Abstract
One of the hallmarks of alcohol dependence is the presence of a withdrawal syndrome during abstinence, which manifests as physical craving for alcohol accompanied by subjective feelings of anxiety. Using a model of chronic intermittent ethanol (CIE) vapor in mice, we investigated the role of serotonin2c signaling in the BNST as a neural substrate underlying ethanol-induced anxiety during withdrawal. Mice were subjected to a 5-day CIE regimen of 16 hours of ethanol vapor exposure followed by an 8 hour “withdrawal” period between exposures. After the 5th and final exposure, mice were withdrawn for 24 hours or 1 week before experiments began. Anxiety-like behavior was assessed in the social approach, light dark, and open field test with mice showing deficits in social, but not general anxiety-like behavior that was alleviated by pretreatment with the 5HT2c-R antagonist SB 242,084 (3 mg/kg, i.p.) 24 hours and 1 week post-CIE. Using immunohistochemistry and whole cell patch clamp electrophysiology, we also found that CIE increased FOS-IR and enhanced neuronal excitability in the ventral BNST (vBNST) 24 hrs into withdrawal in a 5HT2c-R dependent manner. This enhanced excitability persisted for 1 week post-CIE. We also found that mCPP, a 5HT2c/b agonist, induced a more robust depolarization in cells of the vBNST in CIE mice, confirming that 5HT2c-R signaling is upregulated in the vBNST following CIE. Taken together, these results suggest that CIE upregulates 5HT2c-R signaling in the vBNST, leading to increased excitability. This enhanced excitability of the vBNST may drive increased anxiety-like behavior during ethanol withdrawal.
Keywords: Bed nucleus of stria terminalis, Serotonin, Ethanol, Anxiety, Withdrawal
1. Introduction
Chronic alcohol use can induce persistent changes in circuit function in the brain (Kash et al., 2006, Holmes et al., 2012), which may contribute to subjective feelings of stress and anxiety during alcohol withdrawal. The impact of anxiety on the developmental course of alcoholism has been well-documented in the literature and is especially prominent in Type II alcoholics, who use alcohol as a means of mitigating anxiety symptoms (Lesch and Walter, 1996; Bleich et al., 2004; Kiefer, 2005; Leggio et al., 2009). In preclinical studies of chronic ethanol exposure anxiety-like behaviors have been observed in a variety of behavioral paradigms, including the elevated plus maze, the open field, and the light-dark test (see Kliethermes 2005 for review). Recently, the social interaction test was utilized as an index of anxiety-like behavior in rats during chronic ethanol withdrawal, and this behavior was found to depend in part on serotonin receptor signaling in the limbic system (Overstreet et al., 2003; Knapp et al., 2004; Overstreet et al., 2006).
Alcohol dependence is associated with alterations in serotonin (5HT) systems in the brain, including increased expression of tryptophan hydroxylase (TPH) in the dorsal raphe (DR) and 5HT transporter (5HTT) binding sites in the amgydala and the paraventricular nucleus of the hypothalamus (PVN), which may contribute to anxiety-like states (Bonkale et al., 2006; Storvik et al., 2008). In particular, there is ample evidence to suggest that serotonin type 2 receptors (5HT2-Rs) may play a critical role in anxiety states arising from chronic stress and ethanol withdrawal in alcohol dependence disorders (Lal et al., 1993; Krystal et al., 1994). The 5HT2-R family includes 3 subtypes; 5HT2a, 2b and 2c, all of which are present in varying degrees in the brain (Roth et al., 1998). Our focus is on the 5HT2c receptor (5HT2c-R), a seven transmembrane G-protein coupled receptor (GPCR) that is distributed through the limbic system, where it modulates release of other neurotransmitters such as GABA and dopamine (Di Matteo et al., 2001). Activation of the 5HT2c-R is thought to contribute to the anxious features of several SSRI antidepressants (Ni and Miledi, 1997), whereas antagonism of these receptors may actually relieve dysphoria via disinhibition of dopamine signaling (Dremencov et al., 2005). In preclinical studies, mouse strains with a low anxiety phenotype tend to have reduced 5HT2c-R functionality resulting from post-transcriptional editing relative to other more anxiety-prone mouse strains (Englander et al., 2005). Indeed, anxiety is strongly associated with 5HT2c-R levels in the brain, as overexpression tends to increase anxiety (Kimura et al., 2009), whereas deletion of the 5HT2c receptor (5HT2c-R KO) results in a low anxiety phenotype relative to wild-type mice (Heisler et al, 2007). These findings are supported by pharmacological studies in rodents, which demonstrate that inhibitors of central 5HT2c-R function have anxiolytic effects (Wood et al., 2001), whereas 5HT2-R agonists (i.e. mCPP) potentiate anxiety in the social approach test (Mongeau et al., 2010).
Anxiety is a significant contributing factor to the negative reinforcing properties of ethanol during withdrawal, which was previously shown to be depending on 5HT2c-R signaling (Overstreet et al., 2003; Knapp et al., 2004; Overstreet et al., 2006). Both peripheral injection of 5HT2c-R antagonists and site-specific injection of 5HT2c-R inverse agonists into the central amygdala can mitigate withdrawal-induced anxiety, suggesting a potential site of action for the anxiogenic effects of 5HT2c-R signaling. Additional studies demonstrate that FOS immunoreactivity (FOS-IR) in response to an anxiogenic stimulus is reduced in both the central amygdala (CeA) and bed nucleus of stria terminalis (BNST) of 5HT2c-R knockout mice (Heisler et al., 2007), which implicates both structures in the pathophysiology of anxiety. The BNST is both highly interconnected with and functionally analogous to the CeA, and it’s particular role in anxiogenic states associated with withdrawal and relapse has been extensively characterized in the literature (Aston-Jones & Harris, 2004; Huang et al., 2010; Silberman et al., 2013). The BNST contains a high density of both 5HT2c-Rs (Pompeiano et al., 1994; Wright et al., 1995), which mediate excitatory postsynaptic effects in the anterolateral aspect (Guo et al, 2009), a region heavily populated with GABAergic and CRF neurons. The 5HT2c/b agonist mCPP also induces FOS-IR in the BNST, which is suggestive of increased neuronal activation (Singewald et al., 2003). Together, these data support a role for 5HT2c-Rs in the BNST in anxiogenesis.
The ethanol vapor inhalation model has been used to achieve ethanol intoxication in mice over a 14–16 hour period with blood ethanol concentrations stabilizing at 150–200 mg/dl (Melendez et al., 2012; Lopez et al., 2012; Kroener et al., 2012). Delivered in this fashion, CIE has been shown to elicit robust changes in physiology in the BNST (Kash et al., 2009; Szücs et al., 2012), the hippocampus (Roberto et al., 2002), the basolateral amygdala (Läck et al., 2007), the central amygdala (Roberto et al., 2006) and the mPFC (Holmes et al., 2012; Kroener et al., 2012). In this study, the ethanol vapor inhalation model was used to establish a pattern of chronic ethanol exposure, which is predicted to induce an anxious phenotype in DBA/2J mice. The goal of the present study was to investigate the hypothesis that withdrawal from chronic intermittent ethanol (CIE) exposure induces hyperactivity in the BNST via upregulation of 5HT2c-R signaling, which in turn precipitates anxiety. Using combined behavioral, immunohistochemical, and electrophysiological approaches, we assess activation and neuronal excitability in the vBNST.
2. Methods
2.1 Animals
Male DBA/2J mice were obtained from Jackson laboratories at 6 weeks of age and allowed to acclimate to the animal facility at the UNC Thurston-Bowles Center for 1 week with ad libitum access to food and water before experiments began.
2.2 Drugs
All drugs used in electrophysiological experiments (TTX, mCPP, RS 102221) were purchased from Tocris (Bristol, UK). TTX, and mCPP were dissolved in ddH2O at a stock concentration of 5 mM and 50 mM, respectively, and stored at −20°C. RS 102221 was dissolved to 100 mM in DMSO and stored at −20°C. On the day of the experiment, drugs were diluted to their final concentrations in normal aCSF. For behavioral and immunohistochemical experiments, SB 242,084 was purchased from Caymen Chemical (Ann Arbor, Michigan), suspended in 0.5% carboxymethylcellulose vehicle at a working concentration of 0.3 mg/ml, then injected at 3 mg/kg. This dose was chosen primarily based on results from Breese and colleagues indicating that pretreatment with 3 mg/kg SB 242,084 reduced withdrawal-induced anxiety in rats in the social interaction test (Overstreet et al., 2003) and voluntary ethanol consumption following withdrawal (Overstreet et al., 2007). This highly selective 5HT2c-R antagonist exhibits 158-fold selectivity over 5HT2a receptors and 100-fold selectivity over 5HT1b receptors. There is some evidence that SB 242,084 has locomotor activating effects that follow an inverted U shaped curve peaking at 1 mg/kg, but are not present at 3 mg/kg (Martin et al., 2002). Our use of the 3 mg/kg dose circumvents this potential confound.
