuroendocrine cells of the gut, CHGA (chromogranin), GAST (gastrin), and REG4 (regenerating islet-derived family, member 4), were significantly overexpressed in one non-infected molecular subtype. Henceforth, the two uninfected molecular subtypes are referred to as Neuroendocrine-high (NE-high, n=34) and Neuroendocrine-low (NE-low, n=55) (Fig 1, Fig S1, Supplemental Digital Content 1, http://links.lww.com/AIMM/A57 ).
Figure 1. Differential expression of neuroendocrine (NE) RNAs by molecular subtype of gastric cancer.
CHGA (chromogranin), GAST (gastrin), and REG4 are expressed more highly in NE-high molecular type cancers compared to EBV-positive or NE-low molecular types.
Neuroendocrine RNAs distinguish the three molecular subtypes
Of the 73 human RNAs that were measured, 43 were significantly differentially expressed among the three molecular groups. The 9 human genes more highly expressed in EBV-positive compared with EBV-negative cancers are involved primarily in the anti-viral inflammatory/immune response as previously described45. The neuroendocrine markers CHGA, GAST, and REG4 were expressed at a lower level in the EBV-positive compared to the NE-high molecular type. Levels of these neuroendocrine markers in the NE-low molecular type were intermediate between those in the EBV-positive and NE-high groups, suggesting that the EBV-positive tumors had the least amount neuroendocrine differentiation (Fig 1).
Statistical analysis comparing the NE-high and NE-low molecular types revealed differential expression of 32 RNAs (Table 1). Only three genes were upregulated in NE-high compared with the NE-low molecular subtype, and these were the three neuroendocrine markers. The remaining 29 RNAs were downregulated in the NE-high group, and these downregulated RNAs include adhesion factors and intracellular signaling molecules (ICAM1, FSCN1, SULF1, SPP1, INHBA), and collagen components COL1A1, COL1A2, and COL3A1. Additionally, many inflammatory and immune response genes are downregulated in the NE-high compared to the NE-low molecular types, implicating stromal cells as a factor influencing clustering. The EBV genes EBER1, EBER2, and BLLF1 were relatively underexpressed in NE-high compared to the NE-low molecular types, although the levels of these viral RNAs did not approach the elevated levels seen in the EBV-infected carcinomas. Rare EBV-infected stromal lymphocytes could be a source of these viral RNAs in uninfected cancer tissues.
Table 1.
RNAs significantly differentially expressed in NE-high and NE-low molecular types of gastric adenocarcinomaa
| Gene symbol | Colloquial name | Description or function | Direction of differenceb |
|---|---|---|---|
| SULF1 | Cell signaling, sulfatase | ↓ | |
| CHGA | chromogranin | Neuroendocrine cell, gastrin signaling | ↑ |
| SPP1 | Osteogenesis, secreted phosphoprotein | ↓ | |
| FSCN1 | Cell morphology and motility | ↓ | |
| TNFSF9 | Antigen processing, TNF ligand cytokine | ↓ | |
| INBA | inhibin | Hormonal regulation and cell growth | ↓ |
| FCGR2B | Phagocytosis and antibody production | ↓ | |
| PTGS2 | COX2 | Prostaglandin and gastrin signaling | ↓ |
| GAST | gastrin | Neuroendocrine cell, stimulation of acid secretion | ↑ |
| ICAM1 | Cell adhesion | ↓ | |
| SLC2A1 | Glut 1 | Glucose transporter | ↓ |
| CD4 | Helper T cells, MHC class II antigen processing | ↓ | |
| CD70 | TNF ligand, T and NK cell activation | ↓ | |
| DKK4 | Embryonic development | ↓ | |
| SERPINH1 | Collagen synthesis, peptidase inhibitor, heat shock | ↓ | |
| TRAF1 | TNF receptor | ↓ | |
| CXCL1 | Immune development and homeostasis | ↓ | |
| IGLL1 | CD179b | B cell growth | ↓ |
| SPARC | osteonectin | Protects from apoptosis, docetaxel response | ↓ |
| REG4 | Neuroendocrine cell, regenerative islet-derived family | ↑ | |
| COL1A1 | Type I collagen component | ↓ | |
| EBER1 | Non-coding viral RNA | ||
| GPR183 | EBI2 | G-protein coupled receptor, EBV-induced | ↓ |
| EBER2 | Non-coding viral RNA | ||
| COL1A2 | Type I collagen component | ↓ | |
| BLLF1 | Viral RNA, entry via CD21 receptor | ||
| HIF1A | Systemic response to hypoxia | ↓ | |
| TYMS | Thymidilate synthase, 5FU response | ↓ | |
| THY1 | Control of inflammatory cell recruitment | ↓ | |
| BCL2L11 | BIM | Activator of apoptosis, BCL2-like | ↓ |
| COL3A1 | Type III collagen component | ↓ |
FDR adjusted P values are <0.05 for all RNAs listed in the table; RNAs are ranked with most significant P values near the top
Up arrows indicate RNAs expressed at a higher level in NE-high compared to NE-low subtype, and down arrows indicated RNAs expressed at a lower level in NE-high compared to NE-low subtype.
Histologic analysis
H&E stained sections were analyzed to determine histologic classification, to examine stromal content for the extent of chronic inflammation and fibrosis, and to query for morphologic evidence of neuroendocrine differentiation (Table 2). EBV-positive molecular type cancers were noted to have more chronic inflammation compared to NE-high or NE-low tumors and were much more likely to be designated as “gastric carcinoma with lymphoid stroma” (Fig 2). 7/14 EBV-positive cancers received this designation versus 0/34 NE-high and 1/55 NE-low cancers. Tumors with more lymphocytic infiltrate generally showed less stromal desmoplasia, and no EBV-positive molecular type cancers were noted to have severe stromal fibrosis. EBV-positive molecular type cancers were comprised of sheets or large nests of tumor cells with absent or minimal desmoplastic stroma, characteristic of solid carcinoma of the stomach. Focal gland formation was present in some of the otherwise solid-appearing EBV-positive cancers but the focal glands were not associated with stromal infiltration.
Table 2.
