Abstract
Purpose
To compare and examine the storage stability of compounded bevacizumab in polycarbonate (PC) and polypropylene (PP) syringes over a 6-month period. PC syringes have been used in a recent clinical study and bevacizumab stability has not been reported for this type of syringe.
Methods
Repackaged bevacizumab was obtained from Moorfields Pharmaceuticals in PC and PP syringes. Bevacizumab from the stored syringes was analysed at monthly time points for a 6-month period and compared with bevacizumab from a freshly opened vial at each time point. SDS-PAGE electrophoresis and size-exclusion chromatography (SEC) was used to observe aggregation and degradation. Dynamic light scattering (DLS) provided information about the hydrodynamic size and particle size distribution of bevacizumab in solution. VEGF binding and the active concentration of bevacizumab was determined by surface plasmon resonance (SPR) using Biacore.
Results
SDS-PAGE and SEC analysis did not show any changes in the presence of higher molecular weight species (HMWS) or degradation products in PC and PP syringes from T0 to T6 compared with bevacizumab sampled from a freshly opened vial. The hydrodynamic diameter of bevacizumab in the PC syringe after 6 months of storage was not significantly different to bevacizumab taken from a freshly opened vial. Using SPR, the VEGF binding activity of bevacizumab in the PC syringe was comparable to bevacizumab taken from a freshly opened vial.
Conclusion
No significant difference over a 6-month period was observed in the quality of bevacizumab repackaged into prefilled polycarbonate and polypropylene syringes when compared with bevacizumab that is supplied from the vial.
Introduction
Two recent multi-center randomised controlled clinical trials compared the use of ranibizumab (Lucentis, Genentech) and bevacizumab (Avastin, Genentech) to treat wet age-related macular degeneration (AMD).1, 2, 3, 4 These trials (IVAN and CATT) found there is no difference in visual acuity outcome during 1- and 2-year treatment periods, respectively.2, 3 Both ranibizumab and bevacizumab were developed to bind to vascular endothelial growth factor (VEGF) as a means to inhibit blood vessel growth.5 Ranibizumab is a humanised antibody fragment (Fab) that is licensed for intravitreal injection to treat AMD and other retinal conditions.6, 7, 8, 9, 10
Bevacizumab is a humanised monoclonal full-length antibody that is licensed for administeration by intravenous infusion to treat cancer (metastatic colorectal, NSCLC, renal cell cancer, glioblastoma).11, 12 It is not licensed for intravitreal injection to treat retinal diseases. Bevacizumab is normally provided as a solution in a glass vial containing 400 mg of the antibody at a concentration of 25 mg/ml. For ocular use, bevacizumab is often transferred under aseptic conditions into ready-to-use 1.0-ml syringes for intravitreal injection by compounding pharmacies for local distribution. A shelf life of up to 3 months13, 14 is often specified. To avoid the risks and costs of compounding there have been reports of ‘multiple use' from a vial of bevacizumab to treat patients consecutively. However, there is the risk of infection if the vial is punctured multiple times and an increased incidence of endophthalmitis has been reported.15
The National Institute for Health and Care Excellence (NICE) considers the compounding of bevacizumab into syringes followed by storage prior to ophthalmic use to be unlicensed, rather than off-license use of bevacizumab.16 In spite of head-to-head trials indicating that ranibizumab and bevacizumab are clinically statistically equivalent, some safety results from the CATT study indicate there may be a greater burden of side effects for bevacizumab compared with ranibizumab.
