Abstract
Ribavirin is an important component of interferon-based and direct antiviral treatment regimens for hepatitis C virus (HCV) infection. Immunomodulation, in particular improvement of the host interferon (IFN) response, has been proposed as ribavirin’s mechanism of action. Natural killer (NK) cells are sensitive biomarkers for IFN-α/β receptor signaling, as NK cell cytotoxicity and IFN-γ production are regulated by STAT1- and STAT4-phosphorylation, respectively. Specifically, pSTAT1-dependent NK cell cytotoxicity increases and pSTAT4-dependent IFN-γ production decreases in response to endogenous, virus-induced IFN-α and during IFN-α-based therapy. To assess whether ribavirin has a direct effect on NK cells and/or improves the IFN-γ response of NK cells in the presence of IFN-α, we prospectively studied 22 HCV patients with and 32 patients without 4 weeks of ribavirin pretreatment, who all received subsequent PegIFN/ribavirin combination therapy. During ribavirin pretreatment, the frequency of CD56dim NK cells with cytotoxic effector functions decreased (p=0.049) as did the frequency of CD56bright NK cells with the capacity to produce IFN-γ (p=0.001). In vitro or in vivo exposure of NK cells to ribavirin improved the pSTAT4 (p<0.01) but not pSTAT1 response of NK cells to subsequent stimulation with IFN-α. This was associated with an increase in IFN-γ production but not cytotoxicity of NK cells during subsequent IFN-α-based therapy. The frequency of IFN-γ-producing NK cells was greater in fast second-phase virological responders than in slow responders.
Conclusion
Ribavirin enhances the pSTAT4 and IFN-γ response of NK cells to IFN-α–stimulation.
Keywords: ribavirin, interferon, innate, hepatitis C virus, treatment, natural killer
Introduction
The guanosine analogue ribavirin is an important component not only of interferon-based therapies for HCV but also new therapies with direct-acting antivirals (1). While ribavirin by itself only has a modest effect on HCV RNA levels (<1 log reduction) and does not induce viral clearance (2), it is associated with a faster second-phase virological response when administered together with pegylated interferon alpha 2a (PegIFN) (3, 4). Ribavirin also decreases on-treatment virological breakthrough and post-treatment virological relapse when combined with PegIFN (5, 6) and/or direct-acting antivirals (7, 8). Notably, ribavirin’s ability to suppress the development of viral resistance is not limited to antiviral treatment regimens against HCV but extends to treatment regimens against other viruses such as influenza A virus (9).
Several direct effects on viral replication have been proposed as ribavirin’s mechanism of action. These include (i) inhibition of the HCV NS5B-encoded RNA-dependent RNA polymerase, (ii) depletion of guanosine triphosphates by interference with the host enzyme inosine monophosphate dehydrogenase, and (iii) promotion of viral error catastrophe [reviewed in (10)]. While each of these mechanisms is supported by in vitro data, the in vivo evidence from clinical studies is weak. Ribavirin monotherapy results only in a modest, less than 1 log10 decline in HCV titer (3) and does not increase the rate of nucleotide substitutions (11). In combination with PegIFN, ribavirin does not improve the first phase virological decline (12) arguing against a primarily antiviral mode of action.
An immunomodulatory effect has been suggested as an alternative mechanism (3, 4). Ribavirin accelerates the second-phase (3, 4) and the third-phase (12) virological decline during PegIFN/ribavirin combination therapy, which are both thought to reflect immune-mediated clearance of virus-producing hepatocytes (13). Indeed, exposure of cloned T cells to ribavirin enhances the proliferation of T cells, increases the production of the antiviral cytokine IFN-γ, and inhibits the production of the immunosuppressive cytokine IL-10 in vitro (14). However, these data are not confirmed in vivo because studies on immune responses of patients on PegIFN/ribavirin therapy demonstrate a decrease rather than an increase in the frequency of IFN-γ-producing T cells (15–17).
More recent studies suggest that ribavirin modulates the innate response to type I IFN. Based on both in vitro (18–20) and in vivo data (3, 21) ribavirin may induce a subset of ISGs (18–20) and improve the ability of the HCV-infected liver to response to IFN-based therapy (3). Rather than acting independently from type I IFN ribavirin appears to have a synergistic effect because it preferentially accelerates the second-phase virological response in patients with a suboptimal, but not absent, interferon response (4).
Having established that NK cells are a sensitive readout of a patient’s response to IFN-α (22, 23), we asked whether they can be used to identify a potential immunomodulatory effect of ribavirin. NK cells from patients with chronic HCV infection respond to IFN-α with greater STAT1 phosphorylation and less STAT4 phosphorylation than NK cells from healthy controls (24). This corresponds to an increase in pSTAT1-dependent cytotoxicity and a reduction in pSTAT4-dependent IFN-γ production in response to endogenous and exogenous IFN-α (22, 23). We hypothesized that ribavirin may improve NK cell IFN-γ production, and that this may contribute to the enhancement of the second-phase virological decline, which has been observed when ribavirin is added to IFN-α-based therapy.
To address this question we studied prospectively collected lymphocyte samples from patients with chronic HCV infection who had either received 4-weeks ribavirin pretreatment (n=22) or no pretreatment (n=32) prior to PegIFN/ribavirin combination therapy. While pretreatment with ribavirin did not improve treatment outcome, likely because all patients received ribavirin during the long PegIFN/ribavirin phase (3), the study design allowed us to examine (i) whether ribavirin itself in the absence of IFN-a-based therapy has any effect on NK cells and (ii) whether ribavirin modulates the NK cell response to IFN-α. We show that ribavirin decreases pSTAT4 levels in NK cells both in vitro and in vivo and that this results in improved inducibility of pSTAT4 and of pSTAT4-dependent IFN-γ production in response to IFN-α. We also show that a better second phase response is associated with improved IFN-γ production by NK cells, which is consistent with the finding that ribavirin improves the second phase virological response when added to PegIFN therapy.
