Abstract
Elevated concentrations of serum acetaminophen-protein adducts, measured as protein-derived acetaminophen-cysteine (APAP-CYS), have been used to support a diagnosis of APAP-induced liver injury when histories and APAP levels are unhelpful. Adducts have been reported to undergo first-order elimination, with a terminal half-life of about 1.6 days. We wondered whether renal failure would affect APAP-CYS elimination half-life and whether continuous venovenous hemodiafiltration (CVVHDF), commonly used in liver failure patients, would remove adducts to lower their serum concentrations. Terminal elimination half-lives of serum APAP-CYS were compared between subjects with and without renal failure in a prospective cohort study of 168 adults who had ingested excessive doses of APAP. APAP-CYS concentrations were measured in plasma ultrafiltrate during CVVHDF at times of elevated serum adduct concentrations. Paired samples of urine and serum APAP-CYS concentrations were examined to help understand the potential importance of urinary elimination of serum adducts. APAP-CYS elimination half-life was longer in 15 renal failure subjects than in 28 subjects with normal renal function (41.3 ± 2.2 h versus 26.8 ± 1.1 h [mean ± SEM], respectively, p < 0.001). CVVHDF failed to remove detectable amounts of APAP-CYS in any of the nine subjects studied. Sixty-eight percent of 557 urine samples from 168 subjects contained no detectable APAP-CYS, despite levels in serum up to 16.99 μM. Terminal elimination half-life of serum APAP-CYS was prolonged in patients with renal failure for reasons unrelated to renal urinary adduct elimination, and consideration of prolonged elimination needs to be considered if attempting back-extrapolation of adduct concentrations. CVVHDF did not remove detectable APAP-CYS, suggesting approximate APAP-protein adduct molecular weights ≥ 50,000 Da. The presence of urinary APAP-CYS in the minority of instances was most compatible with renal adduct production and protein shedding into urine rather than elimination of serum adducts.
Keywords: Acetaminophen, Adducts, Pharmacokinetics, Renal failure, Overdose
Introduction
Acetaminophen (APAP) is very safe when taken at therapeutic doses, but acute and chronic overdoses can cause acute liver failure. Hepatotoxicity is explained by metabolism of APAP by cytochrome P450 enzymes to N-acetyl-p-benzoquinone imine (NAPQI), an electrophile that covalently binds to intracellular proteins, most commonly to cysteine residues, to form APAP-protein adducts. Evidence strongly supports NAPQI-binding to intramitochondrial proteins as being essential for or associated with subsequent development of hepatocellular necrosis in animals and man [1, 2].
Measurement of circulating APAP-protein adducts, in the form of protein-derived 3-cysteinyl-acetaminophen, was described more than 25 years ago [3]. Methodology has improved since then, but general principles remain the same. For analysis of the protein adducts, serum or plasma is first subjected to dialysis and/or filtration to remove free APAP-CYS, APAP-CYS-glutathione, and other low molecular weight compounds containing an APAP-CYS moiety. The remaining protein fraction is treated with proteolytic enzymes to release protein-derived APAP-CYS, which is then quantified. The term “APAP-CYS” in this paper will be used to represent protein-derived APAP-CYS unless otherwise specified. The term “adducts” or “APAP-protein adducts” will refer to APAP-protein adducts responsible for what is measured as APAP-CYS.
Animal and cell culture studies have demonstrated that APAP-protein adducts are exported or released from hepatocytes without dramatic falls in intracellular glutathione concentrations and without cellular injury after non-toxic APAP doses [4, 5]. Indeed, serum APAP-protein adducts can be detected in human beings following therapeutic doses of APAP, with serum APAP-CYS concentrations remaining at or below 1 μM [6]. In contrast, serum APAP-CYS concentrations have been reported to rise above about 1 μM in patients with known APAP-induced hepatotoxicity when serum alanine aminotransferase (ALT) activity exceeds 1,000 IU/L, with higher ALT activities associated with higher serum APAP-CYS concentrations [7].
Physicians are commonly confronted with unreliable or incomplete histories concerning APAP use in patients presenting with acute liver injury in which APAP overdose is suspected. The relatively short elimination half-life of APAP allows serum APAP concentrations to be undetectable or relatively low during times of elevated and even rising ALT activity and worsening clinical condition. Serum APAP-CYS concentrations, in contrast, are more closely associated with rises and falls in ALT activity, and have been reported to display a mean elimination half-life of approximately 1.6 days in children, adolescents, and adults [7, 8]. Thus, the presence of elevated serum APAP-CYS concentrations has been used to provide evidence for APAP-induced acute liver failure when histories are unreliable and serum APAP concentrations are undetectable or uninterpretable [9]. Making such a distinction is important, given the opportunity for complete recovery in many patients with APAP-induced hepatic failure and when considering whether a patient with acute liver failure would be an appropriate candidate for liver transplantation.
If concentrations of serum APAP-CYS are to be interpreted correctly, it would be of value to understand what factors influence adduct elimination. Renal failure occurs in patients with APAP-induced hepatic necrosis [10, 11], and we wondered if the presence of renal failure is associated with changes in adduct elimination half-life. We also wondered whether renal replacement therapy with continuous venovenous hemodiafiltration (CVVHDF) meaningfully removes APAP-CYS from the circulation, which could affect serum adduct levels and their interpretation. Thus, we undertook a study of patients with APAP overdose with the primary purpose of comparing APAP-CYS elimination half-life in patients with and without renal failure. Secondary purposes were to characterize concentrations of APAP-CYS in dialysate/ultrafiltrate obtained during CVVHDF, and to describe urinary adduct concentrations in order to help understand explanations for potentially different elimination half-lives during renal failure.