The cost of compounded bevacizumab per intravitreal dose is approximately 5–9% of the cost of a dose of ranibizumab.17 Moderate to severe disabilities in our ageing population, of which diminishing visual function is one, are projected to increase by 32–54% in the UK, by 2022.17 Ranibizumab and bevacizumab are used for other major ophthalmic diseases affecting older patients including diabetic retinopathy and retinal vein occlusion, whereas AMD is the main cause of blindness for these older patients.18 Unfortunately, costs have generally become a constraining factor for the use of expensive medicines in many parts of the world. It is not unreasonable to expect that intravitreal use of bevacizumab will continue in many parts of the world; especially, in resource-limited regions and for older patients whose overall health and social care costs are already high and are expected to increase.17, 18
The reported incidence of IOP spike19, 20 or endophthalmitis that may be associated with bevacizumab injections21, 22, 23, 24 is thought to be related to the presence of particulates or protein aggregates.20, 25 The presence of silicon oil contamination and the type of syringe used for repackaged bevacizumab has also been reported to be associated with an increase in protein aggregates or particulate count.14 As with any therapeutic antibodies, exposure to light, storage temperature, product handling, and syringe components can cause protein misfolding, denaturation, and aggregation. These changes in protein structure can decrease protein activity and may result in immunological responses.26
Ranibizumab has recently become available in ready-to-use glass syringes27, 28 but the cost of this medicine has yet to drop. Unfortunately, the compounding and subsequent storage of bevacizumab in plastic syringes have not been approved by regulatory agencies. One important factor to consider is the different types of syringe that are used for bevacizumab. Reports have been published about bevacizumab being compounded into polypropylene (PP) syringes13, 14, 29, 30 and the effects of storage conditions20, 31 on the stability and efficacy of bevacizumab. Polycarbonate (PC) syringes have also been used, and were used in the IVAN trial.32 There does not, however, appear to be a report about the stability of bevacizumab when repackaged in polycarbonate syringes (Table 1).
Table 1. Example studies of storage stability of repackaged bevacizumab in syringes.
| Purpose of study | Duration of study | Syringe material | Reference |
|---|---|---|---|
| Compare quality of repackaged bevacizumab from 5 different compounding pharmacies in UK | 14 days | Polypropylene syringe | 13 |
| Examine the effect of silicon oil microdroplets and mishandling on protein aggregation level in repackaged bevacizumab | 3 months | Plastic syringe (material not specified) | 14 |
| High molecular weight aggregates in repackaged bevacizumab | Not specified | Plastic syringe (material not specified) | 20 |
| Stability of bevacizumab repackaged in 1 ml polypropylene syringes for intravitreal administration | 3 months | Polypropylene syringe | 30 |
In this study, we examined the stability of compounded bevacizumab in both PC and PP syringes. The PC syringe had a luer-lock to secure the needle. The more commonly used PP syringe had a slip-lock to hold the needle. The bevacizumab-filled syringes were then stored at 5±3 °C. The stored bevacizumab-filled syringes were evaluated monthly over a 6-month period by SDS-PAGE, size-exclusion chromatography (SEC), dynamic light scattering (DLS), and surface plasmon resonance (SPR).
Materials and methods
Materials
Bevacizumab (Avastin, Genentech, 400 mg) solution from a vial (16 ml) was aseptically fractionated into 1.0 ml sterile syringes at Moorfields Pharmaceuticals a day before starting the first time point. The volume of the bevacizumab solution transferred to each syringe was 0.13 ml. Two different syringes (as shown in Table 2) were evaluated, (i) PC with a luer-lock barrel and (ii) PP with a slip-lock barrel (Table 2). A fresh vial of bevacizumab was used for the control data obtained at each time point. The filled syringes and vials were stored at 5±3 °C and the temperature was monitored and recorded at regular intervals at Moorfields Pharmaceuticals. At each sampling time point, syringes were shipped to the UCL School of Pharmacy where stability studies were conducted within 24 h of receipt.
Table 2. Material compositions for Polycarbonate (PC) and Polypropylene (PP) syringes.
| Compositions | Polycarbonate (PC) | Polypropylene (PP) |
|---|---|---|
| Barrel | Luer-lock | Slip-lock |
| Plunger Rod | Polypropylene | Polypropylene |
| Stopper | Latex-free elastomer | Polyisoprene |
| Lubricant | Silicone | Silicone |
| Sterilisation Method | Gamma irradiation | Gamma irradiation |
| Supplier | B. Braun Medical Inc (Cat. No 309628) | Becton Dickinson (Cat. No 9161406V) |
Methods
Study design
The bevacizumab solution in PC and PP syringes was evaluated for its physicochemical stability over a 6-month period at monthly time points and compared with bevacizumab solution obtained from freshly opened vials. Only one vial was used per time point and data generated during 8-h period on the day that vial was open. The time points are designated as T0 (first time point after fractionation procedure), T3 and T6 representing three and 6-month storage period.
