Abstract
Cells are constantly exposed to mechanical forces that play a role in modulating cellular structure and function. The cardiovascular system experiences physical forces in the form of shear stress and stretch associated with blood flow and contraction, respectively. These forces are sensed by endothelial cells and cardiomyocytes and lead to responses that control vascular and cardiac homeostasis. This was highlighted at the Pan American Physiological Society meeting at Iguassu Falls, Brazil, in a symposium titled “Mechanosignaling in the Vasculature.” This symposium presented recent research that showed the existence of a vital link between mechanosensing and downstream redox sensitive signaling cascades. This link helps to transduce and transmit the physical force into an observable physiological response. The speakers showcased how mechanosensors such as ion channels, membrane receptor kinases, adhesion molecules, and other cellular components transduce the force via redox signals (such as reactive oxygen species and nitric oxide) to receptors (transcription factors, growth factors, etc.). Receptor activated pathways then lead to cellular responses including cellular proliferation, contraction, and remodeling. These responses have major relevance to the physiology and pathophysiology of various cardiovascular diseases. Thus an understanding of the complex series of events, from the initial sensing through the final response, is essential for progress in this field. Overall, this symposium addressed some important emerging concepts in the field of mechanosignaling and the eventual pathophysiological responses.
Keywords: Anrep effect, mechanotransduction, NADPH oxidase, revascularization, vasculature
this article is part of a collection on 1st PanAmerican Congress of Physiological Sciences: Physiology Without Borders. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Cells in vivo are constantly exposed to the physical forces associated with their local environment. Although it was well known that forces such as gravity and friction act on organisms and affect their function and structure, it is only in the last two decades that the complex processes by which these forces are sensed by cells is becoming clear (37, 48, 55, 98). Cells and indeed cellular structures respond to external forces in a manner similar to chemical signaling where chemicals (ligands) bind to specific receptors on cells and initiate cellular signaling and an eventual response (24, 26, 36). Likewise, mechanical signaling or mechanotransduction involves the activation of receptors. Analogous to chemical signaling or chemotransduction, physical forces trigger a signaling process by which mechanical stimuli are translated into a biochemical and eventual physiological response (9, 16, 49, 80).
This symposium was organized to present mechanosignaling and related issues at the session titled “Mechanosignaling in the Vasculature: Shear stress, Endothelium and Redox Signaling” at the First Pan American Physiological Conference at Iguassu Falls in August 2014. Sponsored by the American Physiological Society, this symposium focused on various sites of vascular mechanotransduction in the cardiovascular system, primarily the vascular endothelium and the cardiomyocytes (primary sites for sensing blood flow and cardiac contractility respectively). Another focus of this session was to highlight the differences in long-term responses by the pulmonary and systemic vascular beds. The content and conclusion from this session has relevance in understanding the long-term consequences of mechanosensing, specifically in the context of cardiovascular diseases ranging from ischemia and atherosclerosis to cardiac hypertrophy.
Endothelial Mechanosensing and Redox Signaling With Altered Shear Stress (presented by A. B. Fisher)
Physical forces can modulate cellular functions by activating mechanosensitive pathways: the sequence of events consists of the initial sensing of the force by mechanosensors, followed by the onset of intracellular signaling and activation of specific transcription factors that eventually lead to regulation of gene and protein expression (11, 25). Among the physical forces that play a role in biology are shear stress associated with blood flow; indeed blood flow is well established to participate in maintaining endothelial function and vessel wall homeostasis. Because the endothelium by virtue of its location serves as a dynamic interface between blood flow and the vessel wall, sensors on endothelial cells are ideally suited to sense the shear associated with blood flow and to transduce this physical force into biochemical responses that help to maintain vascular structure and tone.
For more than two decades, our group has been engaged in understanding endothelial mechanosignaling and the complex pathways by which shear is “sensed” and transformed into a physiological response (9, 15, 17, 18, 65). Studies on shear signaling have hitherto been studied with onset of shear stress where endothelial cells (EC) in culture grown under zero shear stress conditions (i.e., in static cell culture) are subjected to sudden onset of flow. We reversed this paradigm and investigated shear signaling from the point of removal of shear; we reasoned that this being physiologically similar to vascular obstruction or ischemia is more relevant to an in vivo condition. However, ischemia in vivo does not merely affect the alteration of the mechanical component of blood flow but also causes compromised oxygen delivery to tissue. To study altered blood flow (while avoiding the effects of altered oxygen supply), we established an in situ model of pulmonary ischemia using a rodent isolated perfused lung (2, 82). The lung provided a methodological advantage over other organs since ventilation of the lung allows oxygenation of cells in the absence of blood flow. In our experiments with stopped flow in isolated perfused lungs, the tissue pO2 values remained constant (pO2*140 mmHg), and there was no decline in ATP levels. This model thus enabled us to study the effects of the loss of the mechanical component of flow alone without the effects of tissue hypoxia.
Using this isolated perfused model, we observed that abrupt cessation of flow caused the production of reactive oxygen species (ROS) that occurred by via activation of endothelial cell NADPH oxidase (Nox2) (4, 33, 97). The NADPH oxidase (Nox) family consists of 7 members with different catalytic subunits termed Nox1-5 and Duox1 and Duox2 (for Dual Oxidase); regulatory subunits p22phox, p47phox, Noxo1, p67phox, Noxa1, p40phox; and the major binding partner Rac 1 or 2 and the membrane subunit gp91phox. Nox1, 2, 4, and 5 enzymes are expressed in cardiovascular tissues (54). Of these, Nox2 has traditionally been described as responsible for the respiratory burst in phagocytes and is now accepted to be a vascular oxidase. Nox2 was found by us to be activated with stop of flow (15, 97). On activation, Nox2 uses NADPH to reduce molecular oxygen to superoxide anion, which dismutates to H2O2 (53, 54).
Using the H2O2 sensitive dye dichlolorodifluorofluorescein diacetate (H2DCFDA) or its carboxy-fluorinated derivative difluorofluorescein diacetate (H2DFFDA), which was perfused through the isolated lungs, we observed increased fluorescence (DCF or DFF) with stopped flow. DCF/DFF fluorescence was significantly reduced in lungs from mice with knockout of Nox2 (gp91phox subunit) or wild-type lungs pretreated with catalase or the nonspecific NADPH oxidase inhibitor, diphenyleneiodonium (15, 65, 97).
The earliest event in shear sensing had been reported elsewhere to be a K+ channel. Indeed, onset of flow was observed to cause hyperpolarization via opening of this channel (66). We posited that these channels that are activated by shear could also be closed by removal of the shear stimulus. We selected membrane potential sensitive dyes (di-8-ANEPPS and bisoxonol) that increase fluorescence in a depolarized membrane to assess channel activation with stop of flow (1, 82). Isolated lungs labeled with these dyes showed an increased fluorescence almost immediately upon stop of perfusate flow. Depolarization could be blocked by pretreating isolated lungs with the KATP channel opener (agonist) cromakalim; in the presence of flow, depolarization could be attained by the KATP channel blocker (antagonist) glybenclamide (1, 3, 14). Thus we proposed that a KATP channel of lung endothelium is responsible for maintaining membrane potential with normal shear and is inactivated by loss of shear leading to endothelial membrane depolarization as a key component in the cell signaling cascade.