Methods
This prospective cohort study took place at a single academic medical center with active medical toxicology and liver transplantation services, and which served as a referral center for toxicology patients and for patients with acute liver failure from any cause. The medical toxicology physician group cares for about 200 patients of all ages with suspected APAP toxicity annually.
Inpatients ≥18 years of age under the care of medical toxicologists and in whom APAP toxicity from acute or chronic excessive ingestions were initially suspected as being present were considered for recruitment by convenience sampling if pregnancy had been excluded and if informed consent could be obtained. This study was approved by our center’s Institutional Review Board.
Blood for measurement of serum APAP-CYS was drawn into a clot tube upon study entry, again 12 h later, and then once daily until day ten, after which blood was drawn every 48 h until the time of discharge or for a total of 3 weeks. Serum was separated using centrifugation and then frozen. On rare occasions, plasma was used for APAP-CYS measurement when serum was unavailable, and plasma was separated and frozen in a similar manner. As near as possible to the time of each blood sampling, a sample of urine was collected (freshly voided or fresh urine from a urinary catheter) into a 90-mL container containing 100 mg ascorbic acid. A ten milliliter aliquot was separated and then frozen.
For CVVHDF, a Prismaflex M100 cartridge (Gambro, Lakewood, CO) was used at blood flow rates ranging from 200 to 250 mL/min, and dialysate flow rates of 1000 to 2000 mL/h. The manufacturer reports a sieving coefficient of 55 % for protein at 16,000 Da, with a cutoff at 55,000 Da. During CVVHDF, both dialysate and plasma ultrafiltrate are continuously collected together into a 5-L disposable polyvinyl chloride plastic bag. Five liters of ultrafiltrate/dialysate were collected on a single occasion, and an aliquot was taken and frozen for analysis. Mid-collection, a clot tube of venous blood was drawn and separated serum was frozen so that serum APAP-CYS levels could be compared to those in ultrafiltrate/dialysate collected at the same time.
Data on each subject were prospectively collected into a registry and included demographic information, history of ingestion, whether the ingestion history was considered reliable, laboratory and imaging results, date and time of each specimen collection, treatment, and outcome. Intravenous N-acetylcysteine (NAC) was infusing in most all subjects during the initial blood draw and was continued beyond 24 h in those with worsening liver function tests or with acute liver failure until prothrombin time was clearly improving and less than about 25 s, in the absence of hepatic encephalopathy.
At the time of discharge and before results of APAP-CYS concentrations were available, subjects were classified regarding APAP ingestion. Two authors reviewed a final assemblage of records that included: emergency medical services charts; hospital records; laboratory results, including results of comprehensive urine drug screening and evaluations for other causes of liver failure; imaging studies; prescription records; psychiatric assessments; and interviews with patients, friends, and family members. Subjects were then classified as to whether or not an excessive dose of APAP had definitely been ingested, and whether any ALT rise above normal (>46 IU/L) was definitely from APAP. The treating medical toxicologists for each subject were contacted and any discrepancy in classification was discussed with review of records, and a unanimous agreement was made in every case. The cohort for this study comprised subjects who were classified as definitely ingesting toxic doses of APAP and for whom at least one pair of serum and urine APAP-CYS concentrations were obtained.
Definitions
Renal failure was defined as a serum creatinine concentration >2 mg/dL more than 24 h after hospital admission, or as the necessity for renal replacement therapy in the form of hemodialysis or CVVHDF. An acute APAP overdose meant the history suggested that all APAP was ingested in less than a 3-h period. This included subjects in whom serum APAP concentrations were above the treatment line of the Rumack-Matthew nomogram [12], or in whom the nomogram could not be applied. All other excessive ingestions (>4 g APAP per day) were labeled as being chronic, whether staggered over several hours, days, or weeks. The peak serum APAP-CYS concentration and peak ALT activity were defined as the highest levels measured at any time. The time until peak serum APAP-CYS was calculated as hours between the first blood sampling for any laboratory test, which approximated first presentation for medical care, and the time of measurement of peak serum APAP-CYS concentration, because of lack of confidence in times and amounts of ingestion or last APAP in most of instances.
Measurement of APAP-CYS and Urine Creatinine
Quantification of APAP-CYS in serum, plasma, urine and dialysate/ultrafiltrate was performed using high-performance liquid chromatography—tandem mass spectrometry (HPLC-MS/MS). Biological samples (500 μL) were first dialyzed (Spectrum Labs, Santa Domingo, CA; MWCO 3500), and the dialysate then passed twice through gel filtration columns (Bio-Spin® 6 Tris columns), with a 6-kDa molecular weight cutoff, (Bio-Rad Laboratories, Hercules, CA) to remove APAP and APAP-CYS not covalently bound to proteins. The eluates were then subjected to enzymatic protease digestion (protease type XIV from Streptomyces griseus, 40 U/mL) for 24 h at 37 ° C to liberate APAP-CYS from proteins. Norbuprenorphine-D3 (200 ng/mL) was added as an internal standard. The liberated APAP-CYS and internal standard were recovered from the digested matrix by protein precipitation using cold acetonitrile (600 μL). Sample extracts are injected on to an HPLC column (Poroshell 120SB-C18 50 mm L × 2.0 mm I.D.; 3.0 μm particle size) coupled to a triple quadrupole tandem mass spectrometer (Agilent 6460 TripleQuad LC/MS-MS) operating in electrospray ionization and selected reaction mode. The concentration of the analyte was determined from the ratio of the peak area of the analyte to the peak area of the internal standard, and comparison of this ratio with the calibration curve that was generated from concurrent analysis standards fortified with known concentrations of APAP-CYS and its internal standard. Regression analysis was performed using MassHunter software (Agilent Technologies, Santa Clara, CA) and quadratic fits with 1/× weighting. The dynamic range over which APAP-CYS could be accurately quantified with the above procedure was from 0.01 to 10 μM (2.7 to 2700 ng/mL). Samples which exceeded 10 μM on initial analysis were diluted 20-fold (25-μL sample + 475-μL analyte-free serum or urine, as appropriate) and re-assayed. Final concentrations were determined after adjustment for the dilution factor. The limit of quantification for APAP-CYS was 0.01 μM (2.7 ng/mL). Intra- and inter-assay imprecisions in all matrices were determined to be less than 10 % across the range of the calibration curve. APAP-CYS was determined to be stable in human plasma and serum for three freeze-thaw cycles and 24 h at ambient temperature. Paired serum and plasma samples containing a range of APAP-CYS concentrations yielded statistically indistinguishable results.
