Abstract
To determine the effects of exercise on postprandial glucose metabolism and insulin action in type 1 diabetes (T1D), we applied the triple tracer technique to study 16 T1D subjects on insulin pump therapy before, during, and after 75 min of moderate-intensity exercise (50% V̇o2max) that started 120 min after a mixed meal containing 75 g of labeled glucose. Prandial insulin bolus was administered as per each subject's customary insulin/carbohydrate ratio adjusted for meal time meter glucose and the level of physical activity. Basal insulin infusion rates were not altered. There were no episodes of hypoglycemia during the study. Plasma dopamine and norepinephrine concentrations rose during exercise. During exercise, rates of endogenous glucose production rose rapidly to baseline levels despite high circulating insulin and glucose concentrations. Interestingly, plasma insulin concentrations increased during exercise despite no changes in insulin pump infusion rates, implying increased mobilization of insulin from subcutaneous depots. Glucagon concentrations rose before and during exercise. Therapeutic approaches for T1D management during exercise will need to account for its effects on glucose turnover, insulin mobilization, glucagon, and sympathetic response and possibly other blood-borne feedback and afferent reflex mechanisms to improve both hypoglycemia and hyperglycemia.
Keywords: exercise, postprandial glucose kinetics, insulin mobilization
exercise increases peripheral glucose uptake [rate of glucose disappearance (Rd)] via insulin-dependent and -independent mechanisms. Simultaneously, rates of endogenous glucose production (EGP) increase to minimize risks of hypoglycemia (5, 9, 31, 33). These changes in glucose fluxes are facilitated by falling insulin and rising glucagon and catecholamine (34) concentrations in plasma together with emerging roles for potential blood-borne feedback and afferent reflex mechanisms in stimulating glucose rate of appearance (Ra) (6, 18, 19) and pancreatic islet hormone secretion (24).
However, the increment in EGP may not sufficiently compensate for the increase in glucose disposal, thus predisposing to exercise-induced hypoglycemia in type 1 diabetes (T1D) (27). This could, at least in part, be due to impaired glucagon and/or catecholamine secretion and responsiveness because of concomitant dysfunction of α-cell or autonomic systems, respectively, that often afflicts patients with T1D (8, 17). Furthermore, the increase in insulin sensitivity can persist for several hours after cessation of exercise, according to a study in rats (14), hence, further predisposing individuals with T1D to delayed hypoglycemia (20, 22). Although there have been numerous reports (11, 12, 25) evaluating glucose kinetics during and after exercise in individuals without diabetes, a comprehensive assessment of glucose turnover during and immediately after exercise applying state-of-the-art isotope dilution techniques has, to the best of our knowledge, not been conducted in individuals with T1D. In this context, in a study assessing individuals with and without T1D during exercise, Petersen et al. (21), applying magnetic resonance technology, suggested that, compared with healthy individuals, those with T1D had higher rates of EGP due to increased rates of gluconeogenesis.
However, detailed assessment of carbohydrate turnover when exercise is conducted in the postprandial state has been a challenge to exercise physiologists. This is because accurate estimations of essential components of postprandial glucose turnover, i.e., systemic appearance of meal glucose (MRa) and rates of EGP and Rd, require complex isotope dilution technique to minimize non-steady-state errors in the estimation of these parameters. Although we have recently reported use of the triple-tracer technique (25) to measure the components of postprandial glucose turnover during moderate-intensity exercise (50% V̇o2max) in healthy adults, we describe here, applying the same technique and similar protocol, measurement of postprandial glucose kinetics during and after moderate-intensity exercise in a cohort of C-peptide-negative individuals with T1D on insulin pump therapy. Subjects exercised on a treadmill at 50% V̇o2max for 75 min, 2 h after consuming a mixed-meal breakfast. No subjects developed hypoglycemia during or after exercise. We report estimations of components of postprandial glucose turnover (MRa, EGP, and Rd) in a continuous fashion together with insulin and glucagon excursions before, during, and after exercise, constituting the 6-h duration of the study. Finally, we also compare the responses during this period with those in healthy subjects (25) reported recently.
MATERIALS AND METHODS
After approval from the Mayo Clinic Institutional Review Board and following signed informed consent, subjects with T1D on continuous subcutaneous insulin infusion pumps (CSII) were screened for eligibility. Inclusion criteria were age 18–65 yr, BMI 19–40 kg/m2, Hb A1c ≤10%, creatinine ≤1.5 mg/dl, and normal gastric emptying. Exclusion criteria were significant gastrointestinal symptoms by questionnaire, documented recent gastrointestinal disorders, medications affecting gastric motility, pregnancy, unaccustomed physical activity, or on an active weight loss program or any other comorbidities precluding participation. Medications (except for stable thyroid hormone or hormone replacement therapy) that could influence glucose tolerance were exclusionary. Those with stable diabetic retinopathy were included. Subjects did not engage in vigorous physical activities for 72 h before screen and study visits. Each subject underwent two screen visits. A total of 18 people were recruited. One subject failed the enrollment criteria due to C-peptide level, and another withdrew from the study. Two subjects' data on MRa and Rd were excluded from the analyses of glucose turnover because of a malfunction of the pump infusing [6-3H]glucose.
Experimental Protocol
Screen visit 1.
Subjects reported in the morning after an overnight fast to the Clinical Research Unit (CRU) of the Mayo Clinic Center for Clinical and Translational Research for a history, physical examination, screening laboratory tests, standard urinalysis, and resting ECG to ensure good health. All women with reproductive capacity had a negative urine pregnancy test within 24 h of the study visit. Dietary histories were obtained to ensure adherence to a weight-maintaining diet consisting of ≥200 g/day carbohydrates and meeting the American Diabetes Association guidelines for protein, fat, and carbohydrates. Body composition was also measured using dual-energy X-ray absorptiometry (23). Subjects performed a graded exercise test on a treadmill to determine V̇o2max according to guidelines (American College of Sports Medicine Guidelines for Exercise Testing and Prescription, 7th ed.) and to ensure stable cardiac status. Expired gases were collected and analyzed using indirect calorimetry. V̇o2max was determined when at least two of the following three criteria were met: 1) subjects were too tired to continue exercise based on Borg's perceived exertion scale, 2) respiratory exchange ratio was >1.1, or 3) a plateau was reached in oxygen consumption with increasing workload. The purpose of this test was to use individualized V̇o2max data to determine workload during the moderate-intensity (∼50% V̇o2max) protocol during study day.
Screen visit 2.
Subjects eligible to enroll based on screening results during screen visit 1 were scheduled for screen visit 2. Using scintigraphic techniques (3), gastric emptying to solids and liquids was assessed; the results were summarized as the time required for 50% of solids and liquids separately to empty (t½). Thereafter, subjects who had normal gastric emptying for solids and liquids proceeded to the inpatient study visit within 3 wk of this visit.
Study Visit
All subjects spent ∼40 h in the CRU.
Day 1.
Subjects were admitted to the CRU at ∼4 PM. A point of care urine pregnancy test was performed where appropriate to ensure that the test was negative before proceeding any further. They were then provided a standard 10 kcal/kg meal (55% carbohydrate, 15% protein, and 30% fat) consumed between 5 and 5:30 PM. Subjects administered premeal insulin bolus guided by their customary insulin/carbohydrate ratio. No additional food was given until the next morning unless required in the context of hypoglycemia. However, there was no hypoglycemia during the study period.
