Abstract
Toxic leucoencephalopathy (TLE) is a rare neurological complication of heroin abuse. ‘Chasing the dragon’ is an inhalational mode of heroin abuse that originated in Southeast Asia. Intriguingly, no cases of TLE have been reported from this region, although the inhalational mode of heroin abuse is common. We herein report the case of a middle-aged man with a history of polysubstance abuse who presented with progressive neurological symptoms and progressed to an uncommunicative state. While the initial impression was that of iatrogenic parkinsonism, diffuse leucoencephalopathy with sparing of the cerebellum was noted on magnetic resonance imaging. In view of his history of inhalational heroin abuse close to the onset of the neurological symptoms, a diagnosis of TLE was made. No clinical improvement was noted with administration of a dopaminergic agent. This is the first known case of delayed TLE following heroin inhalation from Southeast Asia with the unusual feature of cerebellar sparing.
Keywords: chasing the dragon, leucoencephalopathy, toxic
INTRODUCTION
Toxic leucoencephalopathy (TLE) is a rare neurological complication of heroin abuse that was first described in 1982.(1,2) Heroin smoking has its origins in Asia. ‘Chasing the dragon’ is an inhalational mode of abuse that involves heating the drug, typically on tin foil above a flame. Although this refinement in heroin abuse originated in Southeast Asia, no cases of TLE secondary to ‘chasing the dragon’ have yet been reported in this region, where this inhalational mode of heroin abuse is common.(3)
CASE REPORT
A 58-year-old immunocompetent Chinese man was referred to Neurology Services, Changi General Hospital, Singapore, for progressive stiffness, blunted affect and impaired attention, which progressed over the next few days till he was in an uncommunicative state. The patient’s medical history was significant for polysubstance abuse (alcohol, benzodiazepines, buprenorphine and heroin) of over more than 30 years, which required multiple hospital admissions. He was known to use heroin via inhalation and was last witnessed using drugs one month ago.
The patient was last hospitalised for drowsiness one month prior to the index presentation. He had been found in a drowsy condition by family members with a piece of burnt aluminium foil in a locked bathroom. Tests for blood gases, serum sodium and glucose done at the time were unremarkable, with no evidence of hypoxia or acidaemia, while computed tomography (CT) did not show any abnormalities. Toxicology tests were not performed in view of a clear history of preceding drug use and early improvement back to baseline status. He was discharged after two days with no evidence of any neurological deficits.
The patient’s current medications included fluvoxamine and hydroxyzine, for depression. Haloperidol had been prescribed six years ago, for a period of three months. There was no history of trauma, head injury or any history suggestive of hypoxia/anoxia. An examination revealed a Glasgow Coma Scale score of 14 (E4V4M6). The patient was afebrile. His pupils were normal and extraocular movements were full. Rigidity, cogwheeling, bradykinesia and resting tremors were noted in all four limbs, in addition to left torticollis. A positive right-sided grasp reflex was noted. Routine blood investigation results, including carboxyhaemoglobin levels, were normal apart from a mild elevation in serum creatine kinase level (Table I). Human immunodeficiency virus test was negative. Urine toxicology detected codeine and morphine, which was consistent with heroin, morphine or opium use.
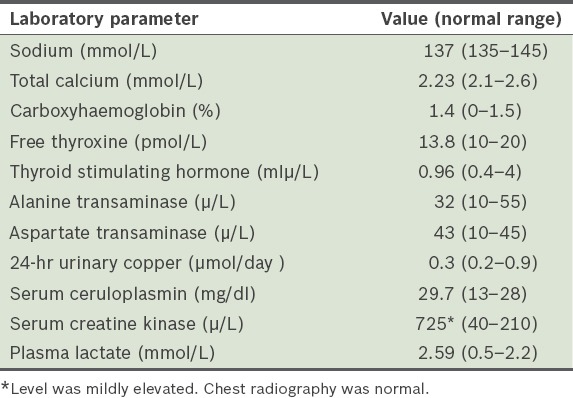
Table I.
The patient’s selected blood test results.
The initial impression was parkinsonism/dystonia, possibly secondary to medications. Magnetic resonance (MR) imaging of the brain showed extensive, symmetrical, periventricular, deep and subcortical white matter signal changes (T2-weighted, fluid-attenuated inversion recovery [FLAIR]) in the cerebral hemispheres, with restricted diffusion (Fig. 1). Mild T2-weighted/FLAIR hyperintensity of the putamen was evident, without associated restricted diffusion. Given the circumstances of the recent admission, these changes were deemed consistent with heroin-induced leucoencephalopathy.
Fig. 1.