Urine creatinine was measured using a colorimetric assay (Cayman Chemical, Ann Arbor, MI), which involved a reaction of creatinine with picric acid and absorbance measured at 492 nm.
APAP-CYS was purchased as the trifluoroacetic acid salt from Toronto Research Chemicals (TRC; North Rock, CA). Norbuprenorphine-D3 was obtained from Cerilliant (Round Rock, TX). Protease type XIV from S. griseus (~4 units/mg solid) was purchased from Sigma/Aldrich Chemical Corp (St. Louis, MO). Sodium acetate was obtained from J.T. Baker (Phillipsburg, NJ). Aqueous solutions were prepared with deionized water filtered by the Mill-Q® Gradient A10® filtration system (Millipore, Boston, MA). Acetonitrile and methanol were LC-MS grade and were purchased from Burdick & Jackson (Muskegon, MI). Concentrated formic acid (88 %) was purchased from Fisher Scientific (Fair Lawn, NJ). Urine creatinine controls were obtained from Sciteck (Arden, NC).
Pharmacokinetic and Statistical Analyses
Interpretation of serum APAP-CYS concentrations is of most interest in patients with elevations in ALT > 1000 IU/L, which has been associated with serum APAP-CYS concentrations greater than approximately 1 μM. Therefore, for a given subject to be included in pharmacokinetic analyses of terminal elimination, the series of terminal APAP-CYS concentrations used in analysis from that subject had to meet the following two criteria: (1) three or more consecutive terminal APAP-CYS levels during which no value was rising; and (2) the highest (first) APAP-CYS concentration in the series had to exceed 1 μM. The time of obtaining the first level in the series of adduct concentrations for each subject was standardized by setting it to time zero. Elimination was fitted using a single-compartment model using Simulation Analysis and Modeling Software (SAAM II) and PopKinetics population analysis software, versions 2.3.1 (The Epsilon Group, Charlottesville, VA). Population analysis approaches are considered to provide a smaller bias in estimation of a group’s pharmacokinetic parameters [13]. With ten subjects in each group and a two-tailed alpha of 0.05, we calculated power >0.95 to detect a 30 % difference in elimination half-life between groups.
Mean values of elimination half-life were compared using independent t tests. To examine potential confounders between normal renal function and renal failure groups, Spearman-rank order correlations were examined between elimination half-life and the following parameters: peak serum APAP-CYS concentration, time until peak serum APAP-CYS concentration, and peak ALT activity. Multiple regression was not used because of risk of over-fitting. A two-tailed p < 0.05 was chosen to represent statistical significance. Descriptive statistics were used to describe demographics and group characteristics and for post hoc subgroup comparisons.
Urine APAP-CYS concentrations were standardized for urine creatinine concentration and are reported as μmol APAP-CYS per gram creatinine (μmol/g creatinine). A urine APAP-CYS concentration was matched to a serum APAP-CYS level if the serum level was obtained within ±6 h of urine sampling.
Results
Over 3.5 years, 220 subjects were recruited, and of these, there were 168 subjects who were considered to have definitely ingested acute or chronic excessive doses of APAP and from whom paired samples of serum and urine APAP-CYS levels were available, composing the study cohort. The mean age was 35.6 years (range 18–79), and 114/168 subjects (68 %) were women. Of these 168 subjects, 128 had acutely and 40 had chronically ingested excessive APAP doses. Peak serum ALT >1000 IU/L developed in 64 subjects (38 %), in whom APAP was definitely considered responsible. Twenty-one subjects (12 %) developed renal failure, and nine subjects were treated with CVVHDF. Acute renal failure resulted from combinations of multiple factors, including APAP, hypotension from coingestants, and rhabdomyolysis. Five of 168 subjects died, and all deaths were from complications of acute liver failure. None were candidates for liver transplantation. In only 28/168 instances (16.7 %), physicians were confident of the time of ingestion or last use. In only 8/128 cases of acute ingestions, 6.3 % were physicians confident in the exact number of grams of excessive APAP that had been ingested. Physicians were never confident as to accurate amounts or times of APAP consumption in chronic overdose subjects.