Materials and Methods
Study cohort
NK cells were studied in 54 patients with chronic HCV infection who were randomized to receive either 4-weeks ribavirin pretreatment (n=22) or no pretreatment (n=32) immediately prior to PegIFN/ribavirin combination therapy (Table 1). PegIFN was administered as a single subcutaneous dose of 180 μg per week. The ribavirin dose depended on the patient bodyweight and the HCV genotype (1000 mg ribavirin for <75 kg bodyweight and 1200 mg for ≥75 kg bodyweight p.o. daily for HCV genotypes 1 and 4; 800 mg p.o. daily for HCV genotypes 2 and 3). The 54 patients were chosen from a larger clinical study (Clinicaltrials.gov registration NCT00718172) (3) based on 3 included: week −4 (start of ribavirin pretreatment), week 0 (start of PegIFN/ribavirin therapy), days 1 and 2, and weeks 1, 2, 4 and 12 after initiation of PegIFN/ribavirin combination therapy. The week 0, 1, 2, 4 and 12 blood samples were drawn prior to the weekly PegIFN injection. In addition, the NK cell response to in vitro stimulation with ribavirin and/or IFN-α was studied using peripheral blood mononuclear cells (PBMC) from 22 untreated patients with chronic HCV infection and 23 healthy subjects. Unless otherwise indicated PBMC were separated from ACD-anticoagulated blood on Ficoll–Histopaque (Mediatech, Manassas, VA) density gradients, washed 3 times with phosphate-buffered saline (PBS, Mediatech) and cryopreserved until use. All subjects gave written informed consent for research testing under protocols approved by the institutional review boards of the NIDDK/NIAMS or the NIH Clinical Center.
Table 1.
Characteristics of studied patient groups.
| Ribavirin Pretreatment (n = 22) | No Ribavirin Pretreatment (n = 32) | p-value | |
|---|---|---|---|
| Early virological responders (EVR), n (%) | 17 (77%) | 29 (88%) | 0.298 |
| Sustained virological responders (SVR), n (%) | 11 (50%) | 18 (56%) | 0.741 |
| Gender (male/female) | 13/9 | 20/13 | 0.911 |
| Age at Start of Therapy, mean (±SD) years | 49.6 (±12.8) | 51.9 (±8.5) | 0.612 |
| Ethnicity (Asian/African-American/Hispanic/Caucasian) | 4/3/0/15 | 6/7/2/18 | 0.979/0.627/0.434/0.503 |
| Body mass index, mean (±SD) | 29 (±6.0) | 29.2 (±6.9) | 0.9314 |
| IL-28B rs12979860 SNP (CC vs. CT/TT) | 9/11 | 14/13 | 0.2974 |
| Genotype (1/2/3/4/6) | 12/3/4/1/2 | 18/7/5/3/0 | 1/0.724/1/0.642/0.173 |
| Serum HCV RNA titer at start of treatment, mean (±SD) log10 IU/ml | 6.4 (±0.6) | 6.3 (±0.7) | 0.843 |
| ALT at start of therapy, mean (±SD) U/L | 104 (±78.6) | 103 (±93) | 0.884 |
| Ishak Inflammatory Score, mean (±SD) | 7.6 (±2.8) | 7.4 (±2.2) | 0.797 |
| Ishak Fibrosis Score, mean (±SD) | 2.2 (±2.2) | 2.1 (±2.1) | 0.768 |
SNP, single nucleotide polymorphism
Kinetics of viral and biochemical parameters
HCV RNA levels were determined using Cobas TaqMan real-time PCR (Roche Diagnostics, Palo Alto, CA). The first-phase virological response after PegIFN injection was defined as the logarithmic decline in viral level from week 0 to the nadir during the first 3 days after PegIFN injection. The second-phase response was the slope of the viral decline from week 1 to week 4, or to the first non-quantifiable measurement if it occurred earlier. A fast second-phase response was defined as > 0.5 log10 reduction in viral titer during that period. Serum alanine aminotransferase (ALT) activity was measured on every visit.
NK cell response to IFN-α stimulation
(i) Phosphorylation of STAT1 and STAT4 in response to IFN-α
Fresh pre-warmed heparinized whole blood was stimulated in vitro with or without 600 ng/mL consensus sequence IFN-α (IFN-α-con1; InterMune, Brisbane, CA) for 5 min at 37°C. A 20-fold excess of Lyse/Fix buffer (BD Biosciences, San Jose, CA) was added for 10 min at 37°C for lymphocyte fixation and erythrocyte lysis. After centrifugation, cells were permeabilized with Perm Buffer (BD Biosciences) for 20 min on ice, washed twice, and resuspended in Staining Buffer (BD Biosciences).
All samples were stained with anti-CD56-PE (Beckman Coulter, Brea, CA) and anti-CD20-PerCP/Cy5.5 (BD Biosciences) to identify NK cells and B cells, respectively, and with anti-CD3-APC (both from BD Biosciences) to exclude T cells. Cells were additionally stained with anti-pSTAT1-Alexa488, or anti-pSTAT4-Alexa488 (BD Biosciences) for 20 min at room temperature. Samples were analyzed on an LSRII with FacsDiva Version 6.1.3 (BD Biosciences) and FlowJo Version 8.8.2 (Tree Star, Ashland, OR) software.
