Abstract
The isolate ATCC® 50979™ is a small amoebozoan whose actin gene was previously characterized, but did not allow a stable phylogenetic placement. This isolate was originally mis-identified upon deposition, and subsequently mis-illustrated in a recent publication. Here, we provide both a detailed morphological description as well as additional molecular analyses in order to clarify the isolate's phylogenetic relationships. The amoeba is minute (less than 5µm), and presents the behavior of staying in a fixed location, while emitting one or two thin pseudopods. Transmission electron-microscopy reveals that the cell is covered in a layer with embedded scales, giving the cell an armored appearance. Molecular phylogenetic analyses of data (actin, alpha- and beta- tubulin, elongation factor 2, and 14-3-3) from transcriptomes of this and four other isolates reveals that ATCC® 50979™ is closely related to the recently described Squamamoeba japonica and in a novel, stable clade. Due to the unique nature of the scale covering, as well as other gross morphological characters and the molecular phylogenetic analyses, we formally describe the isolate as Sapocribrum chincoteaguense n. gen. n. sp.
Keywords: Filamoeba, ATCC PRA-39, phylogenetic analyses, Squamamoeba, Squamamoebidae Transcriptome, Transmission Electron Microscopy, Trichosphaerium
EUKARYOTIC cells often present a covering layer external to the plasma membrane. The cellulose walls in plant cells represent a familiar example, but diverse eukaryotes may possess other types of coverings, such as the fuzzy glycocalyces in animal epithelial cells and the calcified scales of haptophytes. External cell ornamentation is also a common occurrence in the Amoebozoa (Smirnov et al. 2011). Perhaps the most widely known type of covering among amoebozoans are the shells (tests) constructed by arcellinids, which may be secreted, agglutinated or a combination of both. Other lesser-known types of ornamentation include mucous covering as in Gocevia and Endostelium (Olive, Bennett, and Deasey 1984; Lahr et al. 2011) and the leathery coverings in several species of putative arcellinids such as Microcorycia and Amphizonella (Badewitz 2004; Meisterfeld and Badewitz 2006). The presence of scales is also widespread in Amoebozoa, including cochliopodids and dactylopodids (Kudryavtsev, Pawlowski, and Hausmann 2011; Kudryavtsev, Wylezich, and Pawlowski 2011), the protosteloid Ceratiomyxella tahitihensis (Furtado and Olive 1972, Shadwick et al. 2009), and in the recently described genus Squamamoeba (Kudryavtsev and Pawlowski 2013).
Further, several amoebozoans present distinct types of “cell coats” (Smirnov and Brown 2004). Smirnov and Brown (2004), define five types of “cell coats”: filamentous glycocalyces (in Amoebidae), amorphous glycocalyces (in Thecamoebidae), cuticles (such as in Mayorella), scales (eg. Korotnevella and Paramoeba), and finally glycostyles, which are elongated polysaccharide structures embedded within the membrane matrix. In a very broad sense, these “cell coats” might be interpreted as “glycocalyces” as defined by Bennett (1963), referring to polysaccharides external to the cellular membrane. Instead, Smirnov and Brown (2004) justify the non-inclusion of these structures under the category “glycocalyx” either because composition is unknown (as is the case for most scales) or the structures are not embedded in the cellular membrane (as in cuticles). In the current essay, we use the term “cell-coat” as defined by Smirnov and Brown (2004).
Among amoebozoans, the most well developed cell-coats can be found in dactylopodids, thecamoebids, vannellids, pellitids and Dermamoeba (Page 1973; Dykova et al. 1998; Dykova, Figueras, and Peric 2000; O’Kelly et al. 2001; Fiala and Dykova 2003; Smirnov and Kudryavtsev 2005; Michel et al. 2006; Smirnov, Bedjagina, and Goodkov 2011). Cell-coats have been used for classification purposes, but recent results from molecular analyses have mostly jumbled these attempted classifications, as it appears that at least glycostyles have evolved multiple times in the Amoebozoa (Smirnov et al. 2011). Although external cell ornamentations have successfully been used to define monophyletic units within the Amoebozoa (e.g. arcellinids (Lahr et al. 2011)), the evolution and relationships between different types of external ornamentation in Amoebozoa remains an open question.
Here we describe a marine amoeboid isolate from Chincoteague Bay with external ornamentation and affinities with the previously described scaled amoeba Squamamoeba. This isolate has been deposited in the American Type Culture Collection (ATCC®) under accession number ATCC® 50979™, and previously misidentified as belonging to the arcellinid genus Sexangularia. It was subsequently mis-illustrated in Lahr et al. 2011, due to an error in labeling. We show here that the organism does not possess a shell, and cannot therefore be an arcellinid. The actin gene of this isolate has been analyzed previously, and preliminary phylogenetic analyses have placed it close to the dactylopodids (Lahr et al. 2011).
The present work describes the morphological aspects of the isolate ATCC® 50979™ and offers an expanded molecular analysis based on available data. We characterize the taxon as novel based on the unique scale morphology. Additionally, further molecular reconstructions are presented that incorporate relevant species described since the molecular data of the ATCC® 50979™ were first published, as well as adding more genes from next-generation sequencing of transcriptomes from several marine Amoebozoa.
MATERIALS AND METHODS
Cultures
Cultures of ATCC® 50979™ were maintained (2000–2002) in 25 cm2 polystyrene culture flasks under conditions described in Table 1. These were re-grown twice independently in 2008 and 2011. Due to a mistake in image labeling, the figures 1f', g' and h' published in (Lahr et al. 2011) belong to a different isolate which we were not able to identify – an erratum has been published in the PLoS One website (http://www.plosone.org/annotation/listThread.action?root=79421). The purity of the lineage has been verified on all three occasions (i.e. we confirmed that a single morpho-type is present in the lineage), and present here newer images from those verified pure isolates.
Table 1.
Cultures used in this study
| ATCC No. |
Organism | Culture medium | Food source | Temperature |
|---|---|---|---|---|
| 50979 | Sapocribrumchincoteaguense | Cerophyll® in seawater (ATCC medium 1525) with 0.02% FBS | Enterobacter aerogenes(ATCC® 13048™)1 | 20–25 °C |
| 50430 | Filamoebanolandi | Cerophyll® in distilled water (ATCC medium 802) | Enterobactera erogenes ATCC® 13048TM and mixed bacteria | 20–25 °C |
| PRA29 | Pessonella sp. | Cerophyll® in seawater (ATCC medium 1525) | Klebsiella pneumoniae (ATCC® 700831TM) | 20–25 °C |
| 40318 | Trichosphaerium sp. | HESNW medium (ATCC medium 1405) | Heat-killed Chlamydomonas reinhardtii (ATCC® PRA-142TM) | 20–25 °C |
When required, bacterial overgrowth was controlled by treating the culture with an antibiotic cocktail of 250 U/ml penicillin, 250 µg/ml streptomycin, 250 µg/ml neomycin, and 2.5 U/ml bacitracin.