Serum APAP-CYS Terminal Elimination
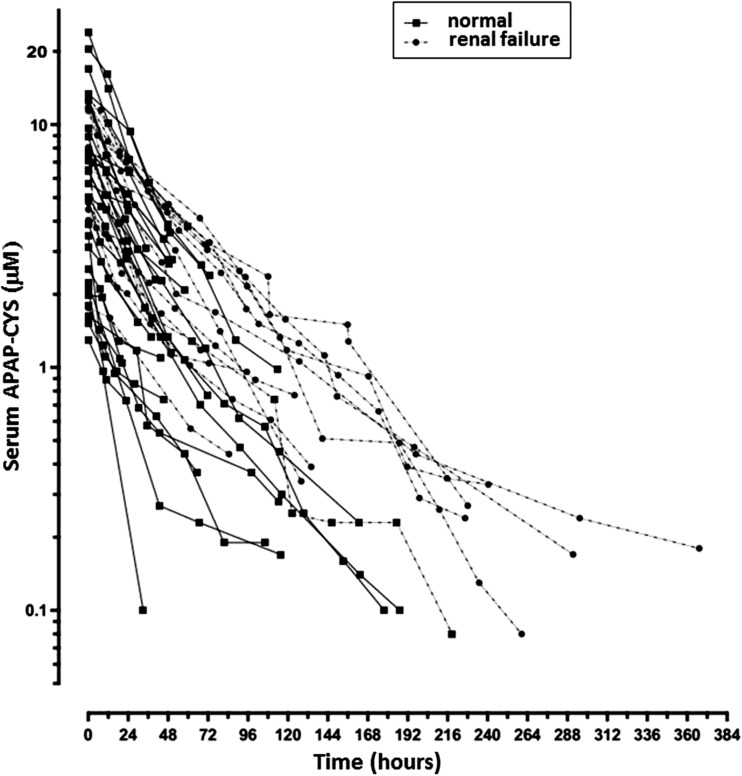
Forty-three of 168 subjects met criteria for inclusion in pharmacokinetic analyses. Twenty-eight subjects with normal renal function and 15 subjects with renal failure provided 123 and 114 serum APAP-CYS levels, respectively, for determinations of elimination half-lives (Table 1 and Fig. 1). Elimination was best characterized as a first-order process, and elimination half-life values were normally distributed. Mean elimination half-life was 54 % longer in subjects with renal failure (41.3 ± 2.2 h [mean ± SEM]) than in those without renal failure (26.8 ± 1.1 h) (p < 0.001). Peak ALT activity, peak serum APAP-CYS concentration, and time until peak serum APAP-CYS concentration appeared similar between groups, and Spearman-rank order correlations between these parameters and elimination half-life were not significant. While ages were similar in both groups, there were fewer females in the group with normal renal function. A greater percentage of chronic ingestions were found in the renal failure group.
Table 1.
Elimination kinetics and group characteristics
| Group | Age year |
Gender % female |
Peak ALT IU/L |
Peak serum APAP-CYS μM |
Time until peak serum APAP-CYS hr |
Hepatic encephalo pathy # (%) |
Ingestion type % acute |
Died # (%) |
Half-life*
h |
|---|---|---|---|---|---|---|---|---|---|
| Normal N = 28 |
37 ± 3 | 46 | 2540 ± 607 | 7.6 ± 1.11 | 31 ± 4 | 5 (18) | 57 | 0 | 26.8 ± 1.1 |
| Renal failure N = 15 |
39 ± 5 | 87 | 2842 ± 898 | 8.76 ± 3.48 | 32 ± 6 | 5 (33) | 33 | 2 (13) | 41.3 ± 2.2 |
Half-life represents serum APAP-CYS terminal elimination half-life. Data for age, peak ALT, peak serum APAP-CYS, time until peak serum APAP-CYS, and half-life are presented as mean ± SEM.
* p < 0.001 (terminal elimination half-life differed between groups)
Fig. 1.
Terminal elimination of serum APAP-CYS in 28 subjects with normal renal function and 15 subjects with renal failure. The first (highest) APAP-CYS value for each subject’s series was standardized to time zero for pharmacokinetic analysis. Mean ± SEM elimination half-life in renal failure group was 41.3 ± 2.2 h compared to 26.8 ± 1.1 h in those with normal renal function (p < 0.001)
Urinary APAP-CYS
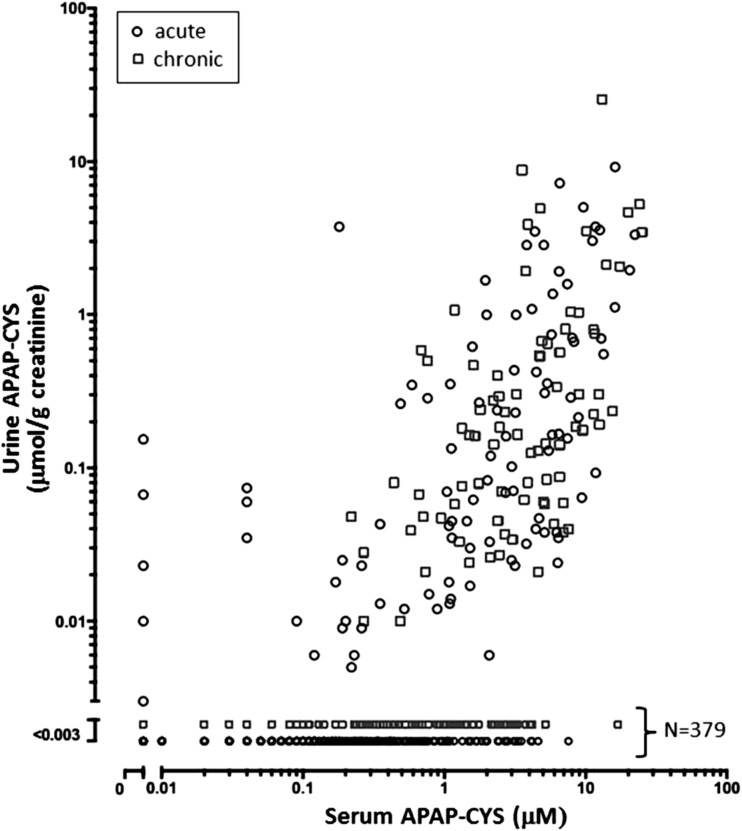
Five hundred fifty-seven paired urine and serum APAP-CYS concentrations from 168 subjects are shown in Fig. 2, with an average of 3.3 paired samples per subject (range 1–14). Data from acute overdoses are distinguished from subjects with chronic ingestions by legend markers, and no apparent pattern differentiates the two groups. Serum APAP-CYS concentrations ranged from undetectable (acute overdoses treated early without development of hepatotoxicity) to 26.36 μM. Three hundred seventy-nine of 557 urine samples (68 %) contained no detectable APAP-CYS, despite corresponding serum APAP-CYS levels ranging up to 16.99 μM. In the remaining 178 of 557 urine samples, urine APAP-CYS concentrations ranged from 0.003 to 25.5 μmol/g creatinine, with all but six values remaining below 5 μmol/g creatinine. Of 76 subjects in whom ALT activity remained within the normal range, 11 subjects had measurable urine adduct concentrations ranging from 0.003 to 0.154 μmol/g creatinine. Five urine specimens contained measurable APAP-CYS, despite undetectable amounts found in corresponding serum.