The KATP channel consists of an inwardly rectified K+ channel pore (KIR6.x) and a regulatory subunit the sulfonylurea receptor (SUR) (8, 10). The KATP channel was observed to be induced in pulmonary microvascular endothelial cells by flow (14). Using in vitro flow systems where endothelial cells were subjected to various periods of flow, we reported that both components of the channel were very low in cells cultured under static conditions but increased during exposure to 24–48 h of flow. KIR currents (as measured by patch clamp) too were low in static cells and increased after adaptation to flow. Real time measurements of KIR currents showed that these currents were shear sensitive, i.e., flow-adapted cells showed characteristics of an activated KIR channel (high inward rectifier currents) under flow that decreased significantly with stop of flow (14, 17).
Induction of the KATP channel by flow suggested to us the presence of a shear responsive element on the endothelium that is upstream and independent of the channel. A cell surface entity would be well suited to be a primary transducer. Based on previous studies on transduction of mechanical forces including shear stress by a mechanosensory complex including platelet endothelial cell adhesion molecule 1 (PECAM-1) (86) and on reports of PECAM-1 activation (tyrosine phosphorylation) upon flow (22) we investigated whether PECAM-1 could, by virtue of its junctional location and cytoskeletal linkage, serve as a mechanosensor for the loss of blood flow. Our investigations revealed that lack of PECAM (PECAM-1 null mice lungs) markedly reduced the response to stop of flow (in terms of ROS production) by the pulmonary endothelium. Immunolabeling of lung sections showed that most of the cellular PECAM (70%) was located in caveolae (65). We had shown earlier that caveolin-1 and (and thus caveolae) participated in the stop of flow response (65).
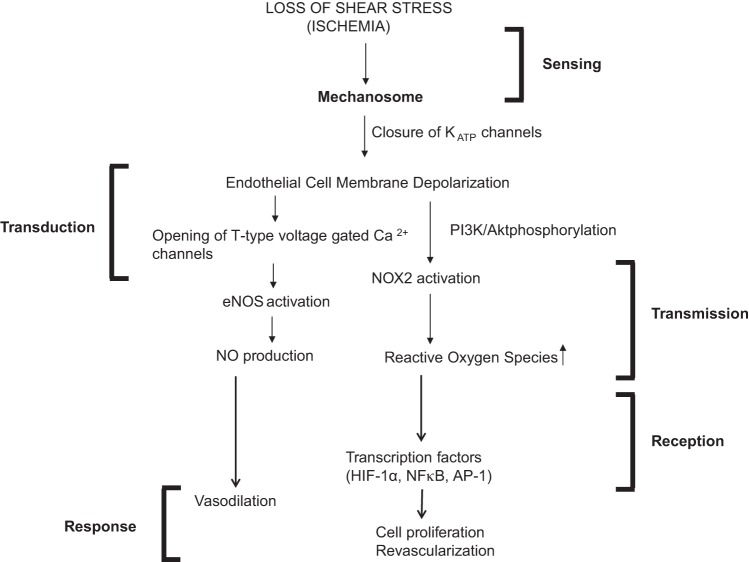
We thus concluded that a PECAM-caveolae mechanosensing complex on the endothelium is able to sense the changes in membrane tension with stop of flow. A multimeric complex comprising PECAM-1, vascular endothelial growth factor (VEGFR2), and vascular endothelial (VE)-cadherin has been reported to be sufficient to confer responsiveness to shear stress in cells. Taken together, we posit a mechanosome hypothesis, i.e., a network of mechanosensors and transducers that transmit a physical force into biochemical and transcriptional activity that can alter cellular structure and function. We conclude that the mechanosome on the endothelial cell membrane consists of caveolae, PECAM, VEGFR2, and VE-cadherin (and possibly other elements). The mechanosome senses changes in membrane tension resulting in the deactivation of KATP channels (15). The altered membrane potential results in the activation of Nox2 and eNOS with consequent production of ROS and nitric oxide (NO) (18) (Fig. 1). ROS is a signal for activation of transcription factors such as hypoxia inducible factor (HIF-1α), NF-κB, and activating protein-1 (AP-1), which reportedly drive revascularization with the homeostatic purpose to restore impeded flow (9).
Fig. 1.
Schematic of the mechanotransduction cascade in pulmonary vascular endothelium with stop of flow. Flow sensing by endothelial cells occurs via the mechanosome complex composed of caveolae-PECAM, VEGFR2, and VE-cadherin and possibly other elements resulting in deactivation of KATP channel. This alteration in membrane potential results in activation of NOX2 and endothelial nitric oxide (NO) synthase (eNOS) with consequent production of reactive oxygen species (ROS) and NO. These mediators result in vasodilation and neovascularization as an attempt to restore the impeded blood flow (modified from Ref. 16). HIF-1α, hypoxia inducible factor-1α; AP-1, activating protein-1.
Mechanism of PECAM-1 Mechanotransduction in Endothelial Cells (presented by K. Fujiwara)
ECs by virtue of their location are uniquely situated to face the physical force associated with blood flow. Shear regulates EC morphology, biosynthetic activity, and gene expression indicating that ECs possess flow sensing machinery that senses and responds to blood flow (21, 28, 41). Thus ECs are considered as a model system to study cellular mechanobiology; indeed studies using ECs have revealed that the mechanoresponse by endothelium triggers signaling that has important implications in the context of the pathophysiology of cardiovascular diseases. For instance, atherosclerosis, a focalized vascular disease caused by a combination of many risk factors, has been observed to be dependent on patterns of blood flow. Atherosclerotic plaques develop in regions where nonlaminar, disturbed blood flow occurs, such as branches, bifurcations, and curvatures of large arteries (28, 41). Besides disturbed flow, stretch patterns in these regions are not uniaxial, i.e., they are not along a single axis; thus both disturbed flow and non-uniaxial stretch are recognized to be pro-atherogenic mechanical forces (21, 83). As it is practically impossible to locally alter the flow patterns associated mechanical forces within blood vessels, a more reasonable approach to controlling atherogenesis would be to regulate EC responses to these mechanical forces. To do so, the processes by which mechanosensing by ECs occurs, must be understood.
The major challenge in studying mechanosensing moieties is that unlike chemo receptors that can be identified based on their binding to specific ligands, and thus purified, cloned, and studied using biochemical and other analytical means, mechanoreceptors cannot be easily identified as they have no ligand equivalent. We thus have to focus on the downstream events that occur post flow and flow sensing to obtain information on the earliest mechanosensing events. Such studies show that ECs are equipped with machinery for sensing mechanical forces, although the entities or moieties (often referred to as mechanosensors) that sense flow are numerous and varied. Analogous to a ligand receptor, a mechanosensor is the first thing that mechanical forces act on and has the ability to convert a mechanical stimulus into a biochemical reaction that can induce mechano-dependent cellular responses.
Candidate mechanosensors are the glycocalyx (34, 69), primary cilia (61, 62), caveolae (9, 10), focal adhesions/integrins (78, 95), ion channels (28, 60), trimeric G proteins (40, 52), nuclei (32), and PECAM-1 (35). It is possible that all of them are, in some way, involved in EC mechanosignaling, but are they sensors? The general consensus is that there is not a single mechanosensor but several which work in concert, possibly as a series of multimeric complexes.
