Abstract
Alzheimer's disease (AD) is a highly prevalent disorder for which there are no effective therapies. Accumulation of amyloid β (Aβ) peptides in the brain is associated with impaired cognition and memory, pronounced inflammatory dysregulation, and subsequent amyloid plaque deposition. Thus, drugs that promote the clearance of Aβ peptides and resolution of inflammation may represent viable therapeutic approaches. Agonists of nuclear receptors LXR:RXR and PPAR:RXR act to ameliorate AD-related cognitive impairment and amyloid accumulation in murine models of AD. The use of an agonist to the nuclear receptor RXR, bexarotene, as monotherapy against AD, presents potential challenges due to the metabolic perturbations it induces in the periphery, most prominently hypertriglyceridemia. We report that the ω-3 fatty acid docosahexaenoic acid (DHA), in combination with bexarotene, enhances LXR:RXR target gene expression of Abca1 and ApoE, reduces soluble forms of Aβ, and abrogates release of pro-inflammatory cytokines and mediators both in vitro and in a mouse model of AD. Moreover, DHA abrogates bexarotene-induced hypertriglyceridemia in vivo. Importantly, dual therapy promotes reductions in AD pathology and resultant amelioration of cognitive deficits. While monotherapy with either bexarotene or DHA resulted in modest effects in vitro and in vivo, combined treatment with both agents produced a significant additive benefit on associated AD-related phenotypes, suggesting that targeted combinatorial agents may be beneficial over single agents alone in treating AD.
Keywords: 5XFAD, ABCA1, ApoE, bexarotene, inflammation, omega-3 fatty acids
Introduction
The most common form of AD occurs sporadically later in life and is characterized by cognitive decline and memory loss. These deficits accompany the impaired clearance of soluble forms of amyloid β (Aβ) peptides, resulting in their accumulation and formation of extracellular plaques. Accumulation of Aβ peptides not only promotes perturbations in neuronal functioning (Mucke and Selkoe, 2012), but also drives secretion of potent pro-inflammatory mediators and cytokines from reactive astrocytes and microglia in the brain (McGeer and McGeer, 2003; Reed-Geaghan et al., 2009).
Apolipoprotein E (ApoE) acts to scaffold the formation of high-density lipoproteins (HDLs), which are responsible for cholesterol and phospholipid trafficking within the brain. Reverse cholesterol transport (RCT) occurs when the lipid transporter Abca1 transfers cholesterol and phospholipids to secreted ApoE to create ApoE-containing HDLs. RCT is under the transcriptional control of liver-X receptor (LXR) and peroxisome-proliferator receptor gamma (PPARγ), which each form obligate heterodimer pairs with retinoid-X receptor (RXR) (Dawson and Xia, 2012). Nuclear receptor agonists targeting PPARγ, RXR, and LXR induce robust expression of Abca1 to form ApoE-HDL particles. Importantly, ApoE-based HDLs promote the clearance of soluble species of Aβ and improved cognition in various mouse models of AD (Jiang et al., 2008; Cramer et al., 2012; Mandrekar-Colucci et al., 2012). Nuclear receptors can also exert potent anti-inflammatory effects through transrepression of pro-inflammatory gene expression (Glass and Saijo, 2010).
The RXR agonist bexarotene has been shown to have salutary effects in mouse models of AD (Cramer et al., 2012; Fitz et al., 2013; Tesseur et al., 2013; Boehm-Cagan and Michaelson, 2014). However, therapeutic use of this drug is accompanied by elevation of plasma triglycerides (Assaf et al., 2006), and this poses a challenge to the implementation of it as a therapy for AD. The ω-3 fatty acid docosahexaenoic acid (DHA) acts to suppress plasma triglyceride levels in both humans (Bernstein et al., 2012) and mice (Sekiya et al., 2003). DHA is the most abundant fatty acid in the brain, and its concentration is significantly lower in AD patients (Lukiw, 2005) versus healthy controls. Importantly, DHA also acts as a nuclear receptor agonist that activates the nuclear receptors RXR (de Urquiza et al., 2000; Lengqvist et al., 2004) and PPARγ (Calder, 2015). Significantly, dietary supplementation with DHA has been effective at decreasing AD-related pathology and behavioral deficits in mouse models (Lim et al., 2005; Perez et al., 2010). DHA also exerts anti-inflammatory effects through activation of PPARs (Wahli and Michalik, 2012; Calder, 2015).
Since DHA can act as a nuclear receptor agonist and can lower triglycerides, we reasoned that coadministration of bexarotene with DHA may provide additional advantages over single agents alone. DHA may enhance both Abca1 and ApoE gene expression and reduce inflammation due to its action on nuclear receptors in the brain. We show here that combinatorial administration of bexarotene and DHA promotes induction of Abca1 and ApoE gene expression, reductions in amyloid pathology and inflammation, and improvements in working memory in the 5XFAD mouse model of AD.
Materials and Methods
Reagents.
DHA, LPS (from Escherichia coli strain 0111:B4), and modified Griess reagent were purchased from Sigma. Aβ1–40 and 1–42 was purchased from American Peptide. Mouse IL-6 and mouse TNFα cytokine ELISA kits were purchased from BD Bioscience.
Mice.
Female 5XFAD mice (B6SJL-Tg(APPSwFlLon, PSEN1*M146L*L286V)6799Vas) aged 4 months and nontransgenic (WT) littermates on the same background were used for all animal experiments (Oakley et al., 2006) with five or more mice randomly divided into treatment groups. Male (M) and female (F) C57BL/6 mice aged 4–6 months were used for plasma triglyceride analysis (N = 12 vehicle-treated mice, with 5M and 7F; N = 7 bexarotene-treated mice, with 2M and 5F; N = 7 DHA-treated mice, with 4M and 3F; and N = 4 dual-treated mice, with 2M and 2F) and liver protein analysis. All animals had access to food and water ad libitum and were placed on a 12 h light/dark cycle. All animal treatments and experimental protocols were approved by the Case Western Reserve University School of Medicine's Institutional Animal Care and Use Committee.