(ii) Effect of in vitro pretreatment with ribavirin on the NK cell response to IFN-α
Frozen PBMC were thawed and incubated in RPMI1640 with 10% FCS with or without 1 μg/ml of ribavirin (Sigma-Aldrich, St. Louis, MO) for 24h, followed by addition of 600 ng/mL IFN-α–con1 (InterMune) for 5 min at 37°C. Cells were then fixed and stained for intracellular pSTAT1 and pSTAT4 as described above.
NK cell function
(i) Degranulation
NK cell degranulation, i.e. upregulation of the degranulation marker CD107a on the NK cell surface in response to target cells that do not express major histocompatibility molecules, was studied as readout for cytotoxicity as described (22). Thawed PBMC were cultured overnight at 37°C, 5% CO2 in RPMI1640 with 10% fetal bovine serum (FBS Serum Source International, Charlotte, NC), 1% Penicillin/Streptomycin, 2 mM L-glutamine, 10 mM HEPES (Cellgro, Manassas, VA) without exogenous IL-2. The next day, PBMC were counted and stimulated in the presence or absence of K562 cells (ATCC, Manassas, VA) to assess NK cell degranulation (22).
(ii) IFN-γ production
Frozen PBMC were thawed and incubated with or without IL-12 (0.5 ng/ml; R&D Systems) plus IL-15 (20 ng/ml R&D Systems) for 14h, followed by addition of brefeldin A for 4h and stained for intracellular IFN-γ as previously described (25).
Statistical Analysis
Statistical analyses were performed with GraphPad Prism Version 5.0 (GraphPad Software Inc, San Diego, CA). D’Agostino & Pearson omnibus normality tests were applied to analyze the data distribution. Paired and unpaired t-tests, Mann-Whitney nonparametric two-sample rank test, Spearman non-parametric tests and repeated measures analysis of variance (ANOVA) were used as indicated in the figure legends. A two-sided p-value of less than 0.05 was considered significant.
Results
Effect of 4 weeks ribavirin pretreatment on HCV RNA and ALT levels
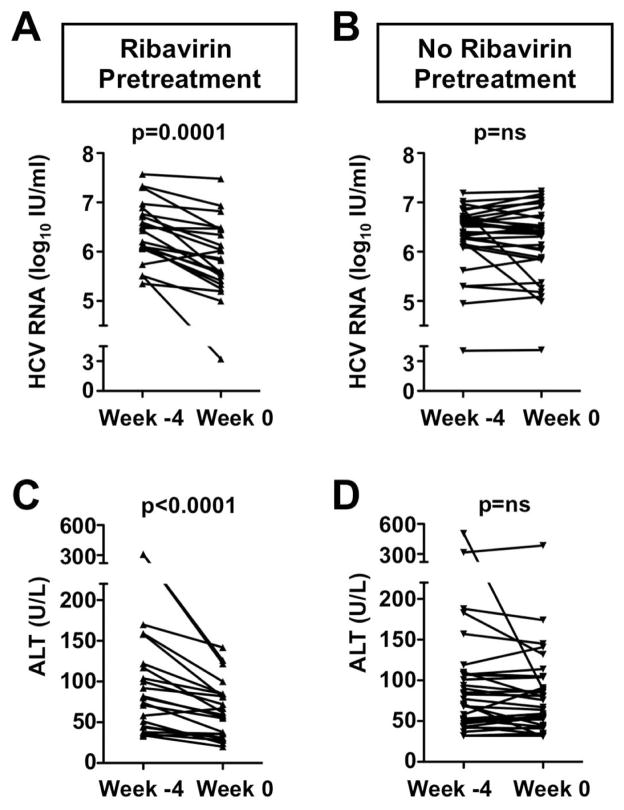
This immunological study included 22 HCV patients who did and 32 patients who did not receive a 4-week course of ribavirin pretreatment. All patients subsequently received a standard course of PegIFN/ribavirin combination therapy. As previously reported (3), ribavirin pretreatment resulted in a modest decrease of HCV RNA levels from 6.3±0.1 log10 IU/ml to 5.8±0.1 log10 IU/ml (p=0.0001, Fig. 1A), but did not improve the overall SVR rate. During the same period, serum ALT activity decreased from 104±16.7 IU/L to 65.3±7.4 IU/L (p<0.0001, Fig. 1C). Both parameters remained stable in the control group that did not receive ribavirin pretreatment (Fig. 1B, D).
Fig. 1. A 4-week course of ribavirin pretreatment results in a modest reduction of serum HCV RNA and ALT levels.
(A–B) Serum HCV RNA levels and (C–D) ALT activity of patients who did (A, C) or did not (B, D) receive a 4-week course of ribavirin pretreatment prior to PegIFN/ribavirin combination therapy. Week −4 marks the start and week 0 the end of ribavirin pretreatment. Statistical analysis: paired t-test. ALT, alanine aminotransferase; ns, not significant.
Effect of 4 weeks ribavirin pretreatment on NK cell function
Two of the major effector functions of activated NK cells are cytotoxicity and IFN-γ production. We previously reported that NK cells from HCV-infected patients display increased cytotoxicity but not IFN-γ production as compared to NK cells from healthy controls (25). The percentage of cytotoxic, i.e. degranulating NK cells increases further, whereas the percentage of IFN-γ-producing NK cells decreases within hours of IFN-α injection (23).