Fig. 1.
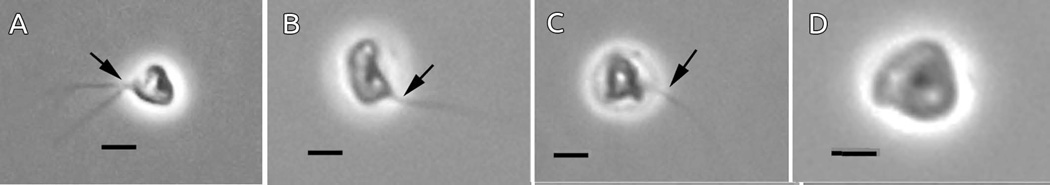
Light microscopic images of living Sapocribrum chincoteaguense n. gen. n. sp. A-C. Motile cells, typically nearly spheroidal (A) to approximately reniform (B) with very thin emergent pseudopodia (arrows), sometimes bifurcating (A). D. A sedentary amoeba exhibiting the angular morphology typically observed for non-motile individuals. (Scale bars = 2 µm).
Additionally, cultures of Filamoeba nolandi (ATCC® 50430™), 'Pessonella' (ATCC® PRA-29™), and Trichosphaerium sp. (ATCC® 40318™) were grown to obtain additional transcriptomes of amoebozoan groups in our analyses, these were already published in Grant and Katz (2014).
Light microscopy
Living cells of ATCC® 50979™ were observed on glass slides with cover slips using an AxioScope compound microscope (Carl Zeiss, Thornwood, New York, U.S.A.) with phase contrast at 1000x magnification under a 100x objective (oil immersion). Digital photomicrographs were obtained with an AxioCam digital camera and Axiovision 4.6 software (Zeiss). The images of locomoting amoebae were captured from individual frames of video recorded behavior of living amoebae that had settled on the slide.
Transmission electron microscopy
Amoebae of ATCC® 50979™ were fixed for transmission electron microscopy as previously published (Anderson, Rogerson, and Hannah 1997). A suspension of the amoebae in culture medium at 25 °C was placed in a 15-ml graduated conical centrifuge tube, allowed to settle briefly, and mixed with an equal volume of 4% (w/v) TEM-grade glutaraldehyde in 0.2 M cacodylate buffer, pH 7.2, to yield a final fixative of 2% (w/v). The tube containing the fixed amoebae was plunged in an ice bath, and after 20 min. at 3 °C, the glutaraldehyde-fixed cells were gently spun down to form a pellet, the supernatant was removed by aspiration, and 2 ml of 2% (w/v) cold osmium tetroxide solution in 0.2 M cacodylate buffer (pH 7.2) were added and the pellet thoroughly dispersed in the fixative. After 1-h post-fixation at 3°C, the cells were again pelleted and the supernatant removed.
The pelleted cells were enrobed in 0.8% (w/v) agar and small cubes (~1 mm) were cut from the agar block, washed in distilled water, dehydrated in a graded acetone/aqueous series, infiltrated with and embedded in low viscosity epon (Energy Beam Sciences, Agawam, MA, U.S.A.), and polymerized at 75°C for 12–18 h. Ultrathin sections were cut with a Porter-Blum MT-2 ultramicrotome (Sorvall, Norwalk, CT, U.S.A.) using a diamond knife, collected on uncoated copper grids, and post-stained with Reynold's lead citrate. Surface coat morphology was observed by depositing small drops of fixed amoebae on a carbon-deposited, collodion-covered grid, air dried at room temperature, and shadowed with a carbon-platinum source in a vacuum evaporator. Preparations of ultrathin sections or the shadowed grids were viewed with a Philips TEM-201 transmission electron microscope (Einthoven, Netherlands) operated at 60 kV accelerating voltage. Surface microscale dimensions were measured on the shadowed TEM preparations.
RNA isolation and Transcriptome data
We compared transcriptome data from Sapocribrum chincoteaguense (ATCC® 50979™), Filamoeba nolandi (ATCC® 50430™), isolate ATCC® PRA-29™, and Trichosphaerium sp. (ATCC® 40318™) obtained from the National Center for Genome Resources (NCGR) as part of the Gordon and Betty Moore Foundation's Marine Microbiology Initiative (http://marinemicroeukaryotes.org/). Total RNA was isolated from live cultures using the RNeasy Mini Kit (Qiagen) and treated with DNase I (New England Biolabs) to eliminate any contaminating DNA. Purity of RNA was determined by measuring the ratio of absorbance at 260 nm and 280 nm. Quantification of RNA was performed using the Quant-iT™ RiboGreen® RNA Assay (Life Technologies). Samples with an OD 260/280 equal to 1.8–1.9 and total RNA concentration ≥3 µg were submitted to the NCGR for transcriptome sequencing. Transcriptome data for ATCC® 50979™ is publicly available at http://camera.crbs.ucsd.edu/mmetsp/details.php?id=MMETSP0437.
We passed the assembled transcriptome data through a series of quality control steps to remove rDNA and bacterial contaminants, as described in Grant et al. (2012). We searched for homologs to five genes of interest – actin, alpha- and beta- tubulin, elongation factor 2, and 14-3-3 – using custom python scripts as in Grant and Katz (2014), which includes a preliminary analysis of S. chincoteaguense (Eukaryota sp. JG2011) as a test-case for development of the pipeline. For F. nolandi we recovered all five genes; for lineage ATCC® PRA-29 and Trichosphaerium sp. we recovered four (actin, beta-tubulin, elongation factor 2 and 14-3-3), and for S. chincoteaguense we recovered two (actin and elongation factor 2.) One actin gene from S. chincoteaguense had previously been acquired by PCR (Lahr, 2011) and deposited in GenBank (accession #JF94319.1), and a nearly identical sequence (1 SNP in 973 base pairs) was also found in the transcriptome data.