In vivo drug administration.
Bexarotene (Targretin; Valaent Pharmaceuticals) was administered at 25 mg/kg by dispersing the contents of the capsule in water to preserve the microcrystalline drug formulation. DHA was delivered at 200 mg/kg in water. Dual formulations were delivered in a single oral gavage. Both drugs were delivered by oral gavage, and water alone served as vehicle treatment. All drugs were administered daily for 7 d.
Aβ extraction, Aβ, and cytokine ELISAs.
Aβ species were extracted from brain homogenates as previously described (Mandrekar-Colucci et al., 2012). Briefly, equal volumes of 0.4% diethylamine (DEA) to brain homogenate were subjected to ultraspeed centrifugation, and, after neutralization, supernatant was collected and used to analyze soluble Aβ species and soluble proteins. The remaining pellet was then dissolved in formic acid (FA), subjected again to ultraspeed centrifugation, and, after neutralization, the supernatant was collected and used to analyze insoluble Aβ species. ELISAs of soluble and insoluble Aβ were captured using 6E10 and detected using either Aβ1–40 or Aβ1–42 conjugated to HRP (Covance) and normalized to total protein levels. Cytokine ELISAs against mouse IL-6 and TNFα were performed using conditioned media from primary microglia according to manufacturer's instructions.
Primary astrocyte and microglia isolation and BV-2 cell culture.
Primary astrocyte and microglia cells were isolated as previously described (Mandrekar-Colucci et al., 2012). In brief, brain tissue from C57BL/6 neonatal mice aged P0–P3 was minced, dissociated with trypsin, and cells were plated in 10% heat-inactivated FBS DMEM/F12 media and maintained at 37°C for ∼2–3 weeks. Both astrocytes and adherent microglia were isolated by sequential trypsin dissociation (with nonadherent microglia combined with adherent population). To remove contaminating microglia from astrocytes, media was aspirated ∼10 min after initial plating of astrocytes and replaced with new serum-containing DMEM/F12 media. The BV-2 murine-transformed microglia cell line was maintained in low-serum containing DMEM media and experiments were performed on cells that had been maintained for no more than 10 passages.
In vitro drug treatment, LPS stimulation, and Griess assay.
Primary astrocytes or microglia were seeded in 10% FBS-containing DMEM/F12 medium overnight. The following day, cell media was replaced with serum-free DMEM/F12 for at least 24 h before performing experiments. Cells were treated with bexarotene, DHA, the combination, or DMSO as vehicle and incubated for 18 h for LXR target gene analysis. For cytokine ELISA measurements microglia were seeded at 2.0 × 105 cells/ml, pretreated with drugs for 1 h, stimulated with 100 ng/ml LPS, and media was collected 6 h after LPS stimulation to assess for cytokine secretion by ELISA. In the case of nitrite production, BV-2 microglia were stimulated with 1.0 μg/ml LPS for 18 h after 1 h pretreatment with drugs, media collected, added in 1:1 volume ratio to modified Griess reagent, wavelength read at 550 nm, and nitrite concentration extrapolated to a sodium nitrite standard curve.
Immunoblotting and native gel electrophoresis.
Protein extracted from cells, cortical and hippocampal, and liver homogenates were resolved on 4–12% Bis-Tris gels (Life Technologies) and transferred to PVDF membranes. Immunoblotting was performed with the following antibodies and dilutions: ApoE (1:2000; Santa Cruz Biotechnology), β-actin (1:10,000; Santa Cruz Biotechnology), Abca1 (1:2500; Novus Biotechnology), SREBP1 (1:5000; Novus Biotechnology), and GAPDH (1:10,000; Santa Cruz Biotechnology). Lipidated ApoE-HDL particles were resolved from astrocyte-conditioned media 24 h after drug treatment or brain homogenates on 4–12% Tris-glycine (Life Technologies) under nondenaturing native conditions. All immunoblots were quantified using the software ImageJ (NIH).
RNA isolation, cDNA synthesis, pre-amplification, and quantitative, real-time RT-PCR.
Total RNA was collected from homogenized brains using chloroform and PureLink RNA Mini Columns (Ambion) following manufacturer's instructions. Contaminating genomic DNA was removed using DNase on the PureLink column. Equal concentrations of RNA were reverse transcribed to cDNA using the High Capacity RNA to cDNA conversion kit (Life Sciences) following manufacturer's instructions. Desired targets in brain homogenates were pre-amplified using the TaqMan PreAmp Master Mix (Applied Biosystems) for 14 cycles, and resultant cDNA was diluted and used as a template for quantitative, real-time RT-PCR using StepOne Plus Real-Time PCR system using TaqMan probes with sequences available on request. Data were analyzed using the comparative Ct method (gene expression represented as relative mRNA to housekeeping gene 18S normalized to wild-type calibrator) and statistics were performed at the delta Ct values (Mandrekar-Colucci et al., 2012).
Immunofluorescence and Aβ plaque analysis.
Frozen, fixed brain hemispheres were processed on a cryostat (Leica) into 10 μm sagittal sections onto slides, and cortical and subicular immunostaining quantifications were performed on six sections per animal on proximally and distally matched hippocampal and cortical regions with respect to the midline. Hippocampal quantifications were performed on four sections per animal. Dense-core plaques were visualized with thioflavin S (ThioS; Sigma) and propidium iodide served as the counterstain. After heat-induced antigen retrieval using citric acid, diffuse plaques were visualized using immunofluorescence staining with 6E10 (Covance) with DAPI as counterstain. The number of ThioS-positive plaques and percentage of 6E10 plaque area were analyzed in a blinded manner. Hippocampal and subicular 6E10 and ThioS plaques were normalized to an area measured in square millimeters.
Plasma triglyceride assay.
Whole blood was collected via cardiac puncture from an anesthetized mouse with sodium citrate as anticoagulant. Blood was centrifuged at 1000 × g for 20 min and plasma was collected. Plasma triglycerides were measured using the Triglyceride Colorimetric Assay Kit (Cayman Chemical) according to the manufacturer's instructions and expressed as mg/dl.