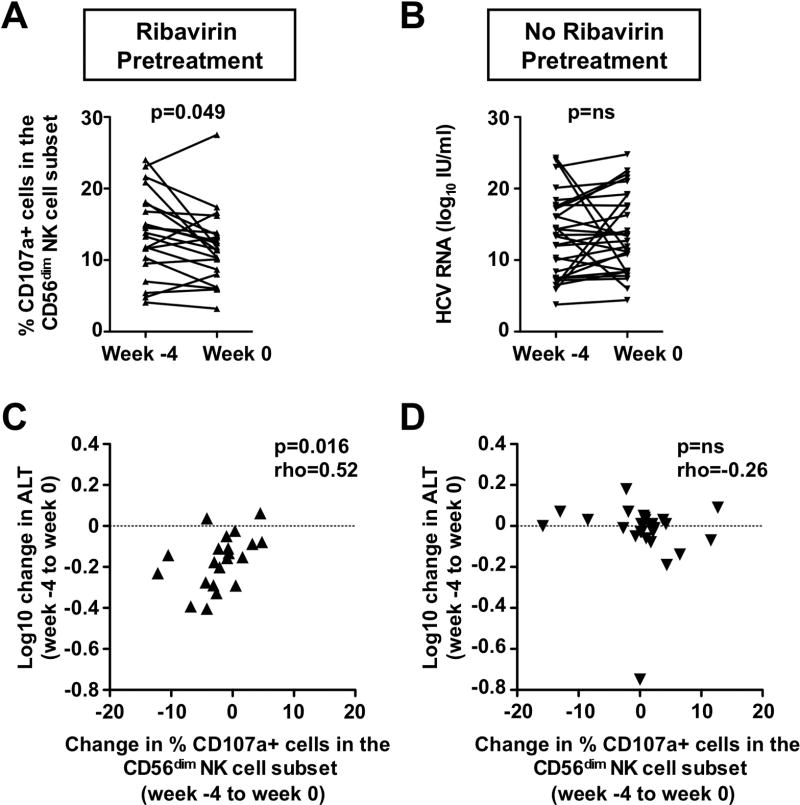
To examine if ribavirin modulates the effector functions of NK cells, PBMC were isolated prior to and after the 4-week course of ribavirin pretreatment, and incubated with MHC class I negative K562 cells, the classical targets for NK cells. After coculture, CD56dim NK cells, which constitute 90% of all NK cells in the circulation and represent the main cytotoxic NK cell population (26), were assessed for cell surface expression of the degranulation marker CD107a, a readout for cytotoxicity and degranulation. As shown in figure 2A, the frequency of CD107a+, i.e. cytotoxic NK cells within the CD56dim population decreased during ribavirin treatment (13.81±1.2% versus 11.8±1.0%, p=0.049). In contrast, there was no such change in untreated patients during the same period (Fig. 2B). Consistent with our previous finding that NK cell degranulation correlates to ALT levels as a marker of liver injury (25), the decrease in the ability of CD56dim NK cells to degranulate correlated with the decrease in ALT levels (Rho=0.52, p=0.0159, Fig. 2C). No such correlation was observed in the untreated control group (Rho=−0.26, p=ns, Fig. 2D). Of note, there was no significant change in either the frequency of TRAIL+ NK cells or their TRAIL expression level.
Fig. 2. The decrease in the frequency of CD56dim cytotoxic NK cells during ribavirin pretreatment correlates with the decrease in ALT levels.
(A–B) Changes in the frequency of CD56dim NK cells, that upregulate the degranulation marker CD107a in vitro in response to K562 target cells, in patients who did (A) or did not (B) receive a 4-week course of ribavirin pretreatment. Week −4 marks the start and week 0 the end of ribavirin pretreatment. Statistical analysis: Wilcoxon signed rank test (left panel) and paired t-test (right panel) based on results of D’Agostino & Pearson omnibus normality test. (C–D) Correlation between changes in the frequency of CD107a+ CD56dim NK cells and ALT levels in patients who did (C) or did not (D) receive a 4-week course of ribavirin pretreatment. Statistical analysis: Spearman non-parametric test. Rho: Spearman correlation coefficient. MFI, mean fluorescence intensity; ns, not significant.