Phylogenetic reconstruction
Two approaches were taken to the phylogenetic reconstruction: 1) an approach combining SSU rDNA and actin genes, which included 140 taxa (130 amoebozoans and 10 opisthokonts); and 2) an approach using 5 protein coding genes and SSU rDNA with 126 taxa (62 amoebozoans and 64 opisthokonts chosen as outgroups):
For strategy 1, taxon sampling for Amoebozoa was identical to the dataset used in (Lahr et al. 2011), with addition of the following relevant taxa: Ceratiomyxella tahitiensis (FJ544419, Shadwick et al. 2009) and the three Squamamoeba species available in GenBank (JN638030-32, Kudryavtsev et al. 2013). Alignments for SSU rDNA and actin were constructed in SeaView (Galtier, Gouy, and Gautier 1996; Gouy, Guindon, and Gascuel 2010) with alignment algorithm MAFFT using the L-INS-I setting (Katoh, Asimenos, and Toh 2009). Alignments were then subjected to automated removal of ambiguously aligned sites using the software GUIDANCE (Penn, Privman, Ashkenazy, et al. 2010; Penn, Privman, Landan, et al. 2010). We performed maximum likelihood phylogenetic reconstruction on the concatenated dataset using RAxML HPC 7.2.7 (Stamatakis 2006; Stamatakis, Hoover, and Rougemont 2008) as implemented in the online server CIPRES (Miller et al. 2009; Miller, Pfeiffer, and Schwartz 2010, 2011). We ran 1000 multiparametric bootstrap searches using among site rate variation following a GAMMA distribution, with the GTR substitution model for the SSU rDNA partition and the LG substitution model with gamma distribution of site heterogeneity for the actin partition. The most appropriate model for amino-acid evolution was determined using model testing implemented in the software ProtTest 3.0 (Darriba et al. 2011). Independently, we ran 100 separate maximum likelihood searches starting from distinct parsimony trees, and a final slow GAMMA optimization of the best scoring tree. Bootstrap values were then plotted on the best tree found by maximum likelihood search for comparative analysis.
For strategy 2, transcriptomes were passed through a custom phylogenomic pipeline searching for homologs for five genes (actin, alpha- and beta- tubulin, elongation factor 2, and 14-3-3) that have relatively deep taxonomic sampling of amoebozoan taxa. The protein genes were concatenated to an SSU alignment representing all taxa except S. chincoteaguense, for which we have been unable to sequence SSU. This resulted in a concatenated alignment with 126 taxa and 3,584 characters, which is available as Supporting Information Matrix S1. We partitioned our data and used the GTRCAT substitution model for the SSU rDNA partition and the LG protein substitution model, which was determined to be the best fitting model for all 5 genes by ProtTest 3.0, for the protein data. We performed maximum likelihood reconstruction on this alignment using RAxML HPC PTHREADS v 7.2.5, which ran 350 independent bootstrap replicates before being stopped by the autoMRE bootstopping algorithm.
RESULTS
Light microscopy
Living amoebae of Sapocribrum chincoteaguense are less than 5 µm when contracted and angular in morphology; motile stages (length = 3.6 ± 0.15 and width = 2.8 ± 0.14, N=21) vary in shape from rounded to elongated (Fig. 1A-C) with thin, filose pseudopodia, sometimes bifurcating (Fig. 1). Amoebae exhibit a squirming or writhing motion during the extension and peripheral elaboration of the pseudopodia, typically either solitary or bifurcated. The latter sometimes become disposed at approximately 180 degrees opposite to one another. Often, a terminal “web” of sub-pseudopodia is produced and attached to the substrate. Sedentary stages are more angular and compacted in appearance (Fig. 1D). See also the shadowed preparations of sedentary amoebae (Fig. 2A-C) exhibiting similar shape. During brief periods of locomotion, lasting several minutes, the body retains a somewhat contracted and angular shape, moving in a meandering path, possibly drawn forward by the contractile action of the adhesive, extended pseudopodia (see Supporting Information Movie S1, S2). Pseudopods are 4.42 ± 0.28 µm long with a range of 2.5 to 9 µm (N = 50).
Fig. 2.
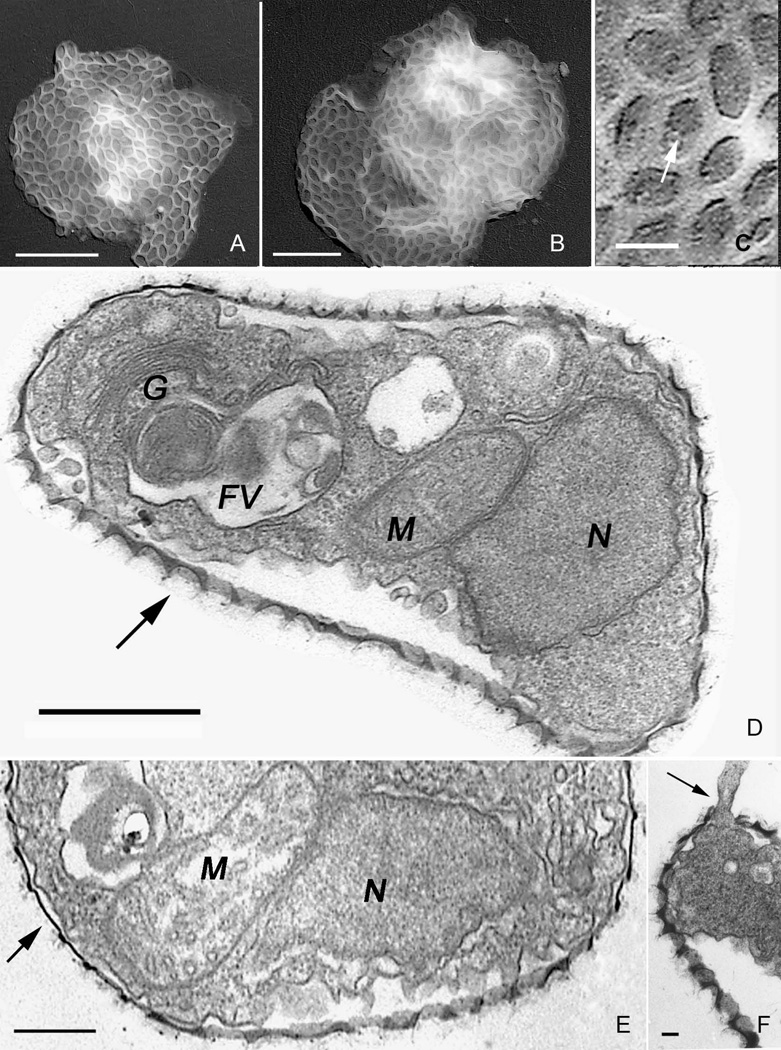
Transmission electron microscopic images of Sapocribrum chincoteaguense n. gen. n. sp. A-C. Shadowed preparations of fixed amoebae showing the oval to rounded scales united within an organic matrix. The amoebae have become somewhat flattened during drying. A, B. whole cells. (Scale bars = 1 µm). C. Details of the scale pore plate showing examples of the scattered pores (arrow). (Scale bar = 150 nm). D-F. Ultrathin sections. D. Cell section showing nucleus (N), mitochondrion (M), food vacuoles (FV) and Golgi apparatus (G). Oblique sections through the scales show the concave profile (arrow) and the electron dense organic matrix. (Scale bar = 1 µm). E. Cytoplasmic details including nucleus (N), tubulo-cristate mitochondrion (M) and enclosing sheath of microscales (arrow). (Scale bar = 0.3 µm). F. Peripheral portion of an amoeba with pseudopodium protruding through the organic matrix and layer of microscales (arrow). (Scale bar = 1 µm).