Y-maze spontaneous alternation task.
Working memory was assessed with the Y-maze as described previously (He et al., 2014). In brief, mice were placed in the center of the Y-maze and allowed to freely explore each arm for 5 min. Number of entrances and number of triad completions were recorded, and percentage of spontaneous alternation was tabulated with the following formula: (# of triads)/(# of entrances − 2) × 100. An entrance was scored when four limbs were placed into an arm, and a triad consisted of consecutive entrances into each arm.
Statistics.
Where appropriate, multiple groups were analyzed by ANOVA followed by a post hoc test defined in the figure legends. Otherwise, pairwise comparisons between groups were made using the two-tailed Student's t test. Statistics were performed using GraphPad Prism 5 with a p <0.05 considered significant. All error bars represent SEM.
Results
Nuclear receptor agonists promote Abca1 and ApoE gene expression and suppress the release of pro-inflammatory cytokines and mediators in vitro
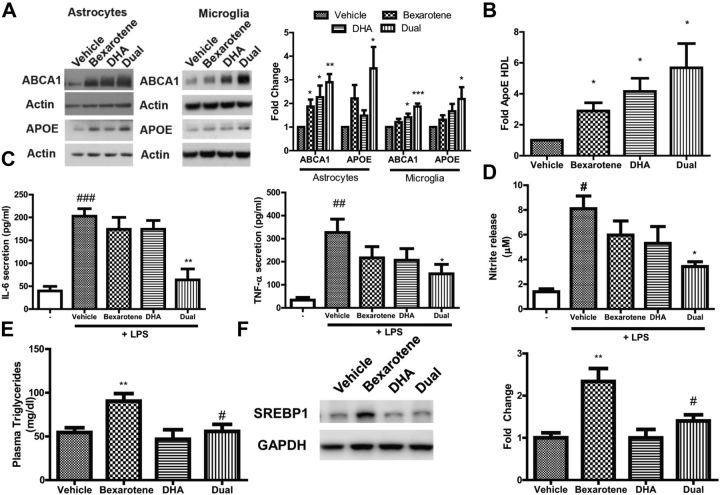
To ascertain whether DHA alone, and in combination with bexarotene, would induce reverse cholesterol transport genes, we treated primary astrocytes and microglia and measured protein expression of Abca1 and ApoE, the LXR target genes involved in RCT. Dose curves were performed with each drug to find dosages that induced maximal expression of LXR target genes (data not shown). We found that the indicated dosages of bexarotene and DHA alone induced expression in Abca1 and ApoE protein in both astrocytes and microglia, and the combined treatment with both bexarotene and DHA promoted significant increases in Abca1 and ApoE greater than vehicle alone when tested in both astrocytes and in microglia (Fig. 1A). As one of Abca1's chief functions is to transfer phospholipids and cholesterol to ApoE, we observed significant increases in the formation of lipidated ApoE-HDL particles in astrocyte-conditioned medium with bexarotene, DHA, and the combination (Fig. 1B).
Figure 1.
DHA augments bexarotene-induced Abca1 and ApoE gene expression, ApoE-HDL secretion, reduction of pro-inflammatory cytokines and mediator release in vitro and suppresses bexarotene-induced hypertriglyceridemia in vivo. A, Primary astrocytes (left) and microglia (right) were treated with either bexarotene, DHA, or both (dual) agents for 24 h, and whole-cell lysate was subjected to Western analysis for reverse-cholesterol genes ABCA1 (upper) and ApoE (lower). Representative immunoblots are shown with actin serving as a loading control. Quantitative protein expression for both astrocytes and microglia is shown as fold change relative to vehicle to the right. B, Quantification of secreted ApoE-HDL particles in astrocyte-conditioned media after 24 h of treatment with indicated drugs. A, B, *p < 0.05; **p < 0.01; ***p < 0.001; one-sample t test respective to vehicle and N = 6–8 independent experiments. C, Primary microglia cytokine ELISA for secreted IL-6 and TNFα. D, Measure of nitrite release into media using the Griess assay following 1 h drug pretreatment and then 18 h of exposure to LPS in BV-2 microglia cells. C, D, #p < 0.05, ##p < 0.01, ###p < 0.001 with respect to untreated cells (represented as -) and *p < 0.05, **p < 0.01, ***p < 0.001 with respect to LPS-stimulated, vehicle-treated cells as measured by one-way ANOVA with Dunnett's post hoc test for multiple comparisons. N = 3–5 independent experiments for C and D. For astrocytes and BV-2 microglia, 1 nm bexarotene, 5 μm DHA, and both dosages were used for all experiments; for primary microglia, 0.1 nm bexarotene, 1 μm DHA, and both dosages were used for all experiments. E, Plasma triglycerides from C57BL/6 mice. F, Whole-liver homogenates analyzed for SREBP1 protein expression via immunoblot with quantification to the right. GAPDH served as loading control. E, F, N ≥ 4 mice per group and **p < 0.01 to vehicle and #p < 0.05 to bexarotene, as measured by one-way ANOVA with Dunnett's post hoc test for multiple comparisons.
DHA is reported to exhibit anti-inflammatory effects, and this effect may be due to its ability to activate nuclear receptors (Wahli and Michalik, 2012; Calder, 2015). The anti-inflammatory potential of RXR agonist bexarotene in combination with DHA in primary microglia has not been explored. To test this, we used lipopolysaccharide (LPS), a component of the cell wall from gram-negative bacteria, which engages Toll-like receptor 4 (TLR4) receptors to promote rapid production of pro-inflammatory cytokines and mediators. We tested whether bexarotene, DHA, or the combination could reduce release of IL-6 and TNFα in the presence of LPS in primary microglia.
