Abstract
Introduction
Phenol is a caustic that may cause cutaneous or gastrointestinal burns depending on the route of exposure. Significant absorption may result in systemic toxicity. We present a case of topical phenol exposure resulting in cutaneous burns and systemic phenol toxicity.
Case report
A 9-year-old girl was exposed to Creolin®, a general-purpose disinfectant containing phenol, when her mother applied this product to her head and upper torso. The patient required endotracheal intubation due to depressed mental status; she had cutaneous erythema in the distribution of contact with the cleanser. An initial EKG revealed sinus tachycardia with brief runs of monomorphic ventricular tachycardia. On hospital day (HD) 1, the area of erythema extended to both upper extremities and hyperpigmentation developed over the affected areas, which continued to darken during the hospital course. The patient was extubated late on HD 1. On HD 2, the patient’s urine was noted to be a dark green color that resolved later that day. On HD 3, areas of desquamation and decreased sensation developed in skin areas of maximal contact with the cleanser. The patient developed a mild transaminitis with peak AST and ALT levels of 84 units/l and 99 units/l, respectively. The patient was discharged to home on HD 4.
Discussion
Our patient presented with signs of cutaneous and systemic phenol toxicity characterized by dermal burns, depressed mental status, cardiac dysrhythmias, and elevated hepatic transaminases. Phenol exposure may cause systemic toxicity following limited dermal exposure.
Keywords: Pediatric, Caustic, Neurotoxicity, Transdermal administration
Introduction
Phenol is a weakly acidic hydrocarbon used as a feedstock chemical for the production of a variety of plastic epoxides as well as in the manufacture of various pharmaceuticals and over-the-counter products [1]. Significant absorption of phenol may result in systemic toxicity as well as cutaneous or gastrointestinal injury, depending on the route of exposure.
Creolin® is a concentrated deodorant cleanser marketed for the cleaning of bathrooms, kennels, and barns and as a shampoo for horses and dogs. This product contains up to 50 % carbolic oil, of which phenol and substituted phenols are primary components, in conjunction with sodium hydroxide and isopropanol [2]. Creolin® is sometimes used in an “off label” manner for delousing human hair.
We present the case of a 9-year-old female who presented to our pediatric emergency department manifesting severe phenol toxicity after her mother attempted to delouse her using undiluted Creolin®.
Case Report
Paramedics responded to a 911 call for an obtunded previously healthy 9-year-old female with respiratory distress. The mother reported that she had poured 8 fl oz (236 ml) of Creolin® over the top of the patient’s head in an attempt to delouse the patient’s hair; some of the fluid splashed down onto the patient’s neck, upper back, left shoulder, and left upper arm. The patient immediately complained of a burning sensation. Over the next 4 min, she rapidly progressed from alert and oriented to stuporous with gurgling respirations and no response to voice or touch, which prompted the mother to call 911.
When the paramedics arrived, the patient was intubated without the need for sedation medications. The paramedics noted multiple areas of erythema where the Creolin® had contacted the patient’s skin. The patient was transported to our pediatric tertiary care center.
Upon arrival to the pediatric emergency department, the patient’s vital signs were temperature (rectal) 34.1 °C, blood pressure 129/105 mmHg, heart rate 148 beats per minute, no spontaneous respirations, and pulse oximetry of 100 % on 100 % oxygen by hand ventilation. Her weight was 40 kg. Physical examination was remarkable for erythema and hyperpigmentation of the scalp, face, neck, upper back, and left arm (Figs. 1 and 2). A strong acrid odor permeated the emergency department, leading some members of the emergency department staff to complain of dizziness, headache, and nausea.
Fig. 1.
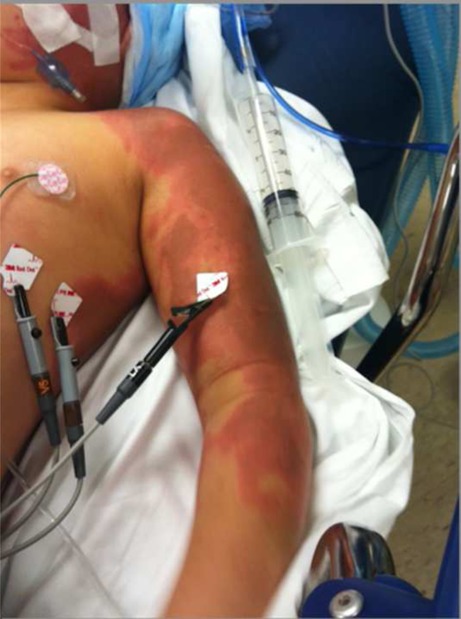
Erythema and hyperpigmentation along arm immediately following cutaneous exposure to Creolin®
Fig. 2.