Day 2.
At 6 AM, an intravenous cannula was inserted in a retrograde fashion into a dorsal hand vein and the hand placed in a heated (55°C) Plexiglas box to enable drawing of arterialized venous blood for glucose, glucose tracer, and hormone analyses. At 7 AM, a triple-tracer mixed-meal study was performed (2). Briefly, a mixed meal containing 75 g of glucose enriched with [1-13C]glucose was ingested at time 0. The meal provided ∼33% of daily estimated calorie intake. Subjects administered premeal insulin bolus with their CSII, taking into account their customary insulin/carbohydrate ratio adjusted for the ambient glucose concentrations and the planned exercise. To be pragmatic and to reflect as much of a real-world scenario as possible and to facilitate incorporation of these data into closed control simulation, as we have done for previous studies in T1D (16, 30), each individual subject was permitted to decide on their prandial insulin dose, as they would have normally done during free-living situation. Basal insulin pump rates were not changed during the study duration. Simultaneously, an intravenous infusion of [6-3H]glucose was started and continued for the next 6 h at variable rates to mimic the anticipated rate of appearance of the ingested [1-13C]glucose. Concurrently, the [6, 6-2H2]glucose infusion rate, started at 4 AM at a primed constant rate until 7 AM, was varied thereafter to mimic the anticipated rate of EGP. At 120 min following the first bite, subjects started a treadmill exercise at 50% V̇o2max, i.e., four bouts of walking at 3–4 miles/h for 15 min, with rest periods of 5 min between each walking bout (total duration 75 min, i.e., 120–195 min) (25). The workload during physical activity was monitored continuously by measurements of oxygen consumption (V̇o2) to maintain a target of 50% of V̇o2max. The [6,6-2H2]glucose and [6-3H]glucose infusion rates were modified from the start of physical activity at 120 min for the next 4 h to mimic the anticipated changes in EGP and MRa during and after physical activity. To determine the optimal tracer infusion rates necessary to minimize changes in tracer/tracee concentration for determination of postprandial EGP and MRa, we analyzed data from the first two subjects and modified the tracer infusion rates accordingly. Before modification of the glucose tracer infusion rates, infusion rates (ml/min) for [6,6-2H2]glucose were as follows: time 0–5 min, 0.1; time 5–10 min, 0.09; time 10–20 min, 0.08; time 20–30 min, 0.06; time 30–210, 0.03; time 210–250 min, 0.04; time 250–290 min, 0.066; time 290–360 min, 0.075. Infusion rates (ml/min) for [6-3H]glucose were as follows: time 0–10 min, 0.2; time 10–20 min, 0.52; time 20–30 min, 0.35; time 30–60 min, 0.2; time 60–120 min, 0.1; time 120–180 min, 0.05; time 180–360 min, 0.02. These above rates were identical to those infused in the healthy controls. After modification, infusion rates (ml/min) for [6,6-2H2]glucose were as follows: time 0–5 min, 0.1; time 5–10, 0.08; time 10–20 min, 0.07; time 20–30 min, 0.05; time 30–120, 0.015; time 120–150 min, 0.04; time 150–170, 0.07; time 170–195 min, 0.085; time 195–250 min, 0.07; time 250–360, 0.09. Infusion rates (ml/min) for [6-3H]glucose were as follows: time 0–10 min, 0.2; time 10–60 min, 0.48; time 60–120 min, 0.38; time 120–180 min, 0.25; time 180–230 min, 0.1; time 230–360 min, 0.02. Following the last blood draw, all tracer infusions were stopped, the hand vein cannulae were removed, lunch was provided, and the study was completed.
Analytical Techniques
Hormone analyses.
C-peptide was measured on the Cobas e411 (Roche Diagnostics, Indianapolis, IN) using a two-site electrochemiluminescence immunometric assay. Insulin was measured by a two-site immunoenzymatic assay performed on the DxI automated system (Beckman Instruments, Chaska, MN) and flucagon by a direct double-antibody radioimmunoassay (Linco Research, St. Charles, MO) (23). Catecholamines were measured by reversed-phase HPLC with electrochemical detection after extraction with activated alumina.
Glucose and glucose tracers.
Plasma samples were placed on ice, centrifuged at 4°C, separated, and stored at −80°C until assay. Plasma glucose concentration was measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma [6-3H]glucose-specific activity was measured by liquid scintillation counting as described (1). Plasma enrichment of [1-13C]glucose and [6,6-2H2]glucose were measured using gas chromatography-mass spectrometry (Thermoquest, San Jose, CA) to simultaneously quantitate C-1 to C-2 and C-3 to C-6 fragments (23).
Calculations.
Glucose turnover using the triple-tracer approach was calculated as described (2, 25). Briefly, [6-3H]glucose was used to estimate the systemic rate of appearance of the ingested [1-13C]glucose in the mixed meal. Thereafter, these rates were divided by the [1-13C]glucose enrichment in the meal to obtain systemic appearance of meal glucose. [6,6-2H2]glucose was used to trace the rate of EGP after estimation of endogenous glucose concentration {calculated by subtracting the concentration of exogenously derived (ingested) glucose (i.e., plasma [1-13C]glucose concentration multiplied by meal [1-13C]glucose enrichment) from total plasma glucose concentration}. Rd were calculated by Steele's non-steady-state equation (28).
Statistical Analyses
The primary scientific goal of the study was to validate the triple-tracer mixed-meal experimental protocol during exercise in patients with T1D. To complement that goal, exploratory analyses were conducted to examine the estimated flux values in terms of clinical relevance and expected directions as a part of the validation of the protocol. To do this, longitudinal models consisting of a random subject effect and a fixed effect for time as a factor were used to quantify changes in hormones and other measurements over time. Post hoc comparisons of mean values at each time point were estimated from this model. These general regression models were also supported by more focused comparisons that related to specific hypotheses of interest. These analyses included the incremental area under the curve (iAUC), which was determined by the difference between the area under the curve relative to zero, as determined by the trapezoidal rule, and baseline area (baseline value × length of time).
In addition, subject characteristics for T1D were compared with previously published data on healthy individuals using Wilcoxon rank sum and chi-square tests, and two-sample t-tests were used to compare the iAUC values between T1D and healthy individuals. Key differences were summarized with 95% confidence intervals in the results. P values <0.05 were considered to be supportive of an initial finding; no correction for multiple testing has been applied to reported P values. All statistical analyses were conducted using the SAS System version 9.4 (SAS Institute, Cary, NC).
RESULTS
Subject Characteristics
Table 1 depicts the anthropometric characteristics of the T1D study subjects. Briefly, 50% of the participants were female, and the mean age was 45 yr. The mean (SD) duration of T1D was 26.9 (12.3) yr. Subjects had a mean (SD) fasting plasma glucose and Hb A1c values of 9.9 (3.4) mM and 7.6 (0.7)%, respectively, at the screen visit.
Table 1.