Gel-electrophoresis SDS-PAGE
Novex bis-tris 4–12% precast gels (Invitrogen, Paisley, UK) were used for PAGE analysis. Solutions were first prepared by taking the bevacizumab solution from a syringe (0.05 ml) and the same volume of bevacizumab solution from the vial (0.05 ml) and adding each to phosphate-buffered saline (PBS), pH 7.2 to make up the volume to 1.0 ml and giving a final concentration of 1.25 mg/ml. PBS was prepared with tablets purchased from Oxoid, UK containing 0.16 M NaCl, 0.003 M KCl, 0.008 M Na2HPO4, and 0.001 M KH2PO4. Samples were then loaded (0.01 ml) onto a gel after mixing with SDS sample buffer (4 × ). Gels were then stained with Instant blue (Expedeon Ltd., Swavesey, UK) staining to visualise the protein lane.
Size-exclusion chromatography
For SEC analysis the bevacizumab solution from a freshly open vial and the stored syringes was diluted with PBS (1.25 mg/ml, 1.0 ml) and transferred to sample vials in an autosampler, which then loaded 950 μl of each sample onto a SEC column, (HiLoad 16/600 Superdex 200 prep grade column, GE Healthcare Life sciences, Buckinghamshire, UK) for separation. SECs were conducted in triplicate for each time point for both syringe and vial samples using a system comprised of a UV detector (Jasco UV-1570, JASCO UK, Great Dunmow, Essex, UK; at 280 nm) and HPLC pump (Jasco PU-980 Intelligent, JASCO UK). Azur software (Great Dunmow, Essex, UK) was used to process chromatographic data.
Dynamic light scattering
Malvern Zetasizer Nano-ZS, Malvern, UK with 633-nm laser source was used for measuring hydrodynamic diameter of bevacizumab. Contaminating particles such as dust in a solution can be detected in DLS and cause interference. Hence, bevacizumab solution from vial and syringe was diluted with 0.22 μm filtered PBS to make 1.25 mg/ml solution. These were then subjected to measurement by DLS in 1.0 ml disposable polystyrene cuvettes. Nano-ZS analysis software was used to analyse the measurements. Each measurement was an average of 25 runs of 10 seconds each, carried out in duplicate. DLS analysis was performed at time points (T0, T3, and T6) for the bevacizumab solution obtained from a freshly opened vial and from the syringe at each time point. Samples from six different PC syringes were evaluated at each time point and three samples were made from the vial.
Active protein concentration using Biacore
Human recombinant VEGF165 (38 kDa MW, purchased from Sigma Aldrich, Dorset, UK) was immobilised on a CM5 (534 RU). The high immobilisation level was selected for concentration assays. The immobilisation was performed using standard carbodiimide mediated coupling (NHS/EDC, 50/50) and ethanolamine (pH 8.5). Samples were prepared in HBS-EP running buffer (10 mM HEPES, pH 7.4, 150 mm NaCl, 3.0 mM EDTA, 0.005% surfactant P20). All binding and concentration measurements were conducted at 25 °C at a flow rate of 10 μl/min. Chip regeneration was accomplished by exposure to 10.0 mM glycine–HCl (pH 1.5) for 1200 s. Double referencing was performed to account for bulk effects caused by changes in the buffer composition or nonspecific binding. Data were evaluated with BIAevaluation software (version 2.1) in Biacore X-100 (Buckinghamshire, UK).
Statistical analysis
Data were analysed for statistical significance using Student's t-test and P-value of <0.05 was considered statistically different. Data are presented as mean±SD for at least triplicate observations.