For more than a decade, our laboratory has investigated PECAM-1-dependent signaling pathways initiated by shear stress. PECAM, a glycoprotein expressed in ECs, platelets, and leukocytes (63), is by virtue of its junctional location and cytoskeletal linkage well suited to be a mechanosensor. In solitary ECs, it is diffusely distributed in the plasma membrane but when cultured ECs make contacts, PECAM-1 aligns at the contact site. This alignment occurs in 2 to 4 h, unlike other contact adhesion molecules such as VE-cadherin, which do so almost immediately (7). This suggests that homophilic binding of PECAM-1 is not essential for cell-cell contact formation and may have some other functions. In vivo, PECAM-1 has several other functions, namely a role in leukocyte trafficking across the endothelium (63), regulation of certain integrin activities (20, 64) [flow-activated integrin activities are PECAM-1 dependent (21)], and interaction with the actin cytoskeleton via β- and γ-catenins (45, 47) and/or by vimentin (27, 46).
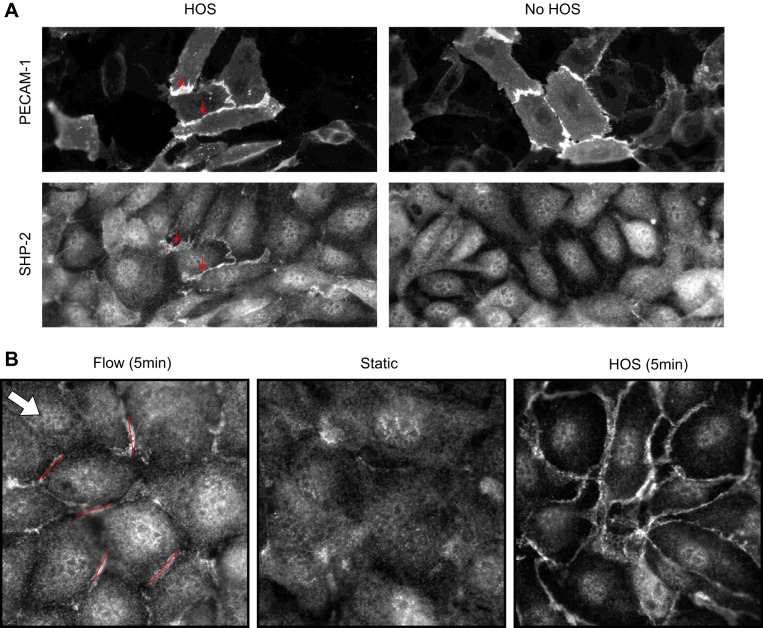
PECAM-1 has been observed to respond to shear or stretch on the endothelial surface by phosphorylation of a tyrosine (Tyr) residue. Indeed, when ECs in a confluent monolayer (instead of a sparse culture) were exposed to physiological levels of shear stress (67) or cyclic stretch (22), PECAM-1 was rapidly Tyr phosphorylated. Tyr phosphorylation of PECAM-1 was also observed with hypo- or hyper-osmotic shock (67), another form of mechanical perturbation. Postphosphorylation PECAM-1 binds to SHP-2 (57), a protein tyrosine phosphatase involved in ERK1/2 activation. Because ERK1/2 is activated by flow (85) and stretch (81) in ECs, this activation may depend on PECAM-1 phosphorylation and subsequent binding to SHP-2 (67). SHP-2 is a cytoplasmic protein, but shortly after ECs are challenged with mechanical stresses, SHP-2 is recruited to the cell-cell contact region where PECAM-1 is localized (67). SHP-2 accumulation at cell contacts is a good readout for observing PECAM-1 activation in ECs (Fig. 2A). With use of SHP-2 as a reporter for PECAM-1 phosphorylation, mechanical force-induced phosphorylation of PECAM-1 inside cells was visualized. When ECs were hyperosmotically shocked, PECAM-1 phosphorylation occurred all around cells while laminar flow activated PECAM-1 signaling at the cell border oriented perpendicular to the direction of flow (Fig. 2B). The flow data suggest that PECAM-1 mechanosignaling is initiated locally, not globally, in a cell.
Fig. 2.
A: bovine aortic endothelial cells (ECs) were transfected with platelet endothelial cell adhesion molecule 1 (PECAM-1) short interfering RNA (siRNA), treated with hyperosmotic shock (HOS) or without hyperosmotic shock (No HOS) for 2 min (a condition known to induce PECAM-1 phosphorylation), and double-stained with anti-PECAM-1 (top) and anti-SHP-2 (bottom). The same field of view is shown for PECAM-1 and SHP-2. Top: several adjacent cells that were not transfected with the siRNA (thus expressing PECAM-1 at their shared cell border; arrows). When such cells were shocked with hyperosmotic medium (HOS), PECAM-1 became phosphorylated, which can be visualized by anti-SHP-2 staining (bottom left). Unshocked cells do not show cell border staining with anti-SHP-2 (right). These images demonstrate that SHP-2 relocalization can be used as a PECAM-1 phosphorylation reporter in ECs. Note that SHP-2 is normally localized throughout the cell. Data provided by Dr. Elena McBeath. B: SHP-2 relocalization to cell-cell contacts in confluent bovine aortic ECs. Anti-SHP-2 staining in unstimulated cells (static) show little cell border associated fluorescence. When ECs were exposed to 1 Pa of laminar shear stress for 5 min (flow), short linear anti-SHP-2 staining associated with cell border can be detected. Some of the stained areas are highlighted by straight lines (roughly 10 μm long). Note that many lines are roughly perpendicular to flow direction (arrow). Five minutes of hypersomotic shock (HOS), which is thought to cause membrane perturbation more equally throughout the entire cell border, causes extensive cell border staining, which surrounds the entire cell. Data provided by Brooke Krovic and Elena Mcbeath.
We reasoned that if PECAM-1 was indeed mechanoresponsive, we may be able to achieve its phosphorylation and downstream signaling by directly pulling on the molecule. To test this, magnetic beads coated with antibodies against the PECAM-1 external domain were attached to the surface of sparsely cultured ECs and pulled by placing a strong magnet over the cells (67). Cell homogenates were assessed for Tyr-phosphorylation, which was detected only when PECAM-1 bound to beads which had been pulled by a magnet. In addition, ERK1/2 was activated when PECAM-1 was pulled by magnetic beads. Although these results suggest that PECAM-1 signaling can be induced in ECs by applying force directly to PECAM-1, it is possible that this phosphorylation is caused by other moieties associated with the plasma membrane (such as ion channel activation) and/or cytosolic proteins, which are activated by pulling on PECAM-1. To evaluate these possibilities, we made a cell model by extracting a monolayer of ECs with a detergent containing solution, which was then stretched in the presence of ATP (22). Under these conditions, PECAM-1 was phosphorylated. Using this system, we identified Fyn kinase (which presumably was associated with the cell model) as the PECAM-1 kinase. Because PECAM-1 phosphorylation occurs by stretching lifeless cell models (that have no cytoplasmic soluble milieu and ionic events involving the plasma membrane), we have concluded that PECAM-1 phosphorylation can be mechanically triggered.
PECAM-1 null mice (29, 56) show no adverse phenotype, but Schenkel et al. subsequently showed that the genetic background of mice against which the PECAM-1 null condition was imposed played a major role in the severity of defective phenotypes (79). There are several lines of evidence that tie PECAM-1 to cardiovascular diseases. It has an anti-inflammatory effect (75), and neointima formation seen in the carotid ligation model depends on PECAM-1 expression (19). When PECAM-1 null mice were crossed with ApoE null mice, reduced atherosclerosis was noted (42), whereas another study found both pro-atherogenic and athero-protective effects of PECAM-1 depending on the vessel types studied (38). There are several known PECAM-1 polymorphisms in the human that have a positive correlation with cardiovascular diseases (30, 31, 39). These recent developments beg for a closer look at the role of PECAM-1 in cardiovascular diseases as well as for further mechanistic studies on the workings of PECAM-1 signaling.