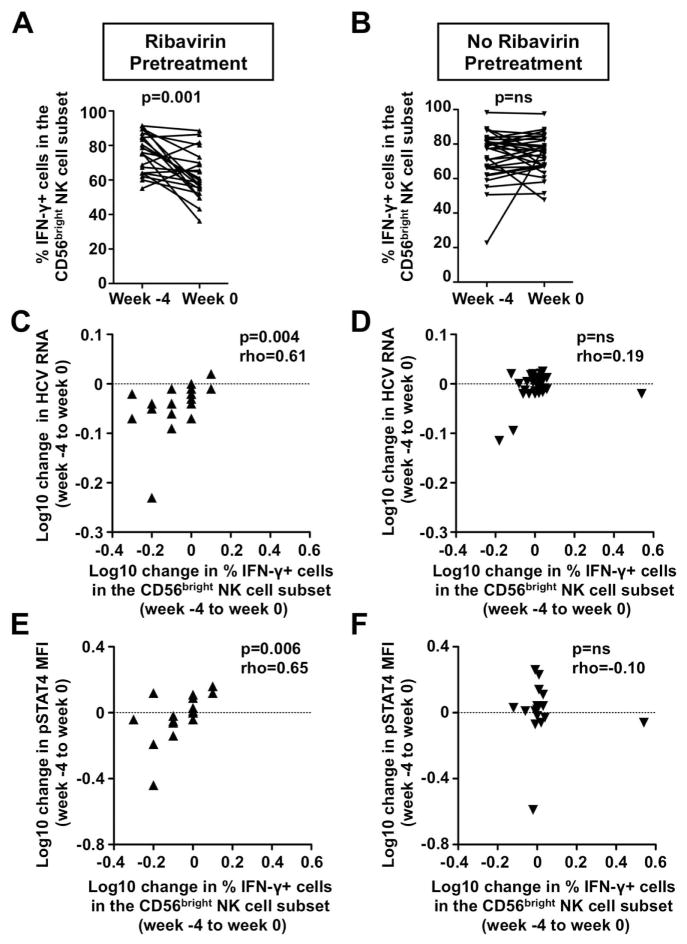
To assess the capacity of NK cells to produce IFN-γ PBMC were in vitro stimulated with IL-12 and IL-15, and the CD56bright NK cell population, the main source of NK cell derived IFN-γ (27), was studied by flow cytometry. As shown in figure 3A the percentage of IFN-γ-producing NK cells in the CD56bright NK cell population decreased during ribavirin pretreatment (75.9±2.5 % at week −4 vs. 62.5±3.0 % at week 0, p=0.001). Significant decreases in the frequency of IFN-γ-producing cells were also observed in the CD56dim (32±3% vs. 22±3%; p=0.001) and the total NK cell population (35±3% vs. 25±3%, p=0.002, not shown). Again, no significant change was observed in the untreated control group during the same time interval (Fig. 3B). The decrease in the frequency of IFN-γ-producing CD56bright NK cells correlated with the decrease in viremia during ribavirin pretreatment (Rho=0.61, p=0.004, Fig. 3C). No such correlation was observed in the untreated control patients (Fig. 3D).
Fig. 3. The decrease in the frequency of IFN-γ-producing CD56bright NK cells during ribavirin pretreatment correlates with the decrease in viremia and in NK cell pSTAT4 levels.
(A–B) Changes in the frequency of IFN-γ-producing CD56bright NK cells in patients who did (A) or did not (B) receive a 4-week course of ribavirin pretreatment. Statistical analysis: Wilcoxon signed rank test (left panel) and paired t-test (right panel) based on results of D’Agostino & Pearson omnibus normality test.
(C–D) Correlation between the change in the frequency of CD56bright NK cells and the change in viremia in patients who did (C) or did not (D) receive a 4-week course of ribavirin pretreatment. Statistical analysis: Spearman non-parametric test. Rho: Spearman correlation coefficient. MFI, mean fluorescence intensity; ns, not significant.
(E–F) Correlation between the change in the frequency of IFN-γ-producing CD56bright NK cells and the change in NK cell ex vivo pSTAT4 levels in patients who did (E) or did not (F) receive a 4-week course of ribavirin pretreatment. Statistical analysis: Spearman non-parametric test.
Consistent with the fact that the ability of NK cells to produce IFN-γ in response to IL-12 depends on STAT4 phosphorylation (28), the decrease in the percentage of IFN-γ-producing CD56bright NK cells correlated with the decrease in ex vivo pSTAT4 levels during ribavirin pretreatment (Rho=0.58, p=0.019, Fig. 3E). No such correlation was observed in the untreated control patients (Fig. 3F).
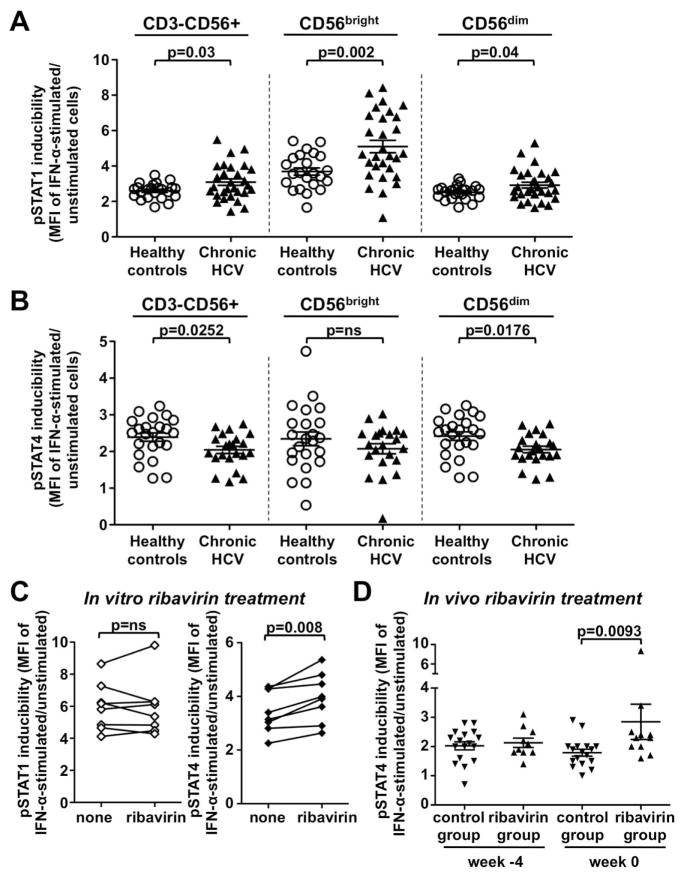
Ribavirin improves the induction of pSTAT4 in NK cells in the presence of IFN-α
As shown in figure 4 NK cells from untreated patients with chronic HCV infection responded to in vitro stimulation with IFN-α with stronger pSTAT1 induction (p=0.03, Fig. 4A) and weaker pSTAT4 induction (p=0.0252, Fig. 4B) than NK cells from healthy controls. When NK cells from chronic HCV patients were pretreated with ribavirin in vitro and then subjected to IFN-α stimulation, there was no further increase in the pSTAT1 response (Fig. 4C, left graph) the pSTAT4 response was improved (p=0.008, Fig. 4C, right graph).