We found that untreated cells released only low levels of IL-6 and TNFα, as measured by ELISA in the culture media, but LPS stimulated robust and significant release of these cytokines into the media (Fig. 1C) after only 6 h. Pretreatment with bexarotene or DHA alone did not significantly inhibit release of LPS-induced IL-6 and TNFα, but combinatorial pretreatment resulted in a statistically significant reduction in release of both IL-6 and TNFα in microglia (Fig. 1C). Additionally, pretreatment with combinatorial bexarotene and DHA significantly reduced the release of nitrite by LPS in BV-2 microglia cells as measured using the Griess assay (Fig. 1D). These data demonstrate that while single agents alone have minimal effect of dampening of LPS-induced cytokine or nitrite release, combinatorial application of bexarotene and DHA effectively and rapidly decrease inflammatory cytokine and mediator release in primary microglia. Additionally, bexarotene and DHA both drive RCT gene production and ApoE-HDL formation in astrocytes and microglia.
DHA treatment ameliorates bexarotene-induced hypertriglyceridemia
Bexarotene induces hypertriglyceridemia in part through activating LXR-target genes involved in lipogenesis, most prominently sterol regulatory element-binding protein 1c (SREBP1c; Lalloyer et al., 2006). DHA has been shown to inhibit activation of SREBP and thus decrease de novo triglyceride synthesis in the liver (Georgiadi and Kersten, 2012). We therefore treated wild-type C57BL/6 mice for 7 d with either vehicle, 25 mg/kg bexarotene, 200 mg/kg DHA, or the combination. Plasma triglycerides were significantly induced in the bexarotene-treated mice (Fig. 1E), but this increase in triglyceride levels was significantly reduced to vehicle levels on cotreatment of DHA (Fig. 1E). DHA alone did not change plasma triglycerides. Bexarotene additionally drove SREBP1 protein expression in the liver, but upon addition of DHA, SREBP1 levels were significantly decreased to vehicle-treated levels (Fig. 1F). Therefore, we conclude that DHA is sufficient to reduce bexarotene-induced hypertriglyceridemia, most likely through inhibition of hepatic SREBP1 protein expression. While bexarotene at 100 mg/kg significantly elevated plasma triglycerides, DHA was unable to suppress plasma triglyceride levels to that of vehicle-treated mice when administered bexarotene (data not shown). Thus, we reasoned that a lower bexarotene dosage (25 mg/kg) would be most beneficial when combined with DHA to normalize plasma triglyceride levels.
Nuclear receptor agonists additively promote RCT gene expression, lipidation of ApoE, and reduction of soluble Aβ1–42 levels in vivo
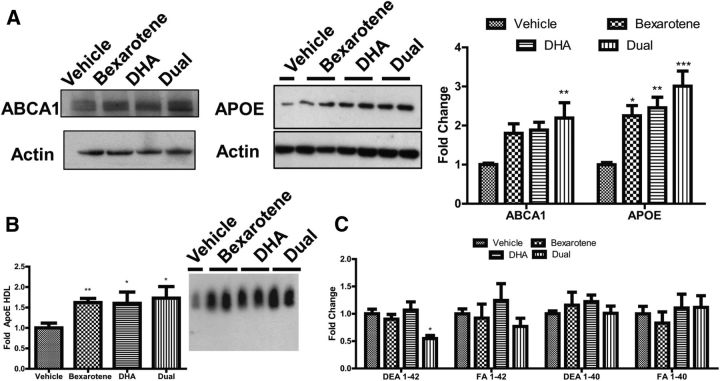
To assess the combinatorial effect of bexarotene and DHA on RCT gene expression, we treated 4-month-old female 5XFAD mice with bexarotene (25 mg/kg), DHA (200 mg/kg), or the combination by oral gavage for 7 d. In these experiments, we administered bexarotene at a dose substantially lower than that used in previous studies. Analysis of brain homogenates revealed that bexarotene and DHA alone promoted significant increases in ApoE protein over vehicle-treated mice, with modest, but not significant, increases in Abca1 protein expression (Fig. 2A). Combination of bexarotene and DHA, however, significantly increased Abca1 and ApoE protein expression in 5XFAD mice versus vehicle-treated animals, similar to our in vitro data with primary astrocytes and microglia (Fig. 1A). In accordance with increased expression of Abca1 protein levels, significantly elevated levels of lipidated ApoE-HDL particles were observed in brain homogenates of 5XFAD treated with bexarotene, DHA, and the combination (Fig. 2B). We evaluated the levels of soluble, DEA-extracted Aβ1–42 in brain homogenates by ELISA and found significant reductions in animals treated with both agents only over vehicle alone (Fig. 2C). No changes were observed in other Aβ species (Fig. 2C). Thus, dual nuclear receptor agonists significantly upregulate LXR target genes Abca1 and ApoE, ApoE-lipidation, and decrease soluble Aβ1–42 levels in the 5XFAD mouse model of AD.
Figure 2.
Short-term bexarotene and DHA combinatorial treatment drive LXR target gene expression, ApoE lipidation, and degradation of soluble Aβ1–42 in 5XFAD mice. Female 5XFAD mice aged 4 months were gavaged daily with bexarotene (25 mg/kg), DHA (200 mg/kg), the combination (dual), or water (vehicle) for 7 d. A, Representative immunoblots on brain lysates for LXR target genes ABCA1 and APOE (left) with quantification of protein induction (right). B, Quantification of ApoE-HDL particles in brain lysates from treated mice, with representative native gel blot. C, ELISA of soluble (DEA) and insoluble (FA) Aβ 1–40 and Aβ 1–42 in brain lysates from treated mice. B, *p < 0.05, **p < 0.01, Student's t test to vehicle. A, C, *p < 0.05, **p < 0.01, ***p < 0.001 with respect to vehicle-treated mice as measured by one-way ANOVA with Tukey post hoc test for multiple comparisons. A–C, N ≥ 5 animals per group.
