Abstract
Introduction
The NBOMes (N-benzyl-oxy-methyl derivatives of known 2C phenylethylamines) are a new and growing class of potent synthetic stimulants. Case reports provide the bulk of available safety and clinical data for clinicians. We report two cases of NBOMe intoxication with 25C-NBOMe (the first lab-confirmed US case) and 25B-NBOMe, respectively, both confirmed via triple quadrapole mass spectrometry.
Case Reports
Case 1: A 16-year-old girl had a generalized seizure after reported use of 25I-NBOMe. She presented with altered mental status, lower extremity rigidity, and elevated CPK (6042 U/L). Despite treatment with benzodiazepines, her lower extremity rigidity persisted and CPK peaked at 47,906 U/L. She was discharged on hospital day 8. Serum and urine specimens confirmed presence of 25C-NBOMe. Case 2: A 15-year-old boy developed bizarre behavior after reported use of 25I-NBOMe. In the ED, he had two generalized seizures and persistent muscle rigidity. CPK peaked at 429 U/L. Seizures were managed with benzodiazepines, and he was discharged within 24 h. Serum specimens revealed 25B-NBOMe.
Discussion
NBOMes are amphetamine derivatives and highly potent 5-HT2A receptor agonists. Clinical manifestations are a product of enhanced central sympathetic and serotonergic tone. We report two cases of NBOMe intoxication in patients who believed they used 25I-NBOME, while lab confirmation proved otherwise. Whether unique clinical manifestations are specific to the NBOMe variant, dose, route of administration, or other factors is unknown. Laboratory confirmation may play a role in identifying unexpected NBOMe variants, while contributing to the epidemiologic data on these novel substances.
Keywords: NBOMe, Substituted phenylethylamines, Serotonin toxicity, New psychoactive substances, Drugs of abuse
Background
In 2010, a potent new synthetic hallucinogen known as “N-Bomb” emerged on the international recreational drug market [1]. First synthesized in 2003 by German chemist Ralph Heim, 2-(4-Iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl)methyl]ethanamine, or 25I-NBOMe, was discovered to be a potent serotonin 2A (5-HT2A) receptor agonist [2]. In 2008, David Nichols furthered this work, creating a radiolabeled form of 25I-NBOMe for use as a high affinity, traceable 5-HT2A ligand, applicable to positron emission tomography and other research purposes [3]. By 2010, 25I-NBOMe began to appear on the Internet [2]. Soon, recreational user experiences were being posted on EROWID, a popular online psychoactive drug and plant library, which recently described NBOMes as “the defining psychedelics of 2013” [4].
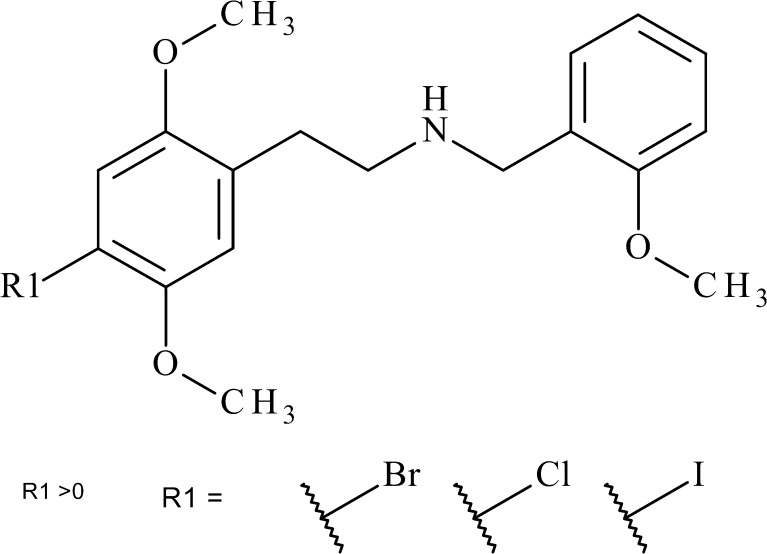
25I-NBOMe was the prototype and remains as one of this now expanding class of NBOMes, which are defined by the addition of a 2-methoxybenzyl (MeOB) functional group to the nitrogen of the phenylethylamine backbone (Fig. 1). While this 2-MeOB addition imparts an increased affinity for the 5-HT2A receptor, up to 16 times that of the well-described 2C-I amphetamine derivative, the impact of the specific halogen substitution on the benzene ring, which differentiates NBOMe variants, is less well-understood [5]. At least seven NBOMe variants seized from the recreational drug market have been described [6]. In November 2013, three, including 25I-NBOMe, 25C-NBOMe, and 25B-NBOMe were placed by the DEA in schedule I.
