Abstract
Purpose
To evaluate changes in ocular surface and central corneal sub-basal nerve fiber layer (SBNFL) after topical cyclosporin therapy in chronic glaucoma patients on long-term topical antiglaucoma therapy.
Methods
A prospective comparative study of ocular surface evaluation of chronic glaucoma patients on long-term topical therapy treated concurrently with a topical cyclosporine 0.05% twice daily for 6 months and controls was done. The study parameters evaluated at recruitment and at the 6-month follow-up included details of topical antiglaucoma medications, visual acuity, intraocular pressure, ocular surface evaluation parameters (TBUT, Schirmers I, ocular surface staining scores and ocular surface disease (OSD) index score (OSDI)), central corneal sensation (Cochet Bonnett aesthesiometer), and central confocal microscopy to study the SBNFL density (SBNFLD).
Results
Thirty-two eyes of 16 patients with chronic glaucoma and 30 eyes of 15 normal subjects as controls were studied. Mean TBUT, pre/post CsA treatment was 8.67±3.01/12.24±1.83 s (P=0.007). Mean conjunctival/corneal staining scores pre/post CsA treatment were 3.38±1.93/1.50±0.718 (P=0.00) /5.19±1.82/1.81±0.78 (P=0.098), respectively. Mean OSDI pre/post CsA treatment scores were 30.63±14.61/14.76±6.06 (P=0.007). Mean corneal sensations scores pre/post CsA treatment were 4.64±0.46/4.94±0.39 (P=0.002). Central corneal SBNFLD pre and post CsA treatment was 8811.35±2985.29/10335.13±4092.064 μm/mm2 (P=0.0001).
Conclusions
Schirmer's test, ocular surface staining scores, OSDI, corneal sensations, and corneal SBNFLD showed a statistically significant improvement following a 6-month concurrent topical CsA therapy.
Introduction
Corneal innervation is vital for the maintainence of corneal epithelial integrity, proliferation function and in corneal wound healing after injury.1
The sub-basal nerve plexus along with stromal keratocytes secrete a number of neuropeptides, which facilitate cell mitogenesis and migration, DNA synthesis, neurite extension and survival, keratocyte proliferation, and the regulation of epithelial stem cells.2 These neuropeptides are diffusible factors that are believed to stimulate the epithelial growth, proliferation, differentiation, and the production of collagen type VII.3, 4
The epithelial cells, in reciprocation, produce the soluble factors neuronal growth factor and glial cell-derived neurotrophic factor with a neurotrophic effect.5 Chronic ocular surface inflammation sensitizes ocular surface nerves and can also disrupt corneal barrier function. Nerve degeneration that occurs in the scenario of chronic ocular surface inflammation, as in cases of dry eye, may lead to altered sub-basal nerve fiber morphology in eyes.6 Several adverse effects on ocular surface and corneal innervation has been reported in patients on long-term antiglaucoma medications.5 The role of in vivo confocal microscopy in ocular surface analysis of dry eye and glaucomatous patients has been elucidated.5, 7, 8, 9, 10, 11, 12, 13, 14 Topical cyclosporine has been proven to have a beneficial effect in cases of dry eye disease (DED).15 In a recent study in rabbit eyes beneficial effects of topical cyclosporine has been demonstrated on adverse ocular surface changes produced by long-term antiglaucoma medications.16
The effect of topical cyclosporine therapy in chronic glaucoma patients on changes in the central corneal sub-basal nerve fiber layer (SBNFL) density in human eyes has not been evaluated. The aim of this study was to evaluate the ocular surface changes and central corneal SBNFL changes in patients on long-term topical antiglaucoma medications induced by the concurrent use of topical cyclosporine therapy.