Subject characteristics of type 1 diabetes (n = 16)
| Variable | Means (SD) |
|---|---|
| Age, yr | 44.9 (12.5) |
| Sex [male, n (%)] | 8 (50.0%) |
| Height, m | 1.7 (0.1) |
| Weight, kg | 84.8 (21.0) |
| Fat-free mass, kg | 54.9 (13.0) |
| %Body fat | 34.9 (8.6) |
| V̇o2max, ml·min−1·kg−1 | 25.5 (5.2) |
| BMI, kg/m2 | 28.6 (5.5) |
| Fasting blood blucose, mg/dl | 177.4 (61.1) |
| Fasting blood glucose, mM | 9.9 (3.4) |
| Hb A1c, % | 7.6 (0.7) |
| Hb A1c, mmol/mol | 60.0 (7.5) |
| Duration of type 1 diabetes, yr | 26.9 (12.3) |
| Hemoglobin, g/dl | 13.9 (1.3) |
| BUN, mg/dl | 15.1 (4.5) |
| Creatinine, mg/dl | 0.8 (0.1) |
| Thyroid-stimulating hormone, mIU/l | 2.2 (1.1) |
BUN, blood urea nitrogen.
Gastric Emptying Rates
For liquid emptying (50% empty, t½), mean (SD) was 19.8 (6.7) min (normal range: 33–75 min), whereas for solids (50% empty, t½), mean (SD) was 98.9 (32.4) min (normal range: 71–198 min).
Glucose, Insulin, and Glucagon Concentrations
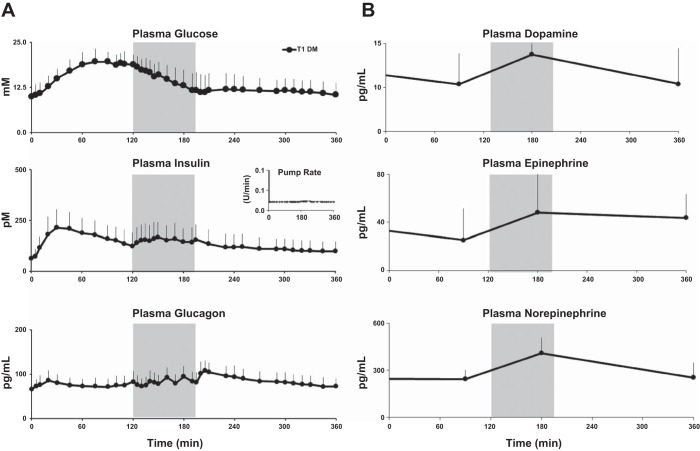
Mean (SD) fasting plasma glucose concentrations were elevated at the start of the meal [9.9 (3.1) mM; Fig. 1A]. Peak mean (range) glucose for all subjects was 21.1 (14.7–27.8) mM. The glucose concentrations gradually fell during exercise, reaching baseline levels at the end of exercise. Thereafter, glucose concentrations remained flat until the end of the study. The plasma glucose concentrations that were elevated after the meal, prior to exercise (time = 20–120 min), and during exercise (120–195 min) were no longer statistically different from baseline (0 min) after exercise (P > 0.07, 240–360 min).
Fig. 1.
A: glucose (top), insulin (middle), and glucagon (bottom) concentrations obtained from time 0 to 360 min in type 1 diabetic (T1D) subjects. Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max. The inset in the middle shows the mean insulin pump infusion rates during the study. B: plasma dopamine (top), epinephrine (middle), and norepinephrine (bottom) concentrations obtained from time 0 to 360 min in T1D subjects. Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max.
As expected, plasma insulin concentrations rose after the prandial bolus, with mean (range) of peak measurements of all subjects being 241.2 (115.8–374.4) pM. The peak concentrations were reached 90 (20–195) min after the bolus given at the start of the meal. Thereafter, insulin concentrations gradually fell until the start of exercise. During exercise (120–195 min), however, plasma insulin concentrations unexpectedly rose (P = 0.005, 150 vs. 120 min) compared with the concentrations prior to the initiation of exercise despite no changes in basal insulin infusion rates. After exercise completion, the concentrations gradually fell and returned to near-baseline levels by the end of the study (P = 0.11).
Glucagon levels, on the other hand, rose 20 min after the meal to a mean level of 84.6 pg/ml, which was an increase from baseline of 18.4 pg/ml (95% confidence interval, 10.7–26.2 pg/ml, P < 0.001). During exercise there was also a gentle rise in glucagon concentrations before they gradually declined to baseline levels (P = 0.18, time = 0 vs. 360 min) at the end of the study (360 min, 71.6 pg/ml).
Dopamine, Epinephrine, and Norepinephrine Concentrations
Plasma dopamine and norepinephrine concentrations increased (P < 0.01), but epinephrine concentrations did not change (P = 0.1) pre- vs. postexercise (Fig. 1B).
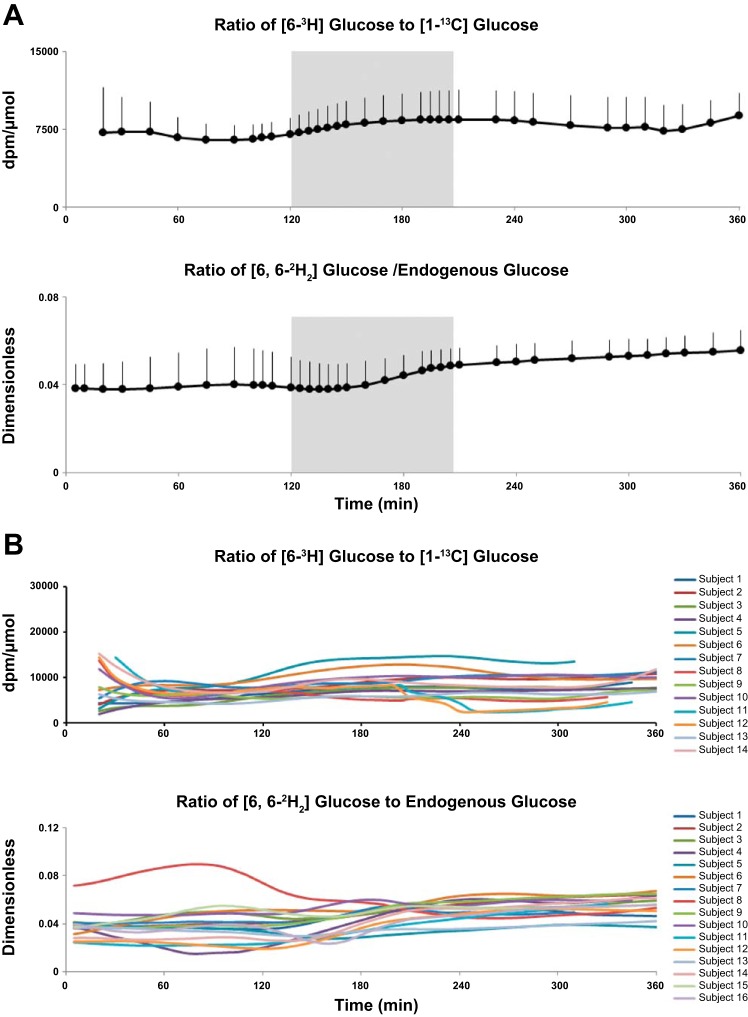
Tracer/Tracee Ratios
The [6-3H]glucose/[1-13C]glucose and [6,6-2H2]glucose/endogenous glucose tracer/tracee ratios rose slightly but not significantly (P = 0.1) during the study, thereby minimizing non-steady-state errors, hence, enabling accurate estimations of MRa, EGP, and Rd throughout the study period (Fig. 2, A and B). Figure 2B represents the individual tracer/tracee ratios during the study.