Fig. 1.
Structures of 25B-NBOMe, 25C-NBOMe, and 25I-NBOMe
In the absence of any reliable safety data regarding NBOMes, case reports, especially those supported by laboratory confirmation, continue to provide the most clinically useful information available. A case series described four patients with adverse effects after the use of 25I-NBOMe, with laboratory confirmation in three cases [7]. These four male teenagers (aged 18–19) simultaneously presented to the ED with altered mental status, tachycardia, and psychomotor agitation after either insufflating or ingesting 25I-NBOMe, sold by a dealer who purchased it from the Internet. Three developed seizures, and required sedation and intubation. One received hemodialysis for severe rhabdomyolysis and acute renal failure.
Another abstract described ten patients who presented to the ED after self-reported use of “25-I,” believed to represent 25I-NBOMe, although only one case was confirmed [8]. Clinical effects included tachycardia, hypertension, agitation, hallucinations, seizures and status epilepticus, intraparenchymal hemorrhage, coma, and acute kidney injury. All patients were discharged from the hospital without sequelae.
A number of deaths associated with 25I-NBOMe have also been reported, attributable either to pharmacologic toxicity, such as status epilepticus producing anoxic brain injury, or behavioral effects resulting in severe traumatic injuries [2, 9].
Here, we report two cases of patients who believed they were taking 25I-NBOMe but were confirmed via mass spectrometry as the chlorine and bromine derivatives, 25C-NBOMe and 25B-NBOMe, respectively.
Case 1
A 16-year-old girl presented to the ED after having a witnessed seizure. Thirty minutes earlier, she had self-administered “25I-NBOME” via blotter paper placed lingually. Her vital signs were as follows: blood pressure, 130/73 mmHg; heart rate, 146 beats/min; respiratory rate, 20 breaths/min; temperature, 97.8 °F; oxygen saturation, 99 %; finger-stick glucose, 170 mg/dl (mg/dL). On examination, she had increased muscle tone in both upper and lower extremities, as well as altered mental status, garbled speech, and visual hallucinations, repeatedly stating, “Look! Everyone is speaking Russian!” Within minutes of arrival, she had a second tonic-clonic seizure, which resolved after a total of lorazepam 8 mg IV. Initial labs were remarkable for white blood cell (WBC) count 19 × 103/mm3, serum sodium 149 milliequivalents/l (mEq/L), serum bicarbonate 11 mEq/L, and anion gap 21 mmol/L. Urine toxicology screen was negative for barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, methadone, and phencyclidine (PCP). CT brain was unremarkable. The patient was admitted to the pediatric ICU. Within 24 hours from exposure, her mental status had returned to baseline, but she complained of persistent stiffness in her arms and legs. On examination, she had increased rigidity in her thighs and 2–3 beats of ankle clonus bilaterally. Creatine phosphokinase (CPK) was 6042 units/l (U/L) (reference range 60–400 U/L) on hospital day (HD) 1 and peaked by HD 3 at 47,906 U/L. The patient’s creatinine remained normal throughout her hospitalization and she was discharged with a down-trending CPK on HD 8. A serum specimen obtained 7 hours post-admission and urine collected ∼9 hours post-admission, contained 25C-NBOMe present at >0.025 nanograms/ml (ng/mL) and 1.0 ng/mL, respectively. 25I-NBOMe was not detected. Confirmatory urine testing for additional drugs of abuse via enzyme immunoassay testing (EMIT) was negative for amphetamines, barbiturates, benzodiazepines, cocaine metabolite, methadone, opiates, oxycodone, PCP, propoxyphene, and marijuana metabolites. Confirmatory serum and urine testing by GC/MS was negative for amphetamines and 3,4-methylenedioxymethamphetamine (MDMA), as well as designer “bath salts” including cathinone, methcathinone, methedrone, methylone, mephedrone, ethcathinone, ethylone, entedrone, pentylone, butylone, and methylenedioxypyrovalerone (MDPV).