Dual nuclear receptor agonists reduce cortical, dense-core plaque burden in vivo
To investigate whether the single agents bexarotene and DHA, or the combination, affected amyloid plaque burden in vivo, we performed immunofluorescence for dense-core plaques using ThioS and the combination of dense, diffuse, and intraneuronal plaques using 6E10 in the cortex, hippocampus, and subiculum of the 5XFAD mice. DHA treatment alone, and the dual treatment with bexarotene, significantly reduced cortical, dense-core ThioS-positive plaques, but total 6E10 immunoreactivity was unchanged (Fig. 3A) and there was a trend toward a reduction with the dual treatment (p = 0.12). Hippocampal and subicular ThioS-positive and 6E10 plaque burdens were unchanged in all treatment groups, leading us to conclude that cortical, dense-core ThioS-positive plaques are most affected by DHA alone and the combination of DHA and bexarotene.
Figure 3.
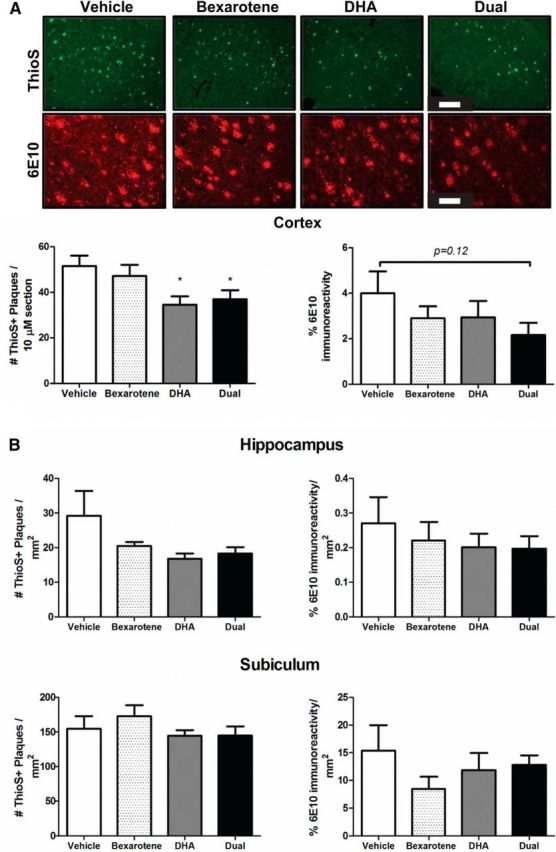
Combinatorial nuclear receptor treatment reduces cortical, dense-core ThioS plaques in vivo. A, Representative cortical images of ThioS and 6E10 staining with quantification directly below. Scale bars: ThioS, 200 μm; 6E10, 100 μm. B, Quantification of ThioS+ plaques and total percentage of 6E10 immunoreactivity in the hippocampus and subiculum normalized to area in square millimeters. *p < 0.05 compared with vehicle-treated mice as measured by Student's t test. N ≥ 5 mice per group.
Single agents alone or in combination reduce inflammation in vivo
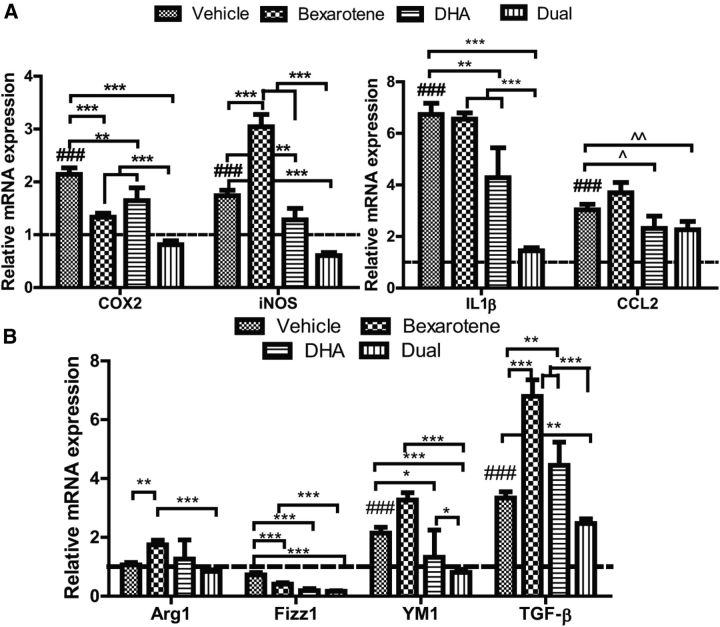
Since the combinatorial treatment with DHA and a nuclear receptor agonist reduced LPS-driven inflammatory mediators and cytokine release in vitro, we wished to determine whether reductions in inflammation were evident in vivo. RNA extracted from brains of 5XFAD mice revealed that relative mRNA expression of pro-inflammatory markers COX2 and iNOS was significantly increased in transgenic vehicle-treated mice versus wild-type mice; both bexarotene and DHA promoted significant decreases in relative mRNA in COX2 expression, with combination treatment resulting in reductions in COX2 expression better than single agents alone (Fig. 4A). While bexarotene drove iNOS expression, combinatorial treatment appeared to reduce iNOS expression significantly to levels lower than both vehicle and either drug treatment alone (Fig. 4A). For both IL-1β and CCL2, dual treatment significantly decreased relative mRNA expression versus vehicle-treated animals alone (Fig. 4A). Additionally, analyses of anti-inflammatory markers Arg1, Fizz1, YM-1, and TGF-β at the mRNA level revealed that levels of Fizz1, YM-1, and TGF-β in the dual-treated mice were significantly decreased compared with vehicle-treated mice. Single agents increased certain markers (Arg1 for bexarotene alone, and TGF-β for both drugs), with the exception of Fizz1, which was decreased with both bexarotene and DHA alone. These data demonstrate that nuclear receptor agonists are effective at decreasing both pro-inflammatory and certain anti-inflammatory genes in vivo.
Figure 4.