Transmission microscopy
Carbon-platinum shadowed preparations of whole cells observed with the transmission electron microscope (Fig. 2A-C) display the organization and fine structure of the surface coat of closely-spaced, approximately oval microscales, embedded within an organic envelope. The microscales are composed of thickened rims and a thin areolate, depressed pore plate. The pore plate varies in the pore pattern. Most pores, when present, are irregularly arranged, occurring in a restricted region of the plate (e.g., Fig. 2 C), or in some cases largely distributed at the perimeter of the depressed plate. The length and width (mean ± s.e., N = 50) of the microscales was 150 ± 2.1 and 100 ± 1.5 nm, respectively. The amoebae were fixed suspended in culture medium and many were undoubtedly floating. The purpose was to describe their overall fine structure, not to obtain evidence of their fine structure during attachment to a substratum. Ultrathin sections through the amoeba exhibit the fine structure details of the scales (Fig. 2D), showing the cup-shaped profiles in vertical, partially oblique sections that emphasize the concavity (arrow). Cytoplasmic organization exhibits the nucleus (N), tubulo-cristate mitochondria (M), Golgi body with 4–5 saccules (G), and food vacuoles (FV). The size of the nucleus is ~ 2×1 µm. Segments of smooth and rough endoplasmic reticulum are distributed throughout the cytoplasm. Higher magnifications of the mitochondrial cristae and nucleus (Fig. 2E) more clearly show the tubular cristae in cross-section. Pseudopodia appear to erupt and protrude through the thin surface envelope of microscales and organic matrix (Fig. 2F). Sections directly through the thickness of the organic matrix and microscales (Fig. 2F; 3), show that they are much more thin-rimmed (arrow) and not as concave as the partially oblique sections suggest. A diagram showing our interpretation of the microscales (Fig. 4) illustrates their general organization as interpreted from the fine structure evidence of the shadowed preparations.
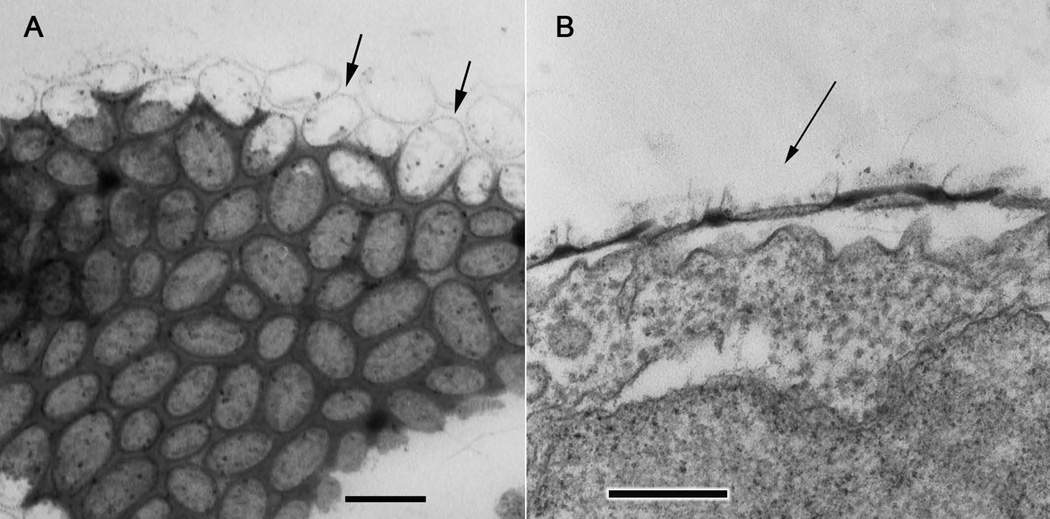
Fig. 3.
Higher magnification images of ultrathin sections through the electron dense organic coat and surface scales of the amoeba. A. Approximately tangential section (slightly oblique to the coat surface) showing the organization of the scales, particularly the oval outlines of the scale perimeters (arrows). B. Transverse section through the vertical thickness of the organic coat showing a more detailed profile of one of the scales (arrow) in vertical profile. Scale bars = 200 nm.
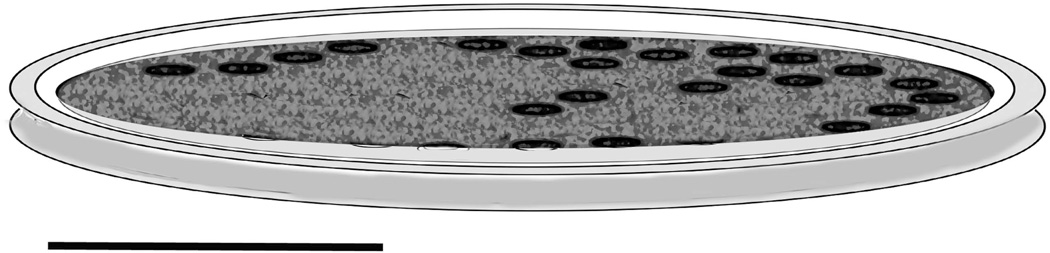
Fig. 4.
Diagram of a microscale showing the thin peripheral rim and the mottled recessed internal plate containing irregularly distributed groups of micropores (Scale bar = 50 nm).
