Abstract
Our review of the literature of the major cardiovascular journals for the past three years showed that for all studies using anesthesia for mouse echocardiography, the predominant anesthetic was isoflurane, which was used in 76% of the studies. The goal of this investigation was to determine if isoflurane is indeed the best anesthetic. Accordingly, we compared isoflurane with 2,2,2-tribromoethanol (Avertin), ketamine-xylazine, and ketamine on different days in the same 14 mice, also studied in the conscious state without anesthesia. A randomized crossover study design was employed to compare the effects on left ventricular (LV) systolic function and heart rate of the four different anesthetic agents assessed by transthoracic echocardiography. As expected, each anesthetic depressed LV ejection fraction and heart rate when compared with values in conscious mice. Surprisingly, isoflurane was not the best, but actually second to last in maintaining normal LV function and heart rate. The anesthetic with the least effect on LV function and heart rate was ketamine alone at a dose of 150 mg/kg, followed by Avertin at 290 mg/kg, isoflurane at 3% induction and 1 to 2% maintenance, and lastly ketamine-xylazine at 100 and 10 mg/kg, respectively. In summary, these results indicate that ketamine alone exerts the least depressant effects on LV function and heart rate, with Avertin second, suggesting that these anesthetics should be used when it is not feasible to study the animals in the conscious state as opposed to the most commonly used anesthetic, isoflurane.
Keywords: echocardiography, mice, conscious state, anesthetics
transthoracic echocardiography is an essential noninvasive tool for the assessment of cardiovascular function in mice. Currently, echocardiography is either performed consciously or under anesthesia. As a general rule, the conscious study is preferred because it eliminates the complicating influences of anesthesia on cardiac function and regulation by the autonomic nervous system. The concept of the superiority of studies in conscious animals as opposed to anesthetized animals are largely based on studies of chronically instrumented animals that were recorded in the conscious state without being touched or manipulated by the investigators (10, 12). While echocardiography also has these advantages in patients, who understand that the transducer is harmless, conscious rodents are wary of a transducer touching their skin. Accordingly, it is possible to argue that light anesthesia affects the rodent's cardiovascular system less than the application of the echocardiographic transducer in the conscious state. Therefore, it becomes important not only to compare echocardiographic studies in mice in the conscious state or during anesthesia but also to compare the effects of the different anesthetics that have been used in mouse echocardiography, which were the goals of the current investigation.
To address these goals, we first reviewed the frequency of mouse echocardiography studies in conscious versus anesthetized animals and then compared the frequency of use of each anesthetic employed in studies published from 2012 to the present in four of the major cardiovascular research journals: Circulation, Circulation Research, American Journal of Physiology-Heart and Circulatory Physiology, and Cardiovascular Research. These data are shown in Table 1 and demonstrate that of the 205 studies using mouse echocardiography, roughly 18.5% of them were conducted in conscious mice, and of the remaining 81.5%, the most frequently used anesthetic was isoflurane (76%). Just because isoflurane was used most frequently does not mean that this anesthetic exerts the least effects on cardiac function. Accordingly, we compared baseline measurements of left ventricular (LV) ejection fraction, fractional shortening, end-diastolic and -systolic diameters, and heart rate in conscious mice trained to be accustomed to being held and having echocardiography with the same mice studied on different days after anesthesia with each of the following four anesthetic agents: isoflurane, ketamine-xylazine, Avertin, and ketamine.
Table 1.
Different anesthetics used in murine echocardiography
| Type of Anesthetic | Studies, % |
|---|---|
| Conscious | 18.5 |
| Isoflurane | 62.4 |
| Avertin | 4.8 |
| Ketamine-xylazine | 4.8 |
| Ketamine | 3.4 |
| Pentobarbital sodium | 2.9 |
| Sevoflurane | 1.9 |
| Etomidate | 0.4 |
| Urethane-chloralose | 0.9 |
| Total | 100 |
MATERIALS AND METHODS
Animals.
Wild-type male and female mice (n = 14) were used in this study at 3 mo of age. Experiments were conducted and compared in three different strains: C57BL/6J, 129SVJ, and FVB/N. All protocols concerning animal use were approved by the Institutional Animal Care and Use Committee of Rutgers University, Rutgers Biomedical Health Sciences, New Jersey Medical School.
Study design.
A randomized crossover study design was employed to compare the cardiovascular effect of four different anesthetic agents assessed by transthoracic echocardiography.