2.3 Chronic Intermittent Ethanol (CIE)
The ethanol inhalation system used in our laboratory was designed by La Jolla Alcohol Research, Inc. (La Jolla, CA) to house mice in a controlled air flow chamber with dimensions of 27.5 cm length × 17 cm width × 13 cm height. Male mice between 7–9 weeks old were given daily injections of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg, i.p.) 30 minutes before ethanol exposure to stabilize blood ethanol concentration (BEC). Volatilized ethanol (95%) was generated by bubbling air through an air stone submerged in 95% ethanol solution at a flow rate sufficient to generate BECs in the range of 150–200 mg/dl in adult male mice. Pyrazole-treated mice were exposed to air (controls) or ethanol vapor for 16 hours per day for 5 days. All biochemical, electrophysiological and behavioral manipulations were carried out 24 hrs or 1 week after the end of the last exposure. This experimental design allowed us to probe the behavioral and physiological effects of acute and protracted withdrawal from CIE. The 24-hour time point was chosen to give the mice sufficient time to fully metabolize the ethanol but still capture the acute effects of ethanol withdrawal, such as handling induced convulsions (Metten and Crabbe, 2005). The 1 week time point was chosen to probe for persistent changes in function. In a subset of the behavioral studies, mice were injected with 3 mg/kg SB 242,084 or vehicle 1 hour prior to testing in the social approach test. For immunohistochemical studies, mice were injected 4 hours after removal from the chambers and then 3 hours before perfusion.
2.4 Electrophysiology
Mice were decapitated under isofluorane anesthesia 24 hours or 7 days after the last ethanol exposure. Brains were rapidly removed and immediately placed in a solution of ice-cold sucrose-artificial cerebrospinal fluid (aCSF) [(in mM): 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, and 26 NaHCO3 saturated with 95% O2/5% CO2. Brains were sectioned at 0.07 (mm/s) on a Leica 1200S vibratome to obtain 300 μm coronal slices of the BNST, which were incubated in a heated holding chamber containing normal, oxygenated aCSF [(in mM):124 NaCl, 4.4 KCl, 2 CaCl2, 1.2 MgSO4, 1 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3] maintained at 30 ± 1°C for at least 1 hour before recording.
Slices were transferred to a recording chamber (Warner Instruments) submerged in normal, oxygenated aCSF maintained at 28–30°C at a flow rate of 2 ml/min. Neurons of the BNST were visualized using infrared-differential interference contrast (DIC) video-enhanced microscopy (Olympus). Slices were bathed in normal aCSF for at least 30 minutes before performing whole-cell patch clamp experiments. Borosilicate electrodes pulled with a Flaming-Brown micropipette puller (Sutter Instruments) with a pipette resistance between 3–6 MΩ filled with internal solution [(in mM): 135 KCl-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 ATP, and 0.4 GTP, pH= 7.35, 290 mOsmol] were used to patch cells in the BNST.
Signals were acquired using a Multiclamp 700B amplifier and analyzed with Clampfit 10.3 software (Molecular Devices, Sunnyvale, CA, USA). All experiments were conducted in current clamp mode with the holding current set to zero. For excitability experiments, a current ramp protocol was used to determine the current required to induce firing (rheobase) and the voltage at first spike. The number of spikes generated by discrete current steps was also determined using a 10 pA current step protocol from 0 to 150 pA (V-I plot). In a subset of experiments, the 5HT2c-R antagonist RS 102221 (10 μM) was bath applied for 10 minutes before rheobase and VI plots were performed.
In another set of experiments, the effects of the 5HT2-R agonist mCPP on neuronal excitability were determined by changes in membrane potential. Tetrodotoxin (TTX, 500 nM) was included in the bath to prevent firing of action potentials and to allow accurate determination of changes in membrane potential. Following establishment of a stable baseline for at least 4 minutes, mCPP (20 μM) was bath applied for 10 minutes.
The ability of mCPP to depolarize cells was calculated by subtracting baseline membrane potential from the average membrane potential over the last 2 minutes of drug application. Only cells with a stable access resistance (less than a 20% change from baseline to the end of the experiment) were used in the data analysis.
In order to calculate membrane conductance, a 25 pA current-step protocol from −75 to 50 pA was used to generate a V-I plot, from which the slope could be computed. The membrane conductance is equal to the inverse slope (1/slope) and the change in conductance was calculated by subtracting baseline conductance from the conductance after 10 min of mCPP application. For analysis of spike amplitude and half-width, we used data generated from the V-I plot. For each cell, these measures were taken at the minimum current injection necessary to induce at least 5 consecutive action potentials. The fast AHP was determined by measuring the minimum Vm during the 7 ms time window after the onset of the action potential in a 7 ms time window after action-potential onset (Senn et al., 2014).
2.5 Behavior
Social approach test
Male DBA/2J mice were tested in the social approach test 24 hr or 1 week after the last exposure to CIE and 1 hour after injection with SB 242,084 or vehicle. The testing apparatus consisted of a 3-chambered Plexiglass container, each 20 cm L × 40.5 cm W × 22 cm H, divided by 2 panels with a small opening at the base to allow movement between the chambers (Moy et al., 2008). At the start of each testing session, the test mouse is placed in the center chamber and was subsequently allowed to move freely between the 3 chambers. Infrared photocell beams at the base of the openings between compartments detected movement across chambers, and the number of entries and time spent in each chamber was automatically recorded by analysis software. During the habituation phase of the test, mice were allowed to freely explore all three empty chambers for a period of 10 minutes. At the end of the habituation phase, the mouse was placed back in the center chamber with the doors closed. A stainless steel holding cage (11 cm high × 10.5 in diameter) was placed in the center of the two outermost chambers. A “stranger” mouse was then placed inside the holding cage on one side (e.g. the right), while the other cage remained empty. During the 10-minute “sociability” phase, the doors were opened and the test mouse was again allowed to freely explore the chambers; however, this time, the time spent sniffing the stranger mouse or the empty cage was recorded manually. Relative levels of anxiety between-groups were determined by the time spent sniffing the stranger mouse during the sociability phase, with less time sniffing the stranger mouse reflecting increased anxiety-like behavior. In the final or “novelty” phase, a second stranger mouse was placed inside of the previously empty cage. The time spent by the test mouse sniffing each stranger mouse was recorded manually over the 10-minute session.
Light-dark test
Male DBA/2J mice were habituated to the procedure room for at least 2 hours prior to testing, which took place 24 hours into CIE withdrawal. Phototaxic exploration was evaluated in photocell-equipped automated open field chambers with dark zone inserts that divide the chamber in half (Chamber: 40 cm × 40 cm × 30 cm; L/D Insert: 20 × 40 × 30 cm). The testing apparatus is divided between a bright chamber and a neutral dark chamber. Mice were placed into the Dark Zone and allowed to freely roam for 15 minutes, given a choice between staying in the dark side of the chamber and entering the lighted side. The chamber was cleaned with 70% EtOH prior to each testing session. Behavior was scored using the Versamax system, Accuscan Instruments. Total time (sec) spent in either the Light Zone was used as a measure of anxiety-like behavior and number of entries into the Light Zone as a measure of locomotor activity.
Open field test
Mice that were 24 hrs into CIE withdrawal were injected with SB 242,084 (3 mg/kg) or vehicle 1 hour before testing in the open field. Briefly, the open field arena (50 cm × 50 cm × 25 cm) was divided into the center, defined as the central 25% of the arena, and the periphery using EthoVison XT 7 (Noldus Information Technologies, Wageningen, The Netherlands). Mice were placed individually in the left front corner of the arena and were allowed to explore the environment for 20 min. The time spent in the center of the box, average velocity, and velocities while moving were scored using EthoVision XT 7.