Results
Gel-electrophoresis (SDS-PAGE)
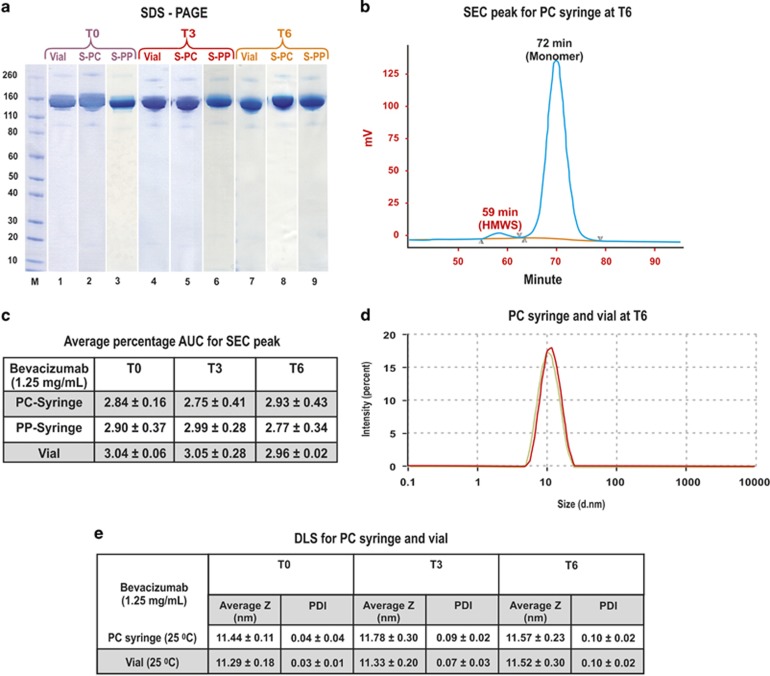
SDS-PAGE analysis was conducted by diluting bevacizumab solution from a syringe (PC and PP, 0.05 ml) and the same volume of bevacizumab solution from the vial (0.05 ml) with PBS buffer (0.95 ml, pH 7.4). Samples (0.01 ml, 1.25 mg/ml) were analysed by SDS-PAGE (Figure 1a). Three individual samples each from the syringe and the vial were evaluated. The band at 150 kDa in Figure 1a is the monomer of bevacizumab. The gels were heavily and equally loaded in an effort to observe any changes in the presence of higher molecular weight species (HMWS) of bevacizumab or degradation products. No change in SDS-PAGE from T0 to T6 was observed for any of the samples.
Figure 1.
(a) SDS-PAGE analysis of bevacizumab solution from the syringes (PC and PP) and vial, at T0, T3, and T6. Novex Bis-Tris 4–12% gels were stained with colloidal blue. Lane M: Protein standards. Lanes 1, 4, 7; bevacizumab (1.25 mg/ml) from vial at T0, T3, and T6, respectively. Lanes 2, 5, 8; bevacizumab (1.25 mg/ml) from PC syringes at T0, T3, and T6, respectively. Lanes 3, 6, 9; bevacizumab (1.25 mg/ml) from PP syringes at T0, T3, and T6, respectively. (b) SECs of bevacizumab from the PC syringe. (c) Average percentage AUC for SEC peak at 58–59 min for bevacizumab solution from the PP and PC syringes and vial at T0, T3, T6 (n=3), no significant difference (P>0.05) between presence of HMWS in vial and syringe over 6 months of storage. (d) Overlay of size distribution curves for PC syringe and vial after 6-month storage, bevacizumab solution from vial and syringe have a similar size distribution. (e) DLS measurements of bevacizumab from PC syringes and vial at T0, T3 and T6 at 25 °C (P>0.05).
Size-exclusion chromatography
Size-exclusion chromatography (SEC) was used in an effort to observe if there was any aggregation of bevacizumab. Six replicates were obtained for samples stored in syringes at each time point. Three replicates were obtained at each time point for the samples obtained directly from freshly opened vials. A representative chromatogram (Figure 1b) shows the HMWS of bevacizumab at a retention time of 59 min and monomer at 72 min for bevacizumab stored in the PC syringe for 6 months. Figure 1c is the area under the curve (AUC) for the HMWS of bevacizumab at different time points (T0, T3, T6) for PP and PC syringes as compared with the vial. There appeared to be no significant change in the AUC of this HMWS over the 6-month period for the PP and PC syringe-stored samples as compared with the vial.
Dynamic light scattering
On examination with SDS-PAGE and SEC, there was no difference in the physical stability of bevacizumab stored in either PP or PC syringes. The result obtained for physical stability of bevacizumab fractionated in PP syringes was found to be in excellent agreement with a previously published extensive report.30 Hence, we decided to focus on the PC syringes for further analysis as these were used for IVAN study and reports were submitted to MHRA without published public records.32
The hydrodynamic diameter of bevacizumab stored in PC syringes was found to be 11.19 with PDI of 0.02 at 25 °C (Figure 1d). There was no significant difference in the size distribution of bevacizumab stored in the syringe after 6 months of storage compared with bevacizumab taken fresh from the vial (Figure 1e).