Fig. 2.
A: [6-3H]glucose/[1-13C]glucose ratio (top) and [6,6-2H2]glucose/endogenous glucose ratio (bottom) obtained from time 0 to 360 min in T1D subjects. Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max. B: individual tracer/tracee ratios during the study.
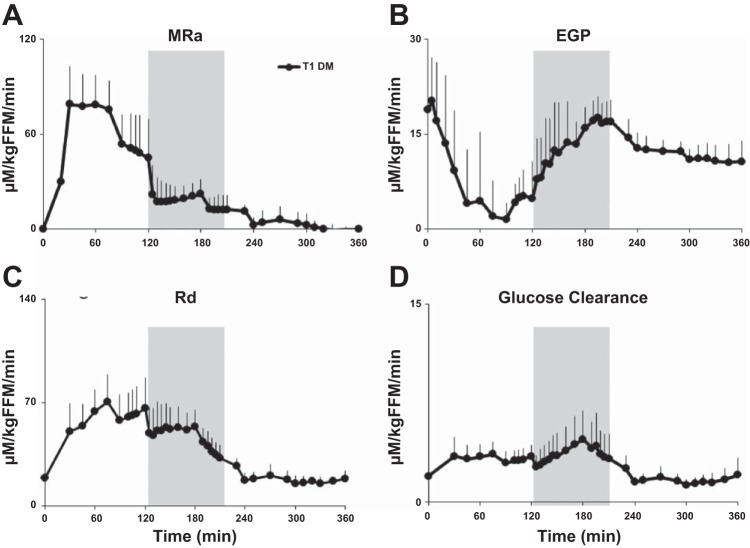
Endogenous Glucose Production
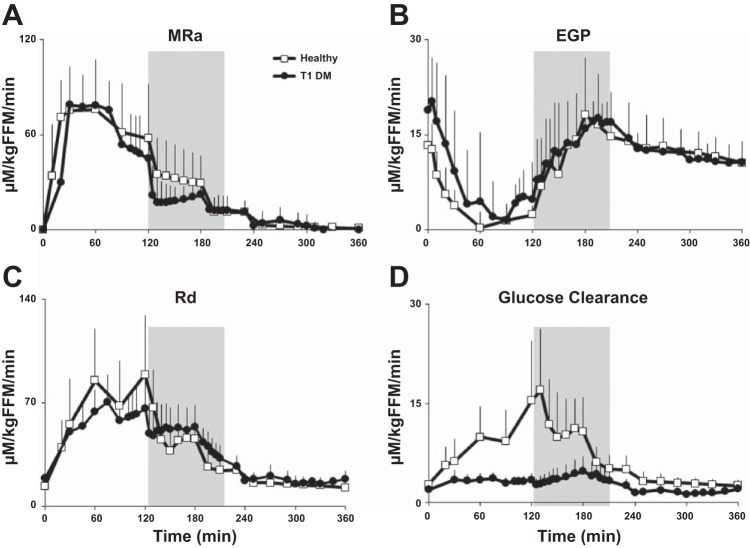
Integrated rates of EGP fell rapidly after the start of the meal (P < 0.003 for all comparisons, time = 30–110 min), reaching a mean (range) time to nadir at 76 (45–130) min (Fig. 3). Thereafter, EGP rose from the nadir time to 200 min after the meal, with values measured between 180 and 210 min not being statistically different from baseline. After 210 min, EGP decreased to less than baseline rates (P < 0.05 for all comparisons).
Fig. 3.
Rates of meal appearance (MRa; A), endogenous glucose production (EGP; B), glucose disappearance (Rd; C), and glucose clearance (D) obtained from time 0 to 360 min in T1D subjects. Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max.
Meal Glucose Appearance, Glucose Uptake, and Glucose Clearance
As explained earlier, due to a malfunction of the [6-3H]glucose infusion pump, data for MRa, Rd, and hence, glucose clearance were unavailable for two subjects. Therefore, on the subset of subjects (n = 14) with complete tracer infusions, MRa reached peak rates, with the mean (range) being 87.7 (70.7–130.6) μM·kg fat-free mass (FFM)−1·min−1 and time = 57 (30–120) min after start of the meal. During exercise (120–195 min), MRa maintained a plateau before rapidly returning close to baseline levels shortly after cessation of exercise (Fig. 3).
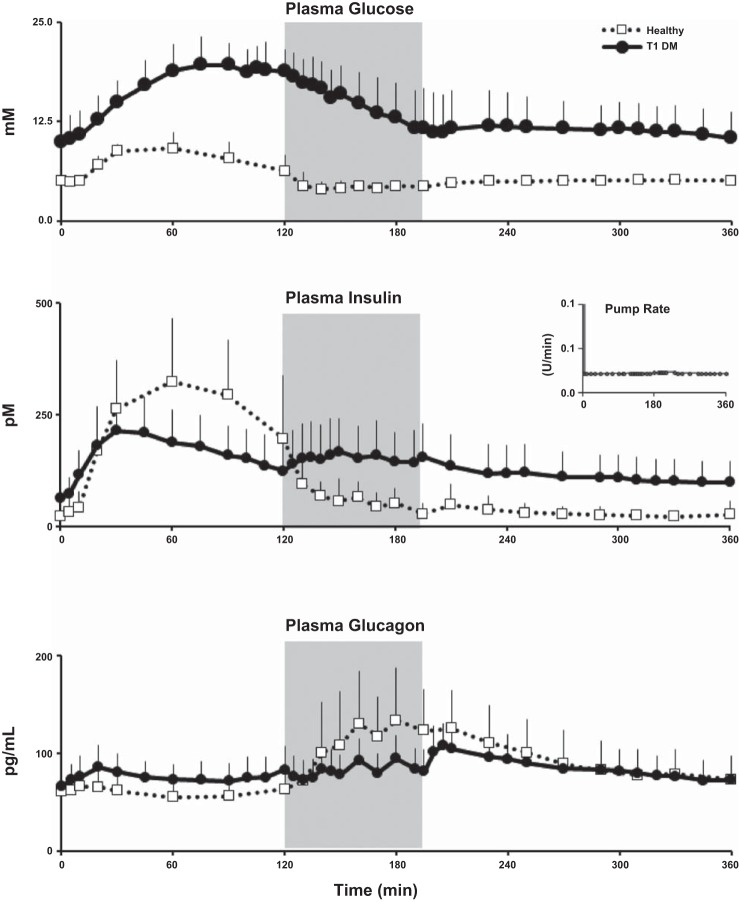
Fig. 4.
Glucose (top), insulin (middle), and glucagon (bottom) concentrations obtained from time 0 to 360 min in T1D subjects (solid line and ●) and healthy controls (dotted line and □). Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max. Inset in middle shows the mean insulin pump infusion rates during the study in T1D subjects.
