Abstract
The advancement of biomolecular techniques has continued to advance in the area of mitochondrial medicine. This has allowed clinicians and researchers to more effectively study the bioenergetics of the mitochondria in various disease states. One potential technique in mitochondrial medicine is the generation of cytoplasmic hybrids. A cytoplasmic hybrid or cybrid are created by introducing mitochondrial DNA (mtDNA) of interest into cells depleted of mtDNA. A cybrid is therefore a hybrid cell that mixes the nuclear genome from one cell with the mitochondrial genes from another cell. Cybrids are currently utilized in mitochondrial research to demonstrate mitochondrial involvement in a wide range of diseases that include diabetes, Parkinson’s disease and inherited diseases. At this time the use of cybrids to study toxicologic poisoning is limited and offers a potential avenue of research in this area.
Keywords: Mitochondria, Toxicology
Introduction
The mitochondria are small structures within the cells that are responsible for over 90 % of energy production. There are complex interactions between the mitochondrial genome and nuclear genome that enables cells to grow and reproduce when the flow of energy is normal [1]. However, when there are disruptions in energy production at the level of the mitochondria, there are consequential effects that manifest in a wide rage of clinical diseases that are observed today. There has been significant advancement in the field of mitochondrial medicine that has been applied to investigate a wide range of clinical problems such as diabetes, cancer, and many neurodegenerative disorders that include Parkinson’s and Alzheimer’s disease [2, 3]. One of the techniques utilized in mitochondrial medicine to study the role of the mitochondrial genome are cytoplasmic hybrids (cybrids). Cybrids have contributed valuable insight into the relationship between mitochondrial DNA and phenotype alterations [4]. The use of cybrids to study the consequences of toxicologic exposures is very limited and offers a powerful tool to study the interaction of the mitochondria and phenotypic expression related to toxicologic poisoning that result in mitochondria inhibition. Mitochondrial inhibition can occur from environmental toxins, occupational exposures (cyanide and carbon monoxide) and a wide range of pharmaceuticals currently used in clinical medicine.
Overview of the Mitochondrial Genome
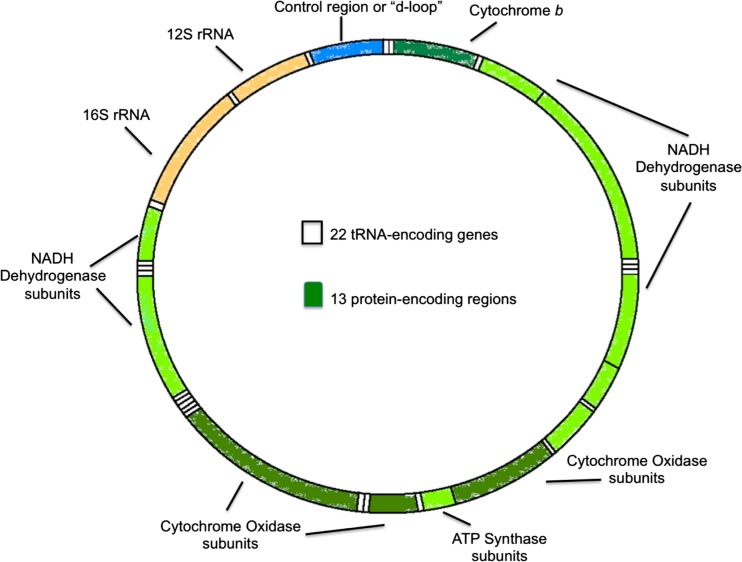
The mitochondria are membrane-bound organelles that contain an outer and inner membrane composed of phospholipid bilayers and proteins. The mitochondria perform a variety of functions that include storage of calcium, cellular metabolism, regulation of membrane potential, and most importantly the production of adenosine triphosphate (ATP) that serves as the primary energy currency of the cell. The mitochondria also contain a separate genome that was first discovered in 1963. The mitochondrial DNA genome is well defined and is comprised of a circular double-stranded DNA containing 16,569 base pairs (Fig. 1). The mitochondrial genome encodes many, but not all, components of the electron transport system (ETS). Specifically, the mitochondrial genome encodes 11 components of the ETS and two subunits of ATP synthase. The mitochondrial genome also contains 24 rRNA and tRNA genes necessary for translation of mitochondrial protein [5]. One of the challenges in studying mitochondrial function is separating the contributions of the nuclear genome from the mitochondrial genome. The use of cybrids offers a method to overcome this limitation.