2.6 Immunohistochemistry
Mice were perfused transcardially using 30 ml phosphate buffered saline (PBS) followed by 30 ml 4% paraformaldehyde in PBS. Brains were dissected out and postfixed for 24 hours in 4% paraformaldehyde at 4°C, t hen transferred to 30% sucrose until fully saturated. Slices of the BNST (45 μm) were prepared on a Leica 1200S vibratome and stored in 50/50 glycerol/PBS at −20°C for FOS i mmunohistochemistry. Slices were first washed in PBS, then incubated in 50% methanol and followed by 3% H2O2. Slices were washed in PBS again followed by a 24 hour incubation at 4°C in 0.5% BSA, 0.3% Triton-X100, and c-fos antibody (1:3000; Santa-Cruz). The next day, slices were washed in Tris-HCl, NaCl, Tween (TNT) buffer, incubated in Tris, NaCl blocking (TNB) buffer, and then incubated in goat anti-rabbit HRP-linked IgG (1:200) in TNB buffer for 30 minutes. Slices were washed in TNT, and tyramine signal amplification (TSA) with Cy3 was performed using a kit (Perkin Elmer).
Slices were mounted with Vectashield on glass slices and imaged using a Zeiss AXIO Zoom V16 microscope with ZEN pro 2012 software. FOS-positive cells from at least 3 sections per animal were counted using NIH image software separately in the dorsolateral BNST (dlBNST), dorsomedial BNST (dmBNST) and vBNST.
2.7 Statistical analysis
Two-way analysis of variance (ANOVA) or Student’s t-tests was used to analyzed behavioral and immunohistochemical data. A two-way ANOVA was also used to analyze rheobase, voltage of first spike, resting membrane potential, spike amplitude and spike half-width data in our electrophysiology experiments, and a 3-way ANOVA was used to analyze V-I plots. Changes in membrane potential and membrane conductance induced by mCPP were analyzed using Student t-tests.
3. Results
3.1 CIE-induced disruption of social approach is mitigated by 5HT2c-R antagonists
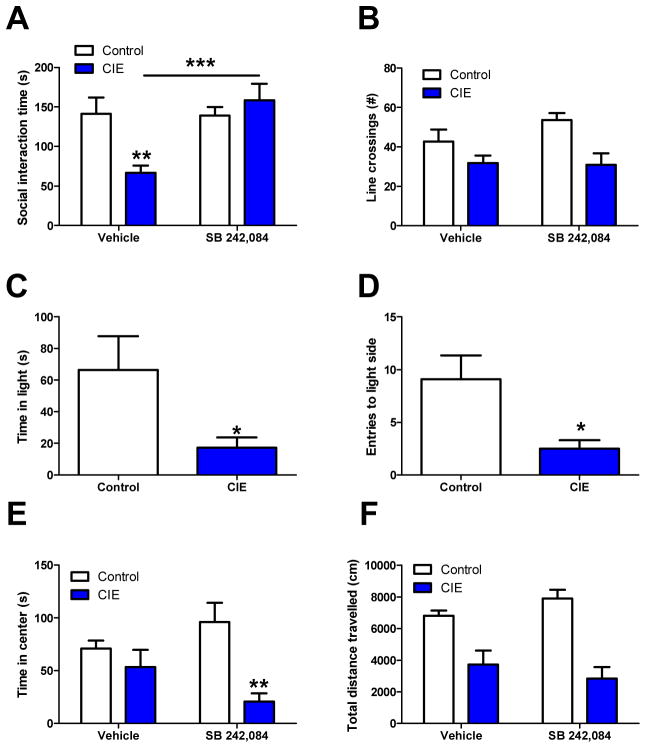
Withdrawal from a chronic ethanol diet was previously shown to elicit a behavioral phenotype in rats that approximates anxiety using the social interaction test that is mitigated by pretreatment with 5HT2c-R antagonists (Overstreet et al., 2003; Knapp et al., 2004; Overstreet et al., 2006). In order to replicate these findings in mice, we exposed 39 male DBA/2J mice to air or CIE vapor for 5 days and then evaluated the impact of the 5HT2c-R antagonist SB 242,084 (3 mg/kg) on anxiety-like behavior in the social approach test 24 hours into withdrawal. A two-way ANOVA revealed that SB 242,084 significantly attenuated the effects of CIE on social approach test (Figure 1A). We found a significant main effect of drug (F(1,35)=7.773, p<0.01) and a significant CIE × drug interaction F(1,35) = 8.576, p<0.01). The main effect of CIE was not significant (F(1,35)=3.055, p>0.05); however, Bonferroni post-hoc comparisons did reveal a significant difference between air- and CIE-exposed mice in the vehicle-treated group only (t=3.263, p<0.001). There was also a significant effect of drug in the CIE exposed group only (t=4.098, p<0.001). These results demonstrate that CIE induces an anxiety-like phenotype in mice that is partly mediated by 5HT2c-R signaling.
Figure 1. CIE-induced anxiety like behavior in the social interaction test is mitigated by 5HT2c-R antagonists.
Social, but not general anxiety-like behavior in the social approach test is mitigated by 5HT2c-R antagonists. Pretreatment with SB 242,084 normalizes CIE induced anxiety-like behavior (A) but does not rescue hypolocomotion in the social approach test (B). CIE effects on time spent in the light (C) and light entries (D) in the light-dark test. SB 242,084 induces an anxiety-like phenotype in the open field test in CIE exposed mice (E) but does not induce locomotor deficits nor rescue CIE induced locomotor deficits (F)
Previous reports regarding the locomotor activating effects of SB 242,084 suggest that this drug may be enhancing social approach by way of increasing locomotor activity (Martin et al, 2002). In order to address this, locomotor activity as measured by the number of crossings between chambers was also significantly impacted by ethanol (F(1,35)=11.87, p<0.01), but not by SB 242,084 (F(1,35)=1.03, p>0.05) and no significant interaction between the two (F(1,35)=1.438, p>0.05) (Figure 1B). The fact that SB 242,084 was able to rescue deficits in social approach but not locomotor activity suggests that these are dissociable features of CIE withdrawal. These findings are in agreement with those previously reported by Breese and colleagues (Overstreet et al., 2003; Knapp et al., 2004).
We also evaluated behavior in the light-dark test to determine if CIE effects on social approach are generalizable to other tests of anxiety-like behavior. We found a trend toward a reduction in time spent in the light that was non-significant (Figure 1C; t=2.004, df=16, p>0.05) in a group of 18 mice. However, there was also a significant reduction in number of entries into the light zone (Figure 1D; t=2.50, df=15, p<0.05), which could reflect an increase in anxiety-like behavior. However, the light-dark test does not allow us to distinguish anxiogenic from locomotor effects, so this decrease in light zone entries may also reflect a CIE induced locomotor deficit in this particular assay.
Finally, we measured performance in the open field test as another measure of general anxiety. Surprisingly, we found that center time was attenuated in the CIE group treated with SB 242,084, suggesting that 5HT2c-R blockade has anxiogenic effects after CIE in this particular assay ((F1,26)=12.17, p<0.01 for main effect of CIE, F(1,26)=0.085, p>0.05 for main effect of SB 242,084 and F(1,26)=4.701, p<0.05 for CIE × SB 242,084 interaction) (Figure 1E). Posthoc comparisons were only significant between the SB 242,084 treated air and CIE-exposed animals (t=3.873, p<0.01). These results indicate that anxiety-like behavior in the social approach test is dissociable from the general anxiety-like behavior observed in the open field test, and that these two forms of anxiety-like behavior are differentially modulated by 5HT2c-R signaling.
Locomotor activity in the open field was also assessed using total distance travelled as a representative measure (Figure 1F). As in the social approach test, there was a main effect of CIE (F(1,26)=38.10, p<0.001) that was not modified by SB 242,084 (F(1,26)=2.166, p>0.05), and SB 242,084 did not have any effect on its own (F(1,26)=0.024, p>0.05). These results indicate that CIE can induce hypolocomotion in the open field test through some mechanism that does not involve 5HT2c-R signaling.