Mice were trained for conscious echocardiographic measurements as previously described (3). The training included three sessions of 10 min each over a period of 3 days. Mice were held by the nape of the neck with the tail held tightly between the last two fingers of the examiner; the probe of the ultrasound transducer was placed lightly on the mouse chest. Before echocardiography, the chest hair was removed by shaving or using hair removal cream.
The mice were randomly anesthetized by either ketamine-xylazine, isoflurane, Avertin, or ketamine; followed by another measurement using a different anesthetic 24–36 h later until all anesthetics were used. The order of the anesthetics was randomized and changed to account for possible sedative effects induced by cumulative anesthetic order.
Anesthesia and sedation.
An induction with 3% isoflurane, followed by maintenance at 1 to 2% was used; a ketamine-xylazine combination dose was 100 and 10 mg/kg ip, respectively; 2,2,2-tribromoethanol (No. T4,840-2, Sigma-Aldrich), commonly known as Avertin, was used at a dose of 290 mg/kg ip; and ketamine alone was used at a dose of 150 mg/kg ip. Isoflurane was administered via a special vaporizer for rodents delivered by a small nose cone. The depth of anesthesia was determined by immobility and assessing the absence of the withdrawal reflex of the right paw.
Echocardiography.
Each mouse was held as previously described (3) and prewarmed echo transmission gel was applied to the hairless chest. The mouse was then placed in decubitus supine position, and the ultrasound transducer was placed slightly left of the sternum over the fourth and sixth ribs. For echocardiographic image acquisition during sedated conditions, the animal was placed in a supine position on a prewarmed platform. The animal's limbs were taped over the metal ECG leads. The mouse body temperature was carefully monitored and maintained as close to 37°C as possible during the entire procedure.
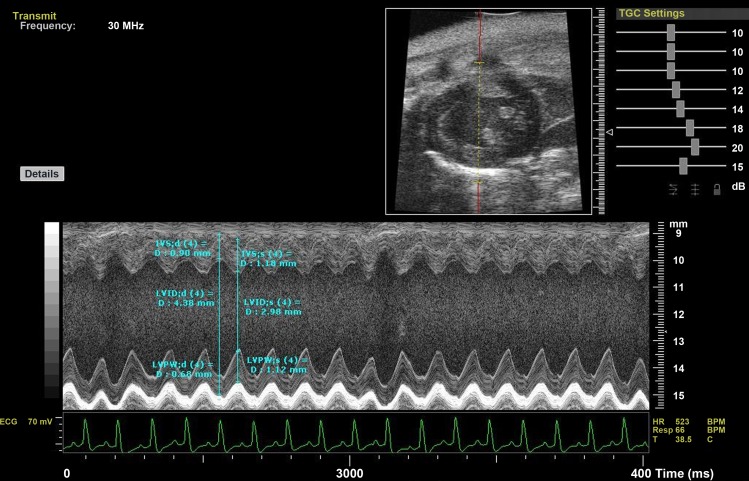
With the use of high-frequency VisualSonics Vevo 770 system (VisualSonics, Toronto, Canada), parasternal short-axis view at the level of the papillary muscles, two-dimensional-guided M-mode images were recorded. Three consecutive beats were measured at the 5th min after anesthesia, and the average of these measurements was taken for analysis (Fig. 1). LV internal systolic and diastolic diameters were measured according to the American Society of Echocardiography leading-edge technique (2, 5, 18). These measurements allowed the determination of fractional shortening (in %) and ejection fraction (in %) using the Vevo 770 software for calculation.
Fig. 1.
M-mode, high-frequency echocardiography: parasternal short-axis view at the level of the papillary muscles. LVIDd and LVIDs, left ventricular (LV) internal dimension in diastole and in systole, respectively; IVSd and IVSs, interventricular septum in diastole and in systole, respectively; LVPWd and LVPWs, LV posterior wall in diastole and in systole, respectively; TGC, time gain compensation; HR, heart rate; BPM, beats/min; Resp, respiratory; T, temperature.
Protocol to assess relationship between heart rate and LV end-diastolic dimensions.
Mice were sedated with ketamine-xylazine (100 and 10 mg/kg, respectively), and a central intravenous line was placed through the left jugular vein for drug administration. Phenylephrine at 50 μg·kg−1·min−1 was infused, followed by infusion of sodium nitroprusside at 10 μg·kg−1·min−1. These drugs increase and reduce arterial pressure with reflexly opposing changes in heart rate. Continuous and simultaneous measurements of LV diameter and heart rate were monitored.