The Anrep Effect: A Redox-Sensitive Phenomenon (presented by N. G. Pérez)
The contractile apparatus of the heart is modulated by potent intrinsic mechanisms in the cardiac muscle that allow for adapting cardiac output to hemodynamic changes; that is, a sudden increase in cardiac muscle length immediately leads to a more powerful contraction. This is termed the Frank-Starling mechanism and describes how stretch of the cardiac muscle increases contractility. The cellular mechanism underlying the Frank-Starling response is an increase in myofilament sensitivity for Ca2+. This allows the heart to increase its output after a rise in preload or to maintain it despite a greater afterload. It also links cardiac ejection (arising from contractility) to cardiac filling (arising from expansion). Interestingly, after this initial rise in contractility myocardial performance slowly continues to increase, reaching a new steady state over the next 10–15 min.
Gleb von Anrep was the first to observe this phenomenon more than a century ago in a whole heart preparation (93) when he observed that clamping the ascending aorta in a dog (acutely decreasing outflow and increasing intraventricular pressures) caused an initial dilation of the heart; however, it progressively returned toward its previous end-diastolic volume unveiling a positive inotropic effect. Several years later the presence of a second slow component of increase in force following a change in cardiac muscle length was found in an isolated cardiac preparation by Parmley and Chuck (70). Afterward, Allen and Kurihara (5) demonstrated that this slow phase was due to a progressive increase in the intracellular calcium transient amplitude. Currently, the term “slow force response” (SFR) to stretch is widely accepted to identify this in vitro equivalent to the “Anrep phenomenon.”
During the last two decades, our laboratory has focused on the signaling pathways that generate the SFR. We have obtained evidence to propose that the SFR is the mechanical expression of a stretch-triggered autocrine/paracrine loop of intracellular signals that involves an increased production of ROS, activation of the redox-sensitive kinase cascade of MEK-ERK1/2-p90RSK, and the consequent phosphorylation (activation) of the cardiac Na+/H+ exchanger (NHE1) (for review see Ref. 23).
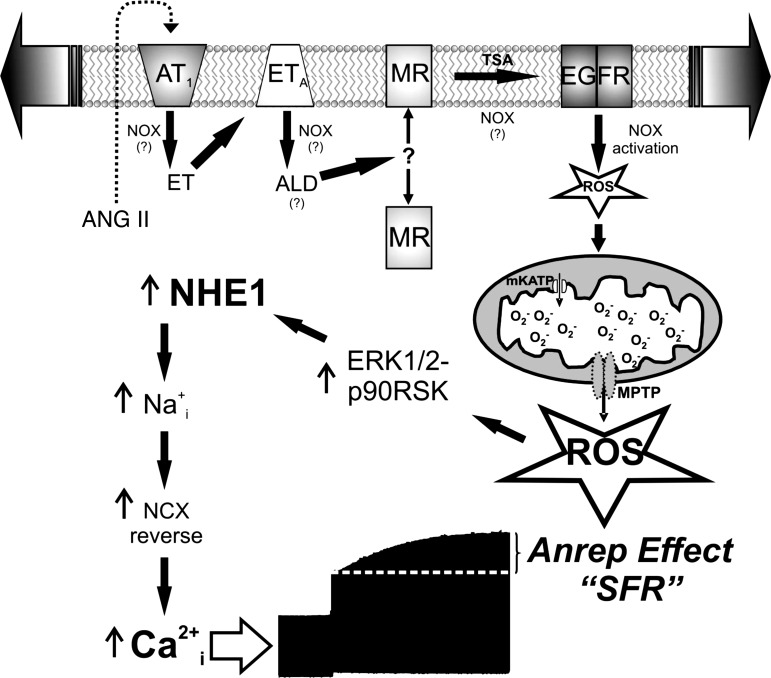
The increased production of ROS is a crucial step in the mechanism leading to the SFR generation. Conversely, suppression of ROS production blunts the SFR (13). These ROS are from mitochondrial origin but induced by a small amount of NADPH oxidase (NOX)-derived ROS, in a way that may be analogous to the so-called “ROS-induced ROS-release” phenomenon described by Zorov et al. (99) years ago and shown more recently in pulmonary microvascular endothelial cells (43). The complete sequence of events triggered by myocardial stretch and leading to the Anrep Effect is schematized in Fig. 3 and comprises the 1) release of the pro-hypertrophic factors angiotensin II and endothelin with the consequent sequential activation of their respective receptors (AT1-ETA), 2) activation of the mineralocorticoid receptor (MR), 3) transactivation of the epidermal growth factor receptor, 4) NOX activation (that may also occur in other steps of the signaling pathway), 5) mitochondrial ROS production, 6) activation of redox-sensitive kinases, 7) NHE1 hyperactivity, 8) increase in intracellular Na+ concentration, 9) increase in Ca2+ transient amplitude through the Na+/Ca2+ exchanger in reverse, and 10) development of the Anrep Effect or SFR.
Fig. 3.
Signaling pathway triggered by myocardial stretch leading to Anrep Effect or slow force response (SFR). To better appreciate the development of the SFR after the initial increase in force following myocardial stretch, an original force record of an isolated papillary muscle stretched suddenly from 92% to 98% of its maximal length is included at the bottom of the figure. ET, endothelin; ALD, aldosterone; TSA, transactivation; mKATP, mitochondrial ATP-sensitive potassium channel; MPTP, mitochondrial permeability transition pore; EGFR, epidermal growth factor receptor; MR, mineralocorticoid receptor; NCX, Na+/Ca2+ exchanger. See text for details and other abbreviations.
Physiologically, the Anrep Effect constitutes a powerful mechanism by which the heart adapts to an abrupt increase in afterload, occurring just after the Frank-Starling mechanism takes place. Interestingly, crucial intracellular signals for the development of the Anrep Effect like increased oxidative stress, NHE1 hyperactivity, and augmented Ca2+ concentration were reported to play critical roles in the progression of pathological cardiac hypertrophy (for review see Refs. 92 and 94).
In this context, it is attractive to hypothesize that mechanical stress may not only trigger immediate intrinsic heart mechanisms to adapt cardiac output to changes in hemodynamic conditions (Anrep phenomenon), but also would constitute the first step toward cardiac hypertrophy and eventually heart failure if the initial events are sustained over time. In this regard, and from a clinical point of view, we are tempted to affirm that among the latest contributions of our group to elucidate the mechanism leading to the SFR, the finding of the crucial role played by the MR was probably the most important one (12, 71). This may provide a reasonable explanation to the remarkably beneficial effects of MR antagonists in the treatment of patients with cardiac hypertrophy and failure (73, 74, 96), and encourage us to suggest that prevention of oxidative stress and NHE1 activation should be considered as potential key factors to reach this salutary effect.