3.2 CIE increases FOS-IR in the vBNST in a 5HT2c-R dependent manner
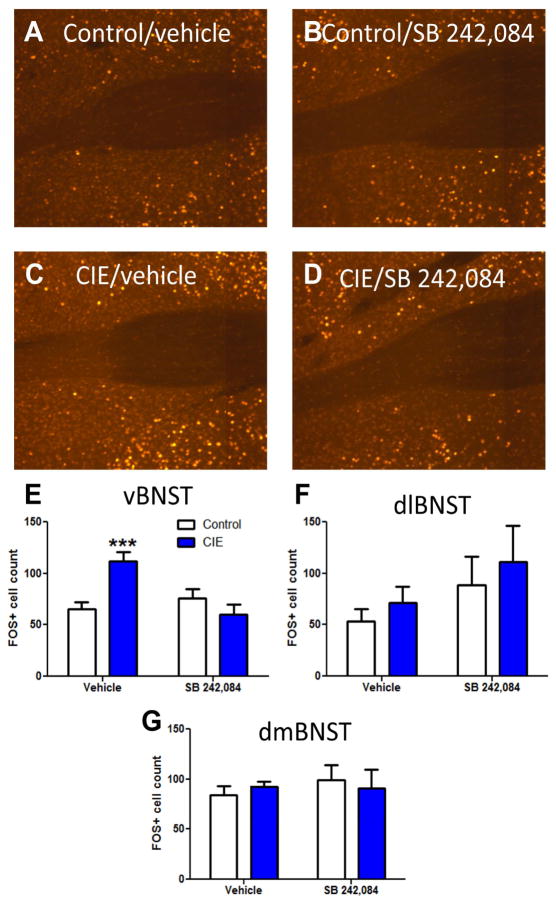
Once we had established a role for 5HT2c-R signaling in CIE induced disruption in behavior in mice, we were interested in identifying a neural mechanism and locus of action. Based on the fact that anxiogenic stimuli elicit an increase in FOS-IR in the BNST, which is attenuated in 5HT2c-R knockout mice, we postulated that the BNST was an important neural substrate mediating anxiety after stress and potentially ethanol withdrawal. This hypothesis is also supported by the fact that peripheral injection with the anxiogenic drug mCPP was shown to increase FOS-IR in the BNST, indicating that 5HT2c-Rs have excitatory effects in the BNST. In order to test this hypothesis, male DBA/2J mice (n=39) were exposed to air or CIE and then injected with the 5HT2c-R antagonist, SB 242,084 or vehicle at 4 and 22 hours post withdrawal. At the 24 hour time point, the mice were perfused and FOS immunohistochemistry was performed to assess CIE effects on neuronal activity in the BNST. In a two-way ANOVA, CIE was found to increase FOS-IR in the ventral portion of the BNST (vBNST), an effect that was attenuated by pretreatment with 5HT2c-R antagonists (Figure 2A–E). We found a significant CIE × drug interaction (F(1,35)=12.81, p<0.01) and a significant main effect of drug (F(1,35)=5.769, p<0.05). Bonferroni post-tests revealed significant increases in FOS-IR in vehicle-treated CIE animals relative to vehicle-treated controls (t=3.937, p<0.001) and relative to drug-treated CIE animals (t=4.15, p<0.001). No other comparisons were significant. These results confirm that withdrawal from CIE activates neurons in the vBNST in a 5HT2c-R dependent manner.
Figure 2.
CIE enhances FOS-IR in the ventral BNST in a 5HT2c-R dependent fashion. FOS-IR in (A) vehicle-injected control (air exposed) mice, (B) SB-242,084-injected control mice, (C) vehicle-injected CIE mice, and (D) SB-242084-injected CIE mice. Graphical representation of FOS cell counts in the (E) ventral, (F) dorsolateral and (G) dorsomedial BNST for all groups
This effect appears to be restricted to the ventral portion of the BNST, as there were no significant differences in FOS+ cell counts in either the dorsolateral or dorsomedial aspects (Figure 2F–G).
3.3 CIE enhances neuronal excitability in the vBNST
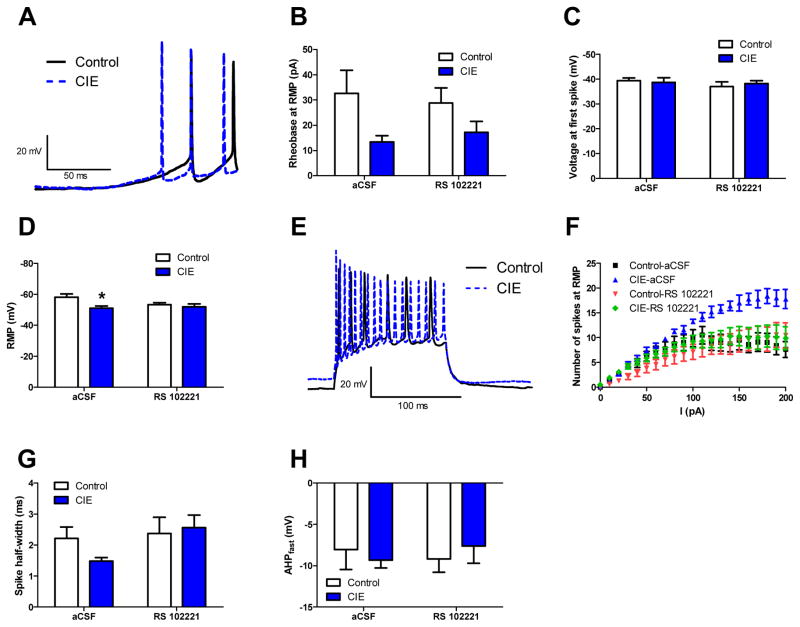
Having found that CIE leads to an increase in FOS-IR in the BNST, we next sought to determine if there were any alterations in neuronal function. In particular, we expected that CIE would increase excitability of neurons in the vBNST in a 5HT2c-R-dependent fashion; consistent with the fact that mCPP was previously found to depolarize neurons in the BNST (Guo et al., 2009). Whole cell patch clamp electrophysiology was used to measure excitability in the vBNST following CIE exposure. The rheobase (Figure 3A–B), the voltage at first spike (Figure 3C), and the resting membrane potential (RMP) (Figure 3D) were used as a measure of basal excitability. These data were collected from 8 control/aCSF cells, 7 control/RS 102221 cells, 9 CIE/aCSF and 9 CIE/RS 102221 cells. Our results indicate a significant main effect of CIE (F(1,29)=6.94, p<0.05), but no effect of RS 102221 (F(1,29)=1.664×10−7, p>0.05) and no significant CIE × RS 102221 interaction (F(1,29)=.417, p>0.05) on rheobase. These data suggest that although CIE reduces the rheobase (and hence increased membrane excitability), this effect is not 5HT2c-R dependent. The voltage at first spike was not significantly affected by CIE or RS 102221, as revealed by a two-way ANOVA (F(1,29)=0.018, p>0.05 for main effect of CIE, F(1,29)=.8841, p>0.05 for main effect of RS 102221, and F(1,29)=0.3511, p>0.05 for CIE × RS 102221 interaction). On RMP, a two-way ANOVA revealed a significant main effect of CIE (F(1,29) = 6.197, p<0.05), but not RS 102221 (F(1,29)=1.202, p>0.05) and no CIE × RS 102221 interaction (F(1,29)=2.788, p>0.05). Input resistance was also measured; however, there was no significant difference between the groups (Data not shown).
Figure 3.
Neuronal excitability in the vBNST increases 24 hours after CIE in a 5HT2c-R dependent fashion. Representative traces of voltage ramp used to determine rheobase (A), the average rheobase (B), the average voltage at first spike (C), and the average RMP (D). Representative traces from control and CIE cells of the current step protocol used to determine VI plot (E) and the average VI plot across all groups (F). The average spike width (G) and the average AHP amplitude (H) across all groups
Next we looked at the number of action potentials generated over a range of current injections (VI plot) as a measure of inducible excitability (Figure 3E–F). In these experiments, we pooled data from 8 control/aCSF cells, 7 control/RS 102221 cells, 5 CIE/aCSF and 10 CIE/RS 102221 cells. No more than 2 cells were used per mouse. vBNST neurons in CIE mice fired significantly more action potentials during current stimulation, relative to air controls, and this effect was absent in the presence of RS102221 (RS102221 × CIE × stimulation interaction: F(20,520)=6.13, p<0.001), suggesting that vBNST neurons can be induced to fire more action potentials following CIE, and that this effect is likely mediated by an upregulation of 5HT2c-R signaling. Activation of 5HT2c-Rs is associated with inhibition of G-protein coupled potassium channels (GIRKs), which may account for the increased spiking frequency observed in these experiments. Interestingly, we also observed a trend toward a decrease in spike half-width (Figure 3G) that did not reach significance but did appear to be modified by RS 102221. Given that inhibition of K+ channels would be expected to increase spike half width (Vincent et al., 2000), these data suggest that other channels contribute to this effect of 5HT2c-Rs on spike half width. We also analyzed fast AHP (Figure 3H) and found no significant differences between groups (F(1,21)=0.04, p>0.05 for main effect CIE, F(1,21)=0.02, p>0.05 for main effect RS 102221 and F(1,21)=0.189, p>0.05 for CIE × RS 102221 interaction).