Two-agent combination bexarotene and DHA decrease pro-inflammatory and anti-inflammatory mRNA transcription in 5XFAD mice. A, mRNA collected from 5XFAD brain homogenates was evaluated for relative expression of pro-inflammatory markers COX2 and iNOS and the cytokines IL-1β and CCL2. B, mRNA collected was also evaluated for anti-inflammatory markers Arg1, Fizz1, YM-1, and TGF-β. All mRNA values are relative to housekeeping gene 18S and normalized to wild-type mRNA levels (indicated by dashed line). A, B, *p < 0.05, **p < 0.01, ***p < 0.001 indicated by brackets and ###p < 0.001 with respect to wild-type as measured by Tukey post hoc ANOVA for multiple comparisons. ∧p < 0.05 and ∧∧p < 0.01 indicated by brackets as measured by Student's t test. N ≥ 7 mice per treatment group.
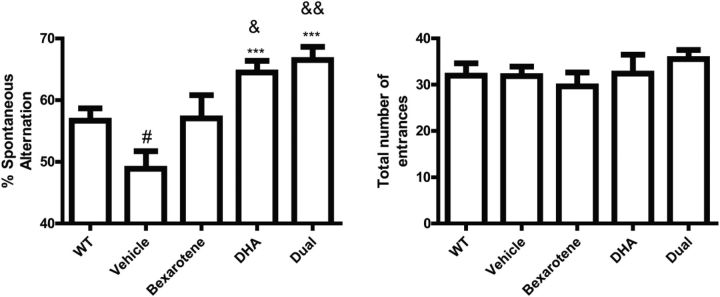
Combinatorial bexarotene and DHA restores working memory
The 5XFAD mouse model exhibits impaired working memory in the Y-maze task at ∼4 months of age (Ohno et al., 2007). To investigate the possible effects of nuclear receptor agonists on working memory, we evaluated the behavior of the 5XFAD mice treated for 7 d with bexarotene, DHA, or the combination in the Y-maze. We observed a transgenic effect of diminished working memory (Fig. 5), an effect that was similar to previous published literature (Ohno et al., 2007). Treatment with bexarotene alone did not significantly (p = 0.09) increase working memory, but DHA treatment alone and the combination resulted in a significant improvement in memory compared with the vehicle-treated mice (Fig. 5). Additionally, both DHA alone and combinatorial treatment improved working memory to a greater extent than nontransgenic counterparts. The number of entrances for each treatment group was unchanged, suggesting that the effect on working memory due to drug treatment was not because of increases in hyperactivity alone. These data suggest that DHA alone and DHA in combination with bexarotene ameliorate deficits in working memory in the 5XFAD mouse model.
Figure 5.
Combinatorial RXR agonists improve working memory in 5XFAD mice. Percentage of mice performing a correct spontaneous alternation (left) and total number of entrances made (right). #p < 0.05 with respect to WT mice and **p < 0.01 and ***p < 0.001 with respect to vehicle-treated mice as measured by Student's t test. &p < 0.05 and &&p < 0.01 with respect to WT mice as measured by Student's t test. No statistical difference was observed for number of entrances. N ≥ 8 mice/treatment group.
Discussion
Our study aimed to determine whether combinatorial treatment with the nuclear receptor agonists bexarotene and DHA would be effective at reducing inflammation and increasing RCT transport genes to promote soluble Aβ reductions both in vitro and in vivo. To the best of our knowledge, this is the first description of the effects of nuclear receptor agonist bexarotene and the ω-3 fatty acid DHA on amyloid pathology and inflammation both in vitro and in vivo. The 5XFAD mouse overexpresses both human APP and presenilin1 (PS1) genes, collectively harboring five mutations associated with familial AD. We chose the 5XFAD mouse model because Aβ production from APP processing is predominantly skewed due to Florida and London mutations on the PS1 gene, which encode the γ-secretase enzyme, resulting in rapid and massive accumulations of the Aβ1–42 isoform in the brain and concomitant deficits in working memory by 4 months of age (Oakley et al., 2006; Ohno et al., 2007). Due to robust expression of Aβ and its engagement of TLRs and their coreceptors (among others; Reed-Geaghan et al., 2009; Stewart et al., 2010), the 5XFAD mice exhibit a robust inflammatory response in the brain, allowing evaluation of heretofore unexplored anti-inflammatory effects of nuclear receptor agonists.
Considerable attention has focused on the effects of RXR-agonist bexarotene in mouse models of AD; in particular, the inability of other laboratories to reduce plaque burden has generated controversy (Fitz et al., 2013; Price et al., 2013; Tesseur et al., 2013; Veeraraghavalu et al., 2013). While we did observe reductions in plaque burden with DHA, bexarotene alone did not reduce plaque burden in the current study (Fig. 3). This discrepancy may be due to a lower bexarotene dosage (25 mg/kg) and a different mouse model in the present study. Indeed, 100 mg/kg bexarotene drives phagocytosis of fibrillar Aβ in vivo (Savage et al., 2015), but 25 mg/kg/d bexarotene may be insufficient to drive genes involved in phagocytosis of fibrillar Aβ. While single agents significantly increased ApoE protein levels in 5XFAD mice, they did not significantly elevate Abca1 protein levels. However, treatment with both agents significantly drove Abca1 protein, lipidation of ApoE particles, and significant decreases in soluble Aβ1–42 in 5XFAD brains. We thus postulate that RCT gene expression requires a sufficient threshold of protein induction to induce efficient decreases in soluble Aβ1–42 that differ from bexarotene dosages reported previously (Cramer et al., 2012).
However, a significant decrease in cortical, dense-core ThioS-positive plaques was observed in DHA-alone and combination-treated 5XFAD mice (Fig. 3A). These effects in the 5XFAD mouse are consistent with previous literature reporting that AD mouse models supplemented with DHA-enriched diets displayed decreased plaque burden (Lim et al., 2005; Perez et al., 2010). While there are several key differences between the other studies and ours, we postulate that provision of DHA as a free fatty acid delivered by oral gavage in our study, either alone, and in combination with bexarotene, may promote clearance of dense-core plaques by catalyzing target genes of LXR and PPARγ involved in phagocytosis (Terwel et al., 2011; Yamanaka et al., 2012). Indeed, DHA has been shown to increase phagocytosis of Aβ1–42 and reduce inflammation in microglia (Hjorth et al., 2013); whether this was through nuclear receptors was unexplored.