Stop of Blood Flow Drives Neovascularization Via Reactive Oxygen Species-Induced Recruitment of Bone Marrow Stem Cells (presented by M. Ushio-Fukai)
Stop of blood flow or ischemia has been reported to drive neovascularization. In the case of ischemic heart and peripheral artery diseases, where blood flow is stopped or compromised, neovascularization is critical in eventual restoration of blood flow. Indeed treatment strategies are focused on accelerating processes that restore impeded blood flow. Restoration or reinstatement of flow depends on angiogenesis that occurs as stem and progenitor cells released from the bone marrow (BM) home into regions of ischemia and promote blood vessel growth. The difference between stem cells and progenitor cells is that while the former exhibit multipotency or pluripotency and the capacity for self-renewal, and clonal expandability, the latter, although possessing their self-renewal capacity, are committed to selected lineages. Although both BM stem and BM progenitor cells have been used for regenerative therapies, their reduced angiogenic and regenerative function under pathological conditions such as aging and diabetes mitigate their regenerative function. Thus identifying the key factors that can regulate their regenerative function would be of great value for therapeutic applications of stem/progenitor cells.
The stem/progenitor cells in BM are distributed in distinct microenvironments also called niches, such as the stromal niche, the endothelial niche, and niches in the extracellular matrix (59). Undifferentiated hematopoietic stem cells that reside within the BM niche are maintained in a quiescent state. With stimulation, these quiescent cells can undergo proliferation, migration, and differentiation. With ischemia, the BM niche is regulated such that stem and progenitor cell function and activity are affected. This is because ischemia associated injury causes an increase in cytokines and growth factors such as VEGF in BM and in the circulation. Cytokines and VEGF activate matrix metalloproteinase (MMP-9) and release soluble kit ligand in the BM microenvironment (77). MMP-9, which is secreted mainly by neutrophils in BM (68), and MT1-MMP (Membrane Type 1 MMP) (50), which is anchored on the cell surface, affect stem/progenitor cell mobilization and thus drive angiogenesis. Our laboratory is focused on understanding how ischemia regulates the BM niches and thus stem/progenitor cell function as this is critical in the development of novel therapeutic strategies to enhance regeneration of diseased tissues.
Recent evidence from our laboratory shows that ROS such as superoxide anion (O2·−) and hydrogen peroxide (H2O2) can determine stem/progenitor cell function (89, 91). Induction of ROS in stem cells is regulated by various factors such as cytokines, growth factors, hypoxia, etc. Studies show that ROS levels in BM stem and progenitor cells correlate with their function and differentiation. ROShigh cells show higher differentiation while ROSlow cells show greater capacity for self-renewal (51). BM niches include hypoxic or normoxic (less hypoxia) niches. Because oxygen is required for production of ROS, hynoxic niches exhibit low ROS levels as compared with normoxic niches (89). Overall, the state of the cells and their micro environment is critical for determining stem and progenitor cell fate and function (44, 89). One of the major sources of ROS involved in BM niche signaling is the enzyme NADPH oxidase 2 (Nox2). Nox 2 is expressed in phagocytes and nonphagocytic cells including stem/progenitor cells (89, 91) and differentiated myeloid cells such as neutrophils and monocytes/macrophages. These cells generate O2·− via activation of Nox2 (also known as gp91phox, the membrane subunit of the NADPH oxidase complex). Nox isoforms expressed in stem/progenitor cells (72) and Nox-derived ROS are involved in differentiation, proliferation, senescence, or apoptosis (89, 90). Under conditions of oxidative stress BM niches or environment may have excess amounts of ROS, which can induce apoptosis of stem and progenitor cells (89).
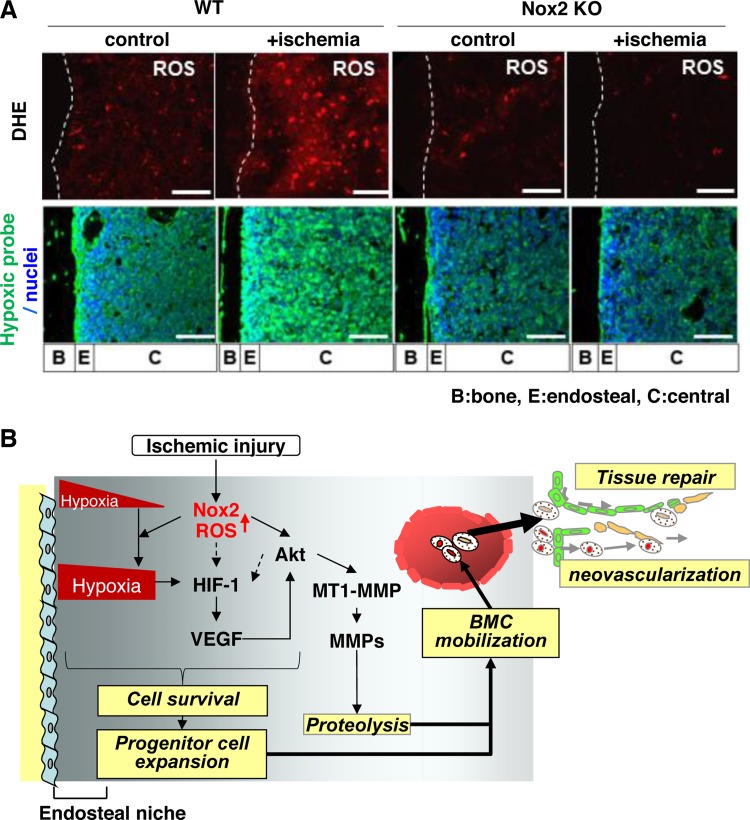
Using a model of hindlimb ischemia (in wild-type and Nox2−/− mice), we demonstrated that stop of blood flow to the femoral artery increases Nox2-dependent ROS production in BM-derived mononuclear cells (BMCs). We also showed that postischemic neovascularization and mobilization of BM progenitor cells are impaired in Nox2−/− mice (84, 87). Injection of wild-type BMCs, but not Nox2−/− BMCs, enhanced angiogenesis in the limb (as evidenced by increased perfusion) after ischemia (87). Moreover, in vivo injection of O2·− reactive dye into wild-type and Nox2−/− mice before euthanasia demonstrated that Nox2-dependent ROS production is markedly increased in entire BM following ischemic injury (88). We found that ischemia induced alteration of the BM microenvironment was Nox2 dependent and occurred via VEGF expression and Akt phosphorylation as also via MT1-MMP expression and MMP-9 activity in BM tissue (88). Furthermore, these ROS-mediated, hypoxic BM microenvironment alterations induced by ischemia regulate progenitor cell survival and expansion, thereby promoting their mobilization from BM (89) (Fig. 4). Thus our study suggests that Nox2-derived ROS play an important role in redox regulation of the BM microenvironment, thereby promoting progenitor cell function and mobilization that is required for enhancing postnatal neovascularization and tissue repair in response to injury.
Fig. 4.
