Abstract
Epidemiological evidence suggests that exposure to ozone increases cardiovascular morbidity. However, the specific biological mechanisms mediating ozone-associated cardiovascular effects are unknown. To determine whether short-term exposure to ambient levels of ozone causes changes in biomarkers of cardiovascular disease including heart rate variability (HRV), systemic inflammation, and coagulability, 26 subjects were exposed to 0, 100, and 200 ppb ozone in random order for 4 h with intermittent exercise. HRV was measured and blood samples were obtained immediately before (0 h), immediately after (4 h), and 20 h after (24 h) each exposure. Bronchoscopy with bronchoalveolar lavage (BAL) was performed 20 h after exposure. Regression modeling was used to examine dose-response trends between the endpoints and ozone exposure. Inhalation of ozone induced dose-dependent adverse changes in the frequency domains of HRV across exposures consistent with increased sympathetic tone [increase of (parameter estimate ± SE) 0.4 ± 0.2 and 0.3 ± 0.1 in low- to high-frequency domain HRV ratio per 100 ppb increase in ozone at 4 h and 24 h, respectively (P = 0.02 and P = 0.01)] and a dose-dependent increase in serum C-reactive protein (CRP) across exposures at 24 h [increase of 0.61 ± 0.24 mg/l in CRP per 100 ppb increase in ozone (P = 0.01)]. Changes in HRV and CRP did not correlate with ozone-induced local lung inflammatory responses (BAL granulocytes, IL-6, or IL-8), but changes in HRV and CRP were associated with each other after adjustment for age and ozone level. Inhalation of ozone causes adverse systemic inflammatory and cardiac autonomic effects that may contribute to the cardiovascular mortality associated with short-term exposure.
Keywords: ozone, inhalational exposure, heart rate variability, systemic inflammation, airway inflammation
over the past two decades a large body of epidemiological evidence has linked exposure to ambient particulate matter to increased risk of mortality that is thought to be due to cardiovascular disease (40). Less attention has been paid to acute cardiovascular responses to ozone, at least in part due to the notion that ozone causes primarily local effects on the respiratory tract. However, with several recent epidemiological studies reporting increased risk of mortality associated with ambient exposure to ozone (10, 29, 53), new concerns about harmful cardiovascular effects of this pollutant have been raised.
Air pollution likely promotes cardiovascular events by multiple mechanisms as described previously (9). Of these, induction of oxidative stress along with systemic inflammation and alterations in autonomic balance are the most plausible pathways through which ozone may affect the cardiovascular system. Initial responses to oxidant injury and inflammation could ultimately lead to endothelial dysfunction, acute arterial vasoconstriction, and procoagulant activity. Stimulation of nociceptive fibers in the airways may lead to changes in sympathetic and/or parasympathetic tone, which could lead to arrhythmias. However, the available data regarding the cardiovascular effects of ozone in humans remain limited (17, 24, 47, 49). Recently, a controlled human exposure study in young healthy subjects reported short-term exposure to 300 ppb ozone to cause adverse changes in markers of inflammation, fibrinolysis, and heart rate variability (HRV) (13), providing some biological plausibility for the epidemiological studies that associate ozone exposure with mortality. However, another recent controlled human exposure study did not find an effect of a similar dose of ozone on HRV (8). In addition to these conflicting results, systemic inflammatory, coagulability, and cardiac autonomic effects of exposure to lower levels of ozone have not been adequately investigated.
Examination of systemic effects of exposure to lower levels of ozone is particularly important since efforts to improve air quality have led to a slight but steady decline in ozone levels seen in many regions of the world (18). For example, daily maximum 8-h average concentrations of ozone in urban regions of California and China in 2010 have reported to be 57 to 94 ppb and 181 to 209 ppb, respectively (18, 20). Although airway inflammation with exposure to levels of ozone as low as 60 ppb has been documented (35), it is possible that the systemic inflammation and autonomic effects of ozone have a higher threshold below which little or no systemic responses may be observed.
The goal of this study was to determine whether exposure to current medium and high ambient levels of ozone (at 100 and 200 ppb, respectively) causes adverse systemic responses. Our hypothesis was that ozone-induced systemic responses vary linearly in a dose-dependent manner with the level of exposure in the 0 to 200 ppb concentration range. To evaluate this hypothesis, we examined systemic markers of cardiovascular disease, namely HRV and biomarkers of inflammation (e.g., C-reactive protein, or CRP, inflammatory cytokines), volume control dysfunction (e.g., angiotensin converting enzyme), and coagulability (e.g., plasminogen activator inhibitor-1 and fibrinogen) after exposure to ozone at 0, 100, and 200 ppb. A secondary hypothesis of our study was that because airways are the portal of entry for ozone, any observed ozone-induced systemic inflammation is mediated by ozone-induced airway inflammation, and, in turn, any observed ozone-induced autonomic responses are at least in part mediated by ozone-induced systemic inflammation. To evaluate this secondary hypothesis, we explored the association among local airway inflammation, systemic inflammation, and HRV responses to ozone inhalation.
METHODS
Ethics statement.
The University of California San Francisco (UCSF) Institutional Review Board (IRB), the Committee on Human Research, approved this study. Written IRB-approved informed consent was obtained from all study participants. All subjects received financial compensation for their participation.
Study design.