Fig. 1.
Mitochondrial DNA (mtDNA)
Background of Cytoplasmic Hybrids
Cytoplasmic hybrids or cybrids are cell lines that can incorporate human mitochondria and perpetuate its mitochondrial DNA (mtDNA)-encoded components. It is a cell line produced by introducing mtDNA of interest into cells depleted of mtDNA. Cells that lack mitochondrial DNA are referred to as rho zero cells (ρ(0) cells). Cybrids serve as a very useful tool in studying the effects of mtDNA alteration or mutation by excluding the influence of the nuclear genome.
ρ(0) cells are commonly utilized to produce cybrids cells. ρ(0) cells are cell lines, often immortal, that have been depleted of mtDNA prior to being fused with enucleated cells. The mechanism of mtDNA depletion occurs in two important steps. The first step is to prevent mtDNA replication. The next step involves dividing the existing cells that will lead to dilution of the existing mtDNA. This will eventually create a population of cells that lack mtDNA, now referred to as “ρ0.” Table 1 provides a list of currently reported ρ0 cell lines [6].
Table 1.
Current ρ0 cell lines
| 143B osteosarcoma |
| A549 lung carcinoma |
| Fibroblast |
| HeLa cervical carcinoma |
| LL/2-m21 mouse |
| Lymphoblastoid, Wal2A |
| molt-4 |
| NT2 teratocarcinoma |
| SH-SY5Y neuroblastoma |
| T-cell lymphoblastic leukemia |
| U251 glioma |
Many techniques have been developed over the years to deplete cells of mtDNA. One of the earlier techniques used to deplete mtDNA involved the use of ethidium bromide (EtBr). EtBr is a positively charged compound that binds with negatively charged mtDNA, resulting in failure of mtDNA replication. EtBr has some limitations with respect to successfully generate ρ(0) cells in vertebrate cells. One limitation with the use of EtBr is the mutagenic effects on the nuclear DNA that may affect the ability to isolate the effects of mtDNA change [7]. Another technique to deplete cells of mtDNA is the use of mitochondrial DNA polymerase inhibitors. An inhibitor used to deplete mtDNA is ditercalinium that is also used as antitumor agent. Rhodamine 6-G and dideoxynucleoside analogues are other agents used to generating ρ(0) cells [8].
Verification of ρ0 status in cell lines is an important aspect in the generation of cybrids. There are several techniques available to verify mtDNA depletion. Techniques include measurement of oxygen consumption, cytochrome c oxidase activity, PCR of mtDNA, and southern blotting. All of the following techniques rely on demonstration of a negative result so will vary depending on the sensitivity of the test being used [6]. Another technique used to verify ρ0 status is testing for uridine/pyruvate auxotrophy. Auxotrophy is the inability of an organism to synthesize a particular organic compound required for growth. Cells that are lacking mtDNA lack the ability to grow without uridine. One of the mitochondrial enzymes, dihydroorotate dehydrogenase, is needed for the synthesis of pyrimidines. This enzyme requires an intact ETS to function; so when depletion of mtDNA occurs, the ETS fails to function and will result in subsequent disruption in pyrimidine synthesis. Supplying uridine to mtDNA-depleted cells will bypass the ETS-dependent dihydroorotate dehydrogenase pyrimidine production allowing mtDNA-depleted cells to grow [9].