A: Nox2 is involved in superoxide (O2·−) production in bone marrow (BM) in situ in response to hindlimb ischemia. Representative images of O2·− production in BM of femur from wild-type (WT) or Nox2 knockout (KO) mice subjected to hindlimb ischemia [+ischemia (day 3)] or without hindlimb ischemia (control). To detect O2·− in situ, dihydroethidium (DHE) was injected 60 min before tissue harvest. Long bone surface is shown by dotted lines. Bottom: hindlimb ischemia increases hypoxic area in BM, in a Nox2-dependent manner. Mice were injected with pimonidazole 3 h before euthanization. Femur sections from mice subjected to hindlimb ischemia [+ischemia (day 3)] or without ischemia (control) were stained with anti-pimonidazole antibody (green) and 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclei (blue). Bars show 100 μm. Bone (B), endosteal (E) (defined as 50 μm apart from the bone surface), and central (C) regions are indicated. B: Nox2 regulates BM microenvironment involved in progenitor cell function and mobilization in response to ischemia. After ischemic injury, ROS production is increased in entire BM in a Nox2-dependent manner, which is required for increasing hypoxic niche and its downstream HIF-1α and VEGF expression in BM. Nox2-derived ROS also increase Akt phosphorylation and its downstream membrane type1-matrix metalloproteinase (MT1-MMP) expression and MMP-9 activity in BM. It is possible that ROS and Akt pathway are involved in HIF-1α expression through hypoxia-independent mechanism (dotted arrows). These Nox2-dependent alterations of BM microenvironment promote progenitor cell survival and expansion, thereby promoting their mobilization, leading to reparative neovascularization and tissue repair (modified from Ref. 88).
Mechanosensing Drives Neovascularization in vivo That is KATP Channel Dependent (presented by S. Chatterjee)
It is now well established that mechanotransduction by the endothelium associated with shear stress arising from luminal blood flow is transduced to signals that help to maintain vascular function and homeostasis (6, 76).
Our research showed that stop of flow caused KATP channel closure (Fig. 5A) that led to ROS production, and this ROS caused endothelial cell proliferation (9, 58). Does this proliferation lead to remodeling or is it a nonpurposeful response? To answer this question, we assessed if the KATP-dependent mode of Nox2 activation had any relevance to vascular remodeling. Using in vitro (Matrigel coated dishes) and in situ (Matrigel plug in nude mice) models, we found that the postischemia angiogenic potential of wild-type endothelial cells was significantly higher than that for KATP channel null and Nox2 null cells (9). The implications of proliferation and angiogenesis were clear when we assessed the effect of stopped flow in vivo using a model of systemic ischemia, i.e., femoral artery ligation. To achieve this, we did not cut or excise the femoral vessel but merely tied it; we reasoned that this approach would minimize the injury arising from the experimental procedure and would limit the complex signaling cascade activated by inflammation and tissue damage.
Fig. 5.
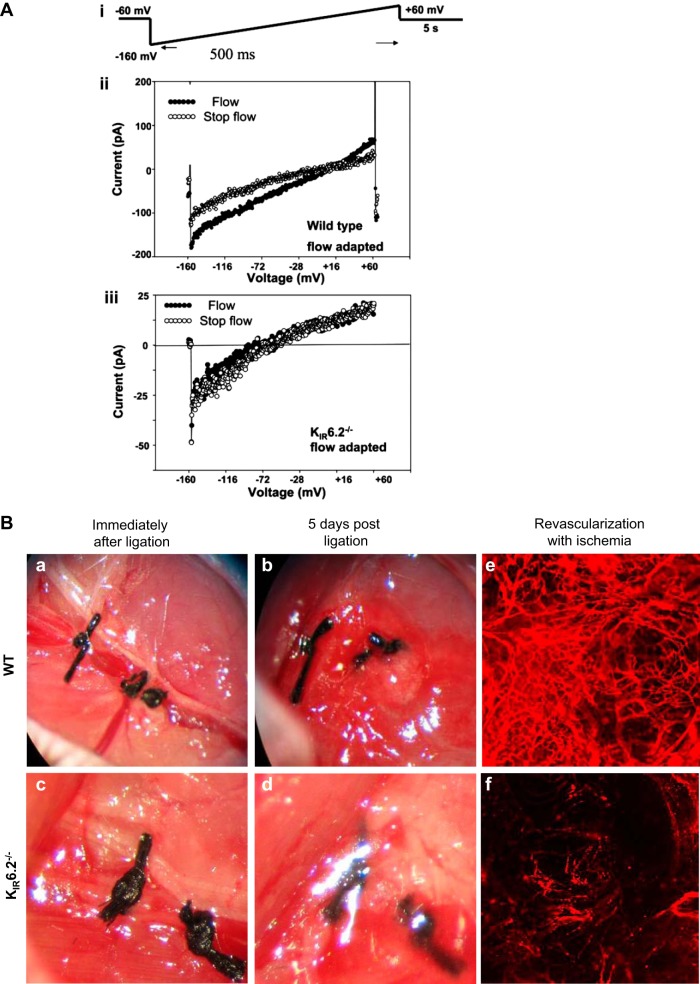
KATP channel in flow sensing in vitro and in vivo. A: KIR currents decrease with stop of shear. Inward rectifying whole cell K currents (KIR) were measured in mouse pulmonary microvascular endothelial cells (PMVEC). i: voltage protocol is shown above the experimental tracings. ii and iii: representative recordings obtained from flow-adapted pulmonary microvascular endothelial cells of wild-type and KIR 6.2 null mice. Current measurement from a single (ii) wild-type cell and (iii) KIR6.2−/− cell, during flow and with stop of flow. The currents recorded are the inwardly rectifying K+ currents (KIR). The flow protocol generated an estimated shear stress of 2 dynes/cm2. Stop flow indicates recording immediately following the abrupt cessation of flow. B: KATP channel-dependent revascularization in vivo. Stop of flow in vivo was studied using a hindlimb ischemia model. The femoral artery was ligated, and the region between the ligatures is excised and photographed (a–d) or imaged (e and f) by confocal microscopy. For e and f, fluorescent microbeads (40 nm) injected into the aorta were used to visualize microvessels in the region distal to the vascular obstruction (modified from Refs. 9 and 17).
Wild-type mice showed revascularization 5 days post femoral artery ligation, whereas mice with knockout of the KATP channel (i.e., the KIR6.2 subunit of the channel) or of Nox2 showed less revascularization post ischemia (Fig. 5B). Revascularization postischemia also was compromised in mice fed with the KATP agonist cromakalim or the NADPH oxidase inhibitor apocynin, as well as in mice where the channel function was compromised by a genetic approach (SUR1−/−, deletion of the SUR subunit is reported to compromise KATP channel function) (9). Blocking or deletion of other elements of the cascade (PECAM-1−/− and Akt-1−/− that do not produce ROS with ischemia) resulted in less revascularization as compared with wild-type mice. We concluded that KATP-channel induced Nox2 activation and subsequent ROS production drives revascularization in this hindlimb model. Nox2 is expressed in several cell types including endothelial cells, bone marrow-derived cells, and inflammatory cells (such as neutrophils, macrophages, etc.) and Nox2 in bone marrow-derived cells was involved in the vascular remodeling (88). To assess this role of endothelial Nox2, we studied endothelial targeted Nox2 expressing mice (endoNox2Tg) in which Nox2 is expressed only in endothelium (9). Revascularization in these mice was somewhat intermediate between wild-type and either Nox2 or KATP null mice, indicating a role for endothelial Nox2 in revascularization. Although endothelial Nox2 generated driven ROS is important, it does not play an exclusive role in vascular remodeling (9).
We discovered that the initiating signal for revascularization postischemia was dependent on HIF-1α and VEGF, based on Nox2-dependent regulation of HIF-1α and VEGF expression. The evidence for VEGF dependence was revascularization in the hind limb was seen only in those mouse models that showed increased VEGF expression and revascularization could be restored in the null phenotype (KATP null, i.e., KIR6.2 null) by delivery of VEGF into the ischemic region postligation.