3.4 CIE augments 5HT2c-R signaling in the vBNST
Given the fact that enhanced excitability in the vBNST post-CIE appears to be mediated by 5HT2c-R signaling, we hypothesized that the 5HT2c/b-R agonist would elicit a greater excitatory response in the vBNST of CIE-relative to air-exposed mice. Although mCPP is nonselective for the 5HT2c receptor, it displays a preference for 5HT2c and 2b receptors (pEC50 = 7.09 and 7.2, respectively) over 5HT2a (pEC50 = 6.65 and 5HT1a receptors (pEC50 = 5.9). Given that 5HT2b receptors are not highly expressed in the BNST, we treated mCPP as a preferential 5HT2c-R agonist. Previously, mCPP has been reported to depolarize neurons in the BNST (Guo et al., 2009), the lateral amygdala (Yamamoto et al., 2014) and in hypothalamic nuclei (Qiu et al, 2007; Sohn et al., 2011; Roepke et al., 2012). Chronic ethanol exposure, by way of increased 5HT2c-R signaling, would be expected to augment this response to mCPP. Consistent with this, our results indicate that bath application of mCPP to neurons of the vBNST depolarized the membrane potential (MP) by an average of 2.07 ± 1.53 mV in control (n=9 cells) and 6.67 ± 1.32 mV in CIE mice (n=7 cells) (Figures 4A–B). T-tests comparing the change in MP induced by mCPP (delta or Δ MP) between control and CIE groups were statistically significant (t(14)=2.19, p<0.05).
Figure 4.
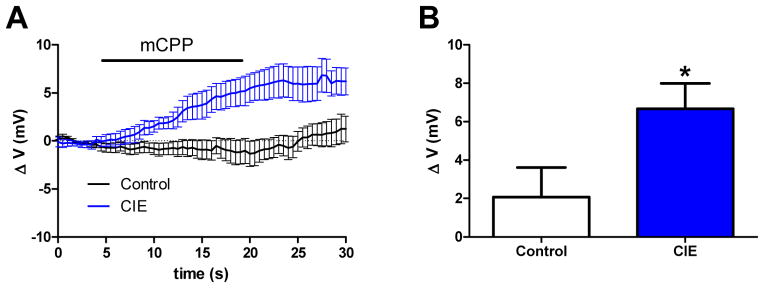
CIE augments 5HT2c-R signaling in the vBNST. The average trace of a neuron in the vBNST during application of mCPP (20 μM) (A) and a graphical representation of the average change in membrane potential by mCPP (B)
3.5 CIE induces persistent alterations in behavior and neural function in the vBNST
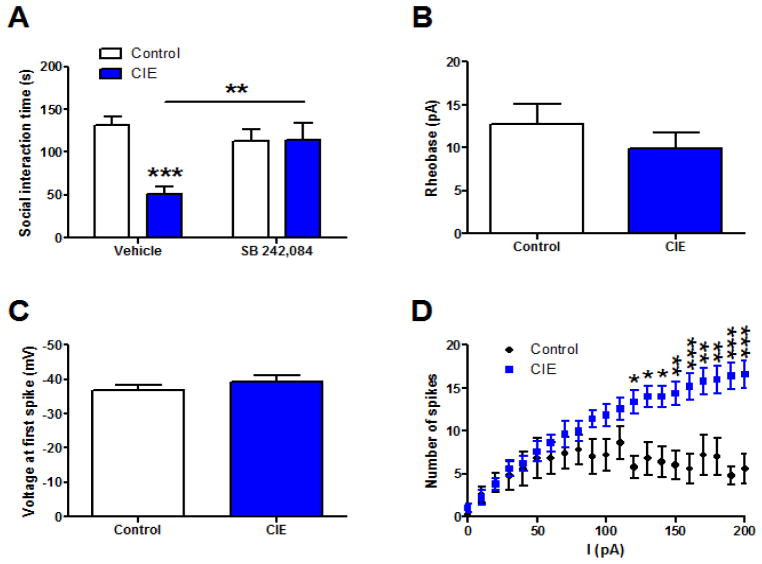
In order to evaluate if there were persistent changes in anxiety-like behavior and physiology following CIE withdrawal, another cohort of mice were exposed to the social approach test 1 week after removal from the ethanol chambers. Again, mice were injected with 3 mg/ml SB 242,084 1 hour before testing. Our results indicate a significant main effect of CIE (F(1,36)=9.097, p<0.01) and a significant interaction between CIE and SB 242,084 (F(1,36)=9.573, p<0.01), indicating that CIE increases anxiety like behavior 1 week post-CIE in a 5HT2C-R dependent fashion (Figure 5A). Bonferroni posttests comparing vehicle-treated control and CIE mice were also significant (t=4.321, p<0.001), as were post-tests comparing vehicle-treated and SB 242,084-treated CIE mice (t=3.389, p<0.01).
Figure 5. Persistent adaptations in 5HT2c-R sensitive behavior and physiology at 1 week post-CIE.
Ethanol-induced adaptations in behavior and physiology persist 1 week after withdrawal from CIE. CIE induced deficits in social approach persist 1 week after withdrawal and are mitigated by 5HT2c-R antagonists (A). Excitability measures 1 week after CIE exposure including rheobase (B), voltage at first spike (C), and spiking frequency as a function of current (D).
We also assessed persistent changes in excitability in the vBNST after 1 week of withdrawal from CIE, since this is proposed to be the mechanism underlying elevations in anxiety. Although we found no changes in rheobase (Figure 5B) or voltage at first spike (Figure 5C) between controls and CIE-treated mice, there was a significant increase in spiking frequency as a function of current (Figure 5D). We pooled data from 5 control cells and 5 CIE cells (one cell in the control group was excluded as an outlier after performing a Grubb’s test). A two-way repeated measures ANOVA revealed a significant main effect of CIE (F(1,160)=9.969, p<0.05), a significant main effect of current (F(20,160)=15.78, p<0.001), and a significant interaction between CIE and current (F(20,160)=6.556. p<0.001). Bonferroni post-tests were performed to make pairwise comparisons between control and CIE mice at different current injections and we found significant differences starting at 120 pA (t=3.519, p<0.05) and continuing through the final 200 pA (t=5.093, p<0.001). Taken together, these data suggest that CIE can induce persistent changes in behavior which may be attributed to enhanced excitability in the vBNST.
4. Discussion
Acute ethanol acts to stimulate 5HT release in discrete brain regions known to be involved in affect regulation including the ventral hippocampus (Bare et al., 1998; Kheirbek and Hen, 2011) and nucleus accumbens (Yoshimoto et al., 1991). Withdrawal, on the other hand, appears to cause 5HT depletion in the hippocampus (Kempf et al., 1990), the mPFC and the amygdala (Carlson and Drew, 2006). Given that 5HT2c-Rs are enriched in these areas (Pompeiano et al., 1994; Eberle-Wang et al., 1997; Liu et al., 2007; Kheirbek and Hen, 2011), it stands to reason that repeated cycles of ethanol exposure and withdrawal may induce long-lasting neuroadaptations in 5HT2c-R systems by way of dysregulated 5HT signaling (Pandey and Pandey, 1996). These, in turn, may contribute to anxiety during ethanol withdrawal. Here we present evidence that withdrawal from chronic ethanol disrupts social approach behavior which is attenuated by pretreatment with 5HT2c-R antagonists. To our knowledge, this is the first study documenting effects of ethanol withdrawal on social approach behaviors in mice, although previous reports have established this in rats (Overstreet et al., 2003; Knapp et al., 2004; Overstreet et al., 2006). Previous studies have failed to detect anxiety in C6 or D2 mouse strains after a 72 hr exposure to ethanol vapor (Finn et al., 2000), although it should be noted that these mice were tested during peak withdrawal (5.5–9.5 hr after removal from the chambers), which may not have been sufficient time for ethanol to be completely metabolized. Our mice were tested 24 hrs and 1 week after a 5-day CIE exposure. These behavioral outcomes of CIE were associated with an increase in neuronal activation and excitability in the vBNST, suggesting hyperexcitability in the vBNST as a potential mechanism for the anxiety-promoting effects of chronic ethanol. This hyperexcitability was attenuated by 5HT2c-R blockade, indicating that CIE may augment 5HT2c-R signaling in the vBNST. Further evidence comes from the fact that following CIE exposure, neurons in the vBNST exhibit a greater depolarization in response to mCPP. In sum, these data indicate that chronic ethanol promotes anxiety-like behavior by enhancing 5HT2c-R signaling in the vBNST, an important neural output to brainstem and hypothalamic nuclei that control behavioral and neuroendocrine responses to stress.