Surface plasmon resonance (SPR)
Binding of bevacizumab was evaluated by SPR (Biacore) to calculate the active concentration of bevacizumab in the syringe compared with the vial. If the binding of bevacizumab to VEGF decreases owing to storage in a syringe, then differences in the active concentration of bevacizumab from a freshly opened vial should be apparent when evaluated by SPR.33 The binding of bevacizumab fractionated into PC syringe was studied during 6-month storage period and compared with bevacizumab in freshly opened vial.
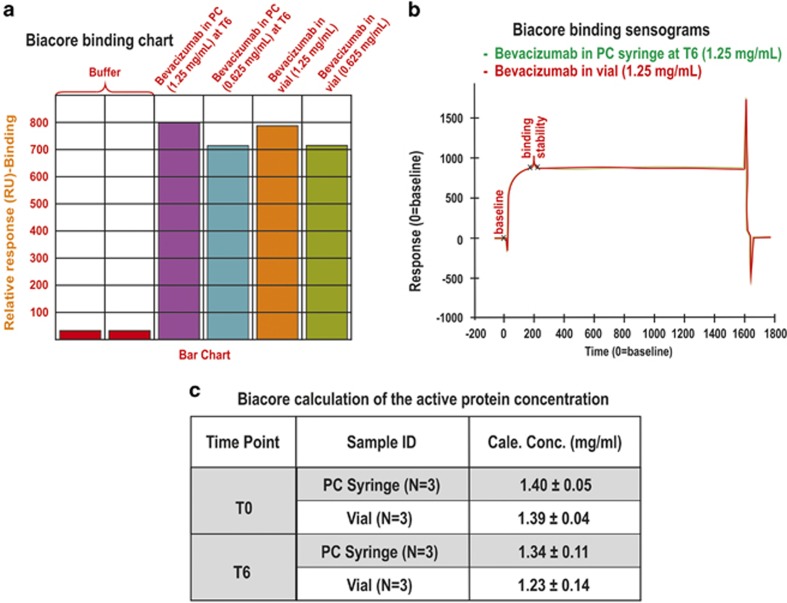
A CM5 chip was functionalized with VEGF (534 RU) to conduct the binding assay. The binding study was then performed on bevacizumab that was aliquoted from a freshly opened vial and from the syringe at each time point. Figure 2a shows a representative bar chart of the binding for bevacizumab in two concentrations (1.25 and 0.625 mg/ml) from a PC syringe after 6 months storage and from a freshly opened vial. There did not appear to be any difference in the binding response of bevacizumab from the syringe at the different time points compared with the bevacizumab from a freshly opened vial (Figure 2a). For reference, a superposition is shown in Figure 2b of the sensograms for the bevacizumab from both the vial and the syringe at the 6-month time point.
Figure 2.
(a) The representative binding chart for bevacizumab in PC syringe at T6 (N=3) and freshly opened vial at 1.25 and 0.625 mg/ml concentration, (b) Binding sensograms of PC syringe at 1.25 mg/ml at T6 overlaid with bevacizumab from freshly opened vial, (c) Biacore calculation of the active protein concentrations; bevacizumab obtained from syringes and the vial (N=3) at T0 and T6.
To calculate the active concentration of bevacizumab from the syringe, a calibration standard curve was generated with bevacizumab (2.0–0.25 mg/ml, four dilutions) obtained from a freshly opened vial. Bevacizumab (1.25 mg/ml) from the PC syringe was prepared from 0.05 ml protein solution into 1.0 ml buffer and was passed over the CM5 chip to interact with immobilised VEGF. In parallel, 0.05 ml of bevacizumab from the vial (1.25 mg/ml) was added to 1.0 ml of buffer and evaluated. The calibration responses were then used to calculate the active concentration of bevacizumab in the syringe and vial (Figure 2c). The amount of bevacizumab in the syringe did not change compared with that observed for the vial and no difference was observed at T6 compared with T0.
