Abstract
Purpose. Experimental data suggest that tumour cells can reversibly transition between epithelial and mesenchymal states (EMT and MET), a phenomenon known as cellular plasticity. The aim of this review was to appraise the clinical evidence for the role of cellular plasticity in prostate cancer (PC) bone metastasis. Methods. An electronic search was performed using PubMed for studies that have examined the differential expression of epithelial, mesenchymal, and stem cell markers in human PC bone metastasis tissues. Results. The review included nineteen studies. More than 60% of the studies used ≤20 bone metastasis samples, and there were several sources of heterogeneity between studies. Overall, most stem cell markers analysed, except for CXCR4, were positively expressed in bone metastasis tissues, while the expression of EMT and MET markers was heterogeneous between and within samples. Several EMT and stemness markers that are involved in osteomimicry, such as Notch, Met receptor, and Wnt/β pathway, were highly expressed in bone metastases. Conclusions. Clinical findings support the role of cellular plasticity in PC bone metastasis and suggest that epithelial and mesenchymal states cannot be taken in isolation when targeting PC bone metastasis. The paper also highlights several challenges in the clinical detection of cellular plasticity.
1. Introduction
Despite advances in the early diagnosis and management of prostate cancer (PC), bone metastasis of PC cells causes significant morbidity and is associated with four to six times higher mortality rates than localized PC [1, 2]. Furthermore, it is not uncommon for PC patients with bone metastasis who initially respond to androgen deprivation therapy to progress to castration resistant PC (CRPC) [3, 4]. It has been recently recognised that the development of successful therapeutic targets against metastasis is challenged by the presence of a subpopulation of cells within tumours characterised by increased resistance to standard radiotherapy, chemotherapy, and hormonal therapy. These cells, referred to as cancer stem cells (CSCs), have tumour-initiating and self-renewal abilities and are believed to be critical drivers of tumour progression [5].
CSCs have first been described in human leukaemia [6] and subsequently demonstrated in solid tumours, such as those of the breast, brain, and prostate [7–9]. Prostate cancer stem cells have been characterised by a CD44+/α 2 β 1 hi/CD133+ phenotype [9]. Importantly, studies have indicated a connection between CSCs and the transition from epithelial to mesenchymal state (EMT) [10–12]. In EMT, polarized immobile epithelial cells convert into spindle-shaped motile mesenchymal cells. This process facilitates detachment of cancer cells from the primary tumour via loss of intercellular adhesion and acquisition of migratory and invasive characteristics. One of the hallmarks of EMT is loss of the transmembrane cell adhesion glycoprotein E-cadherin and an increase in the expression of the mesenchymal markers N-cadherin and vimentin [13, 14]. Several transcription factors have been shown to regulate E-cadherin expression including Snail1, Snail2, Slug, TWIST1, ZEB1, and ZEB2 [13–16]. In addition, maintenance of cellular adhesion between epithelial cells requires the proper interaction between the intracellular domain of E-cadherin and the β-catenin protein. Disruption of this E-cadherin/β-catenin complex in EMT results in translocation of β-catenin into the nuclear compartment and activation of the Wingless (Wnt) signalling pathway [13, 15]. Preclinical models have shown that activation of the Wnt pathway and other signalling pathways such as Notch and transforming growth factor-β (TGFβ) induces EMT and generates stem cell-like phenotypes [17–21].
There is evidence that a transition from a mesenchymal to an epithelial (MET) state, which is the reverse process of EMT, occurs when tumour cells colonise distant sites [22]. The reexpression of E-cadherin facilitates intercellular adhesion between metastatic cells and subsequent tumour growth. This ability of cancer cells to switch between epithelial and mesenchymal phenotypes is referred to as cellular plasticity [23]. An emerging paradigm, based mostly on experimental evidence, proposes that cellular plasticity plays a critical role during the metastatic process, where EMT is critical in the initial invasive and migratory stages, while MET enhances the latter stages of metastatic colonization and growth [22, 24].
The role of cellular plasticity in PC bone metastasis formation is not yet fully understood [25]. In two experimental studies, interaction between the androgen refractory cancer of the prostate (ARCaP) cell lines and bone stromal cells resulted in a mesenchymal phenotype with a switch from E-cadherin to N-cadherin expression [26, 27]. While in another study, similar interactions resulted in an epithelial phenotype with increased expression of E-cadherin [28]. In addition, there have been inconclusive data from experimental studies on the connection between EMT/MET and cancer stem cell states in PC. In one report, pluripotent PC stem cells that express SOX2 and OCT3/4 were found to express E-cadherin [29], while, in two other studies, an EMT phenotype was associated with reduced E-cadherin expression and increased expression of stem cell markers Sox2, Nanog, Oct4, Lin28B, CD44, and/or Notch-1 [12, 30].
These, and other preclinical studies for other types of cancers, suggest that the relation between mesenchymal, epithelial, and CSC states may be more complex than previously viewed and that an epithelial phenotype could promote tumour aggressiveness. For example, breast CSCs have been demonstrated to have the ability to reversibly transition between mesenchymal-like and epithelial-like stem cell states, and it has been shown that the colonization of breast CSCs in bone induces a phenotypic switch from CD44+/CD24− to CD44−/CD24+ cells and co-expression of both epithelial and mesenchymal markers [31, 32]. This ability of CSCs to dynamically transition between epithelial, mesenchymal, and intermediate states facilitates their adaptation to altered microenvironmental stimuli [30]. Hypoxia seems to have a central role in promoting phenotypic transitions and cellular plasticity [33], and E-cadherin has been recently reported to play a role in regulating the response of cancer cells to hypoxia by inducing the expression of hypoxia-inducible factor-1α (HIF-1α) [34]. These experimental data support a context-dependent role for the epithelial phenotype in enhancing the survival of cancer cells. In addition, E-cadherin has been shown to have a central function in establishing and maintaining the pluripotent and self-renewal properties of prostate CSCs and metastatic tumour-initiating cells [29, 35, 36]. Taken together, these experimental data demonstrate that both epithelial and mesenchymal phenotypes endow metastatic cells with structural and functional properties that allow their survival and growth within the altered environment.
Despite the wealth of in vitro and experimental data suggesting the plasticity of cancer cells, it has been difficult to validate cellular plasticity of metastatic tumours in the clinical setting. This is in part because samples taken from patients represent tumour status at a static point in time and do not capture the dynamic phenotypic alterations that have been demonstrated in experimental studies. However, it is imperative that these findings be clinically validated before their translation into potential therapeutic approaches is considered. Therefore, this paper focuses on the clinical evidence of the role of cellular plasticity in PC bone metastasis by reviewing studies that have analysed the differential expression of epithelial, mesenchymal, and stem cell markers in clinical PC bone metastasis tissues compared to primary PC or nonskeletal metastasis tissues.