Rd increased, with mean (range) for all subjects being 78.3 (50.1–107.3) μM·kg FFM−1·min−1 after the meal, reaching a peak rate at 94 (60–150) min. During exercise, Rd rose gently for most (125–180 min) of the exercise period before rapidly falling to near-baseline levels shortly after cessation of exercise (240 min). When considered in the light of the high plasma glucose concentrations, rates of glucose clearance rose gently during exercise, peaking at ∼170 min before returning to baseline levels shortly after completion of exercise. The glucose turnover data from the first two subjects did not differ from the rest (MRa: P = 0.53; EGP: P = 0.14; Rd: P = 0.52).
Comparisons Between T1D and Healthy Participants
Table 2 presents the differences between the healthy individuals and T1D participants (also see Figs. 4 and 5) (18). T1D subjects had a higher BMI, fasting plasma glucose, and Hb A1c but a lower level of fitness (as measured by V̇o2max) than healthy participants. However, the age, FFM, and percent body fat did not differ statistically between groups. There were striking differences in the integrated excursions and patterns of plasma glucose, insulin, glucagon, and Rd and glucose clearance between T1D and healthy subjects, as detailed in Table 3.
Table 2.
Subject characteristics for healthy (n = 12) and type 1 diabetic (n = 16) participants
| Variable | Healthy Participants# [Means (SD)] | Type 1 Diabetes [Means (SD)] | P Value§ |
|---|---|---|---|
| Age, yr | 37.1 (13.0) | 44.9 (12.5) | 0.1313 |
| Sex [male, n (%)] | 5 (41.7%) | 8 (50.0%) | 0.6617 |
| Height, m | 1.7 (0.1) | 1.7 (0.1) | 1.0000 |
| Weight, kg | 70.9 (16.6) | 84.8 (21.0) | 0.1090 |
| FFM, kg | 50.9 (13.5) | 54.9 (13.0) | 0.4718 |
| V̇o2max, ml·min−1·kg−1 | 32.2 (7.2) | 25.5 (5.2) | 0.0114 |
| %Body fat | 29.1 (7.0) | 34.9 (8.6) | 0.1041 |
| BMI, kg/m2 | 24.1 (3.7) | 28.6 (5.5) | 0.0485 |
| Fasting blood glucose, mg/dl | 84.3 (9.9) | 177.4 (61.1) | 0.0001 |
| Fasting blood glucose, mM | 4.7 (0.5) | 9.9 (3.4) | 0.0001 |
| Hb A1c, % | 5.1 (0.2) | 7.6 (0.7) | <0.0001 |
| Hb A1c, mmol/mol | 31.9 (2.6) | 60.0 (7.5) | <0.0001 |
| Hemoglobin, g/dl | 13.9 (1.5) | 13.9 (1.3) | 0.8526 |
| BUN, mg/dl | 14.4 (4.8) | 15.1 (4.5) | 0.5758 |
| Creatinine, mg/dl | 0.8 (0.1) | 0.8 (0.1) | 0.9038 |
| Thyroid-stimulating hormone, mIU/l | 2.4 (1.2) | 2.2 (1.1) | 0.6093 |
FFM, fat-free mass.
Data on the healthy participants have been published previously (18);
P values are from Wilcoxon rank sum/chi-square tests.
Fig. 5.
MRa (A), EGP (B), Rd (C), and glucose clearance (D) obtained from time 0 to 360 min in T1D subjects (●) and healthy controls (□). Shaded box between 120 and 195 min represents exercise period at 50% V̇o2max.
Table 3.
iAUC for hormones and measurements of glucose turnover in type 1 diabetic and healthy participants
| Type 1 Diabetes |
Healthy Participants# |
Type 1 Diabetic vs. Healthy Individuals |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration, min | n | Means | SD | 95% CL of mean | n | Means | SD | 95% CL of Mean | Means | 95% CL of Mean | P Value§ |
| Glucose, mM/min | |||||||||||
| 0–120 | 16 | 823.1 | 233.0 | 698.89, 947.20 | 12 | 326.4 | 121.3 | 249.30, 403.47 | 496.7 | 356.26, 637.06 | <0.0001 |
| 0–360 | 16 | 1,384.2 | 1,182.6 | 754.02, 2,014.39 | 12 | 280.0 | 166.2 | 174.36, 385.56 | 1,104.3 | 394.04, 1,814.46 | 0.0036 |
| 120–195 | 16 | 381.2 | 299.9 | 221.36, 540.99 | 12 | −46.2 | 29.9 | −65.15, −27.21 | 427.4 | 247.88, 606.83 | <0.0001 |
| 195–360 | 16 | 180.0 | 733.1 | −210.66, 570.63 | 12 | −0.3 | 90.7 | −57.87, 57.38 | 180.2 | −259.31, 619.78 | 0.4070 |
| Insulin, pM/min | |||||||||||
| 0–120 | 16 | 11,835.5 | 5,015.3 | 9,163.01, 14,507.89 | 12 | 26,328.8 | 9,729.5 | 20,146.91, 32,510.59 | −14,493.3 | −21,035.41, −7,951.18 | 0.0003 |
| 0–360 | 16 | 26,322.9 | 10,253.5 | 20,859.18, 31,786.54 | 12 | 31,164.0 | 12,492.7 | 23,226.51, 39,101.49 | −4,841.1 | −13,676.22, 3,993.94 | 0.2703 |
| 120–195 | 16 | 6,891.5 | 3,739.1 | 4,899.10, 8,883.91 | 12 | 3,470.8 | 3,252.8 | 1,404.00, 5,537.50 | 3,420.8 | 640.78, 6,200.73 | 0.0178 |
| 195–360 | 16 | 7,595.9 | 4,731.5 | 5,074.66, 10,117.14 | 12 | 1,364.5 | 3,047.8 | −571.99, 3,300.99 | 6,231.4 | 3,009.62, 9,453.18 | 0.0005 |
| Glucagon, pg·ml−1·min−1 | |||||||||||
| 0–120 | 16 | 1,039.8 | 1,132.0 | 436.66, 1,643.02 | 12 | −129.0 | 699.2 | −573.18, 315.27 | 1,168.8 | 405.30, 1,932.30 | 0.0041 |
| 0–360 | 16 | 5,291.1 | 4,560.1 | 2,861.20, 7,720.99 | 12 | 8,944.6 | 5,753.8 | 5,288.79, 12,600.38 | −3,653.5 | −7,656.31, 349.33 | 0.0719 |
| 120–195 | 16 | 1,145.5 | 1,083.5 | 568.12, 1,722.82 | 12 | 3,751.9 | 2,751.6 | 2,003.60, 5,500.15 | −2,606.4 | −4,411.71, −801.10 | 0.0080 |
| 195–360 | 16 | 3,105.8 | 2,809.1 | 1,608.90, 4,602.66 | 12 | 5,321.7 | 3,739.7 | 2,945.55, 7,697.78 | −2,215.9 | −4,755.79, 324.02 | 0.0846 |
| Meal rate of appearance, μM·kg FFM−1·min−1 | |||||||||||
| 0–120 | 14 | 5,712.8 | 1,702.3 | 4,729.98, 6,695.69 | 12 | 7,310.4 | 2,684.4 | 5,604.79, 9,015.96 | −1,597.5 | −3,389.75, 194.67 | 0.0782 |
| 0–360 | 14 | 7,898.6 | 2,471.7 | 6,471.50, 9,325.75 | 12 | 10,445.7 | 4,150.5 | 7,808.57, 13,082.83 | −2,547.1 | −5,264.93, 170.77 | 0.0650 |
| 120–195 | 14 | 1,433.5 | 746.8 | 1,002.29, 1,864.71 | 12 | 2,370.3 | 1,456.1 | 1,445.07, 3,295.43 | −936.7 | −1,924.02, 50.53 | 0.0614 |