Discussion
There have been previous studies to investigate the physical stability of repackaged bevacizumab in PP syringes.13, 14, 30 The results from our study suggest that there is no significant difference in the physical stability of bevacizumab when repackaged in PC or PP syringes when compared with bevacizumab that had been stored in a glass vial. The study was performed at time points over a 6-month period using different techniques.
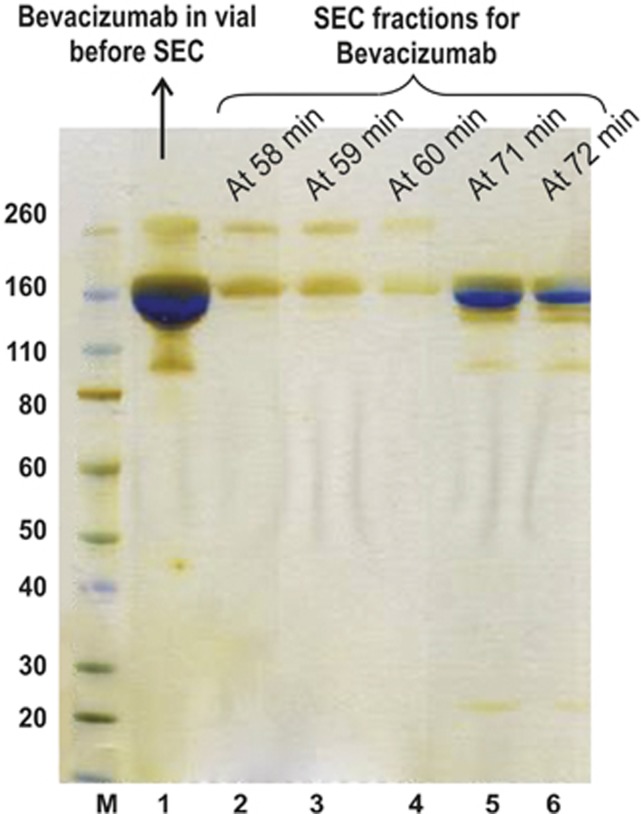
Bevacizumab is a 150 kDa protein that appears as a distinct band (Figure 1a, Lanes 1–9) by SDS-PAGE. There is a faint band seen at about the 260 kDa protein standard band. This may indicate the presence of aggregates of the antibody, which is also consistent with what was observed by SEC30 (Figure 1b, peak at 59 min) with the presence of HMWS. To investigate this further, fractions were collected from 58–59 min and analysed with SDS-PAGE (Figure 3) using silver stain as a detection method. Silver stain is more sensitive than colloidal blue staining and can detect protein in the range of 5–30 ng.34 Fractions were also collected at the main peak (71–72 min; Figure 3, Lanes 5–6). The higher molecular weight band was not observed at the peak of 71–72 min (Figure 3, Lanes 5–6) suggesting that this species was not in equilibrium with bevacizumab. The fractions obtained from the peak at 58–60 min appear to be a heterogeneous population with bevacizumab HMWS. This higher molecular weight band in SDS-PAGE has been reported previously13 for bevacizumab from both the vial and syringe. However, it is important to note that there is lack of significant difference in the level of HMWS between vial and PC syringe at different time points between T0 and T6.
Figure 3.
SDS-PAGE analysis of bevacizumab fractions eluted from SEC. Novex Bis-Tris 4–12% gels were stained with silver stain. Lane M: Protein standards. Lane 1: Bevacizumab from vial. Lane 2–4: Bevacizumab fractions at 58–60 min from SEC represent dimer of bevacizumab. Lane 5,6: Bevacizumab fractions at 71–72 min from SEC represent the monomer content.
There was no significant difference in the hydrodynamic radius of bevacizumab from the vial and the PC syringe over a 6-month period when measured by DLS. A similar result was reported by Paul et al30 for storage stability of bevacizumab fractionated in a PP syringe for a period of 3 months. Paul et al30 also reported that the HMWS present in the bevacizumab solution in the PP syringe was ~360 nm when the DLS measurement was made at 25 °C. However, the hydrodynamic size of the bevacizumab sample stored at ambient temperature overnight was 100.5 nm (PDI=0.46) suggesting that the storage temperature has an impact on the bevacizumab stability profile.