2. Methods
2.1. Literature Search and Eligibility Criteria
A search of the PubMed database for English language studies published up to June 2014 was conducted using the following search algorithms for keywords in the title/abstract: (1) (“prostate cancer” OR “prostate carcinoma”) AND (“bone” OR “skeletal” OR “skeleton” OR “osseous”) AND (“metastasis” OR “metastases”) AND (“cadherin” OR “catenin” OR “vimentin” OR “stem” OR “progenitor” OR “tumor initiating” OR “EMT”) and (2) (“prostate cancer” OR “prostate carcinoma”) AND (“bone” OR “skeletal” OR “skeleton” OR “osseous”) AND (“metastasis” OR “metastases”) AND (“tissue” OR “specimen”). Only studies of human bone metastasis tissues were reviewed. Studies based solely on the use of cell lines or animal models, reviews, case reports, letters to editors, and abstracts with no full reports were excluded. The reference lists from included articles were examined to identity further relevant studies.
2.2. Data Extraction
The following data were extracted from eligible studies: first author and year of publication, number of tissue specimens analysed and their type, tumour grade or Gleason score, treatment status at time of biopsy, marker(s) studied, method of marker detection, criteria used to score marker expression, differential pattern of expression in bone metastasis tissues, and statistical significance of results. Online supplementary files were used to extract relevant data when they were not available in main article.
2.3. Data Synthesis
Descriptive characteristics for studies were summarised. No formal quantitative synthesis (meta-analysis) of the data was performed due to heterogeneity between studies with regard to markers investigated, study population (tumour grade and treatment status), criteria used to score marker expression, and quantitative analysis methods employed.
3. Results
3.1. Literature Search Results
The electronic search identified a total of 720 studies. Of these, 660 were excluded and 60 studies were retrieved based on abstract relevance. Following full-text assessment, 16 studies were deemed eligible, and three additional relevant studies were identified from reference lists. Thus, a total of 19 studies were included in this review. A flowchart of the search and study selection process is shown in Figure 1.
Figure 1.
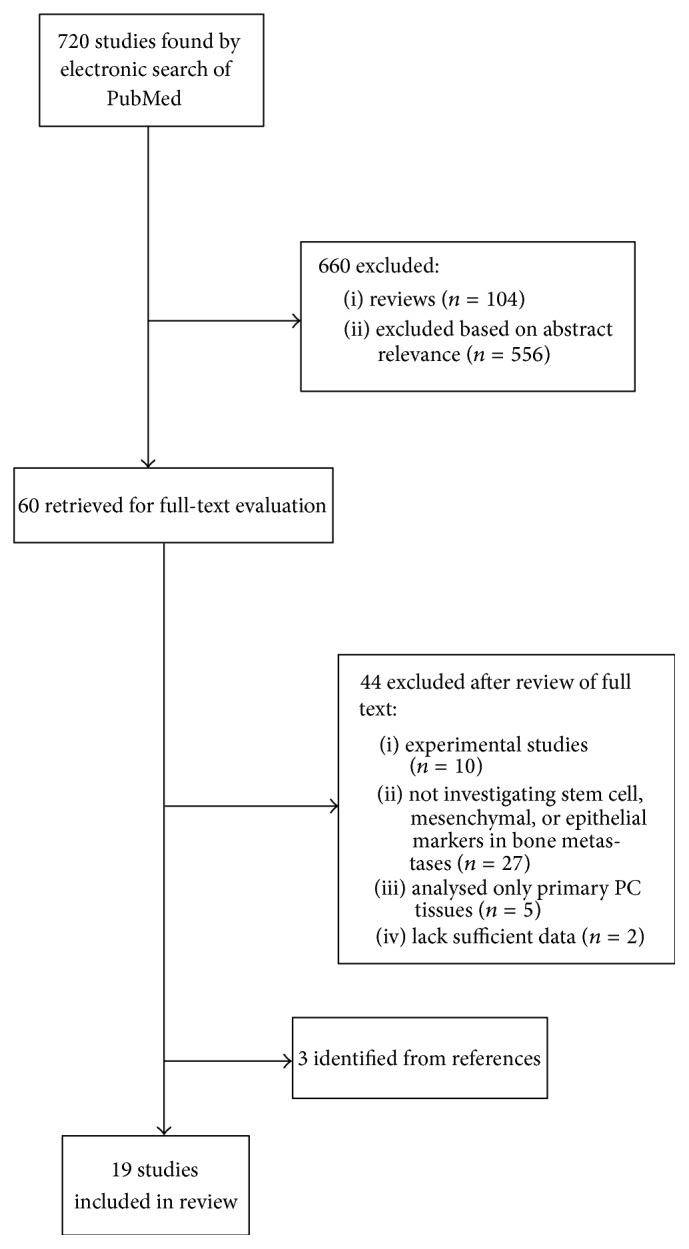
Flow chart of search strategy and study selection.
3.2. Study Characteristics
Table 1 summarises the descriptive characteristics of included studies. More than 60% of the studies were published after 2007. Eight studies [37–44] investigated the differential expression of stem cell markers in bone metastasis tissues, and eleven studies [45–55] analysed the differential expression of epithelial markers (mostly E-cadherin) and/or mesenchymal markers (mostly vimentin). Most studies (74%) compared marker expression between primary PC and bone metastasis tissue samples, and only 7 studies used matched tissue samples from the same patients [37, 39, 41, 42, 46, 51, 52].
Table 1.