Phylogenetic analyses
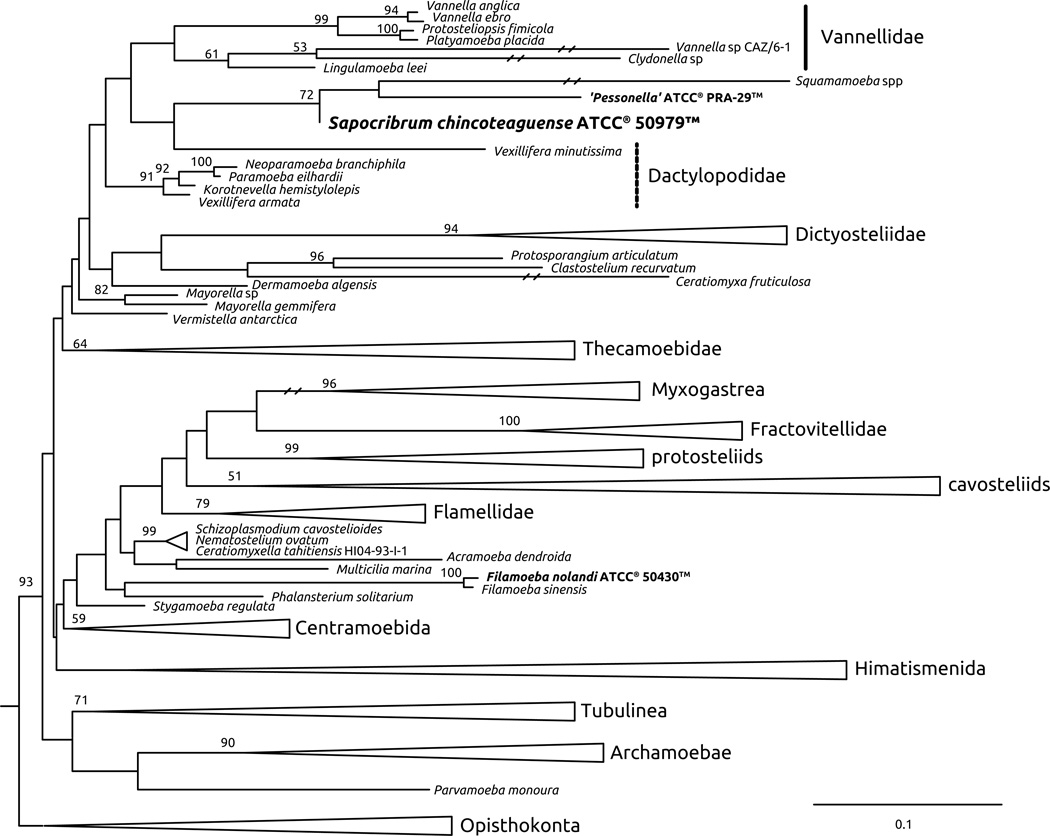
The topologies obtained using both phylogenetic reconstruction approaches were generally concordant with recently published analyses, and place S. chincoteaguense ATCC® 50979™ within the dactylopodids with moderate to strong support. When using an approach with more taxa (strategy 1, Fig. 5), the well-supported groups Dictyosteliidae, Myxogastria, Fractovitellidae, Flamellidae, Centramoebida, Himatismenida, Tubulinea (with embedded Leptomyxida, Echinamoebida, Poseidonida, Tubulinida and Arcellinida), and Archamoebae are recovered with generally moderate to high support (>70), congruent with other recent reconstructions (Kudryavtsev, Wylezich, and Pawlowski 2011; Lahr et al. 2011; Smirnov et al. 2011; Lahr, Grant, and Katz 2013). When using an approach with more genes (strategy 2, Fig. 6), the main established amoebozoan groups are recovered (Archamoebae, Tubulinea, Dictyostelliidae, Cochliopodiidae and Centramoebidae). This approach included the insertae sedis taxon Trichosphaerium sp., which grouped with the myxogastrid representatives most likely due to long branch attraction or other lineage-specific effects as Trichosphaerium sp. gene sequences tend to be divergent from other Amoebozoa.
Fig. 5.
Phylogenetic position of Sapocribrum chincoteaguense from a maximum likelihood reconstruction based on concatenated actin and SSU-rDNA genes and 140 taxa. Major monophyletic groups of Amoebozoa have been collapsed for clarity. Only bootstrap values above 50 are shown. Branches are drawn to scale, except those marked with a double tick have been shortened to half the original length. Molecular data for taxa in bold have been generated in this study.
Fig. 6.
Phylogenetic position of Sapocribrum chincoteaguense from a maximum likelihood reconstruction based on 6 concatenated genes and 126 taxa. Major monophyletic groups of Amoebozoa have been collapsed. Only bootstrap values above 50 are shown. Branches are drawn to scale, except those marked with a double tick have been shortened to half the original length. Molecular data for taxa in bold have been generated in this study.
In both reconstructions, S. chincoteaguense fell within a clade that included ATCC® PRA-29™ and the Squamamoeba species (SSP clade): 72% bootstrap support in the strategy 1 (Fig. 5) and 100% in strategy 2 (Fig. 6). A close-relationship between ATCC® PRA-29™ and Squamamoeba has been indicated in the original description of Squamamoeba (Kudryavtsev and Pawlowski 2013). While the monophyly of the Squamamoeba+Sapocribrum+ ATCC® PRA-29™ clade is robust, the relationships among the three members of the clade cannot be interpreted conclusively, because there are missing data: the SSU rDNA is not available for S. chincoteaguense, and other protein coding genes are not available for Squamamoeba spp.
Deeper relationships between the Squamamoeba+Sapocribrum+ ATCC® PRA-29™ clade and other amoebozoan groups remain elusive. In strategy 1, the Squamamoeba+Sapocribrum+ ATCC® PRA-29™ clade branches sister to the vannellids, and close to the dactylopodids (Fig. 5). These relationships are congruent with the ones proposed for Squamamoeba (Kudryavtsev and Pawlowski 2013). However, in strategy 2, the Squamamoeba+Sapocribrum+ ATCC® PRA-29™ clade is sister to the Cochliopodiidae, and both are sister to the vannellids (without support), instead of being closely related to the dactylopodids (Fig. 6), similar to relationships recovered in Kudryavtsev et al. (2014). It is noteworthy that cochliopodids are a recognized insertae sedis taxon, hence the branching with Squamamoeba+Sapocribrum + ATCC® PRA-29™ may be artifactual (Kudryavtsev, Wylezich, and Pawlowski 2011; Lahr et al. 2011; Smirnov et al. 2011)
DISCUSSION
Morphology
The gross and ultrastructural morphology characterize ATCC® 50979™ as a new genus, and consequently new species. The locomotive form is quite distinct from any other described amoeba, as Sapocribrum chincoteaguense n. sp. typically remains very stationary in the same spot for extended periods of time, and emits only one to two pseudopods at a time (Fig. 1). Sporadic locomotion is by adhesion of the pseudopodia to the substratum and contraction drawing the body of the amoeba forward. Other small amoebae generally have a limax to flabellate locomotive form, such as Nolandella (Page 1983), Flabellula demetica, Vannella aberdonica, Parvamoeba spp., and some Vexillifera spp.
Sapocribrum chincoteaguense presents quite distinct morphologies compared to Squamamoeba and lineage ATCC® PRA-29™, the two most closely-related organisms identified in the molecular analyses. Squamamoeba is a larger organism, presenting a dactylopodid pseudopodial pattern (Kudryavtsev and Pawlowski 2013), clearly distinct from S. chincoteaguense. The genus Pessonella is characterized by much larger organisms (typically reaching 30 µm, (Pussard 1973) that displays a fan-shaped locomotive form (Smirnov and Goodkov 1999). There is uncertainty about the taxonomic placement of isolate ATCC® PRA-29™: it was identified by the original depositor as a member of the genus Pessonella. However, there is very little morphological information available for this isolate, only a light micrograph (Tekle et al. 2008). Hence, it is difficult to establish whether the original identification is accurate without a re-examination of the isolate. The organism displayed in Figure 1A of Tekle et al. (2008) is indeed congruent with the gross-morphology diagnosis of Pessonella. Even if ATCC® PRA-29™ is misidentified, the isolate presents a quite distinct morphology from ATCC® 50979™, justifying the creation of a new genus – Sapocribrum.