4.1 CIE withdrawal disrupts social approach via 5HT2c-R signaling
Withdrawal from chronic ethanol exposure engages neural circuits in the brain that are associated with stress, such that repeated episodes of withdrawal can be conceptualized as a chronic stressor. Anxiety states brought on by stress are accompanied by dysregulation of 5HT2c-R signaling in regions of the extended amygdala including the basolateral amygdala (BLA) (Christianson et al., 2010; Li et al., 2012), the CeA (Hackler et al., 2007; Overstreet et al., 2006), and the BNST (Heisler et al., 2007). Given the similarities between stress and ethanol withdrawal, we predicted that CIE would induce anxiety in mice in a 5HT2c-R dependent fashion, and this was confirmed by a decrease in social approach 24 hours after withdrawal from CIE that was mitigated by pretreatment with 3 mg/kg SB 242,084. The utility of 5HT2c-R antagonists in alleviating deficits in social behaviors during ethanol withdrawal has been previously validated in rats (Overstreet et al., 2003; Knapp et al., 2004; Overstreet et al., 2006), while other studies have shown that the anxiogenic actions of certain antidepressants can be blocked by 5HT2c-R antagonists (Bagdy et al., 2001). Conversely, 5HT2c-R agonists such as mCPP or MK-212 produce subjective feelings of anxiety and cravings for alcohol in human alcoholics (Krystal et al., 1994). Together, these data implicate brain 5HT2c-R systems in the withdrawal-induced anxiety and suggest new avenues for pharmacotherapy in treating alcohol dependence.
The 5HT2c-R antagonist SB 242,084 has been previously reported to increase locomotor activity in rats at 1 mg/kg, but not 3 mg/kg, the dose used in this study (Martin et al., 2002). Nevertheless, we examined locomotor activity in the social approach test and the open field test and found that CIE-induced hypoactivity was not rescued by SB 242,084, indicating that the apparent anxiolytic actions of SB 242,084 are not driven by locomotor deficits.
Contrary to our expectations, there was no change in anxiety in the open field in the vehicle treated CIE group, but the SB 242,084 treated CIE group exhibited a decrease in center time, indicative of anxiety-like behavior. This implies that SB 242,084 actually increases more generalized anxiety as measured by the open field test during CIE withdrawal and that this anxiety is dissociable from specific type of social anxiety determined by the social approach test. These opposing effects of 5HT2c-R antagonism on these two aspects of anxiety may indicate that they are governed by divergent neural circuits. To illustrate this point, a recent study found that optogenetic Gq/5HT2c-R activation of GABAergic interneurons in the DR 5HT2c-Rs actually relieve anxiety in the open field (Spoida et al., 2014), while another study by Breese and colleagues found that a 5HT2c-R inverse agonist (SB 243,213) microinjected into the DR had no effect on CIE induced anxiety in the social interaction test (Overstreet et al., 2006). However, intra-amygdalar injections of SB 243,213 did relieve CIE induced deficits in social interaction, suggesting that 5HT2c-R signaling in the amygdala can modulate the social aspects of anxiety. Taken together, these studies suggest that 5HT2c-R signaling can reduce generalized anxiety via activation of negative feedback circuits in the DR that inhibit firing of 5HT neurons, while social anxiety is actually increased by 5HT2c-R signaling in the extended amygdala. Our data supports this hypothesis by demonstrating a differential effect of 5HT2c-R antagonists on anxiety in the social approach test and the open field test.
4.2 CIE increases cfos expression in the vBNST in a 5HT2c-R dependent fashion
The BNST has reciprocal connections with the dorsal raphe nucleus (DRN) and is particularly sensitive to the effects of drugs selective for 5HT1a, 2a, 2c, and 5HT7 receptors (Guo et al., 2009). Previous studies have shown that 5HT2c-R agonists and anxiogenic stimuli increase FOS-IR in the BNST (Heisler et al., 2007; Singewald et al., 2003), so we anticipated that ethanol withdrawal, as a chronic stressor, would do the same. Our results indicate that FOS-IR is upregulated in the ventral BNST (vBNST) following CIE exposure, and this was attenuated by peripheral injection of a 5HT2c-R antagonist during withdrawal. Interestingly, swim stress also elicits an increase in FOS-IR in the vBNST (Briand et al., 2010), suggesting that ethanol withdrawal and stress engage similar neural circuits, and likely elicit similar behaviors. These results are in agreement with previous studies showing that ethanol withdrawal induces cfos expression in other brain regions associated with anxiety in rats (Bonassoli et al., 2011). Overall, these data suggest that CIE can elicit changes in neuronal activity in the vBNST which may contribute to anxiety-like behavior, and that this behavioral plasticity involves upregulation of 5HT2c-R signaling. While it seems likely that CIE activates vBNST neurons directly via increases in local 5HT2c-R signaling, we did not directly confirm expression of 5HT2c-Rs in these cells, leaving open the possibility that CIE alters FOS levels indirectly via 5HT2c-R dependent adaptations in inputs to the vBNST. However, in light of our electrophysiological data showing enhanced mCPP response in vBNST neurons, we think a direct modulation of FOS-IR is extremely probable.
Surprisingly, these ethanol induced adaptations in neuronal activation were limited to the vBNST, as we found no changes in FOS-IR in either the dmBNST or dlBNST. These results may reflect their divergent roles in modulating network function within the BNST and their efferent projections to brain regions that govern fear and anxiety-related behavior. The dorsal aspect of the BNST, which encompasses the dlBNST and dmBNST, is primarily comprised of GABAergic interneurons which receive dense amygdalar inputs and maintain feedforward inhibition over BNST output neurons, a large population of which resides in the vBNST. These neurons innervate a variety of structures involved in emotional behavior, including the central amygdala (CeA), lateral hypothalamus (LH), PVN, ventrolateral periaquaductal grey (vlPAG) and ventral tegmental area (VTA) (Dong et al., 2001; Dong and Swanson, 2004). Our results suggest that vBNST neurons may be uniquely sensitive to the effects of repeated ethanol exposure, inducing both short-term and long-term adaptations in circuits that regulate emotional expression. Importantly, we cannot exclude that these changes in c-fos in the vBNST are due to circuit effects.
4.3 CIE induces hyperexcitability in the vBNST via upregulation of 5HT2c-R signaling
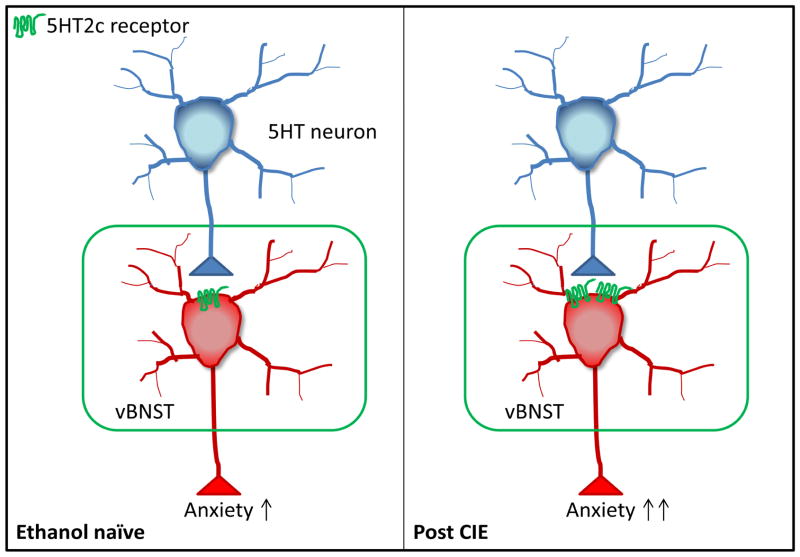
Our electrophysiology results demonstrate that CIE increases membrane excitability in the vBNST on three separate measures; rheobase, RMP and spiking frequency as a function of current. However, only the effect on current induced spiking is 5HT2c-R dependent, suggesting that ethanol induced adaptations in other systems account for the alterations in rheobase and RMP. In the presence of TTX, mCPP was able to evoke a greater increase in membrane potential in the vBNST of CIE mice, indicating that 5HT2c-R signaling within recorded neurons in the vBNST was enhanced after CIE withdrawal. We postulate that ethanol-induced hyperexcitability in the vBNST via enhancement of 5HT2c-R signaling promotes anxiety-like behavior during ethanol withdrawal (Figure 6).