The discrepancy between the robust decrease in soluble Aβ1–42 measured by ELISA (Fig. 2C) and 6E10 plaque burden in the dual-treated animals (Fig. 3) may be due to 6E10 detecting full-length and other fragments of APP, which are the principal species detected by 6E10 in 5XFAD mice (Youmans et al., 2012); thus, a difference between selective decreases in the Aβ1–42 isomer versus all forms of APP via immunohistochemistry may be difficult to discern and explain the discordance between our ELISA and plaque burden data (Fig. 3A). Nevertheless, there was an overall, though statistically insignificant (p = 0.12), trend between vehicle and dual-treated 6E10 cortical levels. Additionally, ELISAs were performed on combined cortical and hippocampal homogenates, thus precluding the ability for the ELISA to detect changes that may be restricted to cortical and/or hippocampal areas alone.
The 5XFAD mice exhibit neuronal loss in the subiculum that is apparent between 2 and 4 months of age (Moon et al., 2012). A preliminary assessment of neuronal number did not reveal any significant changes compared with vehicle-treated mice (data not shown), suggesting that treatment at 4 months of age is insufficient to rescue remaining viable subicular neurons. Plaque burden in either the hippocampus or subiculum was unchanged with either treatment group (Fig. 3B), leading us to conclude that dense-core cortical plaques are most susceptible to dual nuclear receptor treatment.
Importantly, DHA reversed bexarotene-induced hypertriglyceridemia (Fig. 1E), a side effect that greatly diminishes the practicality of administering bexarotene alone to an elderly population. We postulate that this is most likely due to inhibition of SREBP1 protein in the liver (Fig. 1F), which increases de novo fatty-acid synthesis, and this finding is consistent with ω-3 fatty acid's ability to protect against hepatic steatosis due to LXR agonists' upregulation of SREBP1 (Sekiya et al., 2003; Jung et al., 2011; Georgiadi and Kersten, 2012). DHA exerts pleiotropic effects, most likely due to its ability to act broadly at many receptors, but this is the first study to our knowledge reporting that DHA increases RCT gene expression in vitro and in the brain of an AD mouse model. In particular, the anti-inflammatory action of DHA has been shown to be from the stimulation of the free fatty acid receptors Gpr120 and Gpr40 in macrophages (Oh et al., 2010; Yan et al., 2013). We did not observe significant differences in anti-inflammatory capacity of DHA alone in vitro (Fig. 1), suggesting that DHA may not work through Gpr120 or Gpr40 at the dosages we used. Indeed, previous studies concerning anti-inflammatory capacity of ω-3 fatty acids to signal through Gpr40/120 in vitro were between 20- and 100-fold higher than that used in the current study (Oh et al., 2010; Williams-Bey et al., 2014).
Upon ligand binding, nuclear receptors exert potent anti-inflammatory effects through transrepression of NF-κB-dependent genes—such as cytokines and pro-inflammatory enzymes (Glass and Saijo, 2010; Skerrett et al., 2014). Cotreatment with DHA and bexarotene significantly reduced release of pro-inflammatory cytokines in vitro (Fig. 1) and their transcription in the brains of 5XFAD mice treated for 7 d (Fig. 4A). At the mRNA level, significant reductions in COX-2, IL-1β, CCL2, and iNOS were observed with cotreatment with respect to vehicle-treated mice. Bexarotene induced iNOS at mRNA level, but single agents' actions on individual inflammatory markers may be inconsequential considering that dual agents tended to either normalize or even reduce inflammatory mRNA levels below vehicle-treated animals (Fig. 4A), suggesting that nuclear receptor agonists' regulation of inflammatory responses in vivo merits further study. Genes associated with “alternative activation” states, such as Fizz1, YM-1, and TGF-β, were significantly suppressed with both agents (Fig. 4B), suggesting that a suppression of pro-inflammatory markers is not from an elevation in anti-inflammatory markers. Collectively, these results suggest that combined nuclear receptors DHA and bexarotene effectively suppress inflammatory responses both in vitro and in vivo.
We observed a significant working memory deficit in 5XFAD mice in the Y-maze, consistent with previous literature (Oakley et al., 2006; Ohno et al., 2007). While bexarotene alone did not improve working memory, combination with DHA significantly enhanced it (Fig. 5). DHA alone significantly enhanced working memory, which may be unsurprising given the literature on DHA improving cognition in mouse models of AD (Calon et al., 2004; Hooijmans et al., 2009; Arsenault et al., 2011). While the previous literature on DHA proposes different mechanisms, such as improvement of neuronal function, we instead postulate that bexarotene and DHA enhance working memory combinatorially through effective reductions in soluble Aβ1–42 levels through modulation of genes Abca1 and ApoE and lipidated ApoE-HDL particles (Fig. 2). Indeed, soluble species of Aβ have been shown to be linked to impaired cognition, whereas there is a poor correlation of these measures with levels of deposited fibrillar Aβ (Cleary et al., 2005; Selkoe, 2008). The reductions in soluble species correlate with improved behavior in our previous studies with bexarotene (Cramer et al., 2012) with the 5XFAD mouse model requiring a sufficient threshold of Abca1 and induction of lipidated ApoE to promote degradation of Aβ1–42.
In conclusion, this study suggests that AD pathology can effectively be reversed with nuclear agonists, and this combinatorial approach could be harnessed as a potentially beneficial option if single agents fail to work alone in a clinically relevant setting. Additionally, ω-3 fatty acids may act in tandem with bexarotene to drive LXR target genes, decrease Aβ1–42 levels, reduce inflammation, and mitigate bexarotene-induced hypertriglyceridemia. Bexarotene is currently in early stage clinical trials for AD, and the present study may inform the design of subsequent trials.
Footnotes
This work was supported by a grant from the National Institutes of Health (R41-AG048658 to K.G.).