Based on our results, we conclude that mechanotransduction via the KATP channel drives vascular remodeling via ROS generation by endothelial and other cells that subsequently increases VEGF and HIF-1α production. Thus the biochemical response to altered flow generates a signal for growth of collateral vessels and represents an attempt at revascularization to restore the impeded blood flow (16).
In summary, the symposium presentations reflected the novel insights into complexity of mechanotransduction in various systems ranging from the vasculature to the cardiac contractility apparatus, the overarching commonality being the role of Nox2-derived ROS in the transmission and transduction of the mechanosignal into a physiological or pathophysiological response. The main conclusions from these presentations are 1) PECAM-1 is an important component of the mechanosensing complex that senses altered mechanical forces; 2) activation of enzymes (primarily Nox2) that generate ROS play a key role in the propagation on the mechanically induced signal; 3) other key components of the mechanosignaling cascade include ion channels and intracellular kinases that serve as important links between induction of the signal and the physiological response; and 4) an altered mechanotransduction provokes a broad range of physiological and pathophysiological responses.
GRANTS
The research reported in the symposium was supported by National Heart, Lung, and Blood Institute Grants R01-HL-105509 and HL-075587 (to A. B. Fisher) and R01 HL-116976 and R21HL-112293 (to M. Ushio-Fukai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.C., K.F., N.G.P., and M.U.-F. prepared figures; S.C., K.F., N.G.P., and M.U.-F. drafted manuscript; S.C. and A.B.F. edited and revised manuscript; S.C., K.F., N.G.P., M.U.-F., and A.B.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the American Physiological Society for their support of this symposium.
REFERENCES
- 1.Al-Mehdi AB, Ischiropoulos H, Fisher AB. Endothelial cell oxidant generation during K+-induced membrane depolarization. J Cell Physiol 166: 274–280, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mehdi AB, Shuman H, Fisher AB. Intracellular generation of reactive oxygen species during nonhypoxic lung ischemia. Am J Physiol Lung Cell Mol Physiol 272: L294–L300, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Al-Mehdi AB, Shuman H, Fisher AB. Oxidant generation with K+-induced depolarization in the isolated perfused lung. Free Radic Biol Med 23: 47–56, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Al-Mehdi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Ross C, Blecha F, Dinauer M, Fisher AB. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ Res 83: 730–737, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Allen DG, Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol 327: 79–94, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J 73: 1983–1992, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol 126: 247–258, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60: 667–687, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Browning E, Wang H, Hong N, Yu K, Buerk DG, DeBolt K, Gonder D, Sorokina EM, Patel P, De Leon DD, Feinstein SI, Fisher AB, Chatterjee S. Mechanotransduction drives post ischemic revascularization through KATP channel closure and production of reactive oxygen species. Antioxid Redox Signal 20: 872–886, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryan J, Vila-Carriles WH, Zhao G, Babenko AP, Aguilar-Bryan L. Toward linking structure with function in ATP-sensitive K+ channels. Diabetes 53, Suppl 3: S104–S112, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Burke B, Stewart CL. Functional architecture of the cell's nucleus in development, aging, and disease. Curr Top Dev Biol 109: 1–52, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Caldiz CI, Diaz RG, Nolly MB, Chiappe de Cingolani GE, Ennis IL, Cingolani HE, Perez NG. Mineralocorticoid receptor activation is crucial in the signalling pathway leading to the Anrep effect. J Physiol 589: 6051–6061, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldiz CI, Garciarena CD, Dulce RA, Novaretto LP, Yeves AM, Ennis IL, Cingolani HE, Chiappe de Cingolani G, Perez NG. Mitochondrial reactive oxygen species activate the slow force response to stretch in feline myocardium. J Physiol 584: 895–905, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee S, Al-Mehdi AB, Levitan I, Stevens T, Fisher AB. Shear stress increases expression of a KATP channel in rat and bovine pulmonary vascular endothelial cells. Am J Physiol Cell Physiol 285: C959–C967, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Browning EA, Hong N, DeBolt K, Sorokina EM, Liu W, Birnbaum MJ, Fisher AB. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am J Physiol Heart Circ Physiol 302: H105–H114, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee S, Fisher AB. Mechanotransduction in the endothelium: role of membrane proteins and reactive oxygen species in sensing, transduction, and transmission of the signal with altered blood flow. Antioxid Redox Signal 20: 899–913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S, Levitan I, Wei Z, Fisher AB. KATP channels are an important component of the shear-sensing mechanism in the pulmonary microvasculature. Microcirculation 13: 633–644, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Nieman GF, Christie JD, Fisher AB. Shear stress-related mechanosignaling with lung ischemia: lessons from basic research can inform lung transplantation. Am J Physiol Lung Cell Mol Physiol 307: L668–L680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol 29: 1067–1073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiba R, Nakagawa N, Kurasawa K, Tanaka Y, Saito Y, Iwamoto I. Ligation of CD31 (PECAM-1) on endothelial cells increases adhesive function of alphavbeta3 integrin and enhances beta1 integrin-mediated adhesion of eosinophils to endothelial cells. Blood 94: 1319–1329, 1999. [PubMed] [Google Scholar]
- 21.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol 182: 753–763, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cingolani HE, Perez NG, Cingolani OH, Ennis IL. The Anrep effect: 100 years later. Am J Physiol Heart Circ Physiol 304: H175–H182, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol 92: 339–348, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Collins C, Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol 46: 185–188, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connelly T, Yu Y, Grosmaitre X, Wang J, Santarelli LC, Savigner A, Qiao X, Wang Z, Storm DR, Ma M. G protein-coupled odorant receptors underlie mechanosensitivity in mammalian olfactory sensory neurons. Proc Natl Acad Sci USA 112: 590–595, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23: 1024–1030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies PF. Endothelial mechanisms of flow-mediated athero-protection and susceptibility. Circ Res 101: 10–12, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, de la Pompa JL, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 162: 3022–3030, 1999. [PubMed] [Google Scholar]
- 30.Elrayess MA, Webb KE, Bellingan GJ, Whittall RA, Kabir J, Hawe E, Syvanne M, Taskinen MR, Frick MH, Nieminen MS, Kesaniemi YA, Pasternack A, Miller GJ, Humphries SE. R643G polymorphism in PECAM-1 influences transendothelial migration of monocytes and is associated with progression of CHD and CHD events. Atherosclerosis 177: 127–135, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Fang L, Wei H, Chowdhury SH, Gong N, Song J, Heng CK, Sethi S, Koh TH, Chatterjee S. Association of Leu125Val polymorphism of platelet endothelial cell adhesion molecule-1 (PECAM-1) gene and soluble level of PECAM-1 with coronary artery disease in Asian Indians. Indian J Med Res 121: 92–99, 2005. [PubMed] [Google Scholar]