Patients and methods
This study was performed conforming to the Declaration of Helsinki. Informed consent was obtained from all the patients before enrollment in this prospective comparative, longitudinal open label study. The Institute Ethics Committee board approval was sought and obtained for the study. Patients on follow-up with the glaucoma clinic (during the period of January–December 2012), with chronic glaucoma on two or more topical antiglaucoma medications with preservatives for at least 6 months or more, and consenting to participate in the study were included in the study. Patients using combination therapy of topical antiglaucoma agents with BAK preservatives (timolol with brimonidine; ALPHAGAN ophthalmic solution 0.2%, Allergan Inc., Irvine, CA, USA) one drop twice daily at 8 am and 8 pm daily with or without latanoprost (XALATAN latanoprost ophthalmic solution 0.005%, Pfizer Inc., New York, NY, USA) one drop at bed time, were included in the study. Eyes with pre-existing ocular surface morbidities such as trachoma, cicatrizing conjunctivitis, history of intraocular surgery, laser treatment in recent 6 months, contact lens use, recent ocular inflammation/infection, autoimmune diseases, ocular surface diseases (OSDs) like dry eye due to other causes, previous, or current use of other ocular medications like artificial tear therapy were excluded from the study. Patients with previous history of using any other topical medications were excluded from the study. All patients recruited into the study were given topical CsA 0.05% (Restasis, Allergan) one drop twice daily along with their current antiglaucoma treatment for a period of 6 months. Subjects with no ocular problems, who accompanied the glaucoma patients, were recruited as controls.
Demographic data including age, gender, and duration was obtained from all the patients. The baseline examination done at recruitment included visual acuity, intraocular pressure, ocular surface evaluation (tear break-up time (TBUT), Schirmer's I test, ocular surface staining score, OSD index (OSDI), central corneal sensation, and in vivo scanning slit confocal microscopy of the central cornea. Following the baseline evaluation, the patients were started on topical 0.05% cyclosporine therapy (Restasis, Allergan Inc) twice daily concurrent with the antiglaucoma medications. Six months following topical cyclosporine 0.05% therapy, the ocular surface evaluation (TBUT, Schirmer I test, ocular surface staining score, OSDI), central corneal sensation, and in vivo scanning slit confocal microscopy of the central cornea were repeated and the data were recorded on a predesigned proforma.
Clinical evaluation: comprehensive ocular examination was done for all the patients. Best corrected Snellen's visual acuity was recorded. Intraocular pressure was measured by Goldman applanation tonometry. Slit lamp biomicroscopic examination of anterior and posterior segment and ocular adnexa was done. OSDI17 score was calculated using a 12-item OSDI questionnaire, Schirmer I test18 for tear production, fluorescein TBUT18 for tear film stability, and the National Eye Institute ocular surface staining scores18, 19 for ocular surface damage were evaluated.
Aesthesiometry: Central corneal sensation threshold was measured using Cochett Bonnet Aesthesiometer (CBA, Luneau, Paris, France) that stimulates the corneal nerves by direct contact. This was achieved by gently pressing the 0.12-mm nylon thread against the anterior corneal surface. The longest thread (6 cm) was first used since it provides the lowest stimulus intensity. The examination was repeated by reducing the length in steps of 0.5 mm until the stimulus was felt. The criteria for the threshold were the filament length which gives a 50% positive response from four stimuli presentations. Corneal sensation threshold was measured in length of nylon filament (in cm). Manufacturer provided calibration table on conversion of nylon filament length to pressure exerted on eye was used to record the values.
Slit in vivo confocal microscopy of the cornea: scanning slit in vivo confocal microscopy (NIDEK technologies Srl, Italy Confoscan 4) of the central cornea to image the corneal SBNFL was performed in automatic gain mode using a standard setting of four passes with a scanning range of 200 μm at 40X magnification to obtain central corneal imaging of the anterior cornea. The procedure was performed as follows. One drop of lubricating viscous gel was placed on the microscope objective piece and moved to a backward position. After topical anaesthesia and positioning of the patient's head on the adjustable headrest, the confocal microscope with the 40X objective was placed 1–1.5 mm above the apex of the corneal center. The patient was asked to look into the light, so that the optical center aligned with a lateral accuracy of probably <1 mm. Then the microscope was brought into optical contact with the cornea by manual advancement of the micrometer—controlled Z drive. From this point all further x–y–z movements of the instrument were then controlled from the real time picture displayed on the monitor. Images were recorded and checked to ensure that the required images had been captured and were of sufficient quality for investigation. Each eye was scanned three times and the images were saved. At the time of analysis, two best images were selected for analysis, of which the best one containing maximum number of nerves imaged at the sub-basal plexus level was selected for analysis by a masked observer.