Case 2
A healthy 15-year-old boy became agitated approximately 6 hours after self-administering “25I-NBOME” via blotter paper placed on his tongue. Upon arrival, his initial vital signs were as follows: blood pressure, 177/93 mmHg; heart rate, 111 beats/min; respiratory rate, 20 breaths/min; temperature, 99.8 °F; finger-stick glucose, 195 mg/dL. On examination, he was agitated and internally preoccupied, with dilated, reactive pupils, diaphoresis, dry mucous membranes, hypoactive bowel sounds, and generalized muscle rigidity. Within minutes of arrival, the patient had a generalized tonic-clonic seizure, which resolved following the administration of lorazepam 2 mg IV. Approximately 2 hours later, the patient had a second seizure, also controlled with lorazepam 2 mg IV. His initial work up was notable for WBC 17 × 103/mm3, serum bicarbonate 13 mEq/L, glucose 224 mg/dL, CPK 429 U/L, and lactate 7.3 mmol/L. His serum sodium and creatinine were normal. Initial CT brain was unremarkable. The patient was transferred to a tertiary care hospital for pediatric ICU admission and further monitoring. Over the next 8 hours, the patient’s findings gradually improved and vital signs normalized, although episodes of aggressiveness and muscle rigidity continued. A repeat CPK was 230 U/L, and he was discharged within 24 h of presentation. Urine toxicology screen was positive for THC and benzodiazepines (performed after the patient received lorazepam) and was negative for amphetamines, barbiturates, cocaine, opiates, PCP, and tricyclic antidepressants. Serum specimens obtained upon arrival to the ED and 4 hours post-arrival contained 25B-NBOMe at 1.2 and 0.51 ng/ml, respectively. 25I-NBOMe was not detected. Confirmatory serum testing by GC/MS was negative for amphetamines, MDMA, and designer “bath salts” (as in case 1).
Laboratory Analysis
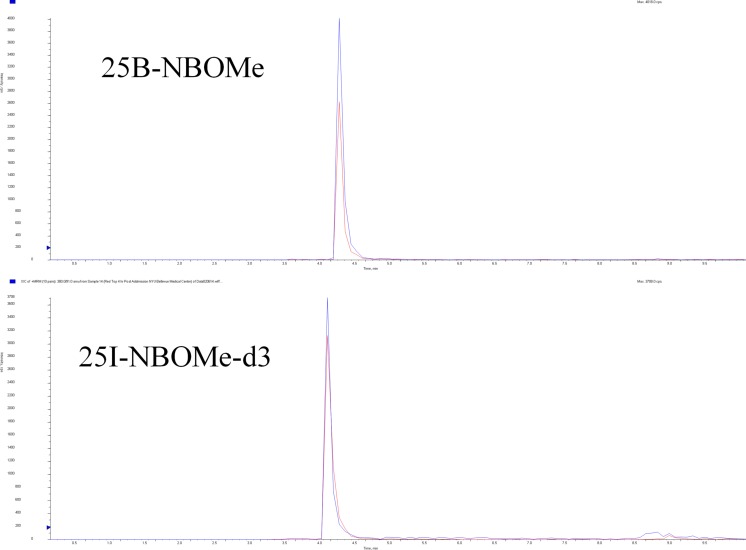
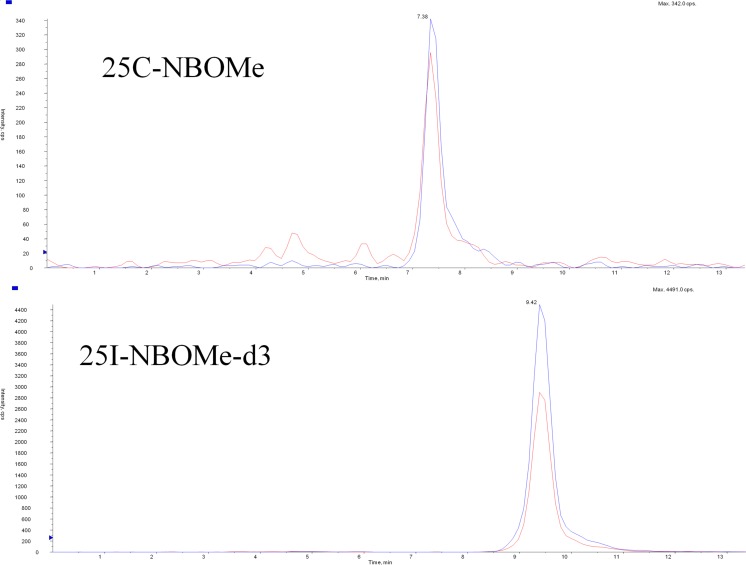