Descriptive characteristics of included studies.
| Year of publication | Number of studies |
|
| |
| 1999 | 1 |
| 2000 | 1 |
| 2002 | 3 |
| 2004 | 1 |
| 2005 | 1 |
| 2008 | 3 |
| 2010 | 3 |
| 2011 | 3 |
| 2012 | 2 |
| 2013 | 1 |
|
| |
| Sample size | Median (range) |
|
| |
| Number of BM specimens | 17 (2–184) |
| Number of primary PC specimens | 22 (6–112) |
| Number of nonskeletal specimens | 23 (7–97) |
|
| |
| Tissue comparisons | Number of studies |
|
| |
| Studies that used matched samples | 7 |
| Comparisons between BM and primary PC | 14 |
| Comparisons between BM and nonskeletal metastases | 7 |
| Comparisons between different BM tissues | 2 |
|
| |
| Markers analysed | Number of studies |
|
| |
| Stem cell markers | 8 |
| EMT/MET markers | 11 |
|
| |
| Main method of marker detection | Number of studies |
|
| |
| Immunohistochemistry (IHC) | 18 |
| In situ hybridisation (ISH) | 1 |
|
| |
| Tumour grade and treatment status | Number of studies |
|
| |
| Studies that reported tumour grade | 12 |
| Studies that reported treatment status | 9 |
|
| |
| Criteria used to score expression | Number of studies |
|
| |
| Pattern of distribution of staining across cell | 6 |
| Percentage of positive cells in each specimen | 5 |
| Staining intensity scoring | 2 |
| Staining intensity multiplied by % of positive cells | 5 |
| Quantitative analysis of % of positive staining areas using Image Pro Plus 6.2 software | 1 |
| Did not report a scoring method | 2 |
|
| |
| Reporting of results | Number of studies |
|
| |
| Statistical analysis of differential expression | 11 |
Note: BM: bone metastasis.
The median number of bone samples analysed was 17, and around 63% of the studies used ≤20 bone samples. All studies used immunohistochemistry (IHC) for protein detection in bone metastasis samples, except for one study [46], which used in situ hybridisation (ISH) for mRNA detection. The study by Armstrong et al. [52] used IHC for marker staining in bone tissues and the FDA-approved CellSearch system for detecting circulating tumour cells. Not all studies reported the tumour grade or Gleason score of the primary or metastasis tissues examined or the treatment status of patients at time of bone biopsy, and among those that did, there was wide heterogeneity in the patient population (details provided in Supplementary Tables 1 and 2) (see Supplementary Material available online at http://dx.doi.org/10.1155/2015/651580).
In general, different criteria were used to score marker expression patterns. Although only two studies did not report scoring criteria, there were variations in the criteria used to categorise positive staining, staining intensity, and staining pattern. Details of these are provided in Supplementary Tables 1 and 2. Eleven studies (58%) reported statistical analysis results of differences in pattern of marker expression between bone metastasis tissues and other tissues, all of which showed statistically significant results. Five out of these eleven studies (45%) used ≤20 bone metastasis specimens.
3.3. Expression Pattern of Stem Cell Markers
Table 2 and supplementary Table 1 show details from eight studies that analysed the differential expression of different stem cell markers in bone metastasis tissues compared to primary PC [37, 38, 40–44] and nonskeletal metastasis [38, 39, 43, 44]. The prostate stem cell antigen (PSCA) was found to be overexpressed in bone metastases compared to both primary PC tissues [37] and nonskeletal (lymph node and liver) metastases [39]. C-kit was found to be overexpressed in bone metastases compared to primary PC tissues [40]. A study that analysed the expression of aldehyde dehydrogenase (ALDH) isoforms in 10 PC tissues and their matched bone metastases found positive staining for the ALDH7A1 isoform in both tissues, with absent staining for ALDH1 [42].
Table 2.
Details of studies analysing expression of stem cell markers in PC bone metastases.
| Study | Marker | No. of BM specimens | Comparison tissue (No. of specimens) | Results | Significance |
|---|---|---|---|---|---|
| Gu et al., 2000 [37] | PSCA | 9 | Normal (25) Primary PC (112, 3 matched) |
All normal tissues were negative. PSCA expression was positive in 105/112 (94%) primary and in 9/9 (100%) BM | NR |
|
| |||||
| Knudsen et al., 2002 [38] | Met receptor | 45 | Primary PC (90) LN (35) Other soft tissues (8) |
High Met expression in 52% of primary, 83% of BM, and 54% of LN metastases | P = 0.0003 for BM versus LN |
|
| |||||
| Lam et al., 2005 [39] | PSCA | 47 | LN (6, 5 matched) Matched liver (3) |
PSCA staining intensity was higher in BM (87%) compared with LN (67%) and liver (67%) metastases | P = 0.014 for BM versus LN. No statistical analysis for liver metastases due to small sample size |
|
| |||||
| Wiesner et al., 2008 [40] | c-kit, SCF | 20 | Primary PC (21) BPH (22) |
Positive staining for c-kit in 5% of BPH, 14% of primary PC, and 40% of BM Positive SCF staining in 95% of BPH and primary PC and in 85% of BM specimens |
P = 0.0077 for BM versus BPH |
|
| |||||
| Eaton et al., 2010 [41] | CD133, CD44, α2β1 integrin, CXCR4, c-met, α6 integrin | 11 | Matched primary PC (11) | 50% of samples positive for CD133. >70% positive for CD44, α2β1, c-met, and α6 integrin. No difference in expression between primary and BM samples for all markers except for CD44. Higher expression of CD44 in BM, with positive staining in bone stoma. Low staining for CXCR4 in both BM and primary samples | NR |
|
| |||||
| van den Hoogen et al., 2010 [42] | ALDH isoforms | 10 | Matched primary PC (10) | No staining for ALDH1 in BM or primary PC ALDH7A1 in 7/10 primary PC and 8/10 BM with no staining in bone stroma | NR |
|
| |||||
| Castellón et al., 2012 [43] | CD133, CD44 | 5 | Primary PC (34) LN (7) |
BM and LN showed lower expression of both CD133 and CD44 compared with primary tissues | P < 0.05 for BM and LN versus medium Gleason grade |
|
| |||||
| Sottnik et al., 2013 [44] | α2β1 | 184 | BPH (43) Primary PC (76) LN (61) Liver (36) |
Higher expression in BM compared to primary PC and nonskeletal metastases | P < 0.042 for BM versus LN |
ALDH: aldehyde dehydrogenase, BM: bone metastasis, BPH: benign prostatic hyperplasia, LN: lymph node, No.: number, NR: not reported, PSCA: prostate stem cell antigen, and SCF: stem cell factor.
Two studies [41, 43] reported different results in relation to the expression of the stem cells markers CD133 and CD44. Castellón et al. [43] found significantly lower expression of both CD133 and CD44 in bone metastases compared to primary tissues. While in the study by Eaton et al. [41], CD44 expression was more frequent in bone metastasis tissues than in primary cancers and CD133 was detected in half of the bone metastases samples. Eaton et al. also found that >70% of bone specimens displayed positive staining for α2β1 integrin, c-met, and α6 integrin, but the staining for CXCR4 was low in both primary and metastases tissues [41]. Sottnik et al. [44] and Knudsen et al. [38] also reported significant overexpression of α2β1 and Met receptor, respectively, in bone metastases compared to lymph node metastases.