Histopathologic features in three molecular subtypes of gastric adenocarcinoma
|
Molecular Subtype |
||||||
|---|---|---|---|---|---|---|
| Histologic feature | # of cases, n=103 | EBV-positive n=14 | NE-high n=34 | NE-low n=55 | 3-way P valued | 2-way P valuee |
| Chronic inflammation | <0.0001f | <0.0001 | ||||
| Mild | 42 | 0 | 18 | 24 | ||
| Moderate | 45 | 5 | 14 | 26 | ||
| Severe | 16 | 9 | 2 | 5 | ||
| Stromal fibrosis | 0.0008 | 0.0001 | ||||
| Mild | 29 | 11 | 5 | 13 | ||
| Moderate | 47 | 2 | 15 | 30 | ||
| Severe | 12 | 0 | 6 | 6 | ||
| Indeterminatea | 15 | 1 | 8 | 6 | ||
| Lauren classification | 0.3f | 0.67 | ||||
| Intestinal | 30 | 3 | 11 | 16 | ||
| Diffuse | 36 | 6 | 15 | 15 | ||
| Unclassifiedb | 37 | 5 | 8 | 24 | ||
| Carneiro histologic groupsc | 0.0003f | 0.0002f | ||||
| 1: isolated-cell | 24 | 0 | 14 | 10 | ||
| 2: gland-forming | 23 | 3 | 7 | 13 | ||
| 3: mixed | 18 | 1 | 7 | 10 | ||
| 4: solid | 17 | 8 | 1 | 8 | ||
| Unclassifieda,b | 21 | 2 | 5 | 14 | ||
| Neuroendocrine morphology | 0.25 | 0.2 | ||||
| Yes | 14 | 0 | 4 | 10 | ||
| No | 89 | 14 | 30 | 45 | ||
| Gastric cancer with lymphoid stroma | <0.0001 | <0.0001 | ||||
| Yes | 8 | 7 | 0 | 1 | ||
| No | 95 | 7 | 34 | 54 | ||
| Signet ring cell carcinoma | 0.002 | 0.12 | ||||
| Yes | 15 | 0 | 11 | 4 | ||
| No | 88 | 14 | 23 | 51 | ||
Due to limited nature of biopsy material
Unclassified cases include tumors with neuroendocrine morphology
Based on Carneiro histopathologic classification
Statistical analysis of all three molecular subtypes
Statistical analysis of the EBV-positive and EBV-negative (combined NE-high and NE-low) cancers
Determined by Chi-square test; all other P values determined by two-tailed Fisher's exact test
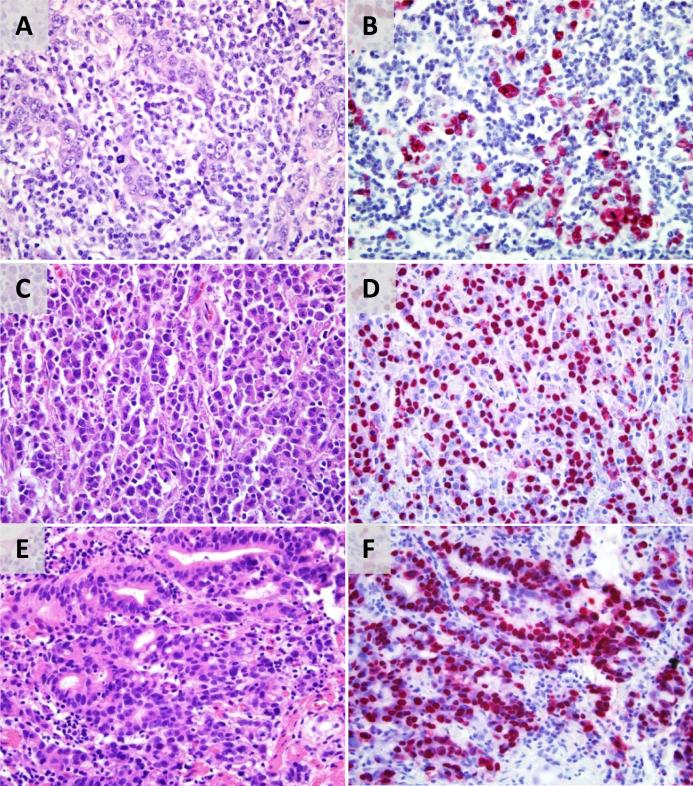
Figure 2. Morphologic range of EBV-positive gastric adenocarcinomas.
(A) Gastric cancer with lymphoid stroma has undifferentiated epithelial nests surrounded by a lymphoplasmacytic infiltrate with lack of stromal desmoplasia; (B) EBER RNA is expressed in the malignant epithelial cells. (C) Solid carcinoma is a sheet-like proliferation of malignant epithelial cells with deep eosinophilic cytoplasm and virtually no intervening fibrous or lymphoid stroma; (D) EBER RNA is expressed in the malignant epithelial cells. (E) Solid carcinoma with areas of gland formation in a lymphoid stroma without significant fibrosis; (F) EBER RNA is expressed in the malignant epithelial cells.
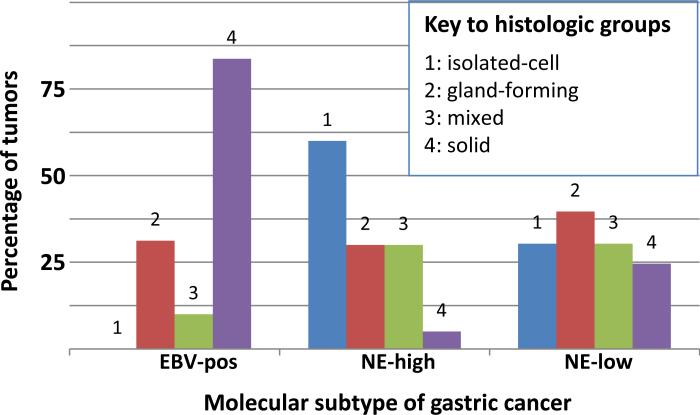
There was a statistically significant association between the four histologic Carneiro groups among the three molecular types (p<0.05) (Table 2, Fig 3). As described above, EBV-positive carcinomas were predominantly group 4 solid. The NE-high molecular type had a greater proportion of group 1 isolated-cell histology (14/34 cases, 41%) compared to the EBV-positive (0/14) and NE-low type (10/55, 18%), and a greater proportion of signet ring cell carcinomas—11/34 (32%) NE-high tumors were signet ring cell carcinomas compared to 0/14 EBV-positive tumors and 4/55 (7%) NE-low tumors. Classification of tumors into Lauren categories did not have a statistically significant association with molecular subtypes.
Figure 3. Distribution of the four histologic groups (relative proportions) among the three molecular types.
EBV-positive carcinomas are predominantly group 4 solid while very few of the NE-high molecular type tumors are classified in that group. Instead, the NE-high molecular type is enriched for tumors having group 1 isolated-cell histology while none of the EBV-positive cancers are classified as isolated-cell morphology (p<0.05, Chi-square test).