SBNFL were identified as unmyelinated fiber bundles consisting of straight and beaded fibers that course in the basal aspect of the basal epithelial cell layer. Confocal image analysis was performed by a masked observer unaware about demographic details of all recruited eyes. The nerve fibers were traced manually using Neuron J plugin of the available online public domain software Image J program (http://www.imagescience.org/meijering/software/neuronj), following which it automatically generates data regarding the total nerve number and total length/frame of sub-basal nerves. The SBNFL branches were counted as a separate nerve if the length of its visible portion was <50 μm. The total number SBNFL was recorded as the mean number per scan for each cornea. The mean sub-basal nerve layer density was calculated as the total length of all the main nerves and their branches imaged divided by the area of the standard frame size 460 μm × 345 μm containing images (area=0.16 mm2).
Statistical analysis: clinical variables included for analysis were Schirmer I test, conjunctival staining score (NEI), corneal staining score (NEI), FTBUT, OSDI results, and corneal sensitivity. Two best imaged nerve fiber layer for each eye were considered for analyses of nerve fiber number, nerve length, and nerve density. Quantitative variables were expressed as mean and SD, and the values obtained before and after topical cyclosporine 0.05% therapy were compared using two sample t-test. P-value <0.05 was considered statistically significant.
Results
A total of 32 eyes of 16 patients of mean age 47.24±16.21 years (range: 22 to 75 years (10 male (mean age of 49.90±15; range 23 to 75 years) and 6 female patients (mean age of 45±18; range 30 to 55 years))) were eligible for topical cyclosporine therapy and further analysis. The control group comprised of 30 normal eyes of 15 subjects (age and sex matched) with normal eyes ((mean age 40.68±13.73 years); males—15, mean age 41.43±10.80 years; females—15, mean age 39.22+13.12 years).The mean duration of topical hypotensives was 7.81±2.88 years (range 0.6–12 years). Amongst the 16 patients, 11 had of bilateral primary open angle glaucoma, two had juvenile open angle glaucoma, and one of normal tension glaucoma , primary angle closure glaucoma and ocular hypertension (OHT) each. All recruited patients were using combination therapy of BAK preservative-containing timolol 0.5% (one drop twice daily) and brimonidine 0.2% (one drop twice daily) along with or without prostoglandin analog—latanoprost 0.005% (one drop once a day). The number of glaucoma medications used was 2±0.5. The mean IOP of all glaucoma patients was 16.74±5.47 mm Hg and that of the controls was 13.52±1.78 mm Hg. All 16 patients had signs and symptoms of OSD with level 2–3 DED (DEWS classification20).
The pre CsA and post CsA treatment parameters are shown in Table 1. A statistically significant improvement was noted in the values of Schirmer I test, FTBUT, conjunctival staining scores, corneal staining scores OSDI, and corneal sensations before and post cyclosporine therapy.
Table 1. Ocular surface evaluation parameters and corneal sensitivity pre/post cyclosporine therapy.
|
Study group |
Controls | |||
|---|---|---|---|---|
| Pre-CsA treatment | Post CsA treatment | P-value | ||
| Schirmer's test (mm) | 7.28±3.48 | 10.78±2.593 | (0.003) | 12.86±1.93 |
| TBUT (s) | 8.67±3.01 | 12.24±1.83 | (0.007 | 11.80±1.88 |
| Conjunctival staining scores | 3.38±1.93 | 1.50±0.718 | (0.00) | 0.84±0.46 |
| Corneal staining scores | 5.19±1.82 | 1.81±0.78 | (0.098) | 1.10±0.58 |
| OSDI | 30.63±14.61 | 14.76±6.06 | (0.007) | 6.02±3.84 |
| Corneal sensations (cm) | 4.64±0.46 | 4.94±0.39 | (0.002) | 5.07±0.37 |
Pre-CsA, parameters before cyclosporine therapy.
Post CsA, parameters after cyclosporine therapy.