25C-NBOMe and 25B-NBOMe were measured in serum using a modification of the method of Poklis et al. [10]. 25-NBOMe-d3 was used as the internal standard. The NBOMes were extracted using solid phase extraction (SPE) with ZSDAU020 Clean Screen Columns (UCT Inc., Bristol, PA). High performance liquid chromatography tandem mass spectrometry (HPLC/MS/MS) analysis was performed with an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system. The separation of the drugs was performed using a Thermo Hypersil Gold C8 100 × 2.1, 3 μm column (Thermo Fisher Scientific Inc., Waltham, MA), (Fig. 2). The following transition ions (m/z) were monitored in multiple reaction monitoring (MRM) mode: 25C-NBOMe; 336 > 121 and 336 > 91, 25B-NBOMe; 381 > 121 and 381 > 91, and 25I-NBOMe-d3; 431 > 124 and 431 > 92. 25C-NBOMe was measured in urine using a previously published method [6]. The NBOMes were extracted using Clean Screen FASt™ extraction columns (UCT Inc., Bristol, PA). The HPLC/MS/MS analysis was performed using the same instrument and MRMs as the serum analysis. Chromatographic separation was performed on a Restek Allure Biphenyl 5 μ 100 × 3.2 mm column (Bellefonte, PA) (Fig. 3).
Fig. 2.
Chromatographic separation of 25B-NBOMe and 25I-NBOMe-d3 in patient’s serum sample
Fig. 3.
Chromatographic separation of 25C-NBOMe and 25I-NBOMe-d3 in patient’s urine sample
Enzyme immunoassay testing (EMIT) was performed using an ADVIA-1200 auto-analyzer in accordance with the manufacturer’s recommendations (Bayer HealthCare Diagnostic Division, Tarrytown, NY). The following were the cutoff concentrations (ng/mL) for each drug: amphetamines, 500; barbiturates, 200; benzodiazepines, 200; cocaine metabolite, 300; methadone 300; opiates, 300; oxycodone, 200; phencyclidine, 25; propoxyphene, 300; and marijuana metabolite, 20.
Discussion
Derived from, and thus similar to, the 2C class of amphetamines made popular by the late self-experimenting chemist Alexander Shulgin, NBOMes are now used recreationally by adolescents and young adults for their sympathomimetic and serotonergic effects. Often sold in microgram doses on blotter paper and marketed as “legal” or “natural LSD,” NBOMes are also available as liquid, powder, capsules, tablets, or sprays [2]. They can be administered sublingually, buccally, or by insufflation; due to low oral bioavailability, they are only rarely ingested. NBOMes are most commonly synthesized outside of the USA but, due to the high potency, are easily smuggled across borders in small batches.