| 195–360 | 14 | 752.3 | 452.2 | 491.18, 1,013.39 | 12 | 765.1 | 480.6 | 459.71, 1,070.45 | −12.8 | −390.71, 365.12 | 0.9449 |
| Endogenous glucose production, μM·kg FFM−1·min−1 | |||||||||||
| 0–120 | 16 | −1,377.2 | 582.3 | −1,687.50, −1,066.93 | 12 | −1,194.9 | 383.4 | −1,438.48, −951.35 | −182.3 | −580.85, 216.26 | 0.3558 |
| 0–360 | 16 | −2,856.2 | 1,771.9 | −3,800.31, −1,911.98 | 12 | −1,377.2 | 1,119.6 | −2,088.57, −665.79 | −1,479.0 | −2,680.16, −277.77 | 0.0178 |
| 120–195 | 16 | −458.3 | 488.1 | −718.36, −198.16 | 12 | −102.1 | 424.0 | −371.50, 167.29 | −356.2 | −718.87, 6.56 | 0.0540 |
| 195–360 | 16 | −1,020.7 | 945.1 | −1,524.2, −517.05 | 12 | −80.2 | 514.6 | −407.11, 246.79 | −940.5 | −1,516.44, −364.58 | 0.0025 |
| Rate of disappearance, μM·kg FFM−1·min−1 | |||||||||||
| 0–120 | 14 | 3,769.1 | 1,346.2 | 2,991.83, 4,546.36 | 12 | 6,016.2 | 2,637.0 | 4,340.77, 7,691.65 | −2,247.1 | −4,033.87, −460.36 | 0.0169 |
| 0–360 | 14 | 6,424.7 | 1,769.1 | 5,403.26, 7,446.11 | 12 | 9,253.3 | 4,462.9 | 6,417.74, 12,088.90) | −2,828.6 | −5,773.03, 115.76 | 0.0584 |
| 120–195 | 14 | 2,360.1 | 742.6 | 1,931.35, 2,788.84 | 12 | 2,597.1 | 1,533.1 | 1,622.99, 3,571.16 | −237.0 | −1,268.78, 794.82 | 0.6320 |
| 195–360 | 14 | 295.5 | 532.6 | −12.02, 603.01 | 12 | 640.0 | 789.3 | 138.51, 1,141.56 | −344.5 | −882.64, 193.55 | 0.1988 |
| Glucose clearance, μM·kg FFM−1·min−1 | |||||||||||
| 0–120 | 14 | 123.1 | 77.2 | 78.53, 167.61 | 12 | 703.2 | 404.1 | 446.43, 959.95 | −580.1 | −806.99, −353.26 | <0.0001 |
| 0–360 | 14 | 254.6 | 256.7 | 106.41, 402.78 | 12 | 1,483.4 | 842.5 | 948.12, 2,018.68 | −1228.8 | −1,775.78, −681.82 | 0.0003 |
| 120–195 | 14 | 132.4 | 113.0 | 67.09, 197.60 | 12 | 649.3 | 389.7 | 401.69, 896.88 | −516.9 | −769.42, −264.46 | 0.0007 |
| 195–360 | 14 | −0.8 | 126.5 | −73.88, 72.24 | 12 | 130.9 | 139.7 | 42.15, 219.69 | −131.7 | −239.51, −23.96 | 0.0187 |
iAUC, incremental area under the curve; CL, confidence level.
Healthy participants;
P values are from 2-sample t-tests.
DISCUSSION
We have demonstrated the successful application of the triple-tracer technique (2) to estimate postprandial glucose turnover during moderate-intensity exercise both in healthy (25) and now in T1D subjects. This was possible with appropriate adjustments to glucose tracer infusion rates of [6,6-2H2]glucose to match anticipated changes in endogenous glucose and of [6-3H]glucose to match anticipated changes in MRa during and after exercise based on results obtained from initial few experiments. The resultant tracer/tracee ratios obtained were constant and unchanging, thereby permitting accurate estimates of glucose turnover while minimizing non-steady-state errors in calculations. This iterative process has for the first time, to the best of our knowledge, enabled us to obtain a unique and virtually continuous temporal profile of the components of postprandial glucose metabolism before, during, and immediately after exercise in T1D subjects. Furthermore, careful and frequent sampling of arterialized venous blood before, during, and after exercise has permitted measurements of glucose, insulin, and glucagon concentrations, thus providing valuable data to further our understanding of glucose-insulin-glucagon interactions under these circumstances. Further analyses of the data obtained in this study in T1D subjects and comparison with those obtained in healthy nondiabetic subjects undergoing a similar exercise protocol reported recently (25) have provided several striking differences discussed below.
Fasting glucose concentrations were significantly higher despite higher fasting insulin concentrations in T1D than in healthy subjects, implying that insulin resistance in T1D subjects in the baseline rested state as has been demonstrated before (4, 15). Detailed examination of the concentration profiles of the underlying hormones (insulin and glucagon) provides interesting perspectives and differences. Despite the fact that T1D subjects administered their customary insulin dose at the start of the meal based on the carbohydrate content adjusted for the degree of planned exercise and their premeal glucose levels, it was clearly inadequate to prevent hyperglycemia in the early postprandial (0–120 min) period. This is corroborated by the observation that the insulin excursion during this period was lower, but glucagon excursion higher, in T1D than healthy subjects, implying that coexisting α-cell dysfunction that has been described in T1D (16, 26), which likely also contributed to postprandial hyperglycemia in these individuals.
There were also striking differences in glucose, insulin, and glucagon profiles during (120–195 min) and after exercise (195–360 min). Although glucose concentrations fell rapidly in T1D subjects throughout exercise, they also fell in healthy subjects before rising to baseline levels and maintained a plateau thereafter. In T1D subjects, plasma glucose concentrations also reached a plateau after the end of exercise before slowly dropping to baseline concentrations. It was noteworthy that in these untrained subjects, plasma glucose concentrations did not continue to fall immediately after the completion of exercise. None of the T1D subjects developed hypoglycemia during or after exercise.