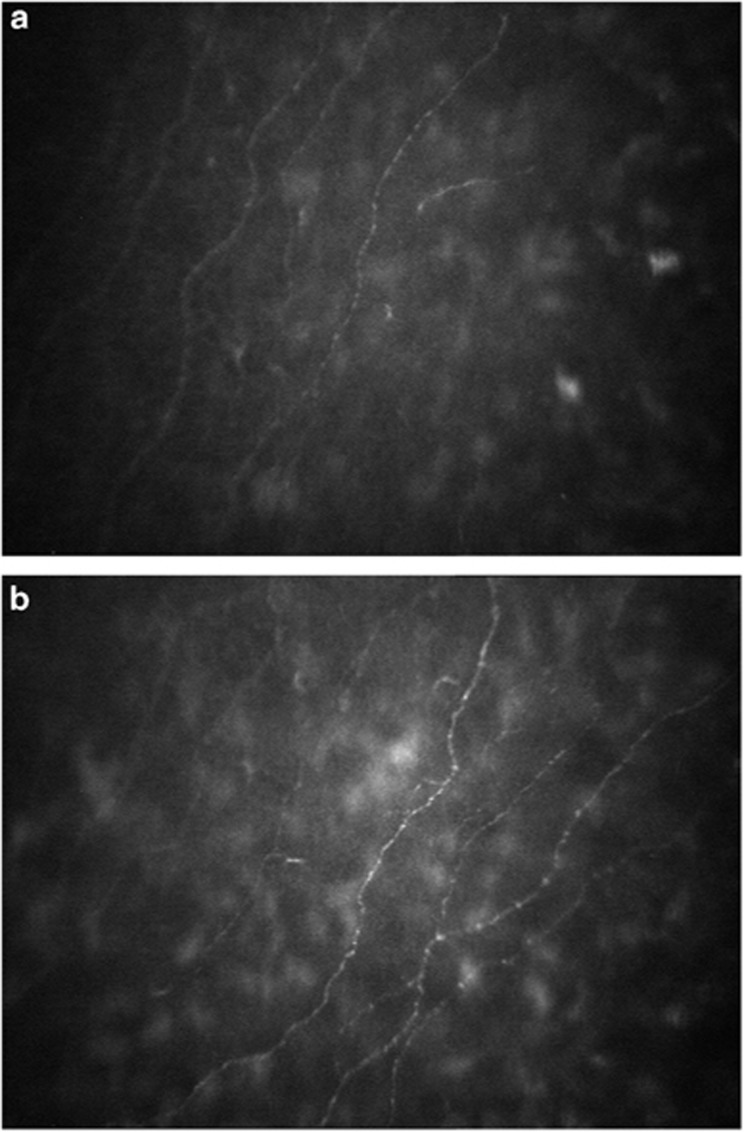
The number of nerves and nerve length in study and control eyes on central corneal confocal microscopy of the SBNFL is given in Table 2. The mean SBNFL density of 30 eyes in the controls was 12273.15±3516.04 μm/mm2. The mean SBNFL density of 32 eyes before cyclosporine therapy was 8811.347±2985.285 μm/mm2. Following 6 months of topical cyclosporine 0.05% therapy there was a statistically significant improvement in the SBNFL density (mean value of 10335.13±4092.064 (P=0.0001)) as shown in Figures 1a and b.
Table 2. Central corneal SBNFL parameters in the study and the control group.
| Study group | Controls | P-value | |
|---|---|---|---|
| Nerve number | 3.58±0.99 | 5.40±1.70 | 0.001 |
| Nerve length | 1101.44±287.64 | 1963.70±562.56 | 0.001 |
| Nerve density | 8811.35+2985.29 | 12 273.15±3516.04 | 0.001 |
Figure 1.
(a) Confocal microscopy imaging of the central cornea using NIDEK Confoscan 4 of the SBNFL showing decrease in the nerve number, branching and density of the SBNFL pretreatment with CsA. (b) Confocal microscopy imaging of the central cornea using NIDEK Confoscan 4 of the SBNFL showing increase in the nerve number, branching and density of the SBNFL post treatment with CsA.