Erythema and hyperpigmentation of the patient’s back immediately following cutaneous exposure to Creolin®
Initial and follow-up laboratory studies are shown in Table 1. Of note, an initial leukocytosis and elevated serum lactate resolved within 24 h of presentation. A urine drug immunoassay was negative for acetaminophen, amphetamine, methamphetamine, barbiturates, benzodiazepines, cocaine, methadone, opiates, phencyclidine, cannabinoids, and tricyclic antidepressants. A chest X-ray and head CT were both normal. The cardiac monitor displayed sinus tachycardia with brief runs of monomorphic ventricular tachycardia. The patient received 30 mg (0.75 mg/kg) of lidocaine intravenously with subsequent resolution of the dysrhythmia.
Table 1.
Initial and follow-up laboratory studies
| Arrival | HD 2 | HD 3 | HD 4 | Reference range | |
|---|---|---|---|---|---|
| Complete blood count | |||||
| White blood cell (thousand/μL) | 20.3 | 12.2 | 4.5–13.5 | ||
| Hemoglobin (g/dL) | 12.7 | 13.6 | 12.0–15.0 | ||
| Hematocrit (%) | 38.1 | 41.1 | 35.0–49.0 | ||
| Platelets (thousand/μL) | 442 | 261 | 150–450 | ||
| Comprehensive metabolic panel | |||||
| Sodium (mmol/L) | 135 | 139 | 138 | 137 | 134–143 |
| Potassium (mmol/L) | 3.3 | 4.0 | 4.1 | 3.7 | 3.3–4.6 |
| Chloride (mmol/L) | 104 | 108 | 106 | 101 | 96–109 |
| Carbon dioxide (mmol/L) | 21 | 23 | 24 | 29 | 20–28 |
| Urea nitrogen (mg/dL) | 12 | 6 | 7 | 6 | 4–16 |
| Creatinine (mg/dL) | 0.35 | 0.42 | 0.41 | 0.40 | 0.3–0.7 |
| Glucose (mg/dL) | 219 | 143 | 105 | 99 | 70–105 |
| Total bilirubin (mg/dL) | 0.30 | 0.19 | 0.18 | 0.30 | 0.0–1.0 |
| Direct bilirubin (mg/dL) | <0.10 | <0.10 | 0.0–0.2 | ||
| Aspartate aminotransferase (U/L) | 84 | 36 | 52 | 53 | <41 |
| Alanine aminotransferase (U/L) | 40 | 36 | 88 | 70 | 5–25 |
| Alkaline phosphatase (U/L) | 215 | 196 | 241 | 223 | 69–325 |
| Urinalysis | |||||
| Specific gravity | >1.035 | 1.025 | 1.003–1.030 | ||
| pH | 5.0 | 5.0 | 5.0–9.0 | ||
| Leukocyte esterase | Negative | Negative | Negative | ||
| Protein | 1+ | 1+ | Negative | ||
| Glucose | 3+ | Negative | Negative | ||
| Urobilinogen | Normal | Normal | Normal | ||
| Bilirubin | Negative | Negative | Negative | ||
| Blood | Negative | 3+ | Negative | ||
| RBC (cells/HPF) | Negative | TNTC | 0–2 | ||
HD hospital day, TNTC too numerous to count, HPF high-power field
The patient was decontaminated in the emergency department using a soap-and-water sponge bath. A solution of low-molecular-weight polyethylene glycol (PEG) mixed with industrial methylated spirits (denatured alcohol) was recommended by the medical toxicology service [2]; however, low-molecular-weight PEG was not available. The patient was admitted to the pediatric ICU.
Due to the concern about possible ingestion of the Creolin® solution while it was dripping down from her head, the patient underwent endoscopy, which was normal. Following endoscopy, she was extubated with a normal mental status approximately 12 h after initial exposure and subsequently transferred out of the ICU to a general pediatric floor bed.
The patient’s vital signs normalized on hospital day (HD) 1. Serum aspartate aminotransferase concentration peaked on HD 1 at 84 units/l while the serum alanine aminotransferase concentration peaked on HD 3 at 88 units/l. On the second hospital day, the patient was noted to have dark green-black urine in her Foley catheter drainage bag. The urinalysis showed 3+ blood. The verdiuria spontaneously resolved later on the second hospital day. Throughout the patient’s hospitalization, the initial erythema extended to involve both upper extremities as well as her face, neck, and back and became progressively more hyperpigmented. The patient sustained partial thickness burns to a total of 17 % of her body surface area. No full thickness burns were noted. By HD 3, the areas that had the maximal contact with Creolin® began to desquamate (Figs. 3 and 4). These areas were noted to be increasingly insensate to palpation.
Fig. 3.