G.E.L. is a cofounder of ReXceptor, Inc., a biotechnology company developing RXR agonists for the treatment of neurodegenerative diseases. K.G. is an employee of ReXceptor, Inc.
References
- Arsenault D, Julien C, Tremblay C, Calon F. DHA Improves Cognition and Prevents Dysfunction of Entorhinal Cortex Neurons in 3xTg-AD Mice. PLoS One. 2011;6:e17397. doi: 10.1371/journal.pone.0017397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf C, Bagot M, Dummer R, Duvic M, Gniadecki R, Knobler R, Ranki A, Schwandt P, Whittaker S. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: an expert opinion. Br J Dermatol. 2006;155:261–266. doi: 10.1111/j.1365-2133.2006.07329.x. [DOI] [PubMed] [Google Scholar]
- Bernstein AM, Ding EL, Willett WC, Rimm EB. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J Nutr. 2012;142:99–104. doi: 10.3945/jn.111.148973. [DOI] [PubMed] [Google Scholar]
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta. 2012;1821:21–56. doi: 10.1016/j.bbalip.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Cronican AA, Lefterov I, Koldamova R. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.”. Science. 2013;340:924-c. doi: 10.1126/science.1235809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadi A, Kersten S. Mechanisms of gene regulation by fatty acids. Adv Nutr. 2012;3:127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang H, Yung A, Villeda SA, Jaeger PA, Olayiwola O, Fainberg N, Wyss-Coray T. ALK5-dependent TGF-β signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci. 2014;17:943–952. doi: 10.1038/nn.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund-Levi Y, Faxen-Irving G, Wahlund LO, Basun H, Eriksdotter M, Schultzberg M. Omega-3 fatty acids enhance phagocytosis of Alzheimer's disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J Alzheimers Dis. 2013;35:697–713. doi: 10.3233/JAD-130131. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lütjohann D, Heerschap A, Kiliaan AJ. DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis. 2009;33:482–498. doi: 10.1016/j.nbd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung UJ, Millman PN, Tall AR, Deckelbaum RJ. n-3 fatty acids ameliorate hepatic steatosis and dysfunction after LXR agonist ingestion in mice. Biochim Biophys Acta. 2011;1811:491–497. doi: 10.1016/j.bbalip.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalloyer F, Fiévet C, Lestavel S, Torpier G, van der Veen J, Touche V, Bultel S, Yous S, Kuipers F, Paumelle R, Fruchart JC, Staels B, Tailleux A. The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2006;26:2731–2737. doi: 10.1161/01.ATV.0000248101.93488.84. [DOI] [PubMed] [Google Scholar]
- Lengqvist J, Mata De Urquiza A, Bergman AC, Willson TM, Sjövall J, Perlmann T, Griffiths WJ. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer's disease. J Neurosci. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Inflammatory processes in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Moon M, Hong HS, Nam DW, Baik SH, Song H, Kook SY, Kim YS, Lee J, Mook-Jung I. Intracellular amyloid-beta accumulation in calcium-binding protein-deficient neurons leads to amyloid-beta plaque formation in animal model of Alzheimer's disease. J Alzheimers Dis. 2012;29:615–628. doi: 10.3233/JAD-2011-111778. [DOI] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 Is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Berg BM, Moore KA, He B, Counts SE, Fritz JJ, Hu YS, Lazarov O, Lah JJ, Mufson EJ. DHA diet reduces AD pathology in young APPswe/PS1ΔE9 transgenic mice: possible gender effects. J Neurosci Res. 2010;88:1026–1040. doi: 10.1002/jnr.22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AR, Xu G, Siemienski ZB, Smithson LA, Borchelt DR, Golde TE, Felsenstein KM. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.”. Science. 2013;340:924-d. doi: 10.1126/science.1234089. [DOI] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and Toll-like receptors 2 and 4 are required for fibrillar Aβ-stimulated microglial activation. J Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JC, Jay T, Goduni E, Quigley C, Mariani MM, Malm T, Ransohoff RM, Lamb BT, Landreth GE. Nuclear receptors license phagocytosis by Trem2+ myeloid cells in mouse models of Alzheimer's disease. J Neurosci. 2015;35:6532–6543. doi: 10.1523/JNEUROSCI.4586-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1053/jhep.2003.09028. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrett R, Malm T, Landreth G. Nuclear receptors in neurodegenerative diseases. Neurobiol Dis. 2014;72:104–116. doi: 10.1016/j.nbd.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Steffensen KR, Verghese PB, Kummer MP, Gustafsson JÅ, Holtzman DM, Heneka MT. Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ phagocytosis. J Neurosci. 2011;31:7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesseur I, Lo AC, Roberfroid A, Dietvorst S, Van Broeck B, Borgers M, Gijsen H, Moechars D, Mercken M, Kemp J, D'Hooge R, Strooper BD. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.”. Science. 2013;340:924-e. doi: 10.1126/science.1233937. [DOI] [PubMed] [Google Scholar]
- Veeraraghavalu K, Zhang C, Miller S, Hefendehl JK, Rajapaksha TW, Ulrich J, Jucker M, Holtzman DM, Tanzi RE, Vassar R, Sisodia SS. Comment on “ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models.”. Science. 2013;340:924-f. doi: 10.1126/science.1235505. [DOI] [PubMed] [Google Scholar]
- Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Williams-Bey Y, Boularan C, Vural A, Huang NN, Hwang IY, Shan-Shi C, Kehrl JH. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS One. 2014;9:e97957. doi: 10.1371/journal.pone.0097957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Kanekiyo T, Stine WB, Jr, Michon SC, Nwabuisi-Heath E, Manelli AM, Fu Y, Riordan S, Eimer WA, Binder L, Bu G, Yu C, Hartley DM, LaDu MJ. Intraneuronal Abeta detection in 5xFAD mice by a new Abeta-specific antibody. Mol Neurodegener. 2012;7:8. doi: 10.1186/1750-1326-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]