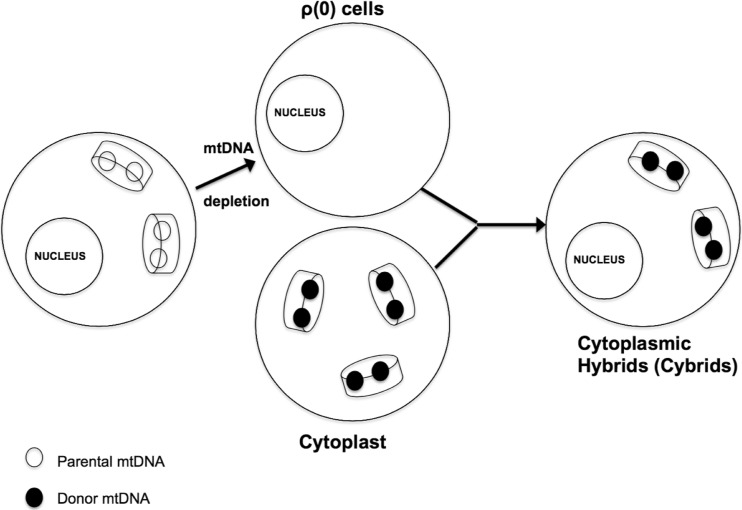
Once ρ0 status is achieved, the next step in the generation of cybrids is mtDNA transfer. Techniques to transfer mtDNA into cells were developed in the 1970s with the first attempt in 1972 that led to the creation of a heterokaryon hybrid cell. Heterokaryon is a hybrid that maintained two or more separate nuclei. The Sendai virus (mice respiratory virus) was used to fuse these cells together [10]. Later, a separate technique was developed to combine nucleated cells with non-nucleated cells (cytoplasts) resulting in cybrids. The term cybrid is used to distinguish them from a hybrid that is a mixture of two nucleated cells. In 1989, a specific cybrid technique was described that fused enucleated cytoplasts with ρ0 cells through the use of polyethylene glycol and also with the direct injection of cytoplast mitochondria [11]. Success of this technique was demonstrated with the loss of uridine auxotrophy and restoration of oxidative phosphorylation. Figure 2 demonstrates this technique.
Fig. 2.
Cytoplasmic hybrid (cybrids) generation techniques: Cybrids are produced in a variety of ways. The nucleated cell can be unaltered or could have undergone endogenous mtDNA depletion as illustrated here before cytoplasmic mixing. The primary purpose in either case is to populate the nucleated cell with mtDNA from the non-nucleated cell
Application of Cybrids to Clinical Medicine
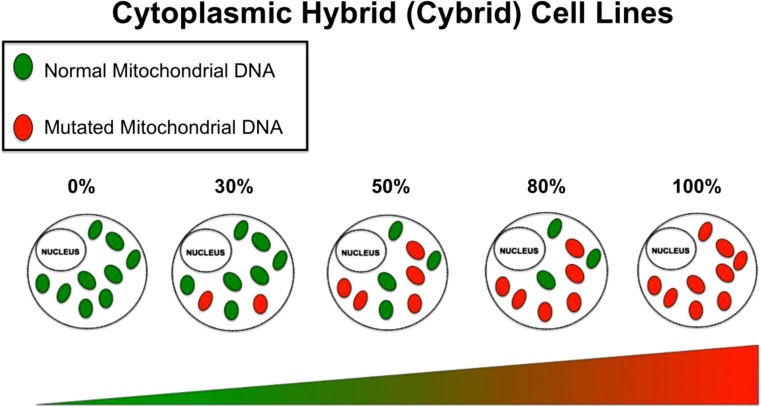
The application of cybrids offers a powerful tool to understand diseases associated with mtDNA mutations. There are important terms to fully understand the implications of utilizing cybrids in mitochondrial studies (Fig. 3):
Homoplasmy—is a state in a cell where all copies of mtDNA are identical. This can apply to cells that contain all normal mtDNA or all mutated mtDNA.
-
Heteroplasmy—is a state in a cell where there is a mixture of normal mtDNA and mutated mtDNA.