Figure 6.
Circuit model of ethanol-induced plasticity in the vBNST. 5HT depolarizes neurons in the vBNST via 5HT2c-Rs. Following CIE, neurons in the vBNST become more excitable due to an upregulation in 5HT2c-R signaling (shown here as an increase in 5HT2c-R surface expression, but could also be due to altered 5HT2c-R editing), more efficient 5HT2c-R-Gαq/11 coupling, etc., leading to increased anxiety-like behavior.
While an in depth analysis of intracellular signaling mechanisms elicited by activation of 5HT2c-Rs is beyond the scope of this paper, we did notice that CIE reduced the spike duration in a 5HT2c-R dependent manner, although this effect was non-significant. This is consistent with a 5HT2c-R elicited increase in Ca2+ entry via opening of TRPC channels (Sohn et al., 2011; Yamamoto et al., 2014).
4.4 CIE induces persistent alterations in behavior and neural function in the vBNST
Having established that acute CIE withdrawal can elicit robust deficits in behavior that are attributable to altered 5HT2cR signaling in the vBNST, we next sought to determine if these changes persist during protracted withdrawal. Our data suggest that deficits in social approach are still present 1 week after withdrawal from CIE and are mitigated by pretreatment with a 5HT2c-R antagonist, suggesting that alterations in 5HT2c-R function are a contributing factor. Next we analyzed the effects of CIE on neural excitability in the vBNST, which confirmed the presence of a persistent change in spiking frequency as a function of current. In sum, these data provide further evidence that CIE can induce long-lasting plasticity in behavior and neural function, particularly in the vBNST. However, as we did not examine if these later changes were sensitive to 5HT2c-R antagonism, it is possible that there are alternate systems that are engaged at this timepoint.
4.5 The vBNST as critical node of interaction between 5HT and stress pathways in the brain
The results of this study highlight the potential significance of the vBNST as a neural interface between serotonin and stress processing centers in the brain. Serotonergic neurons from the DRN (Shin et al., 2008; Phelix et al., 1992) heavily innervate the BNST and preferentially form synapses within the vBNST, which sends outputs to other brain regions involved in emotional processing. The importance of these functional connections is emphasized by the fact that human alcoholics exhibit increased connectivity between the amygdala and BNST during abstinence (O’Daly et al., 2012). The BNST projection to the VTA, but not the LH, also plays a prominent role in stress-induced reinstatement of reward-related behaviors in animal models of addiction (Briand et al., 2010; Sartor and Aston-Jones, 2012), presumably by engaging VTA dopamine neurons (Georges and Aston-Jones, 2001; 2002). Given the functional parallels between stress and ethanol withdrawal, it is likely that CIE withdrawal also engages this neurocircuitry. Repeated cycles of CIE have been shown to promote increased drinking (Griffin et al., 2009), indicating that repeated withdrawal acts in a similar manner to stress in the facilitation of reward-seeking behavior. Interestingly, this vBNST-to-VTA pathway is appetitive and anxiolytic if GABAergic neurons are involved, whereas glutamatergic projections appear to be aversive and anxiogenic (Jennings et al., 2013). These opposing effects on behavior could potentially explain the seemingly contradictory emotional states experienced during ethanol withdrawal, namely anxiety and anhedonia coupled with an increased motivational drive to seek out ethanol. Given that our model of CIE withdrawal is both anxiogenic and increases excitability within the vBNST, it would be interesting to parse out the effects of CIE on glutamatergic vs GABAergic neurons within the vBNST that project to the VTA in a future study.
5. Conclusion
Repeated cycles of ethanol exposure and withdrawal can elicit specific changes in 5HT2c-R signaling that confer hyperexcitability in the ventral BNST, an important neural relay to emotion processing centers of the brain that orchestrate behavioral responses to stress and other stimuli. These findings indicate that 5HT2c-Rs in the vBNST may be an important neural substrate underlying a specific type of anxiety during ethanol withdrawal that may precipitate relapse, and that blocking this receptor may alleviate some of the symptoms of alcohol withdrawal.
Highlights.
Chronic intermittent ethanol (CIE) induces social but not general anxiety-like behavior
Ethanol-induced social anxiety is disrupted by pretreatment with 5HT2c-R antagonists
CIE excites neurons in the ventral BNST in a 5HT2c-R dependent fashion
CIE increases sensitivity of vBNST neurons to 5HT2c-R agonists
CIE-induced adaptations in behavior and physiology persist for 1 week
Acknowledgments
This work was supported by NIAAA grant R01AA019454NIAAA and NIAAA T32 training grant 5T32AA007573-14. We also thank Dr. Andrew Holmes for his assistance with statistical analysis and Sheryl Moy for her assistance with behavioral experiments.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Austgen JR, Dantzler HA, Barger BK, Kline DD. 5-hydroxytryptamine 2C receptors tonically augment synaptic currents in the nucleus tractus solitarii. J Neurophysiol. 2012;108(8):2292–2305. doi: 10.1152/jn.00049.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4(4):399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Bare DJ, McKinzie JH, McBride WJ. Development of rapid tolerance to ethanol-stimulated serotonin release in the ventral hippocampus. Alcohol Clin Exp Res. 1998;22(6):1272–1276. [PubMed] [Google Scholar]
- Bleich S, Bayerlein K, Reulbach U, Hillemacher T, Bönsch D, Mugele B, Kornhuber J, Sperling W. Homocysteine levels in patients classified according to Lesch’s typology. Alcohol Alcoholism. 2004;39(6):493–498. doi: 10.1093/alcalc/agh094. [DOI] [PubMed] [Google Scholar]
- Bonassoli VT, Milani H, de Oliveira RM. Ethanol withdrawal activates nitric oxide-producing neurons in anxiety-related brain areas. Alcohol. 2011;45(7):641–652. doi: 10.1016/j.alcohol.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60(1):81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via activation of 5-HT 2C, 5-HT6, and 5-HT7 receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacol. 2007;32(8):1840–1854. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30(48):16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA. 5-HT2c receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One. 2011;6(6):e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JP, Ragole T, Amat J, Greewood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiat. 2010;67(4):339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMatteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selctive serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38(8):1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Togography of projections from amygdala to bed nucleus of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004;468(2):277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Newman ME, Kinor N, Blatman-Jan G, Schindler CJ, Overstreet DH, Yadid G. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepression treatment. Neuropharmacology. 2005;48(1):34–42. doi: 10.1016/j.neuropharm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Englander MT, Dulawa SC, Bhansali P, Schmauss C. How stress and fluoxetine modulate serotonin 2C receptor pre-mRNA editing. J Neurosci. 2005;25(3):648–651. doi: 10.1523/JNEUROSCI.3895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle-Wang K, Mikeladze Z, Uryu K, Chesselet MF. Pattern of expression of the serotonin2C receptor messenger RNA in the basal ganglia of adult rats. J Comp Neurol. 1997;384(2):233–247. [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther. 2000;292(1):394–405. [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21(16):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22(12):5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201(4):569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JD, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: Molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–1793. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, Gore JC, Sanders-Bush E. 5-Hydroxytryptamine2C receptor contribution to m-chlororphenylpiperazine and N-methyl-beta-carboline-3-carboxamine-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320(3):1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Zhou L, Bajwa P, Hsu J, Tecott LH. Serotonin 5-HT(2C) receptors regulate anxiety-like behavior. Genes Brain Behav. 2007;6(5):491–496. doi: 10.1111/j.1601-183X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, Debrouse L, Colacicco G, Flynn SM, Masneur S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15(10):1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-Releasing Factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: Relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332(1):298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496(7444):224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Winder DG. Neuropeptide Y and corticotrophin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of stria terminalis. Neuropharmacology. 2006;51(5):1013–1022. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Kash TL, Baucum AJ, Conrad KL, Colbran RJ, Winder DG. Alcohol exposure alters NMDAR function in the bed nucleus of stria terminalis. Neuropsychopharmacol. 2009;34(11):2420–2429. doi: 10.1038/npp.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek MA, Hen R. Dorsal vs ventral hippocampal neurogenesis: implications for cognition and mood. Neuropsychopharmacol. 2011;36(1):373–374. doi: 10.1038/npp.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F, Helwig H, Tarnaske T, Otte C, Jahn H, Wiedemann K. Pharmacological relapse prevention of alcoholism: clinical predictors of outcome. Eur Addict Res. 2005;11(2):83–91. doi: 10.1159/000083037. [DOI] [PubMed] [Google Scholar]