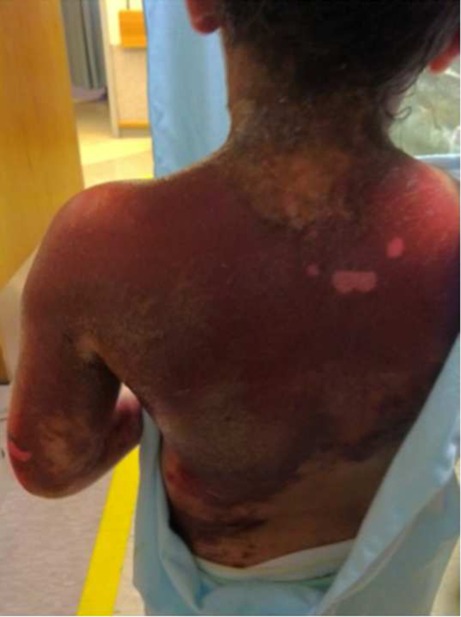
Desquamation of back and arm 3 days after cutaneous exposure to Creolin®
Fig. 4.

Desquamation of arm 3 days after cutaneous exposure to Creolin®
The patient remained stable throughout the remainder of her hospital course and was discharged home on HD 4; follow-up was scheduled with Dermatology and Plastic Surgery as well as with a social worker. The patient failed to attend those follow-up visits. The patient, however, was seen twice in our hospital emergency department for unrelated complaints 2 years after the incident with no residual scarring noted at that time.
Discussion
Phenol was first produced in the early 1800’s as an extraction product from coal tar [3]. Phenol can be found in multiple different products used today. The majority of phenol is used in industry to form plastic resins [3]. It is also the precursor to many pharmaceutical agents, most notably aspirin. It is used as an oral antiseptic and anesthetic in Chloraseptic® spray as well as in Carmex® lip ointment [2]. Propofol and l-dopa are both synthesized from phenolic compounds. Creolin® is comprised of 40–50 % carbolic oil, which in turn is a mixture that predominantly contains phenol and substituted phenols (e.g. cresol, xylenol) [4]. The use of diluted Creolin® in the treatment of head lice is a historical remedy common to inner city communities in the USA that occasionally results in emergency department visits for minor exposures [5, 6].
Systemic phenol toxicity may result from inhalation, ingestion, or cutaneous exposure. Phenol is both water- and lipid-soluble, is readily absorbed through the skin, lungs, and gastrointestinal tract and rapidly and widely distributed. Phenol is primarily metabolized in the liver via glucuronidation, sulfonation, and oxidation by CYP450 2E1 isoenzyme although other pathways may play a role [7]. Phenol metabolites undergo renal excretion.
Systemic toxicity due to phenol exposure has been previously reported in the setting of topical application of phenol for medicinal purposes [8–11]. Altered mental status may occur rapidly following cutaneous exposure as phenol rapidly distributes into the central nervous system. Other manifestations of phenol toxicity include CNS depression, seizures, hypotension, cardiac dysrhythmias, hypothermia, metabolic acidosis, methemoglobinemia, and direct renal and hepatic injury. Phenol ingestion may result in caustic injury to the gastrointestinal tract leading to coagulative necrosis [4]. Verdiuria (green urine) may occur secondary to excretion of the carbolic acid metabolite and may be accompanied by acute renal failure requiring hemodialysis [6].
Chronic phenol exposure may result in liver and kidney damage as well as eye and skin discoloration. Acute phenol exposure is not expected to result in long-term effects once recovery from the acute toxicity has resolved.
Patients with phenol exposure resulting in decreased mental status or seizures may require prompt airway management. After stabilization, decontamination is of critical importance. Exposed skin should be copiously irrigated with soap and water. In one study, 70 % isopropanol (a common formulation of rubbing alcohol) was reported to be superior to water or soap and water for decontamination of phenol [12]. A 3:1 mixture of low molecular weight (LMW) PEG (300–400 g/mol) and ethanol has previously been reported to be effective in phenol decontamination; however, it is unlikely to be immediately available for use [12, 13]. Pharmaceutical grade formulation of LMW PEG is commercially available (KollisolvTM, BASF, Florharm Park, NJ, USA).
Patients with significant dermal toxicity may require referral to a regional burn center. In our case, the pediatric hospital to which the patient was brought also houses the regional pediatric burn center. Partial thickness burns secondary to phenol may become insensate, as in our patient, due to local activity of phenol on cutaneous nerves. Phenol penetrates all barrier gloves that are standard to emergency departments. Neoprene gloves are recommended as appropriate barriers to phenol; however, given that neoprene gloves are typically not immediately available in the healthcare setting, current recommendations to protect healthcare workers are to “double-glove” and change gloves frequently.