Fig. 2.
Urine APAP-CYS versus serum APAP-CYS concentrations with urine values corrected for urinary creatinine concentration. Five hundred fifty-seven data points are plotted from 168 subjects, and 379 samples contained undetectable urinary APAP-CYS
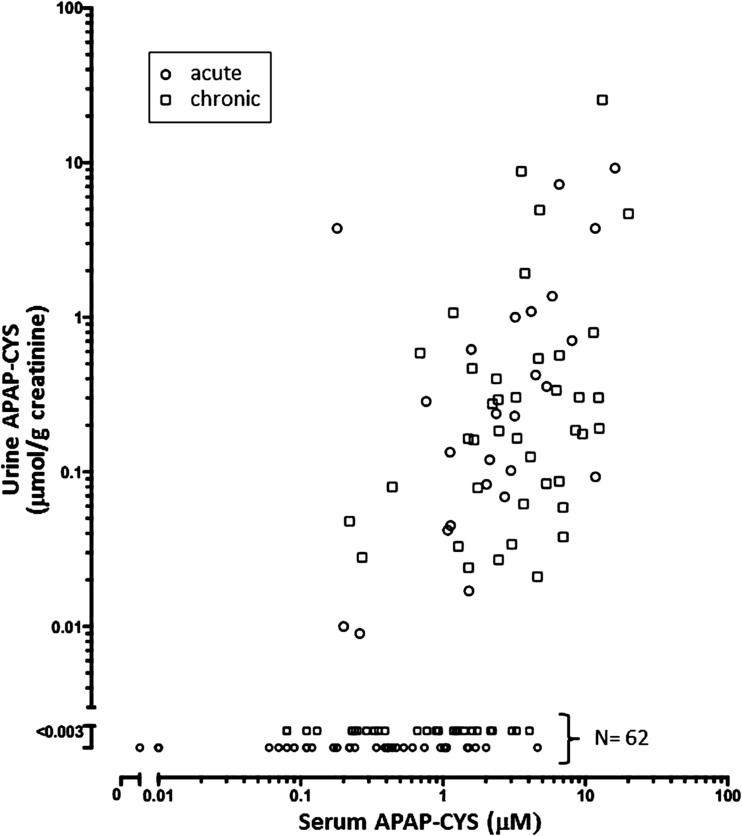
Figure 3 comprises a subset of Fig. 2 and displays 127 data points from the 21 subjects with renal failure. Urine APAP-CYS concentrations were undetectable in 62 specimens despite corresponding serum adduct levels up to 4.61 μM. Inspection of Figs. 2 and 3 show the overall pattern in renal failure patients was similar to all subjects.
Fig. 3.
Urine APAP-CYS versus serum APAP-CYS concentrations from 21 subjects with renal failure. These 127 data points are a subset of those in Fig. 1, and 62 urine specimens contained undetectable APAP-CYS
APAP-CYS Removal by CVVHDF
Table 2 summarizes data on APAP-CYS removal by CVVHDF. Because of mixing of dialysate and ultrafiltrate in the 5-L collection bags during CVVHDF, aliquots of collected ultrafiltrate/dialysate contained between 28 and 68 % plasma ultrafiltrate. In no instance was APAP-CYS detected in dialysate/ultrafiltrate, even when serum APAP-CYS concentrations were as high as 9.77 μM.
Table 2.