3.4. Expression Pattern of EMT/MET Markers
Table 3 and supplementary Table 2 show data extracted from eleven studies that analysed the expression pattern of EMT and MET markers. Only two studies analysed the expression of both epithelial and mesenchymal markers. Sethi et al. [54] found no significant difference in the pattern of expression of E-cadherin or vimentin between primary and bone metastasis tissues. However, in both PC and bone metastasis tissues, reduced expression of E-cadherin was found at the invasive front of the tumour, while it was highly expressed within the tumour centre. Vimentin expression, on the other hand, showed an opposite pattern, where it was overexpressed at the invasive front of the tumour and downexpressed within the tumour centre. In this study, only Notch-1 expression was found to be statistically significantly higher in bone metastasis tissues compared to primary PC [54]. Armstrong et al. [52], on the other hand, found that circulating tumour cells in men with castration resistant prostate cancer (CRPC) coexpress both vimentin and CK (an epithelial marker), while bone metastasis lesions lose vimentin expression and maintain the positive expression of CK. Expression of vimentin was examined in another study [47] and was found to be higher in bone metastasis tissues than in primary PC tissues.
Table 3.
Details of studies analysing expression of EMT/MET markers in PC bone metastases.
| Study | Marker | No. of BM specimens | Comparison tissue (No. of specimens) | Results | Significance for differential expression |
|---|---|---|---|---|---|
| Bryden et al., 1999 [45] | E-Cadherin | 20 | Different grades of BM tissues | Decrease in expression with increasing tumour grade | NR |
|
| |||||
| Bryden et al., 2002 [46] | E-Cadherin, β-catenin | 14 | Matched primary PC (14) | mRNA for both markers was expressed uniformly in 9/14 primary PC. In BM, 6/14 showed negative mRNA for one or both markers and 6/14 expressed both heterogeneously | NR |
|
| |||||
| Lang et al., 2002 [47] | Vimentin | 8 | Primary (54) | 16/54 of primary expressed vimentin with ≤50% staining cells. 7/8 BM expressed vimentin with 100% of cells staining positive in 5 specimens | NR |
|
| |||||
| Chen et al., 2004 [48] | Wnt-1/β-catenin | 23 (from 9 patients) | Primary PC (62, from 49 patients) LN (23, from 9 patients) |
High Wnt-1/β-catenin in 85% of BM and 77% of LN. Highest expression was observed in BM | P < 0.05 for BM (& for LN) versus normal tissues |
|
| |||||
| Saha et al., 2008 [49] | E-Cadherin, β-catenin | 17 | Primary PC (22) BPH (11) |
BM tissues showed higher frequency of homogenous expression of both markers compared to primary PC | P < 0.001 for BM versus primary PC |
|
| |||||
| Saha et al., 2008 [50] | E-Cadherin | 15 | Primary PC (20) BPH (11) |
Higher expression of unmethylated gene and homogenous protein in BM compared to primary tissues | P < 0.001 for BM versus primary PC |
|
| |||||
| Pontes et al., 2010 [51] | E-Cadherin, β-catenin | 28 | Matched primary PC (6) | For E-cadherin & β-catenin, respectively, there was abnormal expression in 86% and 82% of 28 BM specimens. In matched specimens, normal expression was seen in 5/6 and 3/6 of primary PC and abnormal expression was seen in 5/6 of BM for both markers | NR |
|
| |||||
| Armstrong et al., 2011 [52] | Vimentin, CK | 2 | CTCs (10, 2 matched) | Coexpression of vimentin and CK in 10/10 of CTCs Absent vimentin expression in 2/2 of CK-positive BM. Strong vimentin expression in bone stroma | NR |
|
| |||||
| Putzke et al., 2011 [53] | E-Cadherin | 109 | LN (30) Liver (18) Other (8) |
Higher expression in BM compared to all soft tissue metastases | P < 0.01 for BM versus all soft tissue metastases |
|
| |||||
| Sethi et al., 2011 [54] | E-Cadherin, Vimentin, PDGF-D, NF-kB, Notch-1, ZEB1 | 10 | Primary PC (10) | Higher expression of Notch-1 in BM compared to primary PC. No quantitative difference in expression for other markers. In primary and BM, EMT markers (vimentin & NF-kB) had higher expression at invasive tumour front than within tumour centre. The opposite pattern was observed for E-cadherin | For BM versus primary PC: P = 1 for E-cadherin, vimentin, PDGF-D, NF-kB, P = 0.057 for Notch-1, NR for ZEB1 |
|
| |||||
| Wan et al., 2012 [55] | β-catenin | 27 | Association with AR expression in BM tissues | Nuclear localization of β-catenin in 10/27 BM specimens. Localization was inversely associated with AR expression | P = 0.056 for inverse association between nuclear localization of β-catenin and AR expression |
AR: androgen receptor, BM: bone metastasis, BPH: benign prostatic hyperplasia, CK: cytokeratin, CTCs circulating tumour cells, ISH: in situ hybridisation, LN: lymph node, MS-PCR: methylation specific polymerase chain reaction, No.: number, and NR: not reported.
Six studies [45, 46, 49–51, 53] investigated the differential expression of E-cadherin in PC bone metastases. Two of these reported lower expression of E-cadherin in bone metastasis tissues compared to matched primary PC tissues [46, 51]. In contrast, two studies by Saha et al. [49, 50] demonstrated significant upregulation of E-cadherin expression in bone metastases compared to unmatched primary tissue samples, and another study [53] found significantly increased E-cadherin expression in bone compared to soft tissue metastases. Bryden et al. [45] compared different grades of bone metastasis tissues and found an inverse correlation between E-cadherin expression and degree of tumour differentiation, where it was highest in well-differentiated tumours and declined with increasing grade.
Five studies examined changes in the expression of β-catenin in bone metastases [46, 48, 49, 51, 55]. Bryden at al. [46] and Pontes et al. [51] found lower β-catenin expression in bone metastasis tissues compared to matched primary PC tissues, while Saha et al. [49] demonstrated significantly higher membranous β-catenin expression in bone metastases compared to unmatched primary PC samples. It is important to note that in the studies by Pontes et al. [51] and Saha et al. [49], nuclear expression of β-catenin (which would indicate EMT) was considered to be negative, while in the study by Bryden et al. [46] the location of β-catenin could not be evaluated as mRNA was detected using ISH. Chen et al. [48] reported higher nuclear staining for β-catenin and Wnt 1 in bone metastases compared to primary PC and lymph node tissues, with 85% of bone metastases showing strong expression of nuclear β-catenin and Wnt 1. In the study by Wan et al. [55], on the other hand, the proportion of bone metastasis tissues that displayed positive nuclear staining for β-catenin was lower than that reported by Chen et al. (85% versus 37%). Both studies demonstrated an inverse association between nuclear β-catenin expression and androgen receptor status [48, 55].