Intra- and interobserver variability.
For intraobserver variability determination, one examiner performed all measurements twice at a different point in time. For interobserver variability, all measurements were taken by a different examiner.
Statistical analysis.
Data were reported as means ± SE. Statistical significance was assessed using Student's t-test, simple linear regression, and ANOVA with Bonferroni's multiple comparison test for post hoc analysis using GraphPad Prism 5 software. Differences in slopes were assessed by comparison of two independent regression data sets. P < 0.05 was considered statistically significant.
RESULTS
Effects of anesthetics on LV function and heart rate.
All anesthetic agents studied reduced LV function and heart rate significantly (P < 0.05), compared with results in the same animals studied in the conscious state (Table 2). Results for LV function and heart rate for all anesthetics were found to be statistically different from each other (P < 0.05) with the exception of the heart rate measurement between isoflurane and Avertin (Table 2).
Table 2.
Echocardiographic measurements
| Conscious | Ketamine-Xylazine | Isoflurane | Avertin | Ketamine | |
|---|---|---|---|---|---|
| Heart Rate, beats/min | 636 ± 23† | 322 ± 9.2*† | 450 ± 22* | 486 ± 12* | 561 ± 14*† |
| Fractional shortening, % | 51 ± 0.9† | 32 ± 0.6*† | 36 ± 1.1* | 39 ± 0.6*† | 47 ± 0.5*† |
| Ejection fraction, % | 83 ± 0.6† | 60 ± 1*† | 65 ± 1.5* | 69 ± 0.7*† | 79 ± 0.5*† |
| LV internal dimension in diastole, mm | 3.3 ± 0.08† | 4.0 ± 0.12* | 3.9 ± 0.18* | 3.7 ± 0.08 | 3.0 ± 0.09† |
| LV internal dimension in systole, mm | 1.6 ± 0.05† | 2.7 ± 0.09* | 2.5 ± 0.13* | 2.2 ± 0.06* | 1.6 ± 0.06† |
Values are means ± SE; n = 14 for all groups.
LV, left ventricular.
P < 0.05 vs. conscious state;
P < 0.05 vs. isoflurane.
Echocardiographic measurements were obtained 5 min following the administration of each anesthetic to exclude the variation in cardiac function created by time after administration.
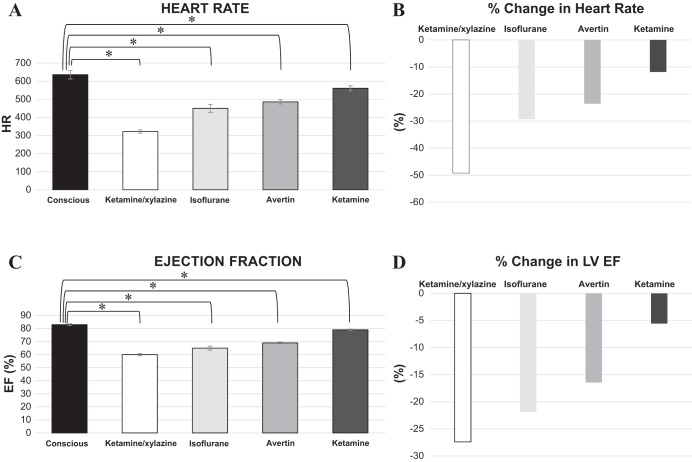
Surprisingly, isoflurane was not the best, but actually second to last in maintaining normal LV function and heart rate (Table 2). The anesthetic with the least effect on LV function and heart rate was ketamine alone at a dose of 150 mg/kg, which induced a surgical level of anesthesia, followed by Avertin (290 mg/kg), isoflurane (3% induction and 1 to 2% maintenance), and lastly ketamine-xylazine (100 and 10 mg/kg, respectively) (Fig. 2). Results were similar in the three different strains studied (Table 3). There were no sex differences observed, as the values between males and females were close and confirmed with two way analysis of variance.
Fig. 2.
A: baseline HR compared among anesthetics vs. conscious state. B: percent change in HR compared among anesthetics vs. conscious state. C: baseline LV ejection fraction (EF) compared among anesthetics vs. conscious state. D: percent change in LV EF compared among anesthetics vs. conscious state. n = 14 for all groups. *P < 0.05.