A unique feature of mtDNA is that, at cell division, the mtDNA replicates and sorts randomly among mitochondria. In turn, the mitochondria sort randomly among daughter cells. Therefore, in cells where heteroplasmy is present, each daughter cell may receive different proportions of mitochondria carrying normal and mutant mtDNA. This is in sharp distinction to nuclear DNA mutations that is binary. In the case of mitochondria DNA, heteroplasmic mutation can be present in varying degrees.
-
Threshold—the mtDNA mutational load that is required within a cell to manifest a particular biochemical or phenotypic manifestation.
Cybrids have been used as early as the 1970s to study cytoplasmic inheritance of antibiotic resistance in various tissue culture cells [12, 13]. Cybrids have also been used to study disease states that involve known mtDNA mutations. These include Leigh’s disease, myoclonic epilepsy, and ragged red fiber disease (MERRF), Kearns-Sayre syndrome and Leber’s hereditary optic neuropathy (LHON). LHON is a mitochondrial-inherited degeneration of retinal ganglion cells and their axons that lead to progressive loss of central vision. LHON is only transmitted through the mother as it is due to mutations in the mitochondrial genome, and only the egg contributes mitochondria to the embryo. LHON is usually due to one of three pathogenic mtDNA point mutations. These mutations are at nucleotide positions 11778, 3460 G to A, and 14484 T to C, respectively, in the ND4, ND1, and ND6 subunit genes of complex I of the oxidative phosphorylation chain in mitochondria [14, 15].
Cybrid studies on LHON reveal that the biochemical manifestation is dependent on the nuclear background and mutation of the ρ0 cell line that is used. For example, LHON mutations can show complex I dysfunction in one nuclear background but not in a separate nuclear background. Even with the difference in various LHON cybrids, there are some consistent findings and that is a reduction in complex I-dependent ATP synthesis and deficits in oxygen consumption. Another important findings from the various LHON cybrid studies are that all phenotypic differences require a very high mutational load [16, 17].
Another important contribution of cybrids is in the understanding of how haplogroups influence diseases with multifactorial components such as LHON. A haplogroup is a group of similar haplotypes that share a common ancestor. A haplotype is a collection of specific alleles (particular DNA sequence) in a cluster of tightly linked genes on a chromosome that are usually inherited together. Haplotype can also mean a set of single-nucleotide polymorphisms (SNPs) on a single chromosome of a chromosome pair [16]. In the case of LHON, haplogroups Uk and J are found at higher levels in patients with LHON when compared to haplogroup H that is underrepresented in patients with LHON. Cybrids from different mitochondrial haplogroups were generated using platelets of different haplogroups fused with the osteosarcoma ρ0 cell line. Cybrids with haplogroups Uk and J were found to have lower ATP production, lower oxygen consumption, and decreased mtDNA when compared to haplogroup H [18, 19].
The A3243G mtDNA mutation is another mitochondrial disorder in which the application of cybrids has furthered our understanding of certain diseases. The mtDNA nucleotide 3243 is located within one of two mtDNA genes that encodes a leucine tRNA (tRNA LeuUUR). This mutation is associated with the syndrome of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS). MELAS is a condition that affects many of the body’s systems, particularly the central nervous system and muscles. The signs and symptoms of this disorder most often appear in childhood following a period of normal development. Early symptoms may include muscle weakness and pain, recurrent headaches, loss of appetite, vomiting, and seizures. Other important clinical findings include hemiparesis, seizures, visual loss, and progressive dementia [19]. At this time, MELAS largely remains an untreatable disease where deaths typically occur by the fourth decade of life from the above complications.