Fig. 4. Ribavirin improves the induction of pSTAT4 in NK cells in the presence of IFN-α.
(A–B) IFN-α-induced pSTAT1 (A) and pSTAT4 (B) expression in CD3-CD56+ NK cells and their CD56bright and CD56dim subpopulations from chronic HCV patients (chronic HCV, n=22) and healthy, uninfected blood donors (healthy controls, n=23). The pSTAT1 and pSTAT4 inducibility reflect the pSTAT1 and pSTAT4 MFI of NK cells after in vitro stimulation of PBMC with IFN-α normalized to the respective MFI of unstimulated cells. Statistical analysis: Mann-Whitney nonparametric two-sample rank test.
(C) IFN-α-induced pSTAT1 and pSTAT4 expression in NK cells from chronic HCV patients (n=8) after in vitro treatment of PBMCs with ribavirin. The pSTAT1 and pSTAT4 inducibility reflects the pSTAT1 and pSTAT4 MFI of NK cells after in vitro stimulation of PBMC with IFN-α normalized to the respective MFI in unstimulated cells. Statistical analysis: paired t-test after analysis with the D’Agostino & Pearson omnibus normality test.
(D) Inducibility of pSTAT4 in CD3-CD56+ NK cells from chronic HCV patients who did or did not receive a 4-week course of ribavirin pretreatment. PBMCs were isolated prior to (week −4) and after (week 0) of a 4-week course ribavirin pretreatment and subjected to in vitro stimulation with IFN-α. The pSTAT1 and pSTAT4 inducibility reflects pSTAT1 and pSTAT4 MFI in NK cells after in vitro stimulation of PBMCs with IFN-α normalized to the respective MFI in unstimulated cells. The comparison remains statistically significant if the outlier in the ribavirin-pretreated group at week 0 is excluded. Statistical analysis: Mann-Whitney nonparametric two-sample rank test after analysis with the D’Agostino & Pearson omnibus normality test. MFI, mean fluorescence intensity; ns, not significant.
The same improvement in pSTAT4 inducibility was observed when NK cells from patients who had received the 4-week course of ribavirin pretreatment were stimulated with IFN-α in vitro and compared to NK cells from not pretreated patients (p=0.0093, Fig. 4D). Again, no such effect was observed for pSTAT1 inducibility (data not shown). These results demonstrate that ribavirin directly affects NK cells rather than indirectly via a reduction in viral titer, and that it improves pSTAT4 inducibility in the presence of IFN-α.
A 4-week course of ribavirin pretreatment increases the pSTAT4-dependent IFN-γ response of NK cells during subsequent PegIFN/ribavirin therapy
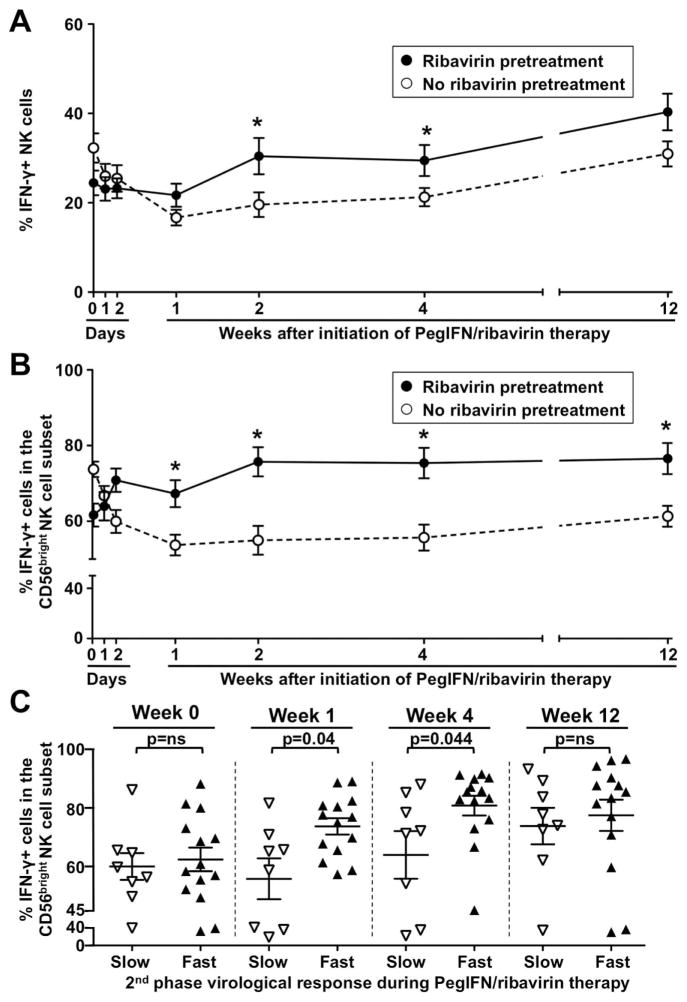
The observation that ribavirin improved the induction of pSTAT4 by IFN-α prompted the question whether it also improved the impaired IFN-γ response of NK cells during IFN-based therapy. To test this hypothesis, we studied NK cell responses of ribavirin-pretreated and non-pretreated patients during the first 12 weeks of PegIFN/ribavirin combination therapy. As shown in figure 5, patients who had been pretreated with ribavirin had a significantly higher percentage of IFN-γ-producing cells in the total (p=0.0206 Fig. 5A) and CD56bright NK cell population (p<0.0001, Fig. 5B) upon in vitro stimulation with IL-12 and IL-15 than patients who were not pretreated with ribavirin. The difference in the percentage of IFN-γ-producing NK cells between both patient groups was apparent by week 1, increased further by week 2, and was maintained in the CD56bright NK cell subset until at least week 12 of PegIFN/ribavirin therapy. It was also observed when the total NK cell population (Suppl. Fig. 1A) or the CD56bright NK cell subset (Suppl. Fig. 1B) were studied ex vivo, i.e. without in vitro stimulation (p<0.0001, serial measures ANOVA).