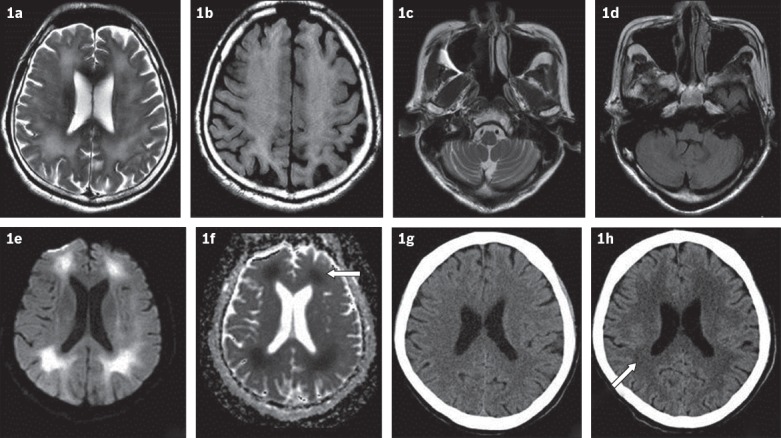
MR images of the brain show widespread, subcortical white matter hyperintensity in (a) T2-W and (b) FLAIR sequences, while (c & d) the cerebellum appears normal. (e) MR image shows areas of diffusion restriction in subcortical white matter with (f) matched defects (arrow) on apparent diffusion coefficient images. (g) CT image one month prior to presentation shows normal white matter, while (h) subsequent CT image shows significant increase in white matter hypodensity (arrow).
The patient was started on madopar, with gradual up-titration to 125 mg thrice daily over one week. A psychiatric opinion was sought and catatonic schizophrenia was deemed unlikely. CT of the brain was repeated after two weeks and revealed a mild increase in periventricular white matter hypodensity without evidence of oedema. There was little clinical improvement and the patient was discharged to a long-term care facility.
DISCUSSION
Heroin abuse results in a range of neurological complications that may be related to the route of intake. Previous reports have shown that intravenous heroin abuse is associated with hypoxic ischaemic leucoencephalopathy (HIE), while inhalation of heroin vapour (‘chasing the dragon’) may result in toxic spongiform leucoencephalopathy, or TLE.(1,3) While there is no conclusive explanation for this difference, various theories have been proposed, including a dose-response relationship and the creation of potentially toxic compounds (from heroin or additives) during the heating process.(4)
The typical clinical picture of heroin-induced TLE is that it evolves over stages, beginning with cerebellar signs and motor restlessness, and progressing to a terminal stage characterised by spasms, hypotonic paresis, bradyphrenia, akinetic mutism and ultimately death.(1) The characteristic lesions, which are also seen on brain imaging, have been described in cerebellar white matter, cerebellar and cerebral peduncles, the posterior limb of the internal capsule and the centrum semiovale, with sparing of U-fibres.(4,5) The frequently perfect symmetry of the involvement points to a toxic and/or metabolic pathophysiology.(4) It has been suggested that sparing of the anterior limb of the internal capsule can aid in distinguishing this condition from other causes of leucoencephalopathy such as toluene toxicity or reversible posterior leucoencephalopathy.(5)
Pathologically, multivacuolar degeneration of oligodendrocytes may explain the restricted diffusion seen on MR imaging in patients with TLE.(6) In contrast, acute myelinopathy with cytotoxic oedema is thought to underlie changes seen in HIE.(5) Grey matter is also often affected in HIE, as it is thought to be more vulnerable to diffuse ischaemic injury than white matter. In HIE, affected cortical areas are prominently hyperintense on FLAIR images between one month and one year after the index event, while basal ganglia changes are often seen early on. Therefore, the absence of lesions in grey matter (cortex and basal ganglia) may differentiate heroin-induced TLE from HIE.(6) In our patient, the absence of grey matter involvement on MR imaging made HIE an unlikely diagnosis.
Delayed post-anoxic leucoencephalopathy has been reported after intravenous heroin use.(3,7) Pathologically, it is characterised by diffuse hemispheric demyelination sparing the subcortical U-fibres, but without vacuolisation.(8) Rizzuto et al reported a case of intravenous heroin abuse in which the clinical picture was consistent with post-anoxic encephalopathy, but a pathological study suggested spongiform leucoencephalopathy.(9) MR imaging findings were also consistent with TLE. This suggests that TLE may have a delayed onset, although the pathogenesis remains unknown.(9) Our patient’s history of heroin inhalation a month prior to progressive neurological deterioration, lack of grey matter involvement on MR imaging, and absence of hypoxic/anoxic episode(s) may be suggestive of delayed TLE.
Radiological sparing of the infratentorial compartment was also noted in our patient, in contrast to previous reports that emphasised cerebellar lesions as typical of this condition. Previously, Blasel et al described MR imaging findings in three patients in which hemispheric subcortical U-fibre involvement was seen with sparing of infratentorial structures; a more recent case report from India also showed total sparing of the infratentorial compartment, although inhalation was the mode of heroin use.(6,10) Paradoxically, cerebellar lesions have been reported even in patients who injected heroin intravenously.(11) The reason for cerebellar sparing is not known. While a vascular basis may be postulated because the cerebellum and brain stem, which are supplied by the posterior circulation (vertebrobasilar system), were spared, occipital involvement weighs against this explanation. Therefore, it remains to be confirmed whether the mode of administration has any bearing on MR imaging findings in heroin-induced leucoencephalopathy.