Discussion
OSD is a common comorbidity finding in glaucoma patients. Vision-related quality of life in patients on chronic long-term glaucoma therapy has been seen to be affected due to the co-existence of OSD and the chronic use of BAK-containing medications.17, 21, 22
Glaucoma patients show a prevalence of OSD in 59% of those using more benzalkonium preservative-containing eye drops,21 and 47.6% of open angle glaucoma patients have been found to have OSD with a significant impact on visual function and quality of life.17 The prevalence of OSD in glaucoma patients increases with age23 and can be compounded with the concurrent use of topical multidose hypotensive therapy containing preservatives. This toxicity has been associated with BAK, which is the most commonly used preservative and causes damage of the conjunctival and corneal epithelial cells and enhances the morbidity of the OSD. Long-term treatment with BAK-containing antiglaucoma medication, multidrop dosage, and beta blocker-containing formulations were found to be responsible for corneal toxicity in eyes on topical antiglaucoma treatment.24, 25 The effects of glaucoma medications on corneal endothelium, keratocytes, and sub-basal nerves among participants in the OHT treatment study revealed that chronic topical hypotensive therapy resulted in a decrease in the number and density of corneal sub-basal nerve fiber bundles but did not affect the keratocyte density or corneal endothelial characteristics.14 Another retrospective study5 on the long-term effects of preservative-free and preservative-containing antiglaucoma eye drops on the tear secretion and ocular surface had divided their study participants into five groups according to the type of topical hypotensive therapy and found that glaucomatous patients with chronic treatment show ocular surface alterations. The described confocal microscopy changes include significant decrease in density of superficial epithelial cells, higher density of basal epithelial cells, activated stromal keratocytes, higher beading of the nerves, lower number of sub-basal nerves and higher SBNFL tortuosity in those with ocular hypotensive medications with preservatives.14, 26
Cyclosporine 0.05% is a topical immunomodulatory compound with anti-inflammatory properties that have been demonstrated to have a benefit in the treatment of dry eye. Topical hypotensive therapy with fixed combinations (prostaglandin analog and beta blocker eye drops) has been observed to increase the expression of the inflammatory markers HLA-DR and interleukin-6 with resultant deterioration in the ocular surface after three months of therapy.27, 28 The anti-inflammatory effect of topical cyclosporine 0.05% administered twice daily has been proven to be beneficial to reduce the inflammatory effects of mild to moderate dry eye disease.15, 20 Our study evaluated the concurrent effect of topical cyclosporine 0.05% therapy on the ocular surface in patients of long-term ocular hypotensives. Patients enrolled in our study were using combination therapy of topical timolol 0.5% and brimonidine 0.2% and prostaglandins analogs (with BAK preservatives). All recruited patients on long-term ocular hypotensive therapy had mild to moderate dry eye disease. Following 6 months of concurrent topical cyclosporine 0.05% therapy, there was a significant improvement noted in all ocular surface evaluation parameters (TBUT, Schirmer's test, conjunctival and corneal staining scores, and OSDI scores). This supports the beneficial effect of concurrent topical cyclosporine therapy in eyes on long-term ocular hypotensive medications.
The corneal nerve fibers are important for corneal trophism and for maintaining a healthy ocular surface.29 A lower nerve density may impair corneal sensitivity,6 as observed in glaucoma patients. A complex relationship seems to exist between the ocular surface changes, DED and decreased SBNFL in these eyes on chronic antiglaucoma medical treatment.5 Sensitization of ocular surface nerves and disruption of corneal barrier function occurs in chronic ocular surface inflammation due to DED leading to nerve degeneration and altered SBNFL characteristics.6 Studies show that topical administration of CsA 0.05% significantly increases tear production, decreases ocular surface damage and dry eye symptoms.30, 31 Conjunctival epithelial biopsies from these patients show decreased molecular markers of immune activation and increased conjunctival goblet cell density.32, 33 Topical cyclosporine therapy by its anti-inflammatory effect results in a decrease in ocular surface inflammation and hence can have a beneficial effect on the corneal nerve regeneration. We therefore evaluated the change in nerve number, length and density of the central corneal SBNFL following 6 months of concurrent topical cyclosporine therapy in eyes on long-term ocular hypotensive therapy.
However, till date no study reported the effect of cyclosporine therapy on SBNFL density in chronic glaucoma patients. In our study we evaluated the changes in ocular surface evaluation parameters, corneal sensations, and SBNFL density following 6 months of cyclosporine therapy in chronic glaucoma patients on long-term topical hypotensive medications. A statistically significant improvement was noted in Schirmer test, TBUT, conjunctival and corneal staining scores, OSDI, and corneal sensations values upon 6 months of topical cyclosporine 0.05% therapy (Table 1).