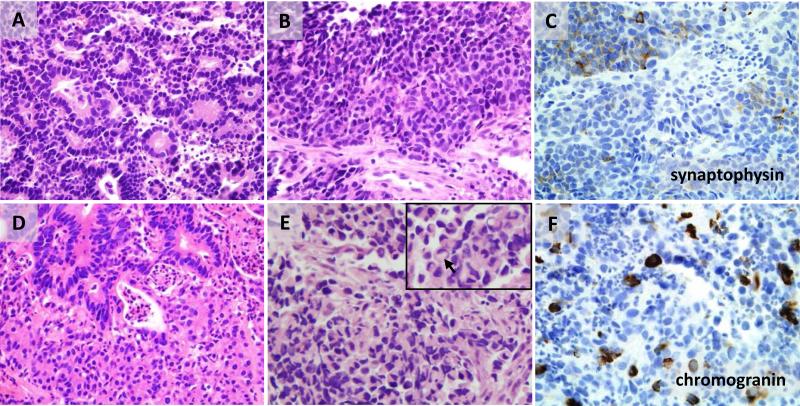
Although all gastric cancers in this study were diagnosed as adenocarcinoma rather than neuroendocrine carcinoma, a number of cases were found to have architectural and cytologic neuroendocrine features on H&E stain, such as nested or rosette-forming architecture, cellular monotony, and evenly dispersed chromatin (Fig 4). In addition, some cases had high-grade neuroendocrine features with increased nuclear/cytoplasmic ratios, hyperchromatic nuclei, and conspicuous mitotic activity. Four biopsy cases additionally showed extensive nuclear molding, single cell apoptosis, and crush artifact, fulfilling the criteria for classic small cell carcinoma (Fig 4E). Two of these small cell carcinoma cases were NE-high and two were NE-low molecular subtype. However, in other cases determination of high-grade vs. low-grade neuroendocrine tumor features could not be made due to the limited size of biopsy material. All tumors with any morphologic neuroendocrine features were separated out for purposes of statistical analysis. While none of the 14 EBV-positive cancers had neuroendocrine features by H&E, such features were found in 4/34 NE-high and 10/55 NE-low molecular type tumors, but this relationship did not reach statistical significance.
Figure 4. Morphologic heterogeneity of carcinomas with neuroendocrine features.
A carcinoma with high-grade neuroendocrine nuclear features such as hyperchromasia and nuclear molding has areas of rosettes (A) and solid nests (B) and shows focal synaptophysin positivity in both areas (C, only nested area is shown). (D) A neuroendocrine tumor with two intermingled morphologies, visible as gland-forming and solid areas, demonstrates cellular monotony, unapparent cellular borders, and evenly dispersed chromatin in both morphologic components but more ample eosinophilic cytoplasm in the solid nests. (E-F) Small cell carcinoma with nuclear molding and crush artifact has rare cells with signet ring morphology (E, arrow in inset) and is focally positive for chromogranin (F).
Genes associated with histologic subtypes
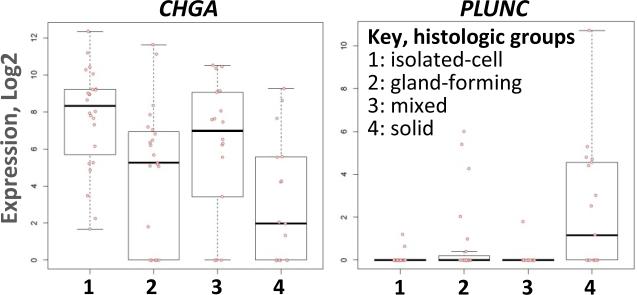
RNAs differentially expressed among the four Carneiro histologic groups included primarily viral RNAs and those human RNAs involved in the inflammatory/immune response. These RNAs were upregulated in group 4 solid carcinomas which includes the majority of EBV-positive molecular type cancers. Four human RNAs not generally considered part of the inflammatory repertoire that were significantly differentially expressed among the histologic groups are CHGA (p=0.007), PLUNC (p=0.008), CYP2W1 (p=0.014), and PPARG (p=0.035). CHGA was expressed at a higher level in group 1 isolated-cell tumors and group 3 mixed tumors, while PLUNC was expressed at higher level in group 4 solid tumors (Fig 5). No RNAs were differentially expressed in association with morphological evidence of neuroendocrine differentiation. Similarly, no RNAs were significantly associated with the Lauren classification or with signet ring cell morphology.
Figure 5. Differential expression of CHGA and PLUNC by Carneiro histologic groupings.
CHGA is expressed at higher levels in histologic groups 1 and 3 while PLUNC is expressed at a higher level in histologic group 4.
Chromogranin RNA level and protein immunohistochemistry
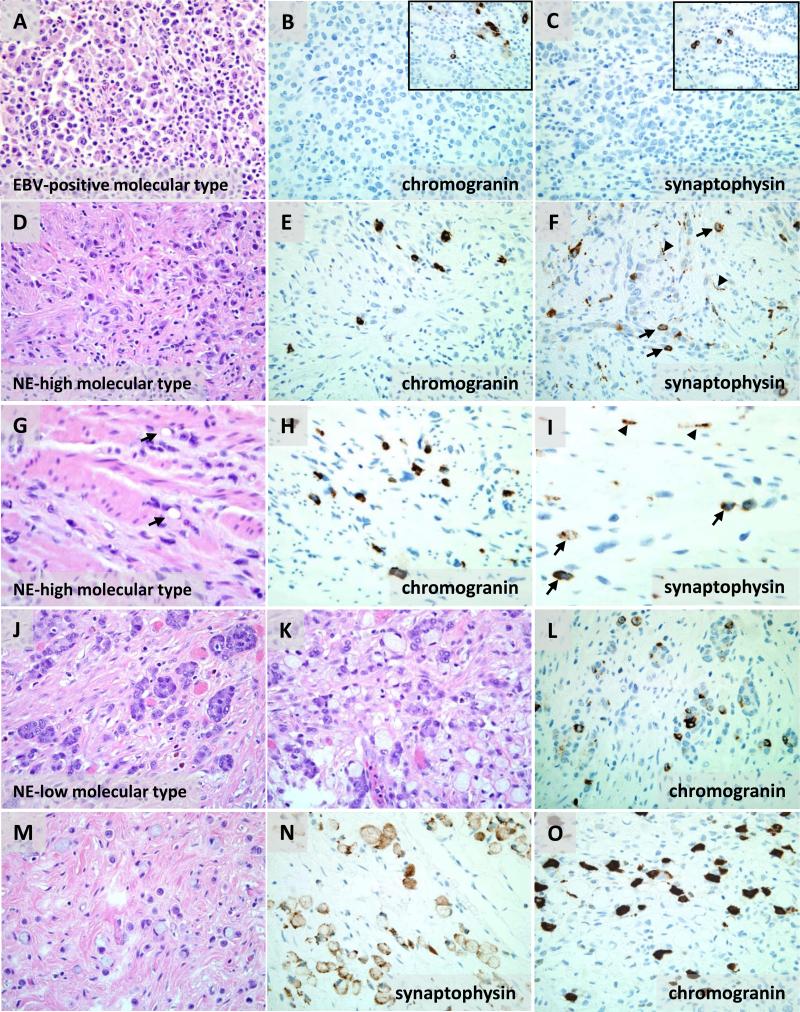
Given the significant association of the molecular groups with the expression of neuroendocrine RNAs, we assessed neuroendocrine differentiation by immunohistochemistry for chromogranin and synaptophysin in representative tumors from the three molecular types (Table S1, Supplemental Digital Content 2, http://links.lww.com/AIMM/A58 ). Immunohistochemistry could be performed only on a small fraction of study samples in part because tissue from many biopsy cases had been exhausted for RNA extraction. Immunohistochemistry was attempted in 16 cases with 14 yielding sufficient tissue for analysis, 5 NE-high cases, 7 NE-low cases, and 2 EBV-positive cases. In the NE-high subtype, chromogranin staining was positive in 4/5 cases (1 NE/small cell carcinoma, 2 isolated-cell carcinomas, and one mixed carcinoma). One NE-high case with neuroendocrine morphology was negative for chromogranin but positive for synaptophysin. In the NE-low subtype, chromogranin staining was positive in 1/7 cases (1 isolated-cell carcinoma) while negative in 2 solid carcinomas and 4 NE carcinomas, including two small cell carcinomas. Three of the four chromogranin-negative NE carcinomas were synaptophysin positive. Neither of the two EBV-positive cases had positive chromogranin or synaptophysin staining. No statistically significant correlation between chromogranin RNA level and protein expression could be established (p=0.06).