SPR was used to evaluate bevacizumab binding to VEGF and no change in binding was observed during the 6-month storage period for the bevacizumab stored in the PC syringes compared with bevacizumab from the vial. SPR is a nonlabelling technique that allows measurement of protein–protein interactions such as antibody–antigen interactions. One of the interacting molecules is immobilised onto a sensor chip and the other molecule is allowed to flow over the functionalised sensor chip. If binding occurs between the analyte and immobilised ligand, a measurable response will be generated. Whereas the BCA assay can be used to determine the total protein content, SPR and ELISA are used to determine the VEGF binding and active protein concentration of bevacizumab. Bakri et al33 studied the VEGF binding of bevacizumab stored in a vial and syringe over 6 months time using ELISA. In our study, Biacore was used to study active protein concentration and VEGF binding of bevacizumab stored in vial and PC syringe for a 6-month time period. A decrease in antibody binding will cause a decrease in relative response units (RU) that are generated. Biacore is a real time-based method and is more sensitive compared to ELISA while no labelling is required.
Our results using several analytical methods and real-time VEGF binding (Biacore) demonstrate that the commercial solution of bevacizumab (25 mg/ml, 16 ml in vial) can be fractionated in PP and PC syringes and stored up to 6 months at 4 °C without any discernible changes in protein physical stability and VEGF binding.
Acknowledgments
HK, GS, PTK and SB are grateful for funding from NIHR Biomedical Research Centre at Moorfields Hospital and the UCL Institute of Ophthalmology, Moorfields Special Trustees, the Helen Hamlyn Trust (in memory of Paul Hamlyn), Fight for Sight, Freemasons Grand Charity, and Michael and Ilsa Katz charity. GS and SB are also grateful for funding from the UK Engineering and Physical Sciences Research Council (EPSRC) for the EPSRC Centre for Innovative Manufacturing in Emergent Macromolecular Therapies. Financial support from the consortium of industrial and governmental users for the EPSRC Centre is also acknowledged.
AF is an employee of Moorfields Pharmaceuticals. The remaining authors declare no conflict of interest.
References
- Ahfat FG, Zaidi FH. Bevacizumab vs ranibizumab-an appraisal of the evidence from CATT and IVAN. Eye. 2013;27 (3:289–290. doi: 10.1038/eye.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119 (7:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Fine SL, Ying G, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119 (7:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman C. CATT and IVAN results. Retina. 2012;17:9. [Google Scholar]
- Ferrara N. Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med. 2010;16 (10:1107–1111. doi: 10.1038/nm1010-1107. [DOI] [PubMed] [Google Scholar]
- Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157 (1:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14:S140–S144. doi: 10.1016/j.ghir.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Boulton M, Foreman D, Williams G, McLeod D. VEGF localisation in diabetic retinopathy. Br J Ophthalmol. 1998;82:561–568. doi: 10.1136/bjo.82.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Fagerholm P, Palmblad J. Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta ophthalmologica Scandinavica. 2004;82 (5:557–563. doi: 10.1111/j.1600-0420.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26 (8:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther. 2006;28 (11:1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, Lowman H, Kim R, Hillan KJ, et al. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333 (2:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Amoaku WM, Kamali F. Quality of bevacizumab compounded for intravitreal administration. Eye. 2013;27 (9:1090–1097. doi: 10.1038/eye.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Ammar DA, Ross LA, Mandava N, Kahook MY, Carpenter JF. Silicone oil microdroplets and protein aggregates in repackaged bevacizumab and ranibizumab: effects of long-term storage and product mishandling. Invest Ophthalmol Vis Sci. 2011;52 (2:1023–1034. doi: 10.1167/iovs.10-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DS, Kwok AK, Chan CW, Li WW. Intravitreal bevacizumab: safety of multiple doses from a single vial for consecutive patients. Hong Kong Med J. 2012;18:488–495. [PubMed] [Google Scholar]
- Bevacizumab (Avastin) for eye conditions. NICE. 2010.