Although marketed as an alternative to LSD, a psychoactive drug well studied and known to have few adverse effects, few studies assess the pharmacologic and safety profiles of NBOMes in either animals or humans. Clinical manifestations, as described in previous case reports and this case series, reflect both sympathomimetic effects (hypertension, tachycardia, diaphoresis, psychomotor agitation, altered mental status, hyperthermia, and/or seizures) and serotonergic toxicity (altered mental status, hallucinations, tremulousness, muscle rigidity, rhabdomyolysis and subsequent renal failure, seizure, hyperthermia, and death).
Without published human safety data describing differences in the potency and toxicity attributable to each NBOMe, we cannot say that one variant is more dangerous or more likely to produce serotonin toxicity than another. Indeed, with a lack of quality controls in illicit drug synthesis, the actual chemical purity, composition, and concentration can vary substantially, even from one dose to the next. Laboratory confirmation helps to identify the presence of undisclosed NBOMe variants, contributing to the limited epidemiologic data of these highly potent new LSD substitutes.
Conclusion
Use of either 25C-NBOMe or 25B-NBOMe can produce similar serotonin toxidromes including seizures. Products containing these derivatives may be sold as 25I-NBOMe, and advanced laboratory analysis can identify the specific drug. This case series highlights both the potential harms of various NBOMes and the inconsistency between a product’s actual content and how it is sold.
Acknowledgments
Sources of Funding
Laboratory analysis was supported in part by the National Institutes of Health (NIH) Center grant P30DA033934.
Conflict of Interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Previous Presentation of Data
Laskowski LK, Calvo J, Exantus-Bernard G, Fong J, Poklis JL, Poklis A, Nelson L. NBOMes Lost in Translation: What You See is Not What You Get [abstract.] NACCT 2014.
References
- 1.Zuba D, Sekuła K, Buczek A. 25C-NBOMe–new potent hallucinogenic substance identified on the drug market. Forensic Sci Int. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 2.Nikolaou P, Papoutsis I, Stefanidou M, Spiliopoulou C, Athanaselis S. 2C-I-NBOMe, an “N-bomb” that kills with “Smiles.” toxicological and legislative aspects. Drug Chem Toxicol. 2014 doi: 10.3109/01480545.2014.911882. [DOI] [PubMed] [Google Scholar]
- 3.Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): a high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg Med Chem. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earth & Fire Erowid. Spotlight on NBOMes – Potent Erowid Extracts. 2013;24:1–8.
- 5.Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(6.51) and Phe340(6.52) with superpotent N-benzyl phenethylamine agonists. Mol Pharmacol. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- 6.Poklis JL, Clay DJ, Poklis A. High-performance liquid chromatography with tandem mass spectrometry for the determination of nine hallucinogenic 25-NBOMe designer drugs in urine specimens. J Anal Toxicol. 2014;38:113–21. doi: 10.1093/jat/bku005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly A, Eisenga B, Riley B, Judge B. 2012 annual meeting of the North American congress of clinical toxicology (NACCT) October 1–6, 2012 Las Vegas, NV, USA. Case series of 25I-NBOMe exposures with laboratory confirmation. Clin Toxicol. 2012;50:702. [Google Scholar]
- 8.Rose SR, Cumpston KL, Stromberg PE, Wills BK. 2012 annual meeting of the North American congress of clinical toxicology (NACCT) October 1–6, 2012 Las Vegas, NV, USA. Severe poisoning following self-reported use of 25-I, a novel substituted amphetamine. Clin Toxicol. 2012;50:707–708. [Google Scholar]
- 9.Mowry JB, Spyker DA, Cantilena LR, Bailey JE, Ford M. 2012 annual report of the American association of poison control centers’ national poison data system (NPDS): 30th annual report. Clin Toxicol. 2013;51:949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 10.Poklis JL, Charles J, Wolf CE, Poklis A. High-performance liquid chromatography tandem mass spectrometry method for the determination of 2CC-NBOMe and 25I-NBOMe in human serum. Biomed Chromatogr. 2013;27:1794–1800. doi: 10.1002/bmc.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]