Splash injuries to the eye with phenol can result in significant corneal injury due to abrasion and denaturation. Copious irrigation with normal saline via either a Morgan lens or through a nasal cannula along with prompt evaluation by an ophthalmologist is mandated. While 3:1 LMW PEG/ethanol has been reported to be safe for ocular decontamination, it’s relative effectiveness for this indication is unknown and it is unlikely to be immediately available [13].
Cardiac dysrhythmias reported in the setting of topical phenol application for face peeling include sinus tachycardia, premature atrial or ventricular contractions, ventricular bigeminy, paroxysmal atrial fibrillation, ventricular tachycardia, and bidirectional ventricular tachycardia [9, 10]. Some authors have previously reported lidocaine or propranolol to be effective in the management of phenol-induced dysrhythmia [8, 10, 11]. However, both the mechanism by which phenol induces dysrhythmias and optimal management of those dysrhythmias remains unknown. Patients who ingest phenol require endoscopic evaluation to determine the extent of caustic injury to the gastrointestinal tract.
Phenol exposure may be confirmed by obtaining either a serum phenol level or a urine phenol level. Because phenol is quickly cleared from the serum, the urine level is more indicative of total body burden of phenol. The urine phenol level represents not only unchanged parent compound but also conjugated metabolites of phenol.
A limitation of our case report is that we did not obtain a serum or urine phenol concentration to confirm and quantify this patient’s exposure. At the time, the corroborating evidence of the empty bottle of Creolin® and the patient’s presentation was deemed sufficient.
Conclusion
Phenol is still used widely in manufacturing processes and is also present in less concentrated formulations such as topical and oral analgesics. Concentrated formulations such as Creolin® are still available and misinformation about their appropriate use can have adverse consequences.
References
- 1.Inder R (1997) Poisons Information Monograph 412 - Phenol. International Programme on Chemical Safety
- 2.Hunter DM, et al. Effects of isopropyl alcohol, ethanol, and polyethylene glycol/industrial methylated spirits in the treatment of acute phenol burns. Ann Emerg Med. 1992;21(11):1303–1307. doi: 10.1016/S0196-0644(05)81891-8. [DOI] [PubMed] [Google Scholar]
- 3.Haddad LM, Dimond KA, Schweistris JE. Phenol poisoning. JACEP. 1979;8(7):267–269. doi: 10.1016/S0361-1124(79)80222-1. [DOI] [PubMed] [Google Scholar]
- 4.Monma-Ohtaki J, et al. An autopsy case of poisoning by massive absorption of cresol a short time before death. Forensic Sci Int. 2002;126(1):77–81. doi: 10.1016/S0379-0738(02)00024-5. [DOI] [PubMed] [Google Scholar]
- 5.Mowry JB, et al. 2012 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th annual report. Clin Toxicol (Phila) 2013;51(10):949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 6.Spiller HA, Quadrani-Kushner DA, Cleveland P. A five year evaluation of acute exposures to phenol disinfectant (26 %) J Toxicol Clin Toxicol. 1993;31(2):307–313. doi: 10.3109/15563659309000397. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann MJ, et al. Metabolism of [14C]phenol in the isolated perfused mouse liver. Toxicol Sci. 1999;49(1):40–47. doi: 10.1093/toxsci/49.1.40. [DOI] [PubMed] [Google Scholar]
- 8.Botta SA, Straith RE, Goodwin HH. Cardiac arrhythmias in phenol face peeling: a suggested protocol for prevention. Aesthet Plast Surg. 1988;12(2):115–117. doi: 10.1007/BF01576925. [DOI] [PubMed] [Google Scholar]
- 9.Gross BG. Cardiac arrhythmias during phenol face peeling. Plast Reconstr Surg. 1984;73(4):590–594. doi: 10.1097/00006534-198404000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Truppman ES, Ellenby JD. Major electrocardiographic changes during chemical face peeling. Plast Reconstr Surg. 1979;63(1):44–48. doi: 10.1097/00006534-197901000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Unlu RE, et al. Phenol intoxication in a child. J Craniofac Surg. 2004;15(6):1010–1013. doi: 10.1097/00001665-200411000-00023. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro-Riviere NA, et al. Efficacy of topical phenol decontamination strategies on severity of acute phenol chemical burns and dermal absorption: in vitro and in vivo studies in pig skin. Toxicol Ind Health. 2001;17(4):95–104. doi: 10.1191/0748233701th095oa. [DOI] [PubMed] [Google Scholar]
- 13.Brown VK, Box VL, Simpson BJ. Decontamination procedures for skin exposed to phenolic substances. Arch Environ Health. 1975;30(1):1–6. doi: 10.1080/00039896.1975.10666623. [DOI] [PubMed] [Google Scholar]