Plasma insulin concentration profiles during exercise reveal intriguing differences between groups. As anticipated, in healthy subjects, plasma insulin concentrations rapidly fell to fasting levels (in appropriate response to ambient glucose concentrations) by the end of exercise and remained at this level for the rest of the study (25). In contrast, in T1D subjects, there was a distinct, nontrivial rise in plasma insulin concentrations during exercise despite the fact that insulin pump infusion rates remained at baseline levels in all subjects. Since, as per inclusion criteria, all enrolled subjects were C-peptide negative, it is safe to assume that the only source of insulin in these subjects was exogenous via their insulin pumps. Furthermore, since the insulin pump basal infusion rates were not altered either before or during exercise, it is reasonable to speculate that there was an increased mobilization of subcutaneously delivered insulin from the insulin pump infusion sites in the abdominal subcutaneous fat depots (since all subjects had their pumps inserted in their abdomen), which was due presumably to increased subcutaneous adipose tissue blood flow during exercise (10). To the best of our knowledge, this increase in plasma insulin concentrations during exercise in T1D subjects has not been described previously and could contribute to enhanced insulin action over and above the independent effects of exercise per se (11, 12, 32) both during and after exercise. However, this important observation needs to be considered by patients, care providers, and scientists responsible for developing the next generation of open-loop and closed-loop therapeutic strategies and algorithms. It is also noteworthy that plasma insulin concentrations at the end of the study remained 50% higher than at the start of the study period. This could be due to continued increased mobilization of insulin from subcutaneous fat depots, reduced insulin clearance, reduced volume of distribution, or a combination thereof despite prior observations that insulin clearance increases during exercise in T1D subjects (29). This observation assumes even more importance because, in sharp contrast to our recent report in T1D subjects (16) where plasma insulin concentrations returned to baseline preprandial levels in the absence of exercise within 3 h after a mixed meal in our current study, plasma insulin concentrations did not return to baseline even after the completion of exercise and remained substantially higher than premeal concentrations.
Plasma glucagon excursions also show intriguing differences between healthy and T1D subjects that support the notion of coexisting α-cell dysfunction in T1D (26). Glucagon concentrations were higher in the postprandial preexercise period (0–120 min) in T1D than in healthy subjects despite the higher plasma glucose concentrations during this time. During exercise, whereas glucagon concentrations rose briskly (∼2-fold) in healthy subjects, in T1D subjects the rise was blunted (∼1.3-fold) and slower, implying persistent α-cell dysfunction during exercise in these individuals. Although this observation, coupled with higher plasma insulin concentrations and lower hepatic glycogen reserves, (17) could expose T1D subjects to greater risk of hypoglycemia during exercise, we did not observe clinical or biochemical hypoglycemia in this study likely because of postprandial hyperglycemia. However, it is possible that if the subjects had administered a higher meal insulin bolus to lower postprandial glucose concentrations and/or exercised for a longer duration or intensity, their risk of hypoglycemia would have increased substantially.
In the postprandial period in vivo, it has been estimated using arterio-venous difference across the leg that greater than 80% of glucose disposal during hyperinsulinemia is accounted for by the skeletal muscle, with other tissues (i.e., adipose or liver) accounting for a much lower percentage (7). However, the arterio-venous difference measurements (7) were made in relatively slim type 2 diabetes subjects that were close to their ideal weight and matched with a control group. The current study reports measurements made in T1D patients with 35% body fat and matched with a control group with 29% body fat with a high likelihood of higher levels of perivascular adipocytes. Therefore, we cannot exclude that adipose tissue could account for a larger percentage of glucose disposal in this study.
There are several intriguing inferences that arose from these observations in this cohort of untrained individuals with and without T1D. First, T1D subjects appeared to be insulin resistant in the postprandial state and particularly during exercise, as has been observed in prior studies (4, 15). This was especially relevant during exercise, when both plasma insulin and glucose concentrations were higher in T1D than healthy subjects. Second, both Rd and glucose clearance rapidly returned to baseline preprandial levels within 30–45 min after the completion of exercise in both groups, thus implying that exercise-induced changes in tissue glucose uptake (both insulin and noninsulin mediated) do not persist for a prolonged period after cessation of exercise. However, this observation will need to be carefully considered in the light of differing insulin concentrations between the two groups, i.e., glucose clearance was numerically same in both groups after exercise in the presence of significantly lower peripheral insulin concentrations in the healthy subjects. Taken together, this implies but does not prove that if one could match the glucose and insulin concentrations in both groups, Rd and clearance would have been higher in healthy than in T1D subjects. Third, there was a brisk rise in rates of EGP during exercise in both groups. In healthy subjects, this was facilitated naturally by falling insulin and glucose levels and rapidly rising glucagon concentrations. Interestingly, the equally rapid increase in EGP in T1D subjects during exercise despite higher glucose levels and lower glucagon concentrations, all of which would normally suppress EGP, implies that robust exercise induced hepatic responsivity despite adverse hormonal and substrate milieu, which necessitates further investigations. Factors that could contribute to this adaptive process in T1D subjects could be related to increased hepatic glucagon sensitivity stimulating glycogenolysis, increased hepatic gluconeogenesis due to enhanced substrate availability (e.g., lactate, free fatty acids), or potential blood-borne feedback and afferent mechanisms that have been shown more recently to modulate glucose Ra (6, 18, 19).
We did not measure lactate turnover nor rates of gluconeogenesis during the study, and hence, we cannot comment on the relative contributions of these metabolic processes to rates of EGP. An additional factor that needs to be considered is that whereas in T1D subjects peripheral and portal insulin concentrations maybe similar, it is different in healthy subjects. Hence, the wide difference in insulin concentrations observed between groups in the peripheral circulation during exercise would be lesser in the portal circulation. In contrast, it is possible that the difference between groups in glucagon concentrations in the peripheral circulation during exercise would be similar to that in the portal circulation since it is likely (but not proven) that hepatic glucagon clearance does not differ between healthy and T1D subjects. Therefore, the insulin/glucagon ratio in the portal circulation, which directly modulates EGP, would be different than that observed in the peripheral circulation between healthy and T1D subjects. Future studies are necessary to elucidate these aspects further in individuals with T1D.
The primary goal of our study was to refine the triple-tracer technique to accurately estimate glucose turnover continuously during and after exercise in the postprandial period under physiologically relevant conditions (i.e., without performing the conventional but nonphysiological insulin clamp technique) in individuals with T1D on insulin pump therapy. Based on the relatively unchanging tracer/tracee ratios that enable accurate estimation of glucose turnover, we were successful in achieving those goals. That said, a limitation of this study is the absence of a no-exercise control visit in the same study cohort. With the current design, it is also not possible to conclude whether both the T1D and controls experience the benefit of exercise with skeletal muscle glycogen depletion leading to increases in insulin-stimulated glucose uptake for up to 48 h after the exercise bout via described mechanisms (13). This implies that the question of whether the T1D subjects benefit from a single bout of exercise as much as healthy individuals cannot be answered without further research. Additionally, the study of the glucose, insulin, and glucagon plasma profiles under the experimental conditions also permitted insights into novel observations that include a suggestion of mobilization of insulin from subcutaneous injection depots and suboptimal glucagon rise during exercise and highlight the possibility of yet to be identified blood-borne feedback or afferent mechanisms that might stimulate Ra during exercise. Furthermore, the maintenance of the “normal” rise in EGP during exercise despite unfavorable glucose and hormonal milieu in these subjects provides material for further investigations in this area.
Although there were no statistical differences between the two groups in age, weight, and percent body fat, these values tended to be higher in T1D subjects. BMI was higher in T1D subjects, although FFM did not differ between groups. These anthropometric differences could have at least in part contributed to the changes in patterns of postprandial glucose turnover between groups.