- Kimura A, Stevenson PL, Carter RN, Maccoll G, French KL, Simons JP, Al-Shawi R, Kelly V, Chapman KE, Holmes MC. Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity. Eur J Neurosci. 2009;30(2):299–306. doi: 10.1111/j.1460-9568.2009.06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28(8):837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland P, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7(5):e375421. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzier H, Charney DS. Specificity of ethanol-like effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiat. 1994;51(11):898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal H, Prather PL, Rezazadeh SM. Potential role of 5HT1C and/or 5HT2 receptors in mianserin-induced prevention of anxiogenic behaviors occurring during ethanol withdrawal. Alcohol Clin Exp Res. 1993;17(2):411–7. doi: 10.1111/j.1530-0277.1993.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Fenton M, Bonenfant E, Swift RM. Typologies of alcohol dependence. From Jellinek to genetics and beyond. Neuropsychol Rev. 2009;19(1):115–129. doi: 10.1007/s11065-008-9080-z. [DOI] [PubMed] [Google Scholar]
- Lesch, Walter Subtypes of alcoholism and their role in therapy. Alcohol Alcoholism Suppl. 1996;1:63–67. [PubMed] [Google Scholar]
- Li Q, Luo T, Jiang X, Wang J. Anxiolytic effects of 5-HT1A receptors and anxiogenic effects of 5-HT2C receptors in the amygdala of mice. Neuropharmacology. 2012;62(1):474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin(2C) receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1667–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendex RI, Becker HC. Repeated cycles of chronic intermittent ethanol exposure leads to the development of tolerance to aversive effects of ethanol in C57BL/6J mice. Alcohol Clin Exp Res. 2012;36(7):1180–1087. doi: 10.1111/j.1530-0277.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Ballard TM, Higgins GA. Influence of the 5-HT2C antagonist, SB-242084, in tests of anxiety. Pharmacol Biochem Behav. 2002;71(4):615–625. doi: 10.1016/s0091-3057(01)00713-4. [DOI] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure an early withdrawal in C57BL/6J mice. Addict Biol. 2012;17(2):351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Crabbe JC. Alcohol withdrawal severity in inbred mouse (Mus musculus) strains. Behav Neurosci. 2005;119(4):911–925. doi: 10.1037/0735-7044.119.4.911. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Martin CB, Chevarin C, Maldonado R, Hamon M, Robledo P, Lanfumey L. 5-HT2c receptor activation prevents stress-induced enhancement of brain 5-HT turnover and extracellular levels in the mouse brain: Modulation by chronic paroxetine treatment. J Neurochem. 2010;115:438–449. doi: 10.1111/j.1471-4159.2010.06932.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restrictive behaviors: relevance to autism. Behav Brain Res. 2008;188(1):178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni YG, Miledi R. Blockage of 5HT2c serotonin receptors by fluoxetine (Prozac) Proc Natl Acad Sci. 1997;94:2036–2040. doi: 10.1073/pnas.94.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacol. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacology. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Angel RA, Navarro M, Breese GR. Reduction in repeated ethanol-withdrawal-induced anxiety-like behavior by site-selective injections of 5-HT1A and 5-HT2C ligands. Psychopharmacology (Berl) 2006;187(1):1–12. doi: 10.1007/s00213-006-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res. 2007;31(9):1473–1481. doi: 10.1111/j.1530-0277.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Pandey GN. Modulation of serotonin 2A/2C receptors and the receptor-linked phosphoinositide system by ethanol. Behav Brain Res. 1996;73(1–2):235–238. doi: 10.1016/0166-4328(96)00103-9. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of the bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28(6):949–965. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2a and 5-HT2c receptors. Mol Brain Res. 1994;23:163–178. doi: 10.1016/0169-328x(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, Kelly MJ. Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: Role in energy homeostasis in females. Mol Pharmacol. 2007;72:885–896. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002;87(5):2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Roberto M, Bajo M, Crawford E, Madamba SG, Siggins GR. Chronic ethanol exposure and protracted abstinence alter NMDA receptors in central amygdala. Neuropsychopharmacol. 2006;31(5):988–996. doi: 10.1038/sj.npp.1300840. [DOI] [PubMed] [Google Scholar]
- Roepke TA, Smith AW, Rønnekleiv OK, Kelly MJ. Serotonin 5-HT2c receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am J Physiol Endocrinol Metab. 2012;302:E1399–E1406. doi: 10.1152/ajpendo.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Willins DL, Kristiansen K, Kroeze WK. 5-Hydroxytryptamine 2-family receptors (5-hydroxytryptamine 2A, 5-hydroxytryptamine2B, 5-hydroyxtryptamine2C): Where structure meets function. Pharmacol Therapeut. 1998;79:231–257. doi: 10.1016/s0163-7258(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci. 2012;36(11):3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlick I, Gründemann J, Fadok JP, Müller C, Letzkus JJ, Lüthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81:428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511(5):628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotrophin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of stria terminalis. J Neurosci. 2013;33(3):950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiat. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71(3):488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoida K, Masseck OA, Deneris ES, Herlitze S. Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proc Natl Acad Sci USA. 2014;111(17):6479–6484. doi: 10.1073/pnas.1321576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storvik M, Haukijärvi T, Tupala E, Tiihonen J. Correlation between the SERT binding densities in hypothalamus and amygdala in Cloninger type 1 and 2 alcoholics. Alcohol Alcoholism. 2008;43(1):25–30. doi: 10.1093/alcalc/agm157. [DOI] [PubMed] [Google Scholar]
- Szücs A, Berton F, Sanna PP, Francesconi W. Excitability of jcBNST neurons in reduced in alcohol-dependent animals during protracted alcohol withdrawal. PLoS One. 2012;7(8):e42313. doi: 10.1371/journal.pone.0042313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Lautermilch NJ, Spitzer NC. Antisense suppression of potassium channel expression demonstrates its role in the maturation of the action potential. J Neurosci. 2000;20(16):6087–6094. doi: 10.1523/JNEUROSCI.20-16-06087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Schmitt A, Wischmeyer E, Döring F. Excitability of pontine startle processing neurons is regulated by the two-pore-domain K+ channel TASK-3 coupled to 5-HT2C receptors. Eur J Neurosci. 2008;28(5):931–940. doi: 10.1111/j.1460-9568.2008.06400.x. [DOI] [PubMed] [Google Scholar]
- Wood MD, Reavill C, Trail B, Wilson A, Stean T, Kennett GA, Lightowler S, Blackburn TP, Thomas D, Gager TL, Riley G, Holland V, Bromidge SM, Forbes IT, Middlemiss DN. SB-243213; a selective 5-HT2c receptor inverse agonist with improved anxiolytic profile: Lack of tolerance and withdrawal anxiety. Neuropharmacology. 2001;41:186–199. doi: 10.1016/s0028-3908(01)00054-5. [DOI] [PubMed] [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Hatano N, Sugai T, Kato N. Serotonin induces depolarization in lateral amygdala neurons by activation of TRPC-like current and inhibition of GIRK current depending on 5-HT2c receptor. Neuropharmacology. 2014;82:49–58. doi: 10.1016/j.neuropharm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9(1):17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]