Acute TLE caused by heroin inhalation may be reversible, as radiologic resolution has been reported after seven months.(11) Some suggest that there is a clear relationship between the clinical course of the disease and its radiological features, although follow-up FLAIR imaging may reveal persistent alterations in periventricular white matter, even in cases in which there is a clear clinical improvement.(12) Delayed TLE, however, may have a poorer prognosis compared with delayed post-anoxic leucoencephalopathy.(11) It has been suggested that the use of antioxidants such as ubiquinone (coenzyme Q) may aid the resolution of clinical and radiologic syndromes.(13) Glucocorticoid has also been tried with no significant therapeutic effect.(14) No significant clinical improvement was noted in our patient.
Recently, the Advisory Council on the Misuse of Drugs (United Kingdom) recommended the use of foil as an intervention aimed at decreasing the high risks of blood-borne viruses and overdose associated with injecting heroin.(15) While such measures have positive aims, they are problematic, as inhalational heroin use may be perceived as being harmless. Given the staggering statistics on the prevalence of heroin abuse, its neurological sequelae deserve greater attention.
In conclusion, we herein report the first known case from Southeast Asia of delayed TLE following heroin inhalation, which had the unusual feature of cerebellar sparing on MR imaging. Although this remains a rare complication of heroin abuse, more case reports from Asia might be expected in the future, given the large number of heroin users in the region and the growing recognition of this condition.
REFERENCES
- 1.Wolters EC, van Wijngaarden Gk, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;2:1233–7. doi: 10.1016/s0140-6736(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 2.Celius EG, Andersson S. Leucoencephalopathy after inhalation of heroin: a case report. J Neurol Neurosurg Psychiatry. 1996;60:694–5. doi: 10.1136/jnnp.60.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang WL, Chang YK, Hsu SY, Lin GJ, Chen SC. Reversible delayed leukoencephalopathy after heroin intoxication with hypoxia: a case report. Acta Neurol Taiwan. 2009;18:198–202. [PubMed] [Google Scholar]
- 4.Au-Yeung K, Lai C. Toxic leucoencephalopathy after heroin inhalation. Australas Radiol. 2002;46:306–8. doi: 10.1046/j.1440-1673.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- 5.Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology. 2001;56:481–5. doi: 10.1212/wnl.56.4.481. [DOI] [PubMed] [Google Scholar]
- 6.Blasel S, Hattingen E, Adelmann M, et al. Toxic Leukoencephalopathy after Heroin Abuse without Heroin Vapor Inhalation: MR Imaging and Clinical Features in Three Patients. Clin Neuroradiol. 2010;20:48–53. doi: 10.1007/s00062-010-0022-9. [DOI] [PubMed] [Google Scholar]
- 7.Barnett MH, Miller LA, Reddel SW, Davies L. Reversible delayed leukoencephalopathy following intravenous heroin overdose. J Clin Neurosci. 2001;8:165–7. doi: 10.1054/jocn.2000.0769. [DOI] [PubMed] [Google Scholar]
- 8.Shprecher DR, Flanigan KM, Smith AG, et al. Clinical and diagnostic features of delayed hypoxic leukoencephalopathy. J Neuropsychiatry Clin Neurosci. 2008;20:473–7. doi: 10.1176/jnp.2008.20.4.473. [DOI] [PubMed] [Google Scholar]
- 9.Rizzuto N, Morbin M, Ferrari S, et al. Delayed spongiform leukoencephalopathy after heroin abuse. Acta Neuropathol. 1997;94:87–90. doi: 10.1007/s004010050676. [DOI] [PubMed] [Google Scholar]
- 10.Verma R, Sharma P, Vidhate MR. A rare case of acute fatal leucoencephalopathy due to heroin exposure. Neurol India. 2011;59:127–8. doi: 10.4103/0028-3886.76863. [DOI] [PubMed] [Google Scholar]
- 11.Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27:1763–5. [PMC free article] [PubMed] [Google Scholar]
- 12.McKinney AM, Kieffer SA, Paylor RT, et al. Acute toxic leukoencephalopathy: potential for reversibility clinically and on MRI with diffusion-weighted and FLAIR imaging. AJR Am J Roentgenol. 2009;193:192–206. doi: 10.2214/AJR.08.1176. [DOI] [PubMed] [Google Scholar]
- 13.Gacouin A, Lavoue S, Signouret T, et al. Reversible spongiform leucoencephalopathy after inhalation of heated heroin. Intensive Care Med. 2003;29:1012–5. doi: 10.1007/s00134-003-1691-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Lu BX, Yin J, et al. Glucocortioid treatment for heroin-induced spongiform leucoencephalopathy: a clinical controlled study. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:172–4. Chinese. [PubMed] [Google Scholar]
- 15.Advisory Council on the Misuse of Drugs. Consideration of the use of foil, as an intervention, to reduce the harms of injecting heroin. [Accessed March 20, 2014]. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/119107/foil-report.pdf .