In cases of glaucoma associated with significant OSD, although corneal staining severity was noted to be related to topical glaucoma therapy, OSDI was not seen as an ideal gauge of assessing OSD.34 However in our study, we found that a significant improvement in OSDI scores following 6 months of topical cyclosporine 0.05% twice daily therapy. This might be attributed to the fact that our study did not evaluate the effect of topical cyclosporine therapy on eyes with of glaucoma associated with significant OSD, but evaluated the effect of concurrent cyclosporine therapy in eyes with ocular hypotensive medications who had mild to moderate dry eye disease.
In normal corneas, the central corneal SBNFL density has been documented to be 10 658±5581 μm/mm2 with NIDEK confoScan4 in vivo slit scanning confocal microscopy.35 In our control group of normal patients the mean central corneal SBNFL density was 12 273.15±3516.04. In the study group, the SBNFL density of the central cornea of chronic glaucoma patients before cyclosporine therapy was 8811.35±2985.29 μm/mm2 that showed a significant improvement to 10335.13±4092.06 μm/mm2 after 6 months of concurrent topical cyclosporine 0.5% therapy.
Our study highlights a strong association of improvement of ocular surface evaluation parameters and SBNFL density with concurrent topical cyclosporine 0.05% therapy in eyes with chronic glaucoma on long-term topical hypotensive therapy, which show evidence of OSD due to drug molecule/BAK toxicity. The measurement of corneal sensation threshold with Cochet–Bonnet aesthesiometer has certain drawbacks as stimulus reproducibility becomes difficult due to practical difficulties in alignment, placement, and replication of the force applied with the nylon filament.
In conclusion, it has been proven that patients with chronic glaucoma show significant ocular surface changes in affected eyes after prolonged usage of topical antiglaucoma medication, with altered morphology of sub-basal nerve fibers. Our study highlights that topical cyclosporine therapy seems to have beneficial effects in DED due to long-term topical hypotensive medications with improvement in ocular surface changes and central corneal SBNFL density. Concurrent topical cyclosporine 0.05% therapy may be considered in eyes with with DED due to long-term topical hypotensive therapy in chronic glaucoma patients as this has a beneficial effect on the ocular surface and corneal SBNFL.

Acknowledgments
This study was funded by the Institute Research Grant of All India Institute of Medical Sciences, New Delhi, India.
The authors declare no conflict of interest.
Footnotes
Presented as a poster in the 4th Eucornea Congress, Amsterdam RAI, Netherlands, October 4–5, 2013.
References
- Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99 (7:1082–1088. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- Benı'tez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45 (9:3030–3035. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- Garcia-Hirschfeld J, Lopez-Briones L, Belmonte C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59 (5:597–605. doi: 10.1006/exer.1994.1145. [DOI] [PubMed] [Google Scholar]
- Baker KS, Anderson SC, Romanowski EG, Thoft RA, SundarRaj N. Trigeminal ganglion neurons affect corneal epithelial phenotype: influence on type VII collagen expression in vitro. Invest Ophthalmol Vis Sci. 1993;34 (1:137–144. [PubMed] [Google Scholar]
- Martone G, Frezzotti P, Tosi GM, Traversi C, Mittica V, Malandrini A, et al. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147 (4:725–735. doi: 10.1016/j.ajo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Benítez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48 (1:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- Qazi Y, Aggarwal S, Hamrah P. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefes Arch Clin Exp Ophthalmol. 2014;252 (6:857–872. doi: 10.1007/s00417-014-2618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E, Baudouin C, Efron M, Hamrah P, Kojima T, Patel SV, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res. 2014;39 (3:213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13 (5:569–576. doi: 10.1097/ACI.0b013e328364ec92. [DOI] [PubMed] [Google Scholar]
- Villani E, Magnani F, Viola F, Santaniello A, Scorza R, Nucci P, et al. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom Vis Sci. 2013;90 (6:576–586. doi: 10.1097/OPX.0b013e318294c184. [DOI] [PubMed] [Google Scholar]