Taken together, information on exercise effects on glucose kinetics could be useful to inform and modify the gain function of an insulin controller of an artificial pancreas system in the future. Finally, comparing the hormonal and glucose turnover data between healthy (25) and T1D subjects undergoing an identical study design offers valuable new hypotheses generating ideas that deserve to be tested in future studies.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-R01-085561 and DK-DP3-094331 and Grant No. UL1-TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. M. Schiavon, C. Dalla Man, and C. Cobelli were partially funded by Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Progetto FIRB 2009).
DISCLOSURES
A. Basu is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. There are no conflicts of interest to declare for any of the authors.
AUTHOR CONTRIBUTIONS
A.M., L.H., Y.C.K., and A.B. performed experiments; A.M., L.H., M.S., C.D.M., V.D., R.B., R.L., C.C., M.L.J., R.C., Y.C.K., and A.B. analyzed data; A.M., L.H., M.S., C.D.M., R.B., R.L., C.C., M.L.J., R.C., Y.C.K., and A.B. interpreted results of experiments; A.M., L.H., R.B., R.L., C.C., R.C., Y.C.K., and A.B. prepared figures; A.M., L.H., R.B., R.L., C.C., R.C., Y.C.K., and A.B. drafted manuscript; A.M., M.S., C.D.M., V.D., R.B., R.L., C.C., M.L.J., R.C., Y.C.K., and A.B. edited and revised manuscript; A.M., L.H., M.S., C.D.M., V.D., R.B., R.L., C.C., M.L.J., R.C., Y.C.K., and A.B. approved final version of manuscript; R.B., R.C., Y.C.K., and A.B. conception and design of research.
ACKNOWLEDGMENTS
We are deeply indebted to the research participants. We give our sincere thanks to the staff of the Mayo Clinic Center for Translational Science Activities CRU, the GI Motility Core, the CRU Mass Spectroscopy Laboratory, the CRU Immunochemical Core Laboratory, Pamela Reich (research assistant), Brent McConahey (research assistant), and Shelly McCrady Spitzer (research assistant). All persons mentioned above are at the Endocrine Research Unit, Mayo Clinic, Rochester, MN.
The data in this article were presented in part at the Annual Scientific Meeting of the American Diabetes Association in San Francisco, CA, June 2014.
REFERENCES
- 1.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 32: 866–872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza R, Cobelli C. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 284: E55–E69, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 70: 415–420, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is “double diabetes” and what are the risks? Diabetologia 56: 1462–1470, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coggan AR, Swanson SC, Mendenhall LA, Habash DL, Kien CL. Effect of endurance training on hepatic glycogenolysis and gluconeogenesis during prolonged exercise in men. Am J Physiol Endocrinol Metab 268: E375–E383, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Coker RH, Koyama Y, Denny JC, Camacho RC, Lacy DB, Wasserman DH. Prevention of overt hypoglycemia during exercise: stimulation of endogenous glucose production independent of hepatic catecholamine action and changes in pancreatic hormone concentration. Diabetes 51: 1310–1318, 2002. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Gunnarsson R, Björkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest 76: 149–155, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enoksson S, Caprio SK, Rife F, Shulman GI, Tamborlane WV, Sherwin RS. Defective activation of skeletal muscle and adipose tissue lipolysis in type 1 diabetes mellitus during hypoglycemia. J Clin Endocrinol Metab 88: 1503–1511, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Felig P, Wahren J. Fuel homeostasis in exercise. N Engl J Med 293: 1078–1084, 1975. [DOI] [PubMed] [Google Scholar]
- 10.Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond) 38: 1019–1026, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol (1985) 82: 1360–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Piacentini MF, Trimmer JK, Brooks GA. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men. J Appl Physiol (1985) 85: 1175–1186, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Frosig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity (Silver Spring) 17, Suppl 3: S15–S20, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab 297: E242–E251, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenbaum CJ. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev 18: 192–200, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Hinshaw L, Dalla Man C, Nandy DK, Saad A, Bharucha AE, Levine JA, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern of insulin action in type 1 diabetes: implications for a closed-loop system. Diabetes 62: 2223–2229, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang JH, Perseghin G, Rothman DL, Cline GW, Magnusson I, Petersen KF, Shulman GI. Impaired net hepatic glycogen synthesis in insulin-dependent diabetic subjects during mixed meal ingestion. A 13C nuclear magnetic resonance spectroscopy study. J Clin Invest 95: 783–787, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaer M, Pollack SF, Mohr T, Weiss H, Gleim GW, Bach FW, Nicolaisen T, Galbo H, Ragnarsson KT. Regulation of glucose turnover and hormonal responses during electrical cycling in tetraplegic humans. Am J Physiol Regul Integr Comp Physiol 271: R191–R199, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Kjaer M, Secher NH, Bangsbo J, Perko G, Horn A, Mohr T, Galbo H. Hormonal and metabolic responses to electrically induced cycling during epidural anesthesia in humans. J Appl Physiol (1985) 80: 2156–2162, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Maran A, Pavan P, Bonsembiante B, Brugin E, Ermolao A, Avogaro A, Zaccaria M. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther 12: 763–768, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J Clin Endocrinol Metab 89: 4656–4664, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 13: 819–825, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Saad A, Dalla Man C, Nandy DK, Levine JA, Bharucha AE, Rizza RA, Basu R, Carter RE, Cobelli C, Kudva YC, Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61: 2691–2700, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samols E, Weir GC. Adrenergic modulation of pancreatic A, B, and D cells alpha-Adrenergic suppression and beta-adrenergic stimulation of somatostatin secretion, alpha-adrenergic stimulation of glucagon secretion in the perfused dog pancreas. J Clin Invest 63: 230–238, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiavon M, Hinshaw L, Mallad A, Dalla Man C, Sparacino G, Johnson M, Carter R, Basu R, Kudva Y, Cobelli C, Basu A. Postprandial glucose fluxes and insulin sensitivity during exercise: a study in healthy individuals. Am J Physiol Endocrinol Metab 305: E557–E566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr J, Tsalikian E, Fox L, Buckingham B, Weinzimer S, Tamborlane WV, White NH, Arbelaez AM, Kollman C, Ruedy KJ, Cheng P, Beck RW. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes Care 37: 1741–1744, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shilo S, Sotsky M, Shamoon H. Islet hormonal regulation of glucose turnover during exercise in type 1 diabetes. J Clin Endocrinol Metab 70: 162–172, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956. [DOI] [PubMed] [Google Scholar]
- 29.Tuominen JA, Ebeling P, Koivisto VA. Exercise increases insulin clearance in healthy man and insulin-dependent diabetes mellitus patients. Clin Physiol 17: 19–30, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Visentin R, Dalla Man C, Kudva YC, Basu A, Cobelli C. Circadian variability of insulin sensitivity: physiological input for in silico artificial pancreas. Diabetes Technol Ther 17: 1–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest 50: 2715–2725, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol (1985) 65: 909–913, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman DH, Williams PE, Lacy DB, Green DR, Cherrington AD. Importance of intrahepatic mechanisms to gluconeogenesis from alanine during exercise and recovery. Am J Physiol Endocrinol Metab 254: E518–E525, 1988. [DOI] [PubMed] [Google Scholar]
- 34.Wolfe RR, Nadel ER, Shaw JH, Stephenson LA, Wolfe MH. Role of changes in insulin and glucagon in glucose homeostasis in exercise. J Clin Invest 77: 900–907, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]