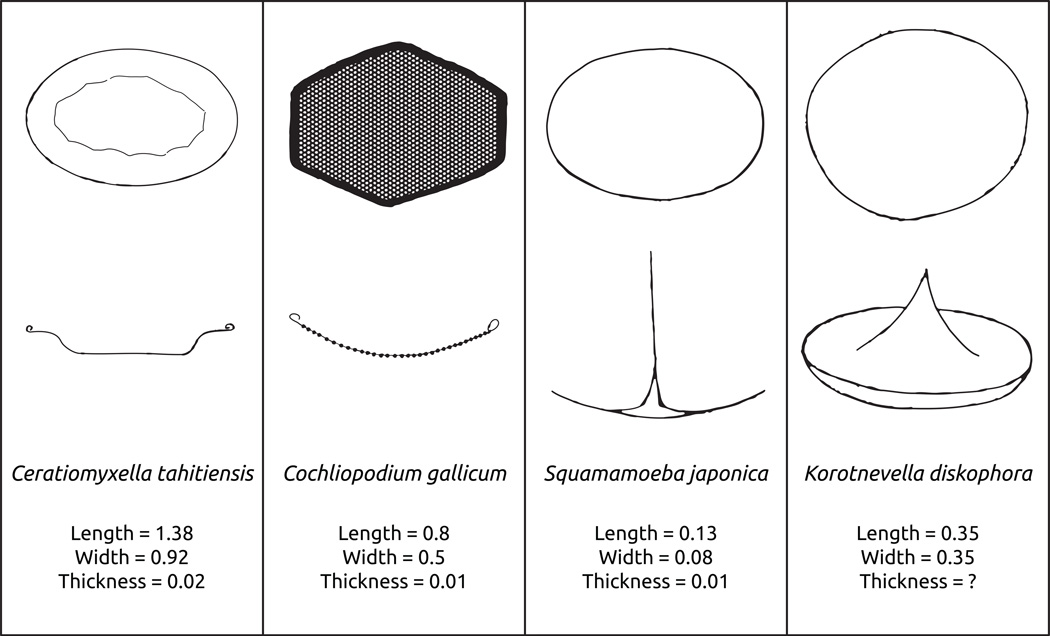
Based on the ultrathin sections and shadowed preparations of whole amoebae, we interpret the oval embedded components to be scales. However, because they are so firmly integrated into the apparently organic electron dense matrix, we have not found evidence of isolated scales. We base our interpretation, moreover, on the same kind of evidence as presented for the description of similar scale-like structures in Squamamoeba sp. (Kudryavtsev and Pawlowski 2013). The organic matrix appears to be rather plastic and deformable based on the more flattened appearance of the air-dried and shadowed amoebae (Fig. 2). Consequently, we interpret this to be a cell covering containing embedded scale-like components rather than a test in the strictest sense of the term. During fixation and dehydration, there is some shrinkage of the cytoplasm. This may account for the widened space between the plasma membrane and the organic coat in some ultrathin sections. The scales present in Sapocribrum chincoteaguense are distinct from both closely- and distantly-related amoebae (Fig. 7). The most similar scales described are the ones in the genus Squamamoeba, as scales in both genera are oval, embedded in a matrix, and the single scale layer is clearly separated from the plasma membrane (Kudryavtsev and Pawlowski 2013). Sapocribrum's scales are 150×100 nm, approximately in the size range of Squamamoeba, but of different fine structure (Fig. 7). Additionally, scales from Squamamoeba posses a central thickening which serves as the base for a long filamentous projection (Kudryavtsev and Pawlowski 2013), while Sapocribrum's scales have a central plate that is perforated, and lacks the central filamentous projection. Interestingly, in both taxa the scales can be pushed aside for pseudopodial protrusion.
Fig. 7.
Comparative morphological attributes of scales similar to those of Sapocribrum chincoteaguense. These drawings represent interpretations on the original descriptions and micrographs for each taxon. Top image represents a frontal view of the scale, bottom image represents a lateral view. Dimensions are given in micrometers for all cases.
The most similar scales among more distantly-related amoebozoans are those in the flagellated stage of the protosteloid genus Ceratiomyxella tahitiensis (Furtado and Olive 1972). These scales are also oval, but differ in having a recurved outer lip, and overlap forming a layer outside the cell, which is not embedded in an organic matrix (Fig. 7). The genus Korotnevella also presents somewhat similar scales. Korotnevella diskophora has oval scales, but these are smaller and present a central projection (Smirnov 1999). Other Korotnevella spp. present quite distinct scales, that may be crown-shaped (O’Kelly et al. 2001), or similar to Paramoeba as in K. nivo (Smirnov 1997). Finally, Cochliopodium gallicum possesses scales with a perforated central plate and an outer, thickened rim, which are closer in size to those of Squamamoeba (Fig. 7). However the shape of scales in C. gallicum is of a stretched hexagon and not oval, and there is an additional layer below the layer of scales that is made of glycostyles (Kudryavtsev and Smirnov 2006). In summary, Sapocribrum chincoteaguense has a unique, novel type of scale, which we consider a key character for justifying the description of a new genus.
Phylogenetic relationships
Sapocribrum chincoteaguense, Squamamoeba spp and isolate ATCC® PRA-29™ group robustly in a clade that potentially shares outer scales as a synapomorphy. The isolates of these three species group together robustly in multiple molecular reconstructions (Fig. 5, 6, (Lahr et al. 2011; Kudryavtsev and Pawlowski 2013). The two organisms that have been morphologically scrutinized, S. chincoteaguense and Sq. japonica, share the striking similarity of having the cell-body covered in an organic matrix embedded with a single layer of non-overlapping scales. The isolate ATCC® PRA-29™ now needs to be morphologically characterized in order to verify the hypothesis that outer-scales are a synapomorphy for this grouping of three isolates. Not many inferences can be made at this point about deeper relationships for the S. chincoteaguense + Sq. japonica + ATCC® PRA-29™ clade besides an affinity with the flabellinids. The flabellinids are a loose assemblage of amoebozoans that receives variable support in distinct phylogenetic reconstructions (see Lahr et al. 2011). They are generally represented by vannelids, dactylopodids and sometimes himatismenids, and are characterized by a flattened locomotive shape, absence of tubular pseudopods and polyaxial cytoplasmic flow (Smirnov et al. 2011). None of the typical characters can be observed in S. chincoteaguense, though the isolate also lacks tubular pseudopods. However, it becomes increasingly clear that scale formation has been a recurrent event in the evolutionary history of Amoebozoa: Sapocribrum + Squamamoeba, dactylopodids, cochliopodids, Ceratiomyxella, among others, must have developed their scales in a non-homologous manner according to current phylogenetic reconstructions.