Neuroendocrine differentiation is observed only in the non-EBV-infected molecular type cancers
Although only a small number of samples were investigated by immunohistochemistry, positive antibody staining for neuroendocrine markers was observed only in non-EBV-infected molecular type cancers. EBV-positive molecular type cancer with solid morphology lacks chromogranin or synaptophysin-expressing malignant cells (Fig 6A-C). In contrast, scattered single tumor cells positive for chromogranin or synaptophysin were seen in NE-high molecular type cancers that were group 1 isolated-cell histology (Fig 6D-F). NE-high tumors with signet ring cells also showed scattered chromogranin or synaptophysin positive cells (Fig 6G-I). NE-low molecular type tumors with isolated-cell histology similarly had neuroendocrine expressing cells. In one such tumor, two differently staining neuroendocrine elements corresponded to two distinct H&E appearances: one with intracellular mucin and signet ring cell appearance and the other with enlarged vesicular nuclei, prominent nucleoli, and eosinophilic cytoplasm (Fig 6J-O). In retrospect, these atypical nuclear features might be consistent with large cell neuroendocrine carcinoma (see Discussion).
Figure 6. Neuroendocrine differentiation is observed in diffuse cancers of the NE-high and NE-low molecular types but not in the EBV-positive type.
(A) Solid carcinoma of EBV-positive molecular type is negative for chromogranin (B) and synaptophysin (C) with positive internal control in the neuroendocrine cells of residual non-neoplastic gastric pits (insets in B and C). (D) A carcinoma of NE-high molecular type and infiltrating isolated-cell histology has scattered individual malignant cells expressing chromogranin (E) and synaptophysin (arrows, F). (G) Similarly, a signet ring cell carcinoma of NE-high molecular type has chromogranin (H) and synaptophysin expressing cells (arrows, I). Synaptophysin also highlights small nerve twigs within the abundant fibrous stroma (arrowheads, F and I). (J-O) An infiltrative carcinoma of NE-low molecular type has focal areas of small nests and short cords of cells with enlarged vesicular nuclei, prominent nucleoli, and eosinophilic cytoplasm (J), other focal areas in which the nests of eosinophilic cells have admixed signet ring cells (K), but predominantly the tumor consists of eosinophilic and signet ring cells infiltrating singly (M). All three of these tumor morphologies are embedded in dense fibrous stroma. Nested areas have individual chromogranin positive cells within the nests (L). The singly infiltrating eosinophilic cells are chromogranin positive and synaptophysin negative while the signet ring cells have the reverse pattern—chromogranin negative and synaptophysin positive (N,O).
Agreement between neuroendocrine differentiation by morphology and immunohistochemistry is imperfect
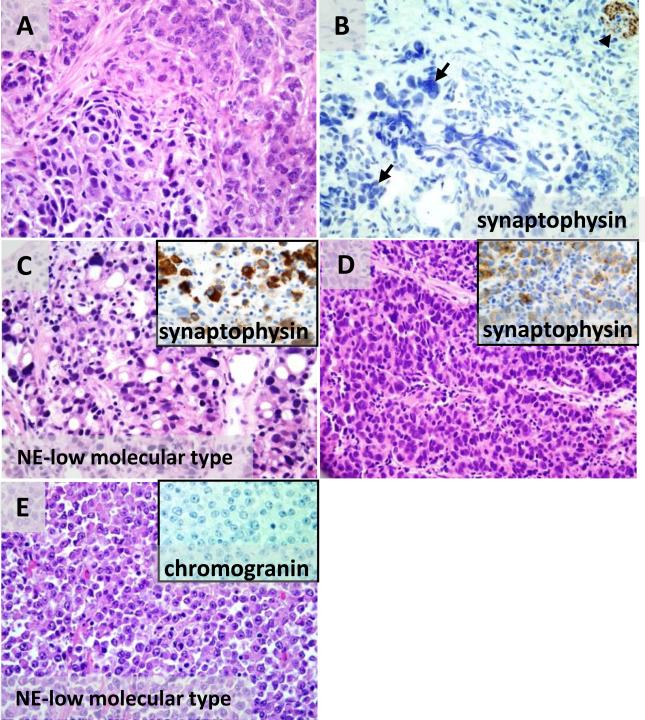
Several tumors with features of neuroendocrine differentiation assessed by H&E showed at least focal synaptophysin and/or chromogranin staining (Fig 4). These tumors often had multiple morphologies within the same tumor evident even in small biopsy specimens. For example, one synaptophysin-positive case showed areas of tumor forming neuroendocrine rosettes and areas of solid nests (Fig 4A-C). The nuclear features were high-grade in both areas with hyperchromasia and nuclear molding. Several other cases had clearly distinct gland-forming/tubular and nested neuroendocrine components (Fig 4D). A small cell carcinoma that was chromogranin-positive had scattered signet ring cells (Fig 4E-F).