4. Discussion
4.1. Summary and Interpretations of Findings
At present, the role of cellular plasticity in bone metastasis formation is not fully clear. Recent preclinical models indicate that the relationship between EMT/MET and CSCs is complex and dynamic. In this paper, we focused on the clinical importance of cellular plasticity in PC bone metastasis by reviewing studies that have comparatively analysed different stemness and EMT/MET markers in bone metastasis and primary tissue samples. We identified methodological limitations and sources of heterogeneity among studies. Sample size in most studies was small, and not all studies used matched primary and metastasis samples from the same patients. In addition, different scoring criteria for defining positive marker expression were used. The study population analysed was heterogeneous with respect to tumour grades and treatment status, which makes direct comparisons between patients difficult. For example, many studies did not report the androgen receptor (AR) status of the tissues analysed. This is important because two studies here showed an inverse association between nuclear β-catenin localization and androgen receptor (AR) status, suggesting that reduced AR expression enables Wnt/b-catenin signalling [48, 55]. This is in line with data from the literature that suggests that low AR expression levels are required for EMT in PC cells [56]. Thus, the heterogeneity in expression of EMT and MET markers observed between studies in this review might be partially attributed to differences in AR status in bone metastasis tissues.
Despite these methodological difference between studies reviewed, in general, most stem cell markers analysed, except for CXCR4, were positively expressed in bone metastasis tissues, with an expression level either similar to or higher than primary PC tissues and soft tissue metastases. On the other hand, the expression levels of EMT and MET markers in bone metastasis tissues showed variability within and between samples. This variation does not simply imply that studies are providing conflicting results but highlights the dynamic and transient nature of cellular plasticity that has been reported by preclinical studies and the challenges in capturing this clinically. Pathological samples represent observations of established masses taken at static points in time and are not capable of reflecting the dynamic plasticity of cancer cells. For example, the expression of markers belonging to the metastasis initiating cell population may gradually decrease as foci progress and the metastatic mass becomes more established [41].
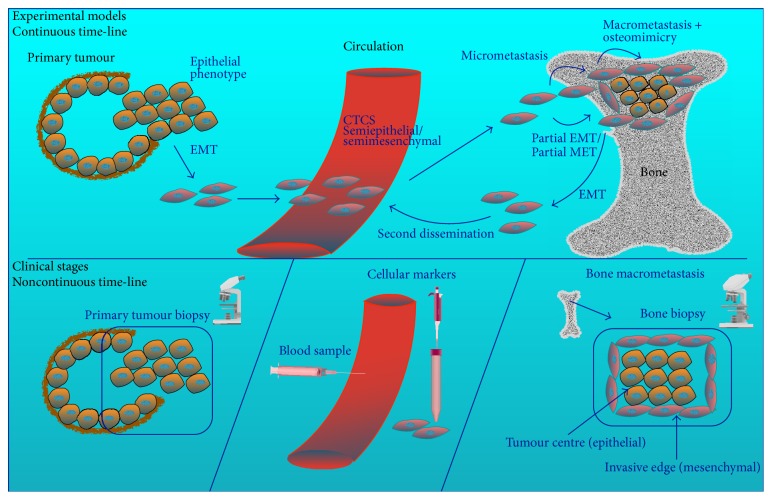
Data from studies in this review and others confirm that temporal and spatial factors as well as degree of metastasis differentiation and metastasis size play a critical role in influencing marker expression status at distant sites. The low expression of CXCR4 in bone metastases reported by Eaton et al. [41] suggests that this marker (with its ligand SDF-1) could be important for cancer cell homing to bone and stem cell trafficking [57, 58], but not for distant tumour growth [41]. Thus, as metastases grow, this marker loses its expression. Bryden et al. [45] reported an inverse link between E-cadherin expression and level of tumour differentiation in bone metastases, where E-cadherin expression was highest in well-differentiated tumours and lowest in poorly differentiated ones. Sethi et al. [54] showed evidence for heterogeneity in the expression of EMT and MET markers within bone metastases, where expression of E-cadherin was reduced at the tumour invasive front and was high within the tumour centre, while the expression pattern for vimentin was the opposite. Armstrong et al. [52] provided evidence that circulating tumour cells can exist in an intermediate state expressing both epithelial and mesenchymal markers. In addition, Chao et al. [59] reported an inverse correlation between E-cadherin expression and metastasis size, where E-cadherin expression decreases as tumour mass gets bigger. Taken together, these data suggest that EMT and MET could be partial and reversible and that an increase in epithelial markers in metastasis tissues does not mean a full reversal into an epithelial state. Chao et al. hypothesise that a second EMT can occur at the distant site allowing for further dissemination into other sites [59] (Figure 2).
Figure 2.
Cellular plasticity of prostate cancer cells over time. The plasticity of cancer cells over time can be delineated from experimental animal and in vitro models. Cells in the primary tumour undergo EMT, which enhances their migratory and invasive potential. These invasive cells enter the circulation and may exist as mesenchymal, epithelial, or semimesenchymal/semiepithelial circulating tumour cells (CTCs). Extravasation of CTCs into the bone gives rise to micrometastases. As the metastatic tumour cells colonise the bone and grow into macrometastasis, cells within the tumour centre undergo MET to enable tumour growth and survival under hypoxia, while cells at the invasive edge retain their mesenchymal phenotype to enable invasiveness and osteomimicry. It is suggested that these phenotypic changes are partial and that metastatic cells can dynamically transition between these two states to allow for adaptation to altered microenvironmental stimuli and for further invasion and secondary dissemination. Clinical samples, on the other hand, represent observations of established primary masses and macrometastases at static points in time. Therefore, they cannot capture the dynamic nature of cellular plasticity. Furthermore, current CTC-detection techniques are epithelial-based and cannot capture CTCs that are mostly mesenchymal and have reduced expression of epithelial markers, which would result in missing a significant fraction of CTCs that are predominantly in the mesenchymal state.