Table 3.
Strain differences in heart rate and LV ejection fraction
| Strain | n | Conscious | Ketamine-Xylazine | Isoflurane | Avertin | Ketamine |
|---|---|---|---|---|---|---|
| Heart rate | ||||||
| C57BL/6J | 5 | 680 ± 14 | 309 ± 15 | 459 ± 27 | 500 ± 25 | 579 ± 15 |
| 129 SVJ | 4 | 650 ± 24 | 347 ± 22 | 457 ± 30 | 494 ± 9 | 543 ± 42 |
| FVB | 5 | 686 ± 16 | 317 ± 9 | 434 ± 26 | 465 ± 24 | 556 ± 18 |
| Ejection fraction | ||||||
| C57BL/6J | 5 | 83 ± 1.0 | 60 ± 1.7 | 62 ± 2.1 | 70 ± 1.5 | 79 ± 1.0 |
| 129 SVJ | 4 | 85 ± 1.5 | 59 ± 1.6 | 65 ± 1.5 | 69 ± 1.1 | 78 ± 1.1 |
| FVB | 5 | 86 ± 0.4 | 62 ± 1.6 | 68 ± 1.0 | 70 ± 1.3 | 80 ± 0.7 |
Values are means ± SE.
The relationship between changes in heart rate and LV end-diastolic diameter was determined by echo during infusions of phenylephrine, which increases pressure and reduces heart rate reflexly, and nitroprusside, which does the reverse. A highly significant, linear relationship was observed, demonstrating the inverse relationship between heart rate and LV end-diastolic diameter.
DISCUSSION
It is generally agreed that cardiovascular physiological measurements are best studied in conscious animals, since anesthesia depresses LV function and heart rate, and even more importantly autonomic control (22, 23). However, there are several reasons why it is often not possible to study conscious animals, e.g., because of time constraints or lack of expertise. It then becomes important to determine the anesthetic agents that exert the least effect on LV function and heart rate. Before embarking on the current investigation, we reviewed the cardiovascular physiological literature to determine the most frequently used anesthetics for mice studies over the last three years. We found that over 75% of studies in mice with anesthesia used isoflurane. Unexpectedly, when comparing this anesthetic with ketamine, Avertin, and ketamine-xylazine, isoflurane was next to most severe in terms of impairment of LV function and heart rate. Ketamine was the best, with Avertin second. Ketamine-xylazine also produced the greatest increase in LV end-diastolic diameter. This could be attributed, in part, to its greater action to depress cardiac function. However, this anesthetic also produced the greatest depression in heart rate. It is well recognized that heart rate is inversely related to LV end-diastolic size (7). To demonstrate this point more clearly in the context of the current study, we altered heart rate over a wide range by infusing phenylephrine and nitroprusside, which alter arterial pressure and induce opposing arterial baroreflex-mediated changes in heart rate (24). This experiment clearly pointed out that part of the greater increase in LV dimensions under ketamine-xylazine could be attributed to the bradycardia that it induced (Fig. 3). It also should be noted that the depressant effects of the anesthetics on cardiac function are due to several mechanisms; the primary one is a direct effect on the heart, but secondary effects on baroreflexes and hormonal as well as neuronal effects on the heart must also be considered (22, 23).
Fig. 3.
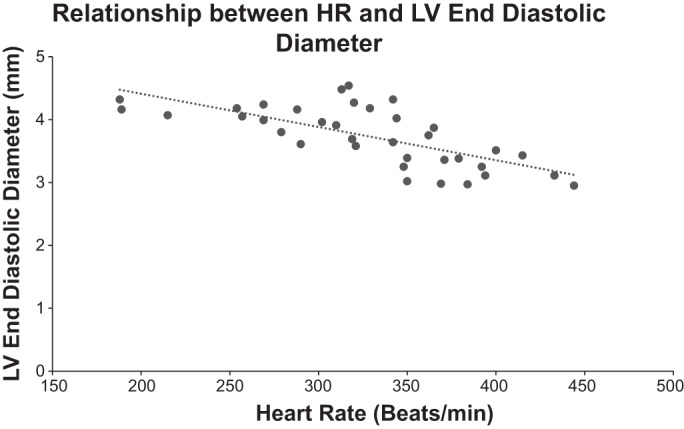
Relationship between HR and LV end-diastolic diameter (n = 5). HR was reflexly increased by infusion of nitroprusside and reflexly decreased by infusion of phenylephrine. An inverse linear relationship was observed (r2 = 0.5002). P = 0.000001.