However, the initial morphologic assessment of neuroendocrine features did not always correlate with positive immunohistochemistry for synaptophysin and chromogranin. At least one case considered to be a high-grade neuroendocrine carcinoma by H&E did not show any synaptophysin or chromogranin reactivity (Fig 7A-B). This tumor had nested architecture and two cytologically distinct cell populations, one with finely clumped, salt-and-pepper chromatin and more abundant cytoplasm, and the other with nuclear hyperchromasia, increased nuclear/cytoplasmic ratio, and nuclear molding. Conversely, an NE-low molecular subtype tumor that was initially not noted to have neuroendocrine features on H&E showed synaptophysin positivity. This tumor was classified as a group 1 isolated-cell carcinoma with pleomorphic signet ring cells although with unusually little stromal fibrosis (Fig 7C). On re-examination there were minor areas of trabecular/nested growth composed of eosinophilic non-signet ring cells with hyperchromatic nuclei, indistinct cell borders, and nuclear molding—features of high grade neuroendocrine carcinoma (Fig 7D). Lack of fibrosis and packed appearance of both signet ring cells and eosinophilic cells can also favor classification as solid carcinoma. In our experience, however, solid carcinomas without neuroendocrine features have a different appearance with round cell shapes, well-defined cellular borders, absent nuclear molding, and sheet-like rather than nested growth. Fig 7E shows one such tumor of the NE-low molecular subtype classified as solid (group 4). This tumor showed no neuroendocrine differentiation by chromogranin or synaptophysin immunohistochemistry.
Figure 7. Agreement between neuroendocrine differentiation by morphology and immunohistochemistry is imperfect.
(A-B) A tumor with two distinct neuroendocrine cytologic patterns on H&E, salt-and-pepper chromatin (A, upper right) and hyperchromasia and nuclear molding (A, lower left) is negative for synaptophysin (B, arrows at tumor cells with crush artifact) with positive internal control synaptophysin staining in the stromal nerve (B, arrowhead). The tumor cells are also negative for chromogranin protein (not shown). (C-D) An NE-high molecular subtype carcinoma has spatially separate zones of pleomorphic signet ring cells (C) and trabecular/solid nests of non-signet ring cells with high grade neuroendocrine morphology (D). In both zones the malignant cells express synaptophysin (insets in G and H). (E) A carcinoma of NE- low molecular type and solid histology has well-defined cellular borders, prominent nucleoli, absent nuclear molding, and sheet-like growth, and is negative for chromogranin (inset) and synaptophysin (not shown).
Discussion
In this study, RNA expression arrays were applied to profile and cluster gastric cancer tissues, which yielded three molecular types of cancer. These three molecular types were distinguished from one another based primarily on EBV status and by the degree of expression of neuroendocrine RNAs. Furthermore, these molecular types correlated in part with histologic features and with histochemical test results for EBV infection and for neuroendocrine differentiation. The EBV-positive molecular type cancers uniformly expressed EBER in the malignant cells by in situ hybridization. We previously showed that these infected cancers expressed multiple EBV genes as well as a subset of human genes involved in inflammatory response (TNFSF9, TRAF1, CXCL11, IFITM1 and FCRL3)45. Overexpression of the B cell markers MS4A1 (CD20) and FCER2 (CD23), and the suppressor T cell marker CD8 are consistent with the chronic inflammatory cell infiltrate in these tumors and their frequent morphologic diagnosis as “gastric carcinoma with lymphoid stroma”. The correlation between the abundant tumor-infiltrating lymphocytes and the molecular evidence of inflammatory response lends validity to the molecular profiling system used in this study.
EBV-positive cancers in this study tended to have solid morphology with minimal fibrosis, features noted by others as well19,51. This morphologic type is distinguished by the Carneiro gastric cancer classification but not by the Lauren classification. EBV-positive cancers also had more chronic inflammation in keeping with lymphoid stroma rather than fibrotic stroma. None of the EBV-positive cancers were signet ring cell type, although more cases must be studies to evaluate whether this findings is significant.
A significant finding in the EBV-positive cancers was the lack of neuroendocrine differentiation as evaluated by H&E histology, immunohistochemistry, and RNA expression. Molecular profiling divided the non-infected cancers in this study into two groups that differed from each other in the expression of three RNAs known to be associated with neuroendocrine cell differentiation in the gut, CGHA, GAST, and REG453,54,55. However, we did not find a significant correlation between RNA expression and neuroendocrine appearance on H&E. There was also no correlation between chromogranin protein positivity assessed by immunohistochemistry and chromogranin RNA expression. Discordance between chromogranin protein and RNA expression has been described in pulmonary small cell carcinomas56,57,58. Immunoreactivity for chromogranin is associated with the presence of neurosecretory granules in tumor cells, and many high-grade neuroendocrine carcinomas have absent or aberrant production of such granules. Among primary pulmonary small cell carcinomas, immunopositivity for chromogranin is generally only around 80%59 and has been reported as low as 23%60. Absent or focal chromogranin staining in gastric neuroendocrine carcinomas is also a known phenomenon with reported chromogranin positivity of 40%61. The lack of correlation between neuroendocrine RNA expression level and neuroendocrine morphology in this study may be due to 1) the small number of tumors with neuroendocrine morphology, 2) poor reproducibility of neuroendocrine carcinoma morphologic diagnosis, 3) expression of neuroendocrine RNAs in non-malignant cells, or 4) biopsy sampling may not be representative of the entire tumor. We conclude that while neuroendocrine RNAs seem to be major drivers of molecular subtype in this study, it was not possible to use H&E nor standard immunostain evidence of neuroendocrine differentiation to predict molecular subtype.
In two-way analysis, NE-high and NE-low molecular types differed not only in neuroendocrine related transcripts but also in the expression levels of several other genes involved in intercellular signaling and adhesion as well as inflammation and metabolism. It should be noted that only 73 human RNAs were profiled, and thus there is limited ability to examine pertinent biochemical pathways in these tumors.
Recent progress towards targeted therapy for gastric cancer is refining the way that cancer is classified. Already implemented in routine patient care are tests for ERBB2(HER2) protein expression or gene amplification as a means to predict therapeutic efficacy of trastuzumab. In our study, ERBB2 RNA was expressed in some tumors but it was not differentially expressed among the three molecular types that we delineated45. Multiple investigators are pursuing strategies for virus-targeted therapy in the subset of cancers that are EBV-infected62,63,64,65. Efforts focused on molecular and histochemical assays will add value to the histopathologic classification of gastric cancer given the inter- and intra-tumoral heterogeneity that confounds histologic evaluation.