Fig. 5. A 4-week course of ribavirin pretreatment increases the pSTAT4-dependent IFN-γ production of NK cells during subsequent PegIFN/ribavirin therapy.
(A–B) Changes in the frequency of IFN-γ-producing CD3-CD56+ NK cells (A) and CD56bright NK cells (B) during therapy with PegIFN/ribavirin of patients who either had (solid lines, n=22) or had not (broken lines, n=31) received a 4-week course of ribavirin pretreatment. The frequency of IFN-γ-producing NK cells was determined after in vitro stimulation. Mean values ± SEM are shown. Asterisks indicate a p≤0.05 by unpaired t-test for the indicated time point. The comparison of the results from both patient groups by serial measures ANOVA is significant (p=0.0206 for panel A, p<0.0001 for panel B).
(C) Frequency CD56bright NK cells that produced IFN-γ in response to in vitro stimulation in ribavirin-pretreated patients who mounted either a slow (open triangles) or fast (filled triangles) 2nd phase virological response to subsequent PegIFN/ribavirin therapy. Statistical analysis: Mann-Whitney nonparametric two-sample rank test after analysis with the D’Agostino & Pearson omnibus normality test. Mean values ± SEM are shown.
Consistent with the results of a previously published larger clinical study (3), ribavirin treatment did not increase the percentage of patients with a sustained virological response in the current study. However, within the ribavirin-pretreated group patients with a fast second-phase virological decline had a greater percentage of IFN-γ-producing CD56bright NK cells than those with a slow second-phase virological decline (73.8±2.8% vs 55.8±7%, p=0.04 at week 1 and 81±3.3% vs 64±8.1%, p=0.044 at week 4, respectively, Fig. 5C). This was confirmed by ex vivo analysis of IFN-γ-producing CD56bright NK cells (4.12% vs 0.69%, p=0.034 at week 4, Suppl. Fig. 1C). In contrast, there was no significant difference in NK cell cytotoxicity between the two groups (not shown). Collectively, these findings suggest that ribavirin ameliorates the IFN-α induced suppression of pSTAT4 and IFN-γ induction.
Discussion
This study demonstrates an immunological effect of ribavirin that results in alleviation of the IFN-α-mediated suppression of the NK cell IFN-γ response. NK cell IFN-γ production is typically decreased in HCV-infected patients, whereas NK cell cytotoxicity is increased (25, 29). This is thought to be due to chronic exposure to virus-induced type I IFN. As first described in a mouse model of lymphocytic choriomenigitis virus infection (30–32) type I IFN increases STAT1 expression, which displaces STAT4 at the IFN-a/b receptor resulting in decreased pSTAT4-dependent IFN-γ production and increased pSTAT1-dependent cytotoxicity.
Our current study extends these findings to HCV infection in humans. Consistent with results by Miyagi et al (24) we demonstrate that NK cells of patients with chronic hepatitis C display increased inducibility of pSTAT1 and decreased inducibility of pSTAT4 upon in vitro stimulation with IFN-α compared to NK cells of healthy controls (Fig. 4A and B). We extend these results by demonstrating that both in vitro and in vivo pretreatment with ribavirin improves the inducibility of pSTAT4 in the presence of IFN-α (Fig. 4C). Accordingly, ribavirin-pretreated patients display an increased frequency of IFN-γ-producing NK cells during PegIFN/ribavirin therapy (Fig. 5A and B).
In contrast, ribavirin pretreatment does not further increase the inducibility of pSTAT1 in vitro cell cultures (Fig. 4C), and ribavirin-pretreated patients do not display an increased frequency of IFN-γ-producing NK cells during PegIFN/ribavirin therapy compared to not pretreated patients (not shown). While the mechanism of ribavirin’s effect on NK cells requires further investigation, the current study demonstrates that ribavirin acts directly on NK cells rather than indirectly via a decrease in viral titer. This is supported by the finding that an improvement of the pSTAT4 response to IFN-α was also observed when PBMC were treated with ribavirin in vitro, i.e. in the absence of the HCV (Fig. 4C).
Within the RBV pretreated patient group, IFN-γ-producing NK cells were more frequent in patients with a fast second-phase virological response than in those with a slow second-phase virological response, which may point to a potential antiviral role of IFN-γ. The conclusion that ribavirin modulates NK cell function is consistent with the results of mathematical modeling, that have led to the concept that the second-phase decrease in viral titer during IFN-based therapy is immune-mediated (13).