- 32.Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog Biophys Mol Biol 115: 76–92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher AB, Al-Mehdi AB, Muzykantov V. Activation of endothelial NADPH oxidase as the source of a reactive oxygen species in lung ischemia. Chest 116: 25S–26S, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 93: e136–e142, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J Intern Med 259: 373–380, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Gardinier J, Yang W, Madden GR, Kronbergs A, Gangadharan V, Adams E, Czymmek K, Dunca RL. P2Y2 receptors regulate osteoblast mechanosensitivity during fluid flow. Am J Physiol Cell Physiol 306: C1058–C1067, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139: 33–44, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel R, Schrank BR, Arora S, Boylan B, Fleming B, Miura H, Newman PJ, Molthen RC, Newman DK. Site-specific effects of PECAM-1 on atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 28: 1996–2002, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman RS, Kirton CM, Oostingh GJ, Schon MP, Clark MR, Bradley JA, Taylor CJ. PECAM-1 polymorphism affects monocyte adhesion to endothelial cells. Transplantation 85: 471–477, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn C, Shchwartz MA. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Nat Rev Mol Cell Biol 10: 53–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harry BL, Sanders JM, Feaver RE, Lansey M, Deem TL, Zarbock A, Bruce AC, Pryor AW, Gelfand BD, Blackman BR, Schwartz MA, Ley K. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 28: 2003–2008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawkins BJ, Madesh M, Kirkpatrick CJ, Fisher AB. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol Biol Cell 18: 2002–2012, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosokawa K, Arai F, Yoshihara H, Nakamura Y, Gomei Y, Iwasaki H, Miyamoto K, Shima H, Ito K, Suda T. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun 363: 578–583, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J Biol Chem 275: 21435–21443, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Ilan N, Madri JA. PECAM1: old friend, new partners. Curr Opin Cell Biol 15: 515–524, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Ilan N, Mahooti S, Rimm DL, Madri JA. PECAM-1 (CD31) functions as a reservoir for and a modulator of tyrosine-phosphorylated beta-catenin. J Cell Sci 112: 3005–3014, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 59: 575–599, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Ingber DE, Wang N, Stamenovic D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys 77: 046603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol 206: 1–8, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110: 3056–3063, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem 272: 1395–1401, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ. NADPH oxidases in vascular pathology. Antioxid Redox Signal 20: 2794–2814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch TM, Lintilhac PM, Domozych D. Mechanotransduction molecules in the plant gravisensory response: amyloplast/statolith membranes contain a beta 1 integrin-like protein. Protoplasma 201: 92–100, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Mahooti S, Graesser D, Patil S, Newman P, Duncan G, Mak T, Madri JA. PECAM-1 (CD31) expression modulates bleeding time in vivo. Am J Pathol 157: 75–81, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masuda M, Osawa M, Shigematsu H, Harada N, Fujiwara K. Platelet endothelial cell adhesion molecule-1 is a major SH-PTP2 binding protein in vascular endothelial cells. FEBS Lett 408: 331–336, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Milovanova T, Chatterjee S, Hawkins BJ, Hong N, Sorokina EM, Debolt K, Moore JS, Madesh M, Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta 1783: 1866–1875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132: 598–611, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol Heart Circ Physiol 274: H1532–H1538, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117: 1161–1171, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newman PJ. The biology of PECAM-1. J Clin Invest 100: S25–S29, 1997. [PubMed] [Google Scholar]
- 64.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol 23: 953–964, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Noel J, Wang H, Hong N, Tao JQ, Yu K, Sorokina EM, Debolt K, Heayn M, Rizzo V, Delisser H, Fisher AB, Chatterjee S. PECAM-1 and caveolae form the mechanosensing complex necessary for NOX2 activation and angiogenic signaling with stopped flow in pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 305: L805–L818, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature 331: 168–170, 1988. [DOI] [PubMed] [Google Scholar]
- 67.Osawa M, Masuda M, Kusano K, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol 158: 773–785, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8: 221–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pahakis MY, Kosky JR, Dull RO, Tarbell JM. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 355: 228–233, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parmley WW, Chuck L. Length-dependent changes in myocardial contractile state. Am J Physiol 224: 1195–1199, 1973. [DOI] [PubMed] [Google Scholar]
- 71.Perez NG, Diaz RG, Morgan PE, Cingolani HE. Silencing of mineralorcorticoid receptors in the heart blunts the Anrep effect. Circulation 126: A19715, 2012. [Google Scholar]
- 72.Piccoli C, D′Aprile A, Ripoli M, Scrima R, Lecce L, Boffoli D, Tabilio A, Capitanio N. Bone-marrow derived hematopoietic stem/progenitor cells express multiple isoforms of NADPH oxidase and produce constitutively reactive oxygen species. Biochem Biophys Res Commun 353: 965–972, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M, Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J, Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 341: 709–717, 1999. [DOI] [PubMed] [Google Scholar]
- 75.Privratsky JR, Tourdot BE, Newman DK, Newman PJ. The anti-inflammatory actions of platelet endothelial cell adhesion molecule-1 do not involve regulation of endothelial cell NF-kappa B. J Immunol 184: 3157–3163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raaz U, Toh R, Maegdefessel L, Adam M, Nakagami F, Emrich FC, Spin JM, Tsao PS. Hemodynamic regulation of reactive oxygen species: implications for vascular diseases. Antioxid Redox Signal 20: 914–928, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rafii S, Avecilla S, Shmelkov S, Shido K, Tejada R, Moore MA, Heissig B, Hattori K. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann N Y Acad Sci 996: 49–60, 2003. [DOI] [PubMed] [Google Scholar]
- 78.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285: H1720–H1729, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Schenkel AR, Chew TW, Muller WA. Platelet endothelial cell adhesion molecule deficiency or blockade significantly reduces leukocyte emigration in a majority of mouse strains. J Immunol 173: 6403–6408, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci 125: 3051–3060, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi F, Chiu YJ, Cho Y, Bullard TA, Sokabe M, Fujiwara K. Down-regulation of ERK but not MEK phosphorylation in cultured endothelial cells by repeated changes in cyclic stretch. Cardiovasc Res 73: 813–822, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song C, Al-Mehdi AB, Fisher AB. An immediate endothelial cell signaling response to lung ischemia. Am J Physiol Lung Cell Mol Physiol 281: L993–L1000, 2001. [DOI] [PubMed] [Google Scholar]
- 83.Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg 59: 1594–1603, 1995. [DOI] [PubMed] [Google Scholar]
- 84.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111: 2347–2355, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res 77: 869–878, 1995. [DOI] [PubMed] [Google Scholar]
- 86.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urao N, McKinney RD, Fukai T, Ushio-Fukai M. NADPH oxidase 2 regulates bone marrow microenvironment following hindlimb ischemia: role in reparative mobilization of progenitor cells. Stem Cells 30: 923–934, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urao N, Ushio-Fukai M. Redox regulation of stem/progenitor cells and bone marrow niche. Free Radic Biol Med 54: 26–39, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ushio-Fukai M. VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9: 731–739, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Ushio-Fukai M, Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal 11: 2517–2533, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viola HM, Hool LC. Targeting calcium and the mitochondria in prevention of pathology in the heart. Curr Drug Targets 12: 748–760, 2011. [DOI] [PubMed] [Google Scholar]
- 93.von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol 45: 307–317, 1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wakabayashi S, Hisamitsu T, Nakamura TY. Regulation of the cardiac Na+/H+ exchanger in health and disease. J Mol Cell Cardiol 61: 68–76, 2013. [DOI] [PubMed] [Google Scholar]
- 95.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116: 1284–1291, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, Group EHS. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Q, Matsuzaki I, Chatterjee S, Fisher AB. Activation of endothelial NADPH oxidase during normoxic lung ischemia is KATP channel dependent. Am J Physiol Lung Cell Mol Physiol 289: L954–L961, 2005. [DOI] [PubMed] [Google Scholar]
- 98.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 346: 950–954, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]