This plasticity and ability to dynamically transition between epithelial, mesenchymal, and intermediate states facilitates adaptation to altered microenvironmental stimuli [30]. One type of plasticity that allows adaptation in the bone microenvironment is osteomimicry [23]. Several clinical studies in this review found increased expression of markers implicated in osteomimicry. In osteomimicry, PC cells acquire an osteoblast-like phenotype [60] and express bone-related markers, such as osteocalcin, bone sialoprotein [61], osteoprotegerin, and RANKL [62]. Expression of Runx2, a major transcription factor of osteoblast differentiation, is a crucial element in the acquisition of osteomimicry [63]. Several experimental studies demonstrated a positive role for EMT and stemness pathways (e.g., TWIST and Notch) in osteomimicry by promoting the expression of Runx2 [64–66]. In addition, interaction between the hepatocyte growth factor (HGF) and its receptor, Met, has been shown to enhance osteomimicry through activation of the Wnt/β-catenin pathway [67, 68]. In this review, positive expression of Met (the stem cell marker and receptor for HGF) in PC bone metastases was demonstrated in two studies [38, 41]; another study reported significant overexpression of the Wnt/nuclear β-catenin pathway in bone metastases [48], and Notch expression was found to be significantly higher expression in bone metastasis tissues compared to primary PC tissues [54].
4.2. Clinical Challenges and Therapeutic Implications
As noted previously, cellular plasticity is difficult to capture clinically. A clear insight into the dynamics of cellular plasticity in time and space in the clinical context would require comparisons between a large number of matched samples that are serially obtained from primary tumour, circulating cancer cells, bone metastasis, and tumour-associated normal tissues at several points in time and space. This could prove extremely difficult and inconvenient. Furthermore, the currently approved technology for the detection of circulating tumour cells (CTCs) poses some limitations. CellSearch, the only FDA-cleared tool for identifying CTCs relies on the antibodies against the epithelial cell adhesion molecule (EpCAM) for capturing these cells and on the epithelial marker CK for their identification. As this is an epithelial-based detection technique, it cannot capture CTCs that are mostly mesenchymal and have reduced expression of EpCAM. Thus, this method would miss a significant fraction of CTCs that are predominantly in the mesenchymal state [69–71] (Figure 2).
The dynamic phenotypic switching of CSCs in bone metastasis tissues poses important therapeutic implications. The link between EMT and stem cell traits previously reported in the literature has motivated suggestions that counteracting the EMT phenotype could hold promise by targeting cells that are resistant to conventional treatments [25, 70, 72]. However, the evidence for the positive expression of both epithelial and mesenchymal markers in bone metastases implies that therapeutic approaches targeting one of those states might result in an unfavourable activation of the other [73, 74]. Thus, cellular plasticity entails that it may be necessary to target both mesenchymal-like and epithelial-like states in order to eliminate these cells [25]. For example, Bitting et al. [23] propose combining antiepithelial drugs, such as AR antagonists, with drugs against mesenchymal targets such as anti-N-cadherin antibodies in the treatment against metastasis.
Targeting the cancer stem cell phenotype might prove to be better than targeting epithelial and mesenchymal states. Emerging insights into the role of microRNAs in cellular plasticity could provide novel therapeutic approaches. MicroRNAs are small noncoding RNAs that mediate posttranscriptional gene regulation. These molecules have been implicated in the network regulating cellular plasticity and stemness in PC progression [75, 76]. For example, Liu et al. [75] demonstrated that microRNA-34a could be a potential therapeutic target against PC stems cells. In addition, microRNA-143 and microRNA-145 have been shown by three studies [77–79] to potentially play a role in PC bone metastasis by targeting the EMT regulator HEF1 [79] and the stem cell markers CD133, CD44, Oct-4, c-myc, and Klf4 [78]. Nevertheless, the microRNA-detection methods employed in these studies were not cell type-specific, and it is yet to be determined whether these microRNAs were expressed by tumour cells or by the surrounding bone stroma [80].
In conclusion, although findings suggest a potential role of CSCs in PC bone metastasis and stemness as a mediator of cellular plasticity, there are major challenges in detecting cellular plasticity in the clinical setting and questions remain unanswered as to whether phenotypic transitions are full or partial, whether nonstem cancer cells undergo phenotypic switching as well, and when and how do these phenotypic transitions occur.
Supplementary Material
The two tables in the supplementary material contain additional details about the 19 studies reviewed. These details pertain to the Gleason score of the primary tumour, the treatment status at the time of bone biopsy, tissue type (e.g. formalin fixed, paraffin embedded, etc), marker detection method used (Immunohistochemistry or In Situ Hybridisation), and the scoring criteria used for defining positive marker expression. Supplementary table 1 contains details for the 8 studies that investigated the differential expression of stem cell markers, and Supplementary table 2 contains details for the 11 studies that analysed the differential expression of EMT/MET markers.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Nørgaard M., Jensen A. Ø., Jacobsen J. B., Cetin K., Fryzek J. P., Sørensen H. T. Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007) Journal of Urology. 2010;184(1):162–167. doi: 10.1016/j.juro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Sathiakumar N., Delzell E., Morrisey M. A., et al. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer and Prostatic Diseases. 2011;14(2):177–183. doi: 10.1038/pcan.2011.7. [DOI] [PubMed] [Google Scholar]
- 3.Feldman B. J., Feldman D. The development of androgen-independent prostate cancer. Nature Reviews Cancer. 2001;1(1):34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 4.Rini B. I., Small E. J. Hormone-refractory prostate cancer. Current Treatment Options in Oncology. 2002;3(5):437–446. doi: 10.1007/s11864-002-0008-1. [DOI] [PubMed] [Google Scholar]
- 5.Li F., Tiede B., Massagué J., Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Research. 2007;17(1):3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D., Dick J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M., Wicha M. S., Benito-Hernandez A., Morrison S. J., Clarke M. F. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S. K., Clarke I. D., Terasaki M., et al. Identification of a cancer stem cell in human brain tumors. Cancer Research. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 9.Collins A. T., Berry P. A., Hyde C., Stower M. J., Maitland N. J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Research. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.can-05-2018. [DOI] [PubMed] [Google Scholar]