Our findings highlight the difficulty in applying neuroendocrine carcinoma designation. Some tumors assessed as having neuroendocrine morphology by H&E were negative for chromogranin or synaptophysin immunohistochemistry. Conversely, some tumors not suspected to have neuroendocrine differentiation showed neuroendocrine marker positivity by immunohistochemistry. Multiple studies have found that neuroendocrine differentiation is under-recognized by morphology alone, and ancillary methods often reveal evidence of it in various tumor types39,47,55,66. Large cell neuroendocrine carcinoma is especially difficult to distinguish from poorly differentiated adenocarcinoma because of overlapping cytologic features and growth patterns, and even accumulation of intracellular mucin is permissible in large cell neuroendocrine carcinoma33,34,35. In our study we observed inter- and intra-tumoral heterogeneity in tumors with neuroendocrine morphologic features as well.
One important question is: does neuroendocrine differentiation in gastric cancer have clinical implications? It seems clear that tumors fulfilling the diagnostic criteria for small cell carcinoma are a distinct group of biologically aggressive malignancies67. Prognostication becomes more difficult when the high grade neuroendocrine component constitutes only part of the tumor. One study set a threshold for tumor designation as large cell neuroendocrine carcinoma if the neuroendocrine component was >50% of the tumor by chromogranin immunostain33. In that study, adenocarcinomas with 20 to 50% chromogranin-positive large cell neuroendocrine component showed reduced survival compared to adenocarcinoma with no neuroendocrine component. There have been conflicting reports of a worse37,39 or better41,68 prognosis associated with any focal neuroendocrine differentiation by immunohistochemistry. Given the uncertainty of the morphologic criteria and the variability of protein immunohistochemistry, it seems logical to ask if an RNA-based molecular test may provide a more objective measure of neuroendocrine differentiation. Further studies are necessary to test if an RNA threshold is clinically significant, and to compare the performance of RNA-based tests to protein immunohistochemistry. It is likely that tumors with a clinically significant level of neuroendocrine differentiation would be defined not by a single RNA but by a panel of several RNA markers such as the ones used in this study. An important limitation of this study is the lack of outcome data to determine if the three molecular subtypes correspond to gastric cancer subsets with clinically significant differences in disease progression or response to therapy.
In conclusion, our study shows molecular evidence of neuroendocrine differentiation in over a third of gastric adenocarcinomas. However, EBV-infected tumors completely lack neuroendocrine differentiation either in their molecular profile or by morphology. EBV-infected are predominantly solid morphologic type which is distinguished by the Carneiro histologic classification but not the Lauren classification. Our study also confirms that molecular profiling has promise as a robust tool, applicable in small fixed biopsies, to add value in classifying gastric cancer.
Supplementary Material
Acknowledgements
We thank Ying Li and members of the University of North Carolina Translational Pathology Laboratory for performing histochemical stains.
This study was sponsored by the University of North Carolina Department of Pathology and Laboratory Medicine, the University Cancer Research Fund, the Alliance for Clinical Trials in Oncology (NCI U10 CA031946), a Clinical Translational Science Award (NIH U54 RR024383), an award for Innovative Technologies for Molecular Analysis of Cancer (NCI R21 CA155543), and the Western Honduras Gastric Cancer Initiative (NCI CA125588, DRM).
Contributor Information
Olga Speck, Department of Pathology & Laboratory Medicine University of North Carolina Chapel Hill, NC 27599-7525 OSpeck@unch.unc.edu.
Weihua Tang, Department of Pathology & Laboratory Medicine University of North Carolina 913 Brinkhous-Bullitt Building Chapel Hill, NC 27599-7525 weihua_tang@med.unc.edu.
Douglas R. Morgan, Division of Gastroenterology Department of Medicine University of North Carolina Chapel Hill, NC 27599-7080 Douglas.morgan@vanderbilt.edu.
Pei Fen Kuan, Biostatistics Lineberger Comprehensive Cancer Center University of North Carolina Chapel Hill, NC 27599-7295 pfkuan@email.unc.edu.
Michael O. Meyers, Surgical Oncology Lineberger Comprehensive Cancer Center P1150 Physician's Office Bldg. 170 Manning Dr. Chapel Hill, NC 27599-7213 michael_meyers@med.unc.edu.
Ricardo L. Dominguez, Department of Medicine Hospital de Occidente Santa Rosa de Copan Honduras ricardoleoneldominguez@yahoo.com.
Enrique Martinez, Department of Gastroenterology Hospital Evangelico Siguatepeque, Honduras vanor77@yahoo.com.
Margaret L. Gulley, Department of Pathology & Laboratory Medicine Lineberger Comprehensive Cancer Center University of North Carolina 913 Brinkhous-Bullitt Building Chapel Hill, NC 27599-7525.
References
- 1.Lauren P. The two histochemical main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 2.Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39(6):2475–2485. doi: 10.1002/1097-0142(197706)39:6<2475::aid-cncr2820390626>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. International Agency for Research on Cancer . Pathology and genetics of tumours of the digestive system. IARC Press; Oxford University Press (distributor,); Lyon: Oxford: 2000. [Google Scholar]
- 4.Kubo T. Histologic appearance of gastric carcinoma in high and low mortality countries: comparison between Kyushu, Japan and Minnesota, USA. Cancer. 1971;28(3):726–734. doi: 10.1002/1097-0142(197109)28:3<726::aid-cncr2820280331>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut. 1992;33(5):606–612. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Japanese Gastric Cancer Association Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1(1):10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 7.Carneiro F, Seixas M, Sobrinho-Simões M. New elements for an updated classification of the carcinomas of the stomach. Pathol. Res. Pract. 1995;191(6):571–584. doi: 10.1016/S0344-0338(11)80878-2. [DOI] [PubMed] [Google Scholar]
- 8.Chiaravalli AM, Cornaggia M, Furlan D, et al. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch. 2001;439(2):158–169. doi: 10.1007/s004280100441. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T, Ikegami N, Hosoda Y, Koide O, Kaneko M. The biological behaviour of gastric cancer. J. Pathol. 1981;134(2):97–115. doi: 10.1002/path.1711340202. [DOI] [PubMed] [Google Scholar]