It could be argued that the difference in LV function in the anesthetics studied was due to differences in anesthesia levels. We did test the adequacy of anesthetic level, ensuring that all animals under each anesthetic had no withdrawal reflex after stimulation of the right paw. In general, there are four stages of anesthesia in animals. We selected stage 3, plane 2, for the current experiments, since it is stable enough for little hemodynamic change during the procedures and reproducible among the anesthetics employed. It is also important to note that the doses of anesthetics used were similar to those used by others for echocardiography (6, 10, 12, 13, 16, 19, 25, 26, 28). For example, the ketamine-xylazine dose ranged from 60–150/2.5–20 mg/kg, respectively (1, 3, 6, 11, 13, 16, 17, 20, 27, 28). The dose we selected, 100 and 10 mg/kg, respectively, was selected on the basis of prior studies (4, 27) and our past experience to allow sufficient time for stable anesthesia to complete the hemodynamic measurements.
Severe side effects have been reported for Avertin (14, 21, 29), although this point is controversial (9). Importantly, we found no adverse side effects for any of the anesthetics used over the course of the 1 to 2 mo during which we studied each animal. However, we only examined four separate anesthetics in each animal using multiple doses (3 per wk) of the same anesthetic. There are advantages and disadvantages for any anesthetic, and some relate to whether the anesthetic is to be used for a surgical level of anesthesia or a level just for sedation. For echo studies isoflurane has been used most commonly (Table 1). This anesthetic induces anesthesia rapidly and its recovery is also rapid. Moreover, the level of anesthesia is easiest to change using isoflurane. One disadvantage to this anesthetic is that it requires a person, different from the surgeon to adjust the anesthesia level. All the other anesthetics studied are generally administered intraperitoneally, which doesn't allow the same flexibility and rapid onset and offset as isoflurane.
Strain differences among mice were also considered. A previous study comparing cardiac function by echocardiography in different strains of mice revealed that FVB mice, under isoflurane anesthesia, had increased LV ejection fraction and fractional shortening with significantly faster heart rate (28). Another study using Avertin did not find any significant difference between FVB and C57BL/6J strains (16). Our results showed no significant difference in LV systolic function among the three strains studied. Another potential source of variance is sex difference, since differences in hormones in males and females may affect the metabolism of anesthetics (8). However, most studies have not found significant sex differences (10, 15). We also did not find any sex differences.
Conclusion.
Cardiac function assessed by echocardiography is affected to a different extent among the commonly used anesthetic agents. Ketamine alone exerts the least depressant effects on LV function and heart rate, with Avertin second. This suggests that ketamine alone or Avertin should be used when it is not feasible to study mice in the conscious state, as opposed to the most commonly used anesthetic, isoflurane.
GRANTS
This work was supported by funding from National Institute of Health Grants 5P01AG027211, 1R01HL102472, 5T32HL069752, 5R01HL095888, 5P01HL069020, R01HL106511, R01HL093481, 1R01HL119464, and 1R01HL106511.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.E.P. performed experiments under guidance of D.E.V. and S.F.V.; R.E.P. analyzed data under guidance of D.E.V. and S.F.V.; S.F.V. interpreted experiments with assistance from R.E.P. and D.E.V.; S.F.V. wrote manuscript with assistance from R.E.P. and D.E.V.; S.F.V. approved final version with assistance from R.E.P., D.E.V., and B.A.S.; S.F.V., R.E.P., and D.E.V. edited and revised manuscript; S.F.V. and D.E.V. were responsible for conception and design of research.