Cybrids have also been utilized to further our understanding of MELAS. Osteosarcoma ρ0 cell lines were used to generate MELAS cybrids and found that a very high mtDNA mutational load of 85 % was required before phenotypic manifestations were observed. This mtDNA mutation is related to translation deficiency of mtDNA-encoded structural proteins. These same studies also demonstrated the important contributions of the nuclear genome where the nuclear background of the ρ0 cells influence the degree of mtDNA mutation required for phenotypic expression. For example, in the osteosarcoma MELAS cybrid model, a 90 % mutational load results in around a 30 % decrease in cytochrome oxidase activity [20]. In an A549 lung carcinoma MELAS cybrid model, a 50 % mutational load results in a 50 % reduction in cytochrome oxidase activity [21]. This illustrates the important role the nuclear genome plays in the mitochondrial genotype-phenotype relationships [22].
Fig. 3.
Heteroplasmy: Cybrids cell lines of increasing heteroplasmy matched for a constant nuclear background can be generated allowing for the phenotypic observation at a particular percentage of heteroplasmy
Application of Cybrids to Toxicologic Poisoning
Cybrids offer a powerful tool to study many inherited mitochondrial defects such as MELAS, LHON, and Leigh syndrome. Cybrids are also utilized to study common clinical diseases such as diabetes, heart disease, autism, and Parkinson’s disease. For example, mtDNA is transferred from an individual with a particular condition of interest such as Alzheimer’s disease generating cell lines [23]. The cybrids generated can now be used to measure an extensive array of biochemical and molecular measurements. Examples of analyses include membrane potential, measurement of respiratory complexes, glycolysis gene expression, and transcriptional profile utilizing RNA sequencing. The measurements obtained from these cybrids can now be compared to control samples. Since the nuclear genome is assumed to be constant, any differences in the indices obtained can be assumed to be from differences in mtDNA.
A potential application of engineered cybrids is to study certain toxicologic exposures, especially those that inhibit the mitochondria preventing oxidative phosphorylation. There are many poisons that inhibit the mitochondria including cyanide, hydrogen sulfide, and carbon monoxide that act predominantly at complex IV. Acute exposure from these toxins often results in multi-organ dysfunction, particular organ systems highly dependent on ATP production from oxidative phosphorylation such as the central nervous system. Consequential exposures often result in death from cardiovascular collapse [24].
There are other poisons that are also mitochondrial inhibitors and have also been linked to certain metabolic disorders with chronic low concentration exposures. Such inhibitors include certain pesticides such as atrazine and rotenone, inhibiting complex III and I, respectively. It has been noted that in agricultural areas where the use of pesticides is prevalent, there is a high incidence of metabolic disorders such as diabetes, cardiovascular disease, and obesity [25, 26]. Chronic exposures to these mitochondrial inhibitors (pesticides) have also been linked to certain neurologic disorders such as Parkinson’s disease and autism [27, 28]. Experimental studies have linked chronic exposures of these toxins to the development of specific metabolic disorders. While there are a variety of reasons for this relationship, it is clear that there is an association between chronic mitochondrial inhibition from these toxins and the various metabolic diseases described above.
Cytoplasmic hybrids represent a method to study the effects of mitochondrial inhibition from specific inhibitors such as pesticides. A series of cybrids with increasing percentages of heteroplasmy (A3243G mutation) have been utilized as a model for the effects of incremental inhibition of mitochondrial function. This results in increasing deficiency of mitochondrial protein synthesis on gene expression which can be assessed with RNA sequencing. An interesting finding is when there is an increase in mitochondrial dysfunction (resulting from increasing mitochondrial DNA mutation); there are four discrete transcriptional phases which corresponds to specific phenotypic expressions. For example, individuals that inherit the most common 3243 mutation and harbor ∼10–30 % 3243G mutant mtDNAs manifest diabetes, whereas the same individuals with ∼50–90 % mutant mtDNA display encephalomyopathies. Given the stepwise nature of these transcriptional phase transitions, significant mitochondrial inhibition can occur before phenotypic expression occurs from an induction of phase transition [21]. It is therefore critical to detect early mitochondrial changes before phenotypic expression can occur. Cybrids offer a potential way to serve as a sensitive assay to detect possible mitochondrial inhibition from select toxins before phenotypic expression can occur.