- 10.Luinetti O, Fiocca R, Villani L, et al. Genetic pattern, histological structure, and cellular phenotype in early and advanced gastric cancers: evidence for structure-related genetic subsets and for loss of glandular structure during progression of some tumors. Hum. Pathol. 1998;29(7):702–709. doi: 10.1016/s0046-8177(98)90279-9. [DOI] [PubMed] [Google Scholar]
- 11.Murayama H, Imai T, Kikuchi M. Solid carcinomas of the stomach. A combined histochemical, light and electron microscopic study. Cancer. 1983;51(9):1673–1681. doi: 10.1002/1097-0142(19830501)51:9<1673::aid-cncr2820510920>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.STEINER PE, MAIMON SN. Gastric cancer; morphologic factors in 5-year survival after gastrectomy. Am. J. Pathol. 1948;24(5):947–969. [PMC free article] [PubMed] [Google Scholar]
- 13.Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am. J. Surg. Pathol. 2003;27(10):1302–1312. doi: 10.1097/00000478-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ushiku T, Uozaki H, Shinozaki A, et al. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci. 2009;100(4):626–632. doi: 10.1111/j.1349-7006.2009.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palli D, Bianchi S, Cipriani F, et al. Reproducibility of histologic classification of gastric cancer. Br. J. Cancer. 1991;63(5):765–768. doi: 10.1038/bjc.1991.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flucke U, Mönig SP, Baldus SE, et al. Differences between biopsy- or specimen-related Laurén and World Health Organization classification in gastric cancer. World J Surg. 2002;26(2):137–140. doi: 10.1007/s00268-001-0195-0. [DOI] [PubMed] [Google Scholar]
- 17.Jónasson L, Hallgrímsson J, Olafsdóttir G. Gastric carcinoma: correlation of diagnosis based on biopsies and resection specimens with reference to the Laurén classification. APMIS. 1994;102(9):711–715. doi: 10.1111/j.1699-0463.1994.tb05224.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol. Int. 2010;60(5):337–350. doi: 10.1111/j.1440-1827.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Ueki T, Yao T, Ueyama T, Tsuneyoshi M. Epstein-Barr virus in gastric carcinoma with lymphoid stroma. Special reference to its detection by the polymerase chain reaction and in situ hybridization in 99 tumors, including a morphologic analysis. Cancer. 1994;73(9):2239–2249. doi: 10.1002/1097-0142(19940501)73:9<2239::aid-cncr2820730902>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Chang MS, Kim WH, Kim CW, Kim YI. Epstein-Barr virus in gastric carcinomas with lymphoid stroma. Histopathology. 2000;37(4):309–315. doi: 10.1046/j.1365-2559.2000.01014.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsunou H, Konishi F, Hori H, et al. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77(10):1998–2004. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1998::AID-CNCR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38(1):232–243. doi: 10.1002/1097-0142(197607)38:1<232::aid-cncr2820380135>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod. Pathol. 2003;16(7):641–651. doi: 10.1097/01.MP.0000076980.73826.C0. [DOI] [PubMed] [Google Scholar]
- 24.Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. 1990;3(3):377–380. [PubMed] [Google Scholar]
- 25.Kijima Y, Ishigami S, Hokita S, et al. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett. 2003;200(1):33–40. doi: 10.1016/s0304-3835(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 26.Van Beek J, zur Hausen A, Klein Kranenbarg E, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J. Clin. Oncol. 2004;22(4):664–670. doi: 10.1200/JCO.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 27.Lee J-H, Kim S-H, Han S-H, et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J. Gastroenterol. Hepatol. 2009;24(3):354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 28.Camargo MC, Kim W-H, Chiaravalli AM, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2013 doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harn HJ, Chang JY, Wang MW, et al. Epstein-Barr virus-associated gastric adenocarcinoma in Taiwan. Hum. Pathol. 1995;26(3):267–271. doi: 10.1016/0046-8177(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 30.Tokunaga M, Land CE, Uemura Y, et al. Epstein-Barr virus in gastric carcinoma. Am. J. Pathol. 1993;143(5):1250–1254. [PMC free article] [PubMed] [Google Scholar]
- 31.Tokunaga M, Uemura Y, Tokudome T, et al. Epstein-Barr virus related gastric cancer in Japan: a molecular patho-epidemiological study. Acta Pathol. Jpn. 1993;43(10):574–581. doi: 10.1111/j.1440-1827.1993.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. International Agency for Research on Cancer . WHO classification of tumours of the digestive system. International Agency for Research on Cancer; Lyon: 2010. [Google Scholar]
- 33.Jiang S-X, Mikami T, Umezawa A, et al. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am. J. Surg. Pathol. 2006;30(8):945–953. doi: 10.1097/00000478-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Shia J, Tang LH, Weiser MR, et al. Is nonsmall cell type high-grade neuroendocrine carcinoma of the tubular gastrointestinal tract a distinct disease entity? Am. J. Surg. Pathol. 2008;32(5):719–731. doi: 10.1097/PAS.0b013e318159371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui K, Jin XM, Kitagawa M, Miwa A. Clinicopathologic features of neuroendocrine carcinomas of the stomach: appraisal of small cell and large cell variants. Arch. Pathol. Lab. Med. 1998;122(11):1010–1017. [PubMed] [Google Scholar]
- 36.Ishida M, Sekine S, Fukagawa T, et al. Neuroendocrine carcinoma of the stomach: morphologic and immunohistochemical characteristics and prognosis. Am. J. Surg. Pathol. 2013;37(7):949–959. doi: 10.1097/PAS.0b013e31828ff59d. [DOI] [PubMed] [Google Scholar]
- 37.Ooi A, Mai M, Ogino T, et al. Endocrine differentiation of gastric adenocarcinoma. The prevalence as evaluated by immunoreactive chromogranin A and its biologic significance. Cancer. 1988;62(6):1096–1104. doi: 10.1002/1097-0142(19880915)62:6<1096::aid-cncr2820620612>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 38.Waldum HL, Aase S, Kvetnoi I, et al. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83(3):435–444. [PubMed] [Google Scholar]
- 39.Staren ED, Lott S, Saavedra VM, et al. Neuroendocrine carcinomas of the stomach: a clinicopathologic evaluation. Surgery. 1992;112(6):1039–1046. discussion 1046–1047. [PubMed] [Google Scholar]