- 10.Mani S. A., Guo W., Liao M.-J., et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel A.-P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3(8) doi: 10.1371/journal.pone.0002888.e2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong D., Banerjee S., Ahmad A., et al. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012445.e12445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery J. P., Sleeman J. P. Complex networks orchestrate epithelial-mesenchymal transitions. Nature Reviews Molecular Cell Biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 14.Thiery J. P., Acloque H., Huang R. Y. J., Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Polyak K., Weinberg R. A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature Reviews Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 16.Peinado H., Olmeda D., Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature Reviews Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 17.He T.-C., Sparks A. B., Rago C., et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 18.Wielenga V. J. M., Smits R., Korinek V., et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. The American Journal of Pathology. 1999;154(2):515–523. doi: 10.1016/s0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J.-H., Luo Y., Jiang Y.-G., He D.-L., Wu C.-T. Knockdown of β-catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α . Cancer Investigation. 2011;29(6):377–382. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 20.Zavadil J., Cermak L., Soto-Nieves N., Böttinger E. P. Integration of TGF-β/Smad and Jagged1/Notch signalling in epithelial-to-mesenchymal transition. The EMBO Journal. 2004;23(5):1155–1165. doi: 10.1038/sj.emboj.7600069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takebe N., Harris P. J., Warren R. Q., Ivy S. P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nature Reviews Clinical Oncology. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 22.Chaffer C. L., Brennan J. P., Slavin J. L., Blick T., Thompson E. W., Williams E. D. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research. 2006;66(23):11271–11278. doi: 10.1158/0008-5472.can-06-2044. [DOI] [PubMed] [Google Scholar]
- 23.Bitting R. L., Schaeffer D., Somarelli J. A., Garcia-Blanco M. A., Armstrong A. J. The role of epithelial plasticity in prostate cancer dissemination and treatment resistance. Cancer Metastasis Reviews. 2014;33(2-3):441–468. doi: 10.1007/s10555-013-9483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ksia̧kiewicz M., Markiewicz A., Zaczek A. J. Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology. 2012;79(4):195–208. doi: 10.1159/000337106. [DOI] [PubMed] [Google Scholar]
- 25.van der Pluijm G. Epithelial plasticity, cancer stem cells and bone metastasis formation. Bone. 2011;48(1):37–43. doi: 10.1016/j.bone.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Xu J., Wang R., Xie Z. H., et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66(15):1664–1673. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 27.Zhau H. E., Odero-Marah V., Lue H.-W., et al. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clinical and Experimental Metastasis. 2008;25(6):601–610. doi: 10.1007/s10585-008-9183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josson S., Sharp S., Sung S.-Y., et al. Tumor-stromal interactions influence radiation sensitivity in epithelial- versus mesenchymal-like prostate cancer cells. Journal of Oncology. 2010;2010:10. doi: 10.1155/2010/232831.232831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae K.-M., Su Z., Frye C., et al. Expression of pluripotent stem cell reprogramming factors by prostate tumor initiating cells. The Journal of Urology. 2010;183(5):2045–2053. doi: 10.1016/j.juro.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deep G., Jain A. K., Ramteke A., et al. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Molecular Cancer. 2014;13, article 37 doi: 10.1186/1476-4598-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S., Cong Y., Wang D., et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2014;2(1):78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amico L., Patanè S., Grange C., et al. Primary breast cancer stem-like cells metastasise to bone, switch phenotype and acquire a bone tropism signature. British Journal of Cancer. 2013;108(12):2525–2536. doi: 10.1038/bjc.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conley S. J., Gheordunescu E., Kakarala P., et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu K., Boley K. M., Moraes R., Barsky S. H., Robertson F. M. The paradox of E-cadherin: role in response to hypoxia in the tumor microenvironment and regulation of energy metabolism. Oncotarget. 2013;4(3):446–462. doi: 10.18632/oncotarget.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celià-Terrassa T., Meca-Cortés Ó., Mateo F., et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of Clinical Investigation. 2012;122(5):1849–1868. doi: 10.1172/jci59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae K. M., Parker N. N., Dai Y., Vieweg J., Siemann D. W. E-cadherin plasticity in prostate cancer stem cell invasion. The American Journal of Cancer Research. 2011;1(1):71–84. [PMC free article] [PubMed] [Google Scholar]
- 37.Gu Z., Thomas G., Yamashiro J., et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19(10):1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- 38.Knudsen B. S., Gmyrek G. A., Inra J., et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60(6):1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 39.Lam J. S., Yamashiro J., Shintaku I. P., et al. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clinical Cancer Research. 2005;11(7):2591–2596. doi: 10.1158/1078-0432.CCR-04-1842. [DOI] [PubMed] [Google Scholar]
- 40.Wiesner C., Nabha S. M., Santos E. B. D., et al. C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia. 2008;10(9):996–1003. doi: 10.1593/neo.08618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eaton C. L., Colombel M., van der Pluijm G., et al. Evaluation of the frequency of putative prostate cancer stem cells in primary and metastatic prostate cancer. Prostate. 2010;70(8):875–882. doi: 10.1002/pros.21121. [DOI] [PubMed] [Google Scholar]
- 42.van den Hoogen C., van der Horst G., Cheung H., et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Research. 2010;70(12):5163–5173. doi: 10.1158/0008-5472.can-09-3806. [DOI] [PubMed] [Google Scholar]
- 43.Castellón E. A., Valenzuela R., Lillo J., et al. Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different Gleason grades and metastasis. Biological Research. 2012;45(3):297–305. doi: 10.4067/s0716-97602012000300011. [DOI] [PubMed] [Google Scholar]
- 44.Sottnik J. L., Daignault-Newton S., Zhang X., et al. Integrin alpha2beta1 (α 2 β 1) promotes prostate cancer skeletal metastasis. Clinical and Experimental Metastasis. 2013;30(5):569–578. doi: 10.1007/s10585-012-9561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryden A. A. G., Freemont A. J., Clarke N. W., George N. J. R. Paradoxical expression of E-cadherin in prostatic bone metastases. BJU International. 1999;84(9):1032–1034. doi: 10.1046/j.1464-410X.1999.00378.x. [DOI] [PubMed] [Google Scholar]
- 46.Bryden A. A. G., Hoyland J. A., Freemont A. J., Clarke N. W., Schembri Wismayer D., George N. J. R. E-cadherin and β-catenin are down-regulated in prostatic bone metastases. BJU International. 2002;89(4):400–403. doi: 10.1046/j.1464-4096.2001.01712.x. [DOI] [PubMed] [Google Scholar]