The finding that ribavirin modulates the NK cell response to IFN-α remains of interest also in the context of new interferon-free antiviral regimens. In the presence of ribavirin, NK cells may be able to mount an improved IFN-γ response to an endogenous type I IFN response, that is stimulated by increased HCV replication during a viral breakthrough. The improved IFN-γ response of NK cells may thus result in better immunosurveillance. This hypothesis is an interesting area for future studies.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by the NIDDK, NIH intramural research program. J. M. W. was supported by grant We-4675/1-1 from the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
The authors thank Birgit Edlich, Leah Hogdal and Aintzane Zabaleta Azpiroz for processing of PBMC; Christopher Koh, Adil Abdalla, Naveen Gara, Souvik Sarkar and Hwalih Han for recruitment and treatment of patients; and Xionce Zhao for help with statistical analysis.
Abbreviations
- HCV
hepatitis C virus
- ribavirin
ribavirin
- PegIFN
pegylated interferon
- ISG
interferon stimulated gene
- NK
natural killer
- STAT
signal transducer and activator of transcription
- PBMC
peripheral blood mononuclear cell
- IFN-γ
interferon γ
References
- 1.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlotsky JM, Dahari H, Neumann AU, Hezode C, Germanidis G, Lonjon I, Castera L, et al. Antiviral action of ribavirin in chronic hepatitis C. Gastroenterology. 2004;126:703–714. doi: 10.1053/j.gastro.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Rotman Y, Noureddin M, Feld JJ, Guedj J, Witthaus M, Han H, Park YJ, et al. Effect of ribavirin on viral kinetics and liver gene expression in chronic hepatitis C. Gut. 2014;63:161–169. doi: 10.1136/gutjnl-2012-303852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feld JJ, Lutchman GA, Heller T, Hara K, Pfeiffer JK, Leff RD, Meek C, et al. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology. 2010;139:154–162. doi: 10.1053/j.gastro.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Conjeevaram HS, Fried MW, Sallie R, Park Y, Yurdaydin C, Swain M, et al. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 8.Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen JT, Hoopes JD, Le MH, Smee DF, Patick AK, Faix DJ, Blair PJ, et al. Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro. PLoS One. 2010;5:e9332. doi: 10.1371/journal.pone.0009332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feld JJ. Is there a role for ribavirin in the era of hepatitis C virus direct-acting antivirals? Gastroenterology. 2012;142:1356–1359. doi: 10.1053/j.gastro.2011.12.064. [DOI] [PubMed] [Google Scholar]
- 11.Dietz J, Schelhorn SE, Fitting D, Mihm U, Susser S, Welker MW, Fuller C, et al. Deep sequencing reveals mutagenic effects of ribavirin during monotherapy of hepatitis C virus genotype 1-infected patients. J Virol. 2013;87:6172–6181. doi: 10.1128/JVI.02778-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann E, Lee JH, Marinos G, Modi M, Zeuzem S. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology. 2003;37:1351–1358. doi: 10.1053/jhep.2003.50218. [DOI] [PubMed] [Google Scholar]
- 13.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 14.Langhans B, Nischalke HD, Arndt S, Braunschweiger I, Nattermann J, Sauerbruch T, Spengler U. Ribavirin exerts differential effects on functions of Cd4+ Th1, Th2, and regulatory T cell clones in hepatitis C. PLoS One. 2012;7:e42094. doi: 10.1371/journal.pone.0042094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman F, Heller T, Sobao Y, Mizukoshi E, Nascimbeni M, Alter H, Herrine S, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 16.Barnes E, Harcourt G, Brown D, Lucas M, Phillips R, Dusheiko G, Klenerman P. The dynamics of T-lymphocyte responses during combination therapy for chronic hepatitis C virus infection. Hepatology. 2002;36:743–754. doi: 10.1053/jhep.2002.35344. [DOI] [PubMed] [Google Scholar]
- 17.Lauer GM, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Schulze Zur Wiesch J, et al. Full-breadth analysis of CD8+ T-cell responses in acute hepatitis C virus infection and early therapy. J Virol. 2005;79:12979–12988. doi: 10.1128/JVI.79.20.12979-12988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu WL, Su WC, Cheng CW, Hwang LH, Wang CC, Chen HL, Chen DS, et al. Ribavirin up-regulates the activity of double-stranded RNA-activated protein kinase and enhances the action of interferon-alpha against hepatitis C virus. J Infect Dis. 2007;196:425–434. doi: 10.1086/518894. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Jamaluddin M, Wang S, Tian B, Garofalo RP, Casola A, Brasier AR. Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol. 2003;77:5933–5947. doi: 10.1128/JVI.77.10.5933-5947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53:32–41. doi: 10.1002/hep.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, Schweigler LM, et al. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–1563. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edlich B, Ahlenstiel G, Zabaleta Azpiroz A, Stoltzfus J, Noureddin M, Serti E, Feld JJ, et al. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C patients. Hepatology. 2012;55:39–48. doi: 10.1002/hep.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, Holz LE, et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–1239. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyagi T, Shimizu S, Tatsumi T, Nishio K, Hiramatsu N, Kanto T, Hayashi N, et al. Differential alteration of CD56(bright) and CD56 (dim) natural killer cells in frequency, phenotype, and cytokine response in chronic hepatitis C virus infection. J Gastroenterol. 2011;46:1020–1030. doi: 10.1007/s00535-011-0408-8. [DOI] [PubMed] [Google Scholar]
- 25.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 27.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 28.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 29.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KB, Cousens LP, Doughty LA, Pien GC, Durbin JE, Biron CA. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nature immunology. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 31.Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. MBio. 2011;2 doi: 10.1128/mBio.00169-11. pii: e00169–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.