- 40.Qvigstad G, Sandvik AK, Brenna E, Aase S, Waldum HL. Detection of chromogranin A in human gastric adenocarcinomas using a sensitive immunohistochemical technique. Histochem. J. 2000;32(9):551–556. doi: 10.1023/a:1004102312006. [DOI] [PubMed] [Google Scholar]
- 41.Fujiyoshi Y, Eimoto T. Chromogranin A expression correlates with tumour cell type and prognosis in signet ring cell carcinoma of the stomach. Histopathology. 2008;52(3):305–313. doi: 10.1111/j.1365-2559.2007.02950.x. [DOI] [PubMed] [Google Scholar]
- 42.Waldum HL, Haugen OA, Isaksen C, Mecsei R, Sandvik AK. Enterochromaffin-like tumour cells in the diffuse but not the intestinal type of gastric carcinomas. Scand. J. Gastroenterol. Suppl. 1991;180:165–169. doi: 10.3109/00365529109093195. [DOI] [PubMed] [Google Scholar]
- 43.Bakkelund K, Fossmark R, Nordrum I, Waldum H. Signet ring cells in gastric carcinomas are derived from neuroendocrine cells. J. Histochem. Cytochem. 2006;54(6):615–621. doi: 10.1369/jhc.5A6806.2005. [DOI] [PubMed] [Google Scholar]
- 44.Bartley AN, Rashid A, Fournier KF, Abraham SC. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: evidence for a common cell of origin in composite tumors. Hum. Pathol. 2011;42(10):1420–1429. doi: 10.1016/j.humpath.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Tang W, Morgan DR, Meyers MO, et al. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect. Agents Cancer. 2012;7(1):21. doi: 10.1186/1750-9378-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carneiro F. Classification of gastric carcinomas. Curr. Diag. Pathol. 1997;4(1):51–59. [Google Scholar]
- 47.Namikawa T, Oki T, Kitagawa H, et al. Neuroendocrine carcinoma of the stomach: clinicopathological and immunohistochemical evaluation. Med Mol Morphol. 2013;46(1):34–40. doi: 10.1007/s00795-012-0006-8. [DOI] [PubMed] [Google Scholar]
- 48.Chang KL, Chen YY, Shibata D, Weiss LM. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn. Mol. Pathol. 1992;1(4):246–255. [PubMed] [Google Scholar]
- 49.Lu DY, Qian J, Easley KA, Waldrop SM, Cohen C. Automated in situ hybridization and immunohistochemistry for cytomegalovirus detection in paraffin-embedded tissue sections. Appl. Immunohistochem. Mol. Morphol. 2009;17(2):158–164. doi: 10.1097/PAI.0b013e318185d1b5. [DOI] [PubMed] [Google Scholar]
- 50.Ryan JL, Morgan DR, Dominguez RL, et al. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Lab. Invest. 2009;89(1):80–90. doi: 10.1038/labinvest.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 1992;140(4):769–774. [PMC free article] [PubMed] [Google Scholar]
- 52.Sentani K, Oue N, Sakamoto N, et al. Gene expression profiling with microarray and SAGE identifies PLUNC as a marker for hepatoid adenocarcinoma of the stomach. Mod. Pathol. 2008;21(4):464–475. doi: 10.1038/modpathol.3801050. [DOI] [PubMed] [Google Scholar]
- 53.Mouland AJ, Bevan S, White JH, Hendy GN. Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression. J. Biol. Chem. 1994;269(9):6918–6926. [PubMed] [Google Scholar]
- 54.Rindi G, Inzani F, Solcia E. Pathology of gastrointestinal disorders. Endocrinol. Metab. Clin. North Am. 2010;39(4):713–727. doi: 10.1016/j.ecl.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Sentani K, Oue N, Noguchi T, et al. Immunostaining of gastric cancer with neuroendocrine differentiation: Reg IV-positive neuroendocrine cells are associated with gastrin, serotonin, pancreatic polypeptide and somatostatin. Pathol. Int. 2010;60(4):291–297. doi: 10.1111/j.1440-1827.2010.02519.x. [DOI] [PubMed] [Google Scholar]
- 56.Abbona G, Papotti M, Viberti L, et al. Chromogranin A gene expression in non-small cell lung carcinomas. J. Pathol. 1998;186(2):151–156. doi: 10.1002/(SICI)1096-9896(1998100)186:2<151::AID-PATH154>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 57.Sumiyoshi Y, Shirakusa T, Yamashita Y, et al. Detection of chromogranin A mRNA in small cell lung carcinoma using a new, highly sensitive in situ hybridization method with a non-radioisotope oligonucleotide probe. Cancer. 1998;82(3):468–473. doi: 10.1002/(sici)1097-0142(19980201)82:3<468::aid-cncr7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 58.Hamid Q, Corrin B, Sheppard MN, Huttner WB, Polak JM. Expression of chromogranin A mRNA in small cell carcinoma of the lung. J. Pathol. 1991;163(4):293–297. doi: 10.1002/path.1711630405. [DOI] [PubMed] [Google Scholar]
- 59.Lyda MH, Weiss LM. Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum. Pathol. 2000;31(8):980–987. doi: 10.1053/hupa.2000.9076. [DOI] [PubMed] [Google Scholar]
- 60.Shy SW, Lee WH, Chou MC, Lai YS, Tu YC. Small cell lung carcinoma: clinicopathological, immunohistochemical, and ultrastructural study. J Surg Oncol. 1990;45(3):146–161. doi: 10.1002/jso.2930450304. [DOI] [PubMed] [Google Scholar]
- 61.Namikawa T, Oki T, Kitagawa H, et al. Neuroendocrine carcinoma of the stomach: clinicopathological and immunohistochemical evaluation. Med Mol Morphol. 2013;46(1):34–40. doi: 10.1007/s00795-012-0006-8. [DOI] [PubMed] [Google Scholar]
- 62.Meng Q, Hagemeier SR, Fingeroth JD, et al. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J. Virol. 2010;84(9):4534–4542. doi: 10.1128/JVI.02487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merlo A, Turrini R, Dolcetti R, Zanovello P, Rosato A. Immunotherapy for EBV-associated malignancies. Int. J. Hematol. 2011;93(3):281–293. doi: 10.1007/s12185-011-0782-2. [DOI] [PubMed] [Google Scholar]
- 64.Claerhout S, Lim JY, Choi W, et al. Gene expression signature analysis identifies vorinostat as a candidate therapy for gastric cancer. PLoS ONE. 2011;6(9):e24662. doi: 10.1371/journal.pone.0024662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao J, Jin H, Cheung KF, et al. Zinc finger E-box binding factor 1 plays a central role in regulating Epstein-Barr virus (EBV) latent-lytic switch and acts as a therapeutic target in EBV-associated gastric cancer. Cancer. 2012;118(4):924–936. doi: 10.1002/cncr.26184. [DOI] [PubMed] [Google Scholar]
- 66.Blumenfeld W, Chandhoke DK, Sagerman P, Turi GK. Neuroendocrine differentiation in gastric adenocarcinomas. An immunohistochemical study. Arch. Pathol. Lab. Med. 1996;120(5):478–481. [PubMed] [Google Scholar]
- 67.Brenner B, Tang LH, Shia J, Klimstra DS, Kelsen DP. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin. Oncol. 2007;34(1):43–50. doi: 10.1053/j.seminoncol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Radi MJ, Fenoglio-Preiser CM, Bartow SA, Key CR, Pathak DR. Gastric carcinoma in the young: a clinicopathological and immunohistochemical study. Am. J. Gastroenterol. 1986;81(9):747–756. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.