- 47.Lang S. H., Hyde C., Reid I. N., et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52(4):253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 48.Chen G., Shukeir N., Potti A., et al. Up-regulation of Wnt-1 and β-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101(6):1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 49.Saha B., Arase A., Imam S. S., et al. Overexpression of E-cadherin and beta-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68(1):78–84. doi: 10.1002/pros.20670. [DOI] [PubMed] [Google Scholar]
- 50.Saha B., Kaur P., Tsao-Wei D., et al. Unmethylated E-cadherin gene expressionis significantly associated with metastatic human prostate cancer cells in bone. The Prostate. 2008;68(15):1681–1688. doi: 10.1002/pros.20836. [DOI] [PubMed] [Google Scholar]
- 51.Pontes J., Jr., Srougi M., Borra P. M., Dall'Oglio M. F., Ribeiro-Filho L. A., Leite K. R. E-cadherin and β-catenin loss of expression related to bone metastasis in prostate cancer. Applied Immunohistochemistry & Molecular Morphology. 2010;18(2):179–184. doi: 10.1097/PAI.0b013e3181640bca. [DOI] [PubMed] [Google Scholar]
- 52.Armstrong A. J., Marengo M. S., Oltean S., et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular Cancer Research. 2011;9(8):997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Putzke A. P., Ventura A. P., Bailey A. M., et al. Metastatic progression of prostate cancer and E-cadherin: regulation by ZEB1 and Src family kinases. American Journal of Pathology. 2011;179(1):400–410. doi: 10.1016/j.ajpath.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sethi S., Macoska J., Chen W., Sarkar F. H. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. American Journal of Translational Research. 2011;3(1):90–99. [PMC free article] [PubMed] [Google Scholar]
- 55.Wan X., Liu J., Lu J.-F., et al. Activation of β-catenin signaling in androgen receptor-negative prostate cancer cells. Clinical Cancer Research. 2012;18(3):726–736. doi: 10.1158/1078-0432.ccr-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu M.-L., Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. The FASEB Journal. 2010;24(3):769–777. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taichman R. S., Cooper C., Keller E. T., Pienta K. J., Taichman N. S., McCauley L. K. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Research. 2002;62(6):1832–1837. [PubMed] [Google Scholar]
- 58.Kucia M., Reca R., Miekus K., et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 59.Chao Y., Wu Q., Acquafondata M., Dhir R., Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenvironment. 2012;5(1):19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koeneman K. S., Yeung F., Chung L. W. K. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39(4):246–261. doi: 10.1002/(sici)1097-0045(19990601)39:460;246::aid-pros562;3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 61.Huang W.-C., Xie Z., Konaka H., Sodek J., Zhau H. E., Chung L. W. K. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Research. 2005;65(6):2303–2313. doi: 10.1158/0008-5472.can-04-3448. [DOI] [PubMed] [Google Scholar]
- 62.Brown J. M., Corey E., Lee Z. D., et al. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57(4):611–616. doi: 10.1016/s0090-4295(00)01122-5. [DOI] [PubMed] [Google Scholar]
- 63.Brubaker K. D., Vessella R. L., Brown L. G., Corey E. Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate. 2003;56(1):13–22. doi: 10.1002/pros.10233. [DOI] [PubMed] [Google Scholar]
- 64.Yuen H.-F., Kwok W.-K., Chan K.-K., et al. TWIST modulates prostate cancer cell-mediated bone cell activity and is upregulated by osteogenic induction. Carcinogenesis. 2008;29(8):1509–1518. doi: 10.1093/carcin/bgn105. [DOI] [PubMed] [Google Scholar]
- 65.Zayzafoon M., Abdulkadir S. A., McDonald J. M. Notch signaling and ERK activation are important for the osteomimetic properties of prostate cancer bone metastatic cell lines. The Journal of Biological Chemistry. 2004;279(5):3662–3670. doi: 10.1074/jbc.m308158200. [DOI] [PubMed] [Google Scholar]
- 66.Sethi N., Kang Y. Notch signaling: mediator and therapeutic target of bone metastasis. BoneKEy Reports. 2012;1, article 3 doi: 10.1038/bonekey.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Previdi S., Maroni P., Matteucci E., Broggini M., Bendinelli P., Desiderio M. A. Interaction between human-breast cancer metastasis and bone microenvironment through activated hepatocyte growth factor/Met and β-catenin/Wnt pathways. European Journal of Cancer. 2010;46(9):1679–1691. doi: 10.1016/j.ejca.2010.02.036. [DOI] [PubMed] [Google Scholar]
- 68.Hassan M. Q., Maeda Y., Taipaleenmaki H., et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. Journal of Biological Chemistry. 2012;287(50):42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bednarz-Knoll N., Alix-Panabières C., Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer and Metastasis Reviews. 2012;31(3-4):673–687. doi: 10.1007/s10555-012-9370-z. [DOI] [PubMed] [Google Scholar]
- 70.Tsai J. H., Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes & Development. 2013;27(20):2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu H., Zhang X., Li J., Sun B., Qian H., Yin Z. The biological and clinical importance of epithelial–mesenchymal transition in circulating tumor cells. Journal of Cancer Research and Clinical Oncology. 2015;141(2):189–201. doi: 10.1007/s00432-014-1752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li P., Yang R., Gao W.-Q. Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer. Molecular Cancer. 2014;13(1, article 55) doi: 10.1186/1476-4598-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai J. H., Donaher J. L., Murphy D. A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao D., Vahdat L. T., Wong S., Chang J. C., Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitionsin cancer. Cancer Research. 2012;72(19):4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu C., Kelnar K., Liu B., et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Medicine. 2011;17(2):211–216. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C., Tang D. G. MicroRNA regulation of cancer stem cells. Cancer Research. 2011;71(18):5950–5954. doi: 10.1158/0008-5472.can-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng X., Guo W., Liu T., et al. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS ONE. 2011;6(5) doi: 10.1371/journal.pone.0020341.e20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang S., Guo W., Tang Y., Ren D., Zou X., Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncology Reports. 2012;28(5):1831–1837. doi: 10.3892/or.2012.2015. [DOI] [PubMed] [Google Scholar]
- 79.Guo W., Ren D., Chen X., et al. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. Journal of Cellular Biochemistry. 2013;114(7):1606–1615. doi: 10.1002/jcb.24502. [DOI] [PubMed] [Google Scholar]
- 80.Kent O. A., McCall M. N., Cornish T. C., Halushka M. K. Lessons from miR-143/145: the importance of cell-type localization of miRNAs. Nucleic Acids Research. 2014;42(12):7528–7538. doi: 10.1093/nar/gku461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The two tables in the supplementary material contain additional details about the 19 studies reviewed. These details pertain to the Gleason score of the primary tumour, the treatment status at the time of bone biopsy, tissue type (e.g. formalin fixed, paraffin embedded, etc), marker detection method used (Immunohistochemistry or In Situ Hybridisation), and the scoring criteria used for defining positive marker expression. Supplementary table 1 contains details for the 8 studies that investigated the differential expression of stem cell markers, and Supplementary table 2 contains details for the 11 studies that analysed the differential expression of EMT/MET markers.