External coverings are the only body part of amoebozoans with fossilization potential. However, information on composition of these parts is key to the putative identification of microfossils (Bosak et al. 2011; Cohen et al. 2011; Dalton et al. 2013). None of the scales discussed here have a known composition. Compositional analyses may illuminate interpretations of the deep fossil record, and the scale-bearing Amoebozoa have thus the potential of being important tools in illuminating early eukaryotic evolution.
TAXONOMIC SUMMARY
Sapocribrum n. gen
Diagnosis
Amoebae circular in outline to angular-elongated, typically with one to a few largely anterior pseudopodia.
Type species
Sapocribrum chincoteaguense n. sp. (Monotypy)
Etymology
A junction of words sapo (m, Lt) meaning soap, and cribrum (n, Lt) meaning sieve, in reference to the scales that are perforated and shaped like soap bars.
Sapocribrum chincoteaguense n. sp
Diagnosis
Amoeba with characters of the genus, less than 5 µm (more usually 3 to 4 µm), with typical locomotive angular form that moves infrequently, and when sedentary emits only one to a few long filose pseudopods at a time. Pseudopods may bifurcate. Pseudopods 4.42 ± 0.28 µm, varying from 2.5 to 9 µm. During brief periods of locomotion, the body remains contracted and angular in shape moving in a meandering path in the direction of the extended adhesive pseudopodia, that appear to adhere to the substratum and pull the amoeba forward. Surface contains microscales (~ 150×100 nm in mean length and width, respectively).
Etymology
toponym with suffix -ense (n, Lt) reference to type locality, Chincoteague Bay (Chincoteague Island, VA).
Type locality
Chincoteague Bay, isolated from moist soil in mud flat approximately 800 yards from the ocean, Chincoteague, VA, 2002.
Type culture
ATCC® 50979™
History of type culture
This culture was isolated, identified and deposited by Thomas A. Nerad in 2002.
Supplementary Material
ACKNOWLEDGMENTS
The authors are thankful for 4 anonymous reviewers for helpful suggestions on earlier versions of the manuscript. DJGL is funded by a FAPESP Young Researcher award (2013-04585-3), LAK is supported by awards from the U.S. National Science Foundation (DEB-1208741, OCE-1129734) and the National Institutes of Health (1R15GM097722-01). This is Lamont-Doherty Earth Observatory Contribution Number 7841.
LITERATURE CITED
- Anderson OR, Rogerson A, Hannah F. Three new limax amoebae isolated from marine surface sediments: Vahlkampfia caledonica n sp, Saccamoeba marina n sp, and Hartmannella vacuolata n sp. J. Eukaryot. Microbiol. 1997;44:33–42. [Google Scholar]
- Badewitz H-J. The genus Microcorycia Cockerell, 1911 (Testacealobosia, Rhizopoda, Protozoa). A critical monograph of the genus including a first description of a new species: Microcorycia scutella n. sp. Lauterbornia. 2004;50:111–146. [Google Scholar]
- Bennett HS. Morphological aspects of extracellular polysaccharides. Journal of Histochemistry & Hytochemistry. 1963;11:14–23. [Google Scholar]
- Bosak T, Lahr DJG, Pruss SB, Macdonald FA, Dalton L, Matys E. Agglutinated tests in post-Sturtian cap carbonates of Namibia and Mongolia. Earth Planet. Sci. Lett. 2011;308:29–40. [Google Scholar]
- Cohen PA, Schopf JW, Butterfield NJ, Kudryavtsev AB, Macdonald FA. Phosphate biomineralization in mid-Neoproterozoic protists. Geology. 2011;39:539–542. [Google Scholar]
- Dalton LA, Bosak T, Macdonald FA, Lahr DJG, Pruss SB. Preservational and Morphological Variability of Assemblages of Agglutinated Eukaryotes in Cryogenian Cap Carbonates of Northern Namibia. PALAIOS. 2013;28:67–79. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics. 2011;27:1164–1165. doi: 10.1093/bioinformatics/btr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykova I, Figueras A, Novoa B, Casal JF. Paramoeba sp., an agent of amoebic gill disease of turbot Scophthalmus maximus. Dis. Aquat. Organ. 1998;33:137–141. doi: 10.3354/dao033137. [DOI] [PubMed] [Google Scholar]
- Dykova I, Figueras A, Peric Z. Neoparamoeba Page, 1987: light and electron microscopic observations on six strains of different origin. Dis. Aquat. Organ. 2000;43:217–223. doi: 10.3354/dao043217. [DOI] [PubMed] [Google Scholar]
- Fiala I, Dykova I. Molecular characterisation of Neoparamoeba strains isolated from gills of Scophthalmus maximus. Dis. Aquat. Organ. 2003;55:11–16. doi: 10.3354/dao055011. [DOI] [PubMed] [Google Scholar]
- Furtado JS, Olive LS. Scale Formation in a Primitive Mycetozoan. Trans. Am. Microsc. Soc. 1972;91:594–596. [Google Scholar]
- Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. CABIOS. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Grant JR, Lahr DJG, Rey FE, Burleigh JG, Gordon JI, Knight R, Molestina RE, Katz LA. “Gene Discovery from a Pilot Study of the Transcriptomes from Three Diverse Microbial Eukaryotes: Corallomyxa tenera, Chilodonella uncinata and Subulatomonas tetraspora”. Protist Genomics. 2012;1:3–18. [Google Scholar]
- Grant JR, Katz LA. Building a Phylogenomic Pipeline for the Eukaryotic Tree of Life - Addressing Deep Phylogenies with Genome-Scale Data. PLoS Curr. 2014:6. doi: 10.1371/currents.tol.c24b6054aebf3602748ac042ccc8f2e9. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3973741/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Asimenos G, Toh H. Bioinformatics for DNA sequence analysis. Springer; 2009. Multiple alignment of DNA sequences with MAFFT; pp. 39–64. Available from: http://link.springer.com/protocol/10.1007/978-1-59745-251-9_3. [DOI] [PubMed] [Google Scholar]
- Kudryavtsev A, Pawlowski J, Hausmann K. Description of Paramoeba atlantica n. sp.(Amoebozoa, Dactylopodida)� a Marine Amoeba from the Eastern Atlantic, with Emendation of the Dactylopodid Families. Acta Protozool. 2011;50:239. [Google Scholar]