Exposure from environmental toxins such as pesticides that inhibit the mitochondria may also exhibit phase transitions similar to what is seen with increased heteroplasmy. Accumulation of pesticides from the environment may also result in increasing inhibition of mitochondrial function with exposure leading to discrete phase transitions such as heart disease, diabetes, and autism. Cybrids may serve as a sensitive assay to detect low concentrations of environmental mitochondrial inhibitors such as pesticides. Cybrids with increasing heteroplasmy can be exposed to certain mitochondrial inhibitors to serve as a sensitive assay for detecting these toxins before phenotypic manifestations occur.
There are some limitations to consider with the use of cybrids. The nuclear background of the ρ0 cells used to produce the cybrid influences the variation in mitochondrial physiology from one cell type to the next. This is a consideration when interpreting study results as cybrid methodology may also vary from one study to the next. Another limitation is the majority of ρ0 cell lines used to produce cybrids are tumor cells so do not necessarily reflect normal cell lines. Tumor cells are anaerobic at baseline that should be taken into account. It is also important to take into account the method used to deplete mtDNA. While the majority of cybrid studies have used EtBr, newer methods are being utilized to deplete mtDNA. This should also be taken into consideration in the interpretation of findings [29].
Summary
In summary, the use of cybrids has allowed research scientists to study disease states that relate to mtDNA mutations. Cybrid studies continue to gain further insight into how altered mitochondrial function affects cell physiology and phenotypic expression and the role the nuclear genome contributes to this relationship. While cybrids have been traditionally utilized to study inherited and sporadic disease states, they also represent a powerful tool to study acquired mitochondrial inhibition from specific poisons such as pesticides, cyanide, and carbon monoxide.
Acknowledgments
We thank Douglas Wallace, PhD, and Deborah Murdock, PhD, for their guidance of this article.
Funding
The project described was supported by award K12 HL109009 from the National Heart, Lung, and Blood Institute. Dr. Jang is a National Heart, Lung, and Blood Institute (NHLBI) K12 Scholar.
References
- 1.Mullins C. The biogenesis of cellular organelles. New York: Kluwer Academic / Plenum Publishers; 2005. [Google Scholar]
- 2.Ritz P, Berrut G. Mitochondrial function, energy expenditure, aging and insulin resistance. Diabetes Metab. 2005;31:5S67–5S73. doi: 10.1016/S1262-3636(05)73654-5. [DOI] [PubMed] [Google Scholar]
- 3.Burté F, Carelli V, Chinnery PF, et al. Disturbed mitochondrial dynamics and neurodegenerative disorders. Nat Rev Neurol. 2014. [DOI] [PubMed]
- 4.Trounce IA, Pinkert CA. Cybrid models of mtDNA disease and transmission, from cells to mice. Curr Top Dev Biol. 2007;77:157–83. doi: 10.1016/S0070-2153(06)77006-5. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335–43. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins HM, Carl SM, Swerdlow RH. Cytoplasmic hybrid (cybrid) cell lines as a practical model for mitochondriopathies. Redox Biol. 2014;2C:619–631. doi: 10.1016/j.redox.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol Cell Biol. 1985;5(5):1163–9. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishikawa K, Hayashi J. Generation of mtDNA-exchanged cybrids for determination of the effects of mtDNA mutations on tumor phenotypes. Methods Enzymol. 2009;457:335–46. doi: 10.1016/S0076-6879(09)05019-8. [DOI] [PubMed] [Google Scholar]
- 9.Grégoire M, Morais R, Quilliam MA. On auxotrophy for pyrimidines of respiration-deficient chick embryo cells. Eur J Biochem. 1984;142(1):49–55. doi: 10.1111/j.1432-1033.1984.tb08249.x. [DOI] [PubMed] [Google Scholar]
- 10.Poste G, Reeve P. Enucleation of mammalian cells by cytochalasin B. II. Formation of hybrid cells and heterokaryons by fusion of anucleate and nucleated cells. Exp Cell Res. 1972;73(2):287–94. doi: 10.1016/0014-4827(72)90050-X. [DOI] [PubMed] [Google Scholar]