REFERENCES
- 1.Chaves AA, Weinstein DM, Bauer JA. Non-invasive echocardiographic studies in mice: influence of anesthetic regimen. Life Sci 69: 213–222, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Devereux RB, Lutas EM, Casale PN, Kligfield P, Eisenberg RR, Hammond IW, Miller DH, Reis G, Alderman MH, Laragh JH. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol 4: 1222–1230, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in mice. Curr Protoc Mouse Biol 1: 71–83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J 53: E55–E69, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ, American Society of Echocardiography. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 17: 1086–1119, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Hart CY, Burnett JC Jr, Redfield MM. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. Am J Physiol Heart Circ Physiol 281: H1938–H1945, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Higgins CB, Vatner SF, Franklin D, Braunwald E. Extent of regulation of the heart's contractile state in the conscious dog by alteration in the frequency of contraction. J Clin Invest 52: 1187–1194, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 49: 17–26, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Hill WA, Tubbs JT, Carter CL, Czarra JA, Newkirk KM, Sparer TE, Rohrbach B, Egger CM. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J Am Assoc Lab Anim Sci 52: 176–179, 2013. [PMC free article] [PubMed] [Google Scholar]
- 10.Janssen BJ, De Celle T, Debets JJ, Brouns AE, Callahan MF, Smith TL. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol 287: H1618–H1624, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara Y, Tanonaka K, Daicho T, Nawa M, Oikawa R, Nasa Y, Takeo S. Preferable anesthetic conditions for echocardiographic determination of murine cardiac function. J Pharm Sci 99: 95–104, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Kiatchoosakun S, Kirkpatrick D, Hoit BD. Effects of tribromoethanol anesthesia on echocardiographic assessment of left ventricular function in mice. Comp Med 51: 26–29, 2001. [PubMed] [Google Scholar]
- 13.Lairez O, Lonjaret L, Ruiz S, Marchal P, Franchitto N, Calise D, Fourcade O, Mialet-Perez J, Parini A, Minville V. Anesthetic regimen for cardiac function evaluation by echocardiography in mice: comparison between ketamine, etomidate and isoflurane versus conscious state. Lab Anim 47: 284–290, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Lieggi CC, Artwohl JE, Leszczynski JK, Rodriguez NA, Fickbohm BL, Fortman JD. Efficacy and safety of stored and newly prepared tribromoethanol in ICR mice. Contemp Top Lab Anim Sci 44: 17–22, 2005. [PubMed] [Google Scholar]
- 15.Reddy DS, Zeng YC. Differential anesthetic activity of ketamine and the GABAergic neurosteroid allopregnanolone in mice lacking progesterone receptor A and B subtypes. Methods Find Exp Clin Pharmacol 29: 659–664, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth DM, Swaney JS, Dalton ND, Gilpin EA, Ross J Jr. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol 282: H2134–H2140, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography 24: 83–89, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer A, Meyer GP, Brand B, Hilfiker-Kleiner D, Drexler H, Klein G. Effects of anesthesia on diastolic function in mice assessed by echocardiography. Echocardiography 22: 665–670, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Tan TP, Gao XM, Krawczyszyn M, Feng X, Kiriazis H, Dart AM, Du XJ. Assessment of cardiac function by echocardiography in conscious and anesthetized mice: importance of the autonomic nervous system and disease state. J Cardiovasc Pharmacol 42: 182–190, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Tarin D, Sturdee A. Surgical anaesthesia of mice: evaluation of tribromo-ethanol, ether, halothane and methoxyflurane and development of a reliable technique. Lab Anim 6: 79–84, 1972. [DOI] [PubMed] [Google Scholar]
- 22.Vatner SF. Effects of anesthesia on cardiovascular control mechanisms. Environ Health Perspect 26: 193–206, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med 293: 970–976, 1975. [DOI] [PubMed] [Google Scholar]
- 24.Vatner SF, Higgins CB, Braunwald E. Sympathetic and parasympathetic components of reflex tachycardia induced by hypotension in conscious dogs with and without heart failure. Cardiovasc Res 8: 153–161, 1974. [DOI] [PubMed] [Google Scholar]
- 25.Vinhas M, Araujo AC, Ribeiro S, Rosario LB, Belo JA. Transthoracic echocardiography reference values in juvenile and adult 129/Sv mice. Cardiovasc Ultrasound 11: 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J, Bu L, Gong H, Jiang G, Li L, Ma H, Zhou N, Lin L, Chen Z, Ye Y, Niu Y, Sun A, Ge J, Zou Y. Effects of heart rate and anesthetic timing on high-resolution echocardiographic assessment under isoflurane anesthesia in mice. J Ultrasound Med 29: 1771–1778, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Ming Z, Dart AM, Du XJ. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin Exp Pharmacol Physiol 34: 499–507, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol Heart Circ Physiol 277: H1967–H1974, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Zeller W, Meier G, Burki K, Panoussis B. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim 32: 407–413, 1998. [DOI] [PubMed] [Google Scholar]