- Wittenberg R, Hu B, Comas-Herrera A, Fernandez JL.Care for older people: projected expenditure to 2022Published on-line in nuffieldtrust2012
- Appleby J.Spending on health and social care over the next 50 years Why think long termPublished by The King's Fund2013
- Sniegowski M, Mandava N, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J. 2010;4:28–29. doi: 10.2174/1874364101004010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahook MY, Liu L, Ruzycki P, Mandava N, Carpenter JF, Petrash JM, et al. High-molecular-weight aggregates in repackaged bevacizumab. Retina. 2010;30 (6:887–892. doi: 10.1097/IAE.0b013e3181d50cea. [DOI] [PubMed] [Google Scholar]
- Yamashiro K, Tsujikawa A, Miyamaoto K, Oh H, Otani A, Tamuara H, et al. Sterile endophthalmitis after intravitral injection of bevacizumab obtained from a single batch. Retina. 2010;30 (3:485–490. doi: 10.1097/IAE.0b013e3181bd2d51. [DOI] [PubMed] [Google Scholar]
- Artunay O, Yuzbasioglu E, Rasier R, Sengul A, Bahcecioglu H. Incidence and management of acute endophthalmitis after intravitreal bevacizumab (Avastin) injection. Eye. 2009;23 (12:2187–2193. doi: 10.1038/eye.2009.7. [DOI] [PubMed] [Google Scholar]
- Goldberg RA, Flynn HW, Isom RF, Miller D, Gonzalez S. An outbreak of streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153 (2:204–208 e201. doi: 10.1016/j.ajo.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RA, Flynn HW, Miller D, Gonzalez S, Isom RF. Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: one-year outcomes and investigative results. Ophthalmology. 2013;120 (7:1448–1453. doi: 10.1016/j.ophtha.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, et al. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98 (4:1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Prediction of aggregation prone regions of therapeutic proteins. J Phys Chem. 2010;114:6614–6624. doi: 10.1021/jp911706q. [DOI] [PubMed] [Google Scholar]
- Salcedo G, Harasawa M, Velez-Montoya V, Mathias MT, Siringo FS, Olson JL, et al. Spectral Domain Optical Coherence Tomographic Analysis of Non-Occluded Retina in Branch Retinal Vein Occlusion And Its Response to Anti-angiogenic Therapy, in ARVO 2014, 2014. [DOI] [PubMed]
- Clunas N, Sigg J, Boado L, Momm J, Weber C, Alete D, et al. Ophthalmology. Novartic Pharma AG: Swizerland; 2014. Ranibizumab pre-filed syringe: recently approved innovation in the eurean union with the protein to reduce infection risk, improve dose accuracy, and enhance efficient treatment administration. [Google Scholar]
- Majumdar S, Ford BM, Mar KD, Sullivan VJ, Ulrich RG, D'Souza AJ. Evaluation of the effect of syringe surfaces on protein formulations. J Pharm Sci. 2011;100 (7:2563–2573. doi: 10.1002/jps.22515. [DOI] [PubMed] [Google Scholar]
- Paul M, Vieillard V, Roumi E, Cauvin A, Despiau MC, Laurent M, et al. Long-term stability of bevacizumab repackaged in 1 mL polypropylene syringes for intravitreal administration. Annales Pharmaceutiques Francaises. 2012;70 (3:139–154. doi: 10.1016/j.pharma.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wu PC, Shiea J, Lo LH, Wu YC, Kuo HK. Evaluation of the sterility, stability, and efficacy of bevacizumab stored in multiple-dose vials for 6 months. J Ocular Pharmacol Ther. 2009;25 (1:65–69. doi: 10.1089/jop.2008.0043. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382 (9900:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- Bakri S, Mellissa R, Pulido JS, Mccannel CA, Weiss W, Singh RJ. Six-month stability of bevacizumab (Avastin) binding to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina. 2006;26 (5:519–522. doi: 10.1097/01.iae.0000225354.92444.7a. [DOI] [PubMed] [Google Scholar]
- Bartsch H, Arndt C, Koristka S, Cartellieri M, Bachmann M. Silver staining techniques of polyacrylamide gels. Methods Mol Biol. 2012;869:481–486. doi: 10.1007/978-1-61779-821-4_42. [DOI] [PubMed] [Google Scholar]