- Mastropasqua L, Agnifili L, Mastropasqua R, Fasanella V, Nubile M, Toto L, et al. In vivo laser scanning confocal microscopy of the ocular surface in glaucoma. Microsc Microanal. 2014;20 (3:879–894. doi: 10.1017/S1431927614000324. [DOI] [PubMed] [Google Scholar]
- Mastropasqua L, Agnifili L, Fasanella V, Curcio C, Ciabattoni C, Mastropasqua R, et al. Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: an in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 2013;91 (5:e397–e405. doi: 10.1111/aos.12131. [DOI] [PubMed] [Google Scholar]
- Agnifili L, Fasanella V, Costagliola C, Ciabattoni C, Mastropasqua R, Frezzotti P, et al. In vivo confocal microscopy of meibomian glands in glaucoma. Br J Ophthalmol. 2013;97 (3:343–349. doi: 10.1136/bjophthalmol-2012-302597. [DOI] [PubMed] [Google Scholar]
- Baratz KH, Nau CB, Winter EJ, McLaren JW, Hodge DO, Herman DC, et al. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25 (9:1046–1052. doi: 10.1097/01.ico.0000230499.07273.c5. [DOI] [PubMed] [Google Scholar]
- Zhou XQ, Wei RL. Topical cyclosporine A in the treatment of dry eye: a systematic review and meta-analysis. Cornea. 2014;33 (7:760–767. doi: 10.1097/ICO.0000000000000123. [DOI] [PubMed] [Google Scholar]
- Cho HK, Park MH, Moon JI. The effect of additional topical cyclosporin or vitamin A on the ocular surface during antiglaucoma medication administration. Ophthalmic Res. 2012;48:139–145. doi: 10.1159/000334248. [DOI] [PubMed] [Google Scholar]
- Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9. doi: 10.1016/j.ajo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Nelson JD.In office diagnostic tests for dry eye diseaseIn: Asbell PA, Lemp MA (eds).Dry Eye Disease: The Clinician's Guide to Diagnosis and Treatment Thieme Medical Publishers, Inc.: New York, NY, USA; 200639–44. [Google Scholar]
- Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dysfunctional tear syndrome study group et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25:900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocularsymptoms and signs with preserved and preservative-free glaucoma medication. Br J Ophthalmol. 2002;86 (4:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaštelan S, Tomić M, Metež Soldo K, Salopek-Rabatić J. How ocular surface disease impacts the glaucoma treatment outcome. Biomed Res Int. 2013;2013:696328. doi: 10.1155/2013/696328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim MK, Choi HJ, Wee WR, Kim DM. Comparative cross-sectional analysis of the effects of topical antiglaucoma drugs on the ocular surface. Adv Ther. 2013;30 (4:420–429. doi: 10.1007/s12325-013-0021-8. [DOI] [PubMed] [Google Scholar]
- Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–2135. doi: 10.2147/OPTH.S41358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iester M, Telani S, Frezzotti P, Motolese I, Figus M, Fogagnolo P, et al. Ocular Surface changes in glaucomatous patients treated with and without preservatives beta-blockers. J Ocul Pharmacol Ther. 2014;30 (6:476–481. doi: 10.1089/jop.2013.0216. [DOI] [PubMed] [Google Scholar]
- Russ HH, Nogueira-Filho PA, Barros Jde N, de Faria NV, Montiani-Ferreira F, Gomes JÁ, et al. Ocular surface evaluation in patients treated with a fixed combination of prostaglandin analogues with 0.5% timolol maleate topical monotherapy: a randomized clinical trial. Clinics (Sao Paulo) 2013;68 (10:1318–1324. doi: 10.6061/clinics/2013(10)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol. 2000;28:113–117. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structures, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter,randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. 2008;2 (4:829–836. doi: 10.2147/opth.s1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120 (3:330–337. doi: 10.1001/archopht.120.3.330. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, De Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27 (1:64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120 (11:2241–2248. doi: 10.1016/j.ophtha.2013.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DV, Tavakoli M, Craig JP, Efron N, McGhee CNJ. Corneal Sensitivity and Slit Scanning In Vivo Confocal Microscopy of the Subbasal Nerve Plexus of the Normal Central and Peripheral Human Cornea. Cornea. 2009;28:735–740. doi: 10.1097/ICO.0b013e318193e0e3. [DOI] [PubMed] [Google Scholar]