- 11.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246(4929):500–3. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 12.Bunn CL, Wallace DC, Eisenstadt JM. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974;71(5):1681–5. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace DC, Bunn CL, Eisenstadt JM. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. Cell Biol. 1975;67(1):174–88. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maresca A, Caporali L, Strobbe D, et al. Genetic basis of mitochondrial optic neuropathies. Curr Mol Med. 2014. [Epub ahead of print] [DOI] [PubMed]
- 15.Brown MD, Voljavec AS, Lott MT, et al. Mitochondrial DNA complex I and III mutations associated with Leber’s hereditary optic neuropathy. Genetics. 1992;130(1):163–73. doi: 10.1093/genetics/130.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jun AS, Trounce IA, Brown MD, et al. Use of transmitochondrial cybrids to assign a complex I defect to the mitochondrial DNA-encoded NADH dehydrogenase subunit 6 gene mutation at nucleotide pair 14459 that causes Leber hereditary optic neuropathy and dystonia. Mol Cell Biol. 1996;16(3):771–7. doi: 10.1128/mcb.16.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MD, Allen JC, Van Stavern GP, et al. Clinical, genetic, and biochemical characterization of a Leber hereditary optic neuropathy family containing both the 11778 and 14484 primary mutations. Am J Med Genet. 2001;104(4):331–8. doi: 10.1002/1096-8628(20011215)104:4<331::AID-AJMG10054>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Brown MD, Sun F, Wallace DC. Clustering of Caucasian Leber hereditary optic neuropathy patients containing the 11778 or 14484 mutations on an mtDNA lineage. Am J Hum Genet. 1997;60(2):381–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell SL, Goodloe R, Brown-Gentry K, et al. Characterization of mitochondrial haplogroups in a large population-based sample from the United States. Hum Genet. 2014;133(7):861–8. doi: 10.1007/s00439-014-1421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thambisetty M, Newman NJ. Diagnosis and management of MELAS. Expert Rev Mol Diagn. 2004;4(5):631–44. doi: 10.1586/14737159.4.5.631. [DOI] [PubMed] [Google Scholar]
- 21.Picard M, Zhang J, Hancock S, et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci U S A. 2014;111:E4033–42. doi: 10.1073/pnas.1414028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunbar DR, Moonie PA, Jacobs HT, et al. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci U S A. 1995;92(14):6562–6. doi: 10.1073/pnas.92.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trimmer PA, Swerdlow RH, Parks JK, et al. Abnormal mitochondrial morphology in sporadic Parkinson’s and Alzheimer’s disease cybrid cell lines. Exp Neurol. 2000;162(1):37–50. doi: 10.1006/exnr.2000.7333. [DOI] [PubMed] [Google Scholar]
- 24.Yeoh MJ, Braitberg G. Carbon monoxide and cyanide poisoning in fire related deaths in Victoria. Aust J Toxicol Clin Toxicol. 2004;42(6):855–63. doi: 10.1081/CLT-200035211. [DOI] [PubMed] [Google Scholar]
- 25.Swaminathan K. Pesticides and human diabetes: a link worth exploring? Diabet Med. 2013;30(11):1268–71. doi: 10.1111/dme.12212. [DOI] [PubMed] [Google Scholar]
- 26.Dirinck E, Jorens PG, Covaci A, et al. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obesity (Silver Spring) 2011;19(4):709–14. doi: 10.1038/oby.2010.133. [DOI] [PubMed] [Google Scholar]
- 27.Shelton JF, Geraghty EM, Tancredi DJ, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect. 2014;122(10):1103–9. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maturana MV, Pinheiro AS, Souza TL, et al. Unveiling the role of the pesticides paraquat and rotenone on α-synuclein fibrillation in vitro. Neurotoxicology. 2014;pii: S0161-813X(14):00189–2. doi: 10.1016/j.neuro.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85(15):3416–28. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]