- Kudryavtsev A, Pawlowski J. Squamamoeba japonica n. g. n. sp. (Amoebozoa): A Deep-sea Amoeba from the Sea of Japan with a Novel Cell Coat Structure. Protist. 2013;164:13–23. doi: 10.1016/j.protis.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Kudryavtsev A, Smirnov A. Cochliopodium gallicum n. sp.(Himatismenida), an amoeba bearing unique scales, from cyanobacterial mats in the Camargue (France) Eur. J. Protistol. 2006;42:3–7. doi: 10.1016/j.ejop.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kudryavtsev A, Wylezich C, Pawlowski J. Ovalopodium desertum n. sp. and the Phylogenetic Relationships of Cochliopodiidae (Amoebozoa) Protist. 2011;162:571–589. doi: 10.1016/j.protis.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kudryavtsev A, Brown MW, Tice A, Spiegel FW, Pawlowski J, Anderson OR. A Revision of the Order Pellitida Smirnov et al., 2011 (Amoebozoa, Discosea) Based on Ultrastructural and Molecular Evidence, with Description of Endostelium crystalliferum n. sp. Protist. 2014;165:208–229. doi: 10.1016/j.protis.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Lahr DJG, Grant J, Nguyen TB, Lin JH, Katz LA. Comprehensive phylogenetic reconstruction of Amoebozoa based on concatenated analyses of SSU-rDNA and actin genes. PLoS ONE. 2011 doi: 10.1371/journal.pone.0022780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr DJG, Grant JR, Katz LA. Multigene Phylogenetic Reconstruction of the Tubulinea (Amoebozoa) Corroborates Four of the Six Major Lineages, while Additionally Revealing that Shell Composition Does not Predict Phylogeny in the Arcellinida. Protist. 2013;164:323–339. doi: 10.1016/j.protis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Meisterfeld R, Badewitz H. A redescription of Amphizonella violacea (Amoebozoa: Arcellinida) Acta Protozool. 2006;45:167. [Google Scholar]
- Michel R, Wylezich C, Hauroder B, Smirnov A. Phylogenetic position and notes on the ultrastructure of Sappinia diploidea (Thecamoebidae) Protistology. 2006;4:319–325. [Google Scholar]
- Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T. The CIPRES portals. CIPRES URL Httpwww Phylo Orgsubsectionsportal. 2009 08–04. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. Gateway Computing Environments Workshop (GCE) 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; pp. 1–8. 2010. Available from: http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=5676129. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. The CIPRES science gateway: a community resource for phylogenetic analyses; Proceedings of the 2011 TeraGrid Conference: Extreme Digital Discovery; 2011. p. 41. Available from: http://dl.acm.org/citation.cfm?id=2016785. [Google Scholar]
- O’Kelly CJ, Peglar MT, Black MND, Sawyer TK, Nerad TA. Korotnevella hemistylolepis N. Sp. and Korotnevella monacantholepis N. Sp. (Paramoebidae), Two New Scale-covered Mesohaline Amoebae. J. Eukaryot. Microbiol. 2001;48:655–662. doi: 10.1111/j.1550-7408.2001.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive LS, Bennett WE, Deasey MC. The New Protostelid Genus Endostelium. Mycologia. 1984;76:884–891. [Google Scholar]
- Page FC. Paramoeba: A Common Marine Genus. Hydrobiologia. 1973;41:183–188. [Google Scholar]
- Page FC. Marine gymnamoebae. Institute of Terrestrial Ecology. 1983 Available from: http://nora.nerc.ac.uk/5144/1/Marine_gymnamoebae.pdf. [Google Scholar]
- Penn O, Privman E, Ashkenazy H, Landan G, Graur D, Pupko T. GUIDANCE: a web server for assessing alignment confidence scores. Nucleic Acids Res. 2010;38:W23–W28. doi: 10.1093/nar/gkq443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn O, Privman E, Landan G, Graur D, Pupko T. An alignment confidence score capturing robustness to guide tree uncertainty. Mol. Biol. Evol. 2010;27:1759–1767. doi: 10.1093/molbev/msq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pussard M. Description d’une amibe de type flabellulien: Pessonella marginata ngn sp. Mayorellidae Amoebaea Protistol. 1973;9:175–185. [Google Scholar]
- Shadwick LL, Spiegel FW, Shadwick JDL, Brown MW, Silberman JD. Eumycetozoa=Amoebozoa?: SSUrDNA Phylogeny of Protosteloid Slime Molds and Its Significance for the Amoebozoan Supergroup. PLoS ONE. 2009;4:e6754. doi: 10.1371/journal.pone.0006754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov AV, Bedjagina OM, Goodkov AV. Dermamoeba algensis n. sp. (Amoebozoa, Dermamoebidae): An algivorous lobose amoeba with complex cell coat and unusual feeding mode. Eur. J. Protistol. 2011;47:67–78. doi: 10.1016/j.ejop.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Smirnov AV, Brown S. Guide to the methods of study and identification of soil gymnamoebae. Protistology. 2004;3:148–190. [Google Scholar]
- Smirnov AV, Chao E, Nassonova ES, Cavalier-Smith T. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa) Protist. 2011;162:545. doi: 10.1016/j.protis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Smirnov AV, Goodkov AV. An illustrated list of basic morphotypes of Gymnamoebia (Rhizopoda, Lobosea) Protistology. 1999;1:20–29. [Google Scholar]
- Smirnov AV, Kudryavtsev AA. Pellitidae n. fam. (Lobosea, Gymnamoebia): a new family, accommodating two amoebae with an unusual cell coat and an original mode of locomotion, Pellita catalonica n.g., n.sp. and Pellita digitata comb. nov. Eur. J. Protistol. 2005;41:257–267. [Google Scholar]
- Smirnov AV. Two new species of marine amoebae: Hartmannella lobifera n. sp. and Korotnevella nivo n. sp.(Lobosea, Gymnamoebida) Arch. Für Protistenkd. 1997;147:283–292. [Google Scholar]
- Smirnov AV. Korotnevella diskophora n. sp.(Gymnamoebia, Paramoebidae)-small freshwater amoeba with peculiar scales. Protistology. 1999;1:30–33. [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Tekle YI, Grant J, Anderson OR, Nerad TA, Cole JC, Patterson DJ, Katz LA. Phylogenetic placement of diverse amoebae inferred from multigene analyses and assessment of clade stability within “Amoebozoa” upon removal of varying rate classes of SSU-rDNA. Mol. Phylogenet. Evol. 2008;47:339–352. doi: 10.1016/j.ympev.2007.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.