APAP-CYS removal by CVVHDF
| Subject | Blood flow mL/min |
Dialysate flow mL/h |
Collection time hr |
Plasma ultrafiltrate percent |
Serum APAP-CYS μM |
Dialysate/ultrafiltrate APAP-CYS μM |
|---|---|---|---|---|---|---|
| 1 | 200 | 1,000 | 2.6 | 48 | 0.46 | < 0.01 |
| 2 | 250 | 2,000 | 1.2 | 47.9 | 1.38 | < 0.01 |
| 3 | 200 | 2,000 | 1.6 | 36 | 6.14 | < 0.01 |
| 4 | 250 | 1,000 | 1.8 | 64 | 6.41 | < 0.01 |
| 5 | 250 | 1,500 | 1.5 | 55 | 6.62 | < 0.01 |
| 6 | 200 | 1,000 | 1.6 | 68 | 7.10 | < 0.01 |
| 7 | 250 | 1,000 | 1.8 | 64 | 7.32 | < 0.01 |
| 8 | 240 | 2,000 | 1.8 | 28 | 9.23 | < 0.01 |
| 9 | 200 | 1,000 | 1.8 | 64 | 9.77 | < 0.01 |
CVVHDF was performed using double-lumen central venous catheters and a Prismaflex® M100 cartridge set (Gambro®, Lakewood, CO.). Collection time refers to time required to collect 5 L of dialysate/ultrafiltrate, from which an aliquot was taken for measurement of APAP-CYS concentration (last column). The percent plasma ultrafiltrate represents the percent of plasma ultrafiltrate in the 5 L collection of ultrafiltrate/dialysate sample used for analysis. Serum APAP-CYS concentrations were measured on blood collected at mid-collection time
Discussion
Elimination kinetics of APAP-protein adducts have been described, previously. James and others reported a mean ± SEM adduct elimination half-life of 35.3 ± 0.64 h in 125 adolescents and children following APAP overdose, and this cohort included those who did and did not develop hepatotoxicity [8]. No prevalence of renal failure was described. In 2009, James and colleagues also reported on APAP-CYS elimination kinetics in a cohort of 53 adults with acute liver failure and found a mean ± SEM elimination half-life of 41.3 ± 1.1 h [7]. All members of this latter cohort were encephalopathic, 23 % died or underwent liver transplantation, and the mean peak serum creatinine concentration was 3.3 mg/dL, indicating a high prevalence of renal failure. However, no formal comparisons of adduct elimination kinetics in patients with and without renal failure in either of these two cohorts were performed, and none have been previously reported. Furthermore, APAP-CYS concentrations in urine and ultrafiltrate/dialysate also have not previously been described.
In this study, we compared terminal serial serum APAP-CYS concentrations in 28 subjects with normal renal function to 15 subjects with renal failure and found serum adduct terminal elimination half-life was significantly longer in subjects with renal failure. The mean elimination half-life of 41.3 h in subjects with renal failure was the same as that reported by James in adults with liver failure and a mean peak serum creatinine of 3.3 mg/dL [7]. Serum APAP-CYS concentrations we found were similar to those reported previously [6–8].
It seems unlikely that impaired renal clearance of APAP-protein adducts is a plausible explanation for prolonged adduct elimination in the renal failure group. The failure to detect any APAP-CYS in plasma ultrafiltrate during CVVHDF at times of elevated serum adduct concentrations indicates that the APAP-protein adducts formed and subsequently measured as APAP-CYS were of high molecular weight, greater than approximately 50,000 Da. This is in keeping with animal studies that have isolated protein adducts and found them to generally range from about 50,000 to more than 100,000 Da [14]. Such high molecular weight proteins do not undergo significant glomerular filtration or other forms of renal clearance [15]. Furthermore, 68 % of 557 urine samples contained no quantifiable APAP-CYS, even when serum adduct concentrations were as high as 16.99 μM (Fig. 2). The relationship between serum and urine APAP-CYS concentrations was also examined only in subjects with renal failure (Fig. 3) to look for a pattern that would suggest different urinary APAP-CYS concentrations accompanying acute renal failure, during times of low glomerular filtration rates and/or tubular dysfunction, but the same general pattern was seen as for all subjects. In only a minority of instances APAP-CYS was measurable in urine, and these included subjects in whom peak ALT values never exceeded 46 IU/L.
The most plausible explanation for the irregular presence of APAP-protein adducts in urine was renal production of NAPQI and subsequent local adduct formation. CYP450 enzymes are expressed in animal and human proximal renal tubular cells [16, 17]. As is the case for animal models of hepatic necrosis, animal models of APAP-induced acute renal failure have demonstrated CYP450-mediated formation of NAPQI and subsequent glutathione depletion, protein adduct formation, and acute tubular necrosis (ATN) [17, 18]. A similar mechanism is believed to explain APAP-induced ATN in humans [19], and IV NAC has been shown to prevent both hepatic and renal injury following APAP overdose [20]. It would not be unexpected that proteinuria comprising tubular proteins would include proteins that had been covalently modified by NAPQI to form APAP-protein adducts. While we did not measure urinary total protein concentrations in urine samples, detection and quantification of the high molecular weight APAP-protein adducts as APAP-CYS in urine would not have been possible unless increased urinary protein excretion was present in positive samples. Higher doses of APAP and subsequent liver injury would be expected to be associated with higher adduct formation both in liver and kidney. In the face of proteinuria from any cause, then, higher serum adduct levels would be expected to be roughly associated with higher urine adduct levels, as reflected in Figs. 2 and 3. That APAP-CYS could be detected and quantified in urine in the absence of measurable serum adducts is also in keeping with renal production of protein adducts that then were subsequently excreted in urine from protein shedding, rather than from filtration of plasma adducts. We did not calculate a correlation coefficient for urine versus serum APAP-CYS concentrations since 68 % of urine values were unmeasurable, and a correlation restricted to non-zero values of urine APAP-CYS would represent a selective post hoc analysis on one-third of data points.
The liver is responsible, in part, for clearance of high molecular weight circulating proteins [21], and more severe hepatic dysfunction in the renal failure group, theoretically, could have contributed to a prolonged adduct elimination half-life in these subjects. However, subjects in both groups appeared similar with regard to peak ALT activity, peak serum APAP-CYS levels, and time until peak serum APAP-CYS concentrations. Spearman rank correlations between these parameters and elimination half-life were insignificant. Both groups also shared similar ages. Hepatic encephalopathy was more common in those with renal failure, and two deaths occurred in this group (Table 1). However, of the five patients in the renal failure group with hepatic encephalopathy, the mean elimination half-life was 42.3 h, similar to the group, overall. The two subjects who died displayed terminal elimination half-lives of 40.3 and 26.1 h, which fail to support changes in hepatic clearance of adducts being responsible for differences between groups in elimination half-life.
The preponderance of women in the renal failure group is in keeping with about 68 % of our total subjects being female, as has been reported previously [22, 23]. The lower fraction of women in the normal renal function groups probably represents a chance occurrence. The mean ± SEM adduct elimination half-life for the 13 women in the normal renal function group, alone, was 27.1 ± 1.7 h, a value similar to that of the group overall (26.8 ± 1.1 h), indicating that gender was unlikely to explain differences in half-life.
Chronic ingestions were more commonly seen in those with renal failure, and this observation has been reported previously, making it an expected finding [11, 24]. Those who are hospitalized following chronic or staggered doses of APAP commonly exhibit evidence of liver and renal failure at time of presentation, which accounts for their hospital admission. In the normal renal function group, the 16 subjects with acute ingestions had a mean ± SEM elimination half-life of 25.8 ± 1.5 h compared to that of 27.9 ± 1.6 h in those with chronic ingestions, which does not suggest that the type of ingestion was important in determining half-life.
Apart from changes in clearance, an increase in the apparent volume of distribution (Vd) would also increase elimination half-life. A plausible explanation for a longer half-life in the renal failure group would be an increased Vd from increased total body water or other changes that may influence distribution of large molecular weight proteins. An increase in body water causing such a dramatic prolongation of elimination half-life would suggest a relatively small Vd for APAP-protein adducts, which would be in keeping with their large molecular weight and reports of Vds for smaller peptides that mainly are limited to extravascular volume [15].
If serum APAP-CYS measurements are used to support or refute a diagnosis of APAP toxicity, confounding factors that influence their concentrations over time, such as hepatic ischemia, may need to be considered [4]. We have shown that an additional factor is the presence of renal failure, which was accompanied by significant prolongation of adduct elimination half-life. When one or two serum adduct levels are measured late in the course of an illness, at which time they may have already fallen from peak levels, back-extrapolation to what concentrations may have been previously would be difficult, given the variation in times when peak serum APAP-CYS levels occur and, in our experience, frequently unreliable histories concerning time of ingestion(s). However, such extrapolations need to consider different elimination half-lives in the presence or absence of renal failure. The correlation between ALT activity and serum APAP-CYS concentration theoretically may differ between patients with and without renal failure, if declines in ALT activity are not affected by renal dysfunction, and this may be a topic for future study. Data from this study also indicate that measurement of urine adduct concentration would not be a reliable predictor of serum adduct concentration.
Limitations
We did not determine the cause of prolonged serum adduct elimination half-life in subjects with renal failure, though we demonstrated that impairment of urinary clearance seemed unlikely. Whether renal failure subjects had a larger Vd, and/or impaired adduct clearance mediated by other organs was not established. While we corrected for urine concentration by reporting urine adduct concentrations in μmol APAP-CYS/g creatinine, we did not report values for urinary adduct clearance. However, since APAP-CYS was undetectable in 68 % of urine samples, all corresponding clearance values would have been zero, making urinary clearance an insignificant contributor to total clearance. Calculating clearance of serum adducts based on protein adducts in urine that may never have been circulating, but were formed in the kidney, would also be unhelpful. As noted, earlier, we did not measure urinary protein concentration in each specimen in which urinary adduct concentrations were measured. However, it was not possible to detect APAP-CYS in the absence of increased urinary protein excretion because of the methodology used, though the amount of proteinuria required for adduct detection would vary, depending on the degree of renal protein adduct formation, and such thresholds were not determined. It is possible that the profile of adducted proteins responsible for detectable APAP-CYS were different from adducted proteins found in serum, and it was not the purpose of our study to characterize the various adducted proteins. Finally, the lack of confidence in ingestion histories surrounding our subjects may reflect, in part, referral bias. That is, many patients were sent to us for admission and care because a toxic ingestion could not be excluded, given unreliable histories or lack of data.
Conclusion
Serum elimination half-life of APAP-protein adducts in adults with APAP overdose was prolonged in the presence of renal failure for reasons that were unrelated to urinary adduct elimination. Renal replacement therapy with CVVHDF did not result in significant removal of adducts, and lack of removal suggested that adducted proteins found in serum were larger than approximately 50,000 Da. The presence of measurable APAP-protein adducts in urine in some samples was most compatible with renal production of protein adducts. Measurement of urinary APAP-protein adducts was not a reliable indicator of serum adduct concentrations. The presence or absence of renal failure may need to be taken into consideration when interpreting a given APAP-CYS concentration, especially if attempts are made at back-extrapolation.
Acknowledgments
The authors thank Mitch McGill, Ph. D and Hartmut Jaeschke, Ph. D., University of Kansas Medical Center, for their review and comments during manuscript preparation.
Conflict of Interest
None.
Footnotes
Michael Levine, Daniel E. Brooks, Rachel Levitan, Joshua Canning, Kimberlie A. Graeme, Aaron B. Skolnik, Carrie A. Truitt, Adam R. Bosak, Frank A. Lovecchio, Robert N. E. French, An Tran, and C. Will Heise.
McNeil Consumer Healthcare provided partial financial support for this study.
References
- 1.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122(4):1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30(9):2174–2187. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts DW, Pumford NR, Potter DW, Benson RW, Hinson JA. A sensitive immunochemical assay for acetaminophen-protein adducts. J Pharmacol Exp Ther. 1987;241(2):527–33. [PubMed] [Google Scholar]
- 4.McGill MR, Lebofsky M, Norris H-R, Slawson MH, Bajt ML, Xie Y, Williams CD, Wilkins DG, Rollins DE, Jaeschke H. Plasma and liver acetaminophen-protein adduct levels in mice after acetaminophen treatment: dose–response, mechanisms, and clinical implications. Toxicol Appl Pharmacol. 2013;269(3):240–249. doi: 10.1016/j.taap.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53(3):974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heard KJ, Green JL, James LP, Judge BS, Zolot L, Rhyee S, Dart RC. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol 2011; doi: 10.1186/1471-230X-11-20 [DOI] [PMC free article] [PubMed]
- 7.James LP, Letzig L, Simpson PM, Capparelli E, Roberts DW, Hinson JA, Davern TJ, Lee WM. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James LP, Caparelli EV, Simpson PM, Letzig L, Roberts D, Hinson JA, Kearns GL, Blumer JL, Sullivan JE, Network of pediatric pharmacology research units, national institutes of child health and human development Acetaminophen-associated hepatic injury: evaluation of acetaminophen protein adducts in children and adolescents with acetaminophen overdose. Clin Pharmacol Ther. 2008;84(6):684–690. doi: 10.1038/clpt.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM, Acute Liver Failure Study Group Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53(2):567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blantz RC. Acetaminophen: acute and chronic effects on renal function. Am J Kidney Dis. 1996;28(1):S3–S6. doi: 10.1016/S0272-6386(96)90561-2. [DOI] [PubMed] [Google Scholar]
- 11.O’Riordan A, Brummell Z, Sizer E, Auzinger G, Heaton N, Grady JG, Bernal W, Hendry BM, Wendon JA. Acute kidney injury in patients admitted to a liver intensive therapy unit with paracetamol-induced hepatotoxicity. Nephrol Dial Transplant. 2011;26(11):3501–3508. doi: 10.1093/ndt/gfr050. [DOI] [PubMed] [Google Scholar]
- 12.Hendrickson RG. Acetaminophen. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfank LR, Flomenbaum NE, editors. Goldfrank’s toxicologic emergencies. 9. New York: McGraw Hill; 2011. pp. 483–499. [Google Scholar]
- 13.Sheiner LB, Beal SL, Evaluation of methods for estimating population pharmacokinetics parameters I. Michaelis-menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm. 1980;8(6):553–571. doi: 10.1007/BF01060053. [DOI] [PubMed] [Google Scholar]
- 14.Cohen SD, Pumford NR, Khairallah EA, Boekelheide Km Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143(1):1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 15.Diao L, Beibohm B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin Pharmacokinet. 2013;52(10):855–868. doi: 10.1007/s40262-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 16.Cummings BS, Lasker JM, Lash LH. Expression of glutathione-dependent enzymes and cytochrome P450s in freshly isolated and primary cultures of proximal tubular cells from human kidney. J Pharmacol Exp Ther. 2000;293(2):677–685. [PubMed] [Google Scholar]
- 17.Hart SGE, Beierschmitt WP, Wyand DS, Khairallah EA, Cohen SD. Acetaminophen nephrotoxicity in CD-1 mice. I. Evidence of a role for in situ activation in selective covalent binding and toxicity. Toxicol Appl Pharmacol. 1994;126(2):267–275. doi: 10.1006/taap.1994.1116. [DOI] [PubMed] [Google Scholar]
- 18.McMurtry RJ, Snodgrass WR, Mitchell JR. Renal necrosis, glutathione depletion, and covalent binding after acetaminophen. Toxicol Appl Pharmacol. 1978;46(1):87–100. doi: 10.1016/0041-008X(78)90139-4. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JR, McMurtry RJ, Statham CN, Nelson SD. Molecular basis for several drug-induced nephropathies. Am J Med. 1977;62(4):518–526. doi: 10.1016/0002-9343(77)90407-7. [DOI] [PubMed] [Google Scholar]
- 20.Prescott LF, Illingworth RN, Critchley JAJH, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcysteine: the treatment of choice for paracetamol poisoning. Br Med J. 1979;2(6198):1097–1100. doi: 10.1136/bmj.2.6198.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeLeve LD. The hepatic sinusoidal endothelial cell: morphology, function, and pathobiology. In: Arias IM, Alter HJ, Boyer JL, Cohen DE, Fausto N, Shafritz DA, Wolkoff AW, editors. The liver. Biology and pathobiology. 5. Wiley-Blackwell: West Sussex; 2009. pp. 373–388. [Google Scholar]
- 22.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976–1985) N Engl J Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 23.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shakil AO, Lee WM, Acute Liver Failure Study Group Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42(6):1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 24.Pakravan N, Simpson KJ, Waring WS, Bates CM, Bateman DN. Renal injury at first presentation as a predictor for poor outcome in severe paracetamol poisoning referred to a liver transplant unit. Eur J Clin Pharmacol. 2009;65(2):163–168. doi: 10.1007/s00228-008-0580-9. [DOI] [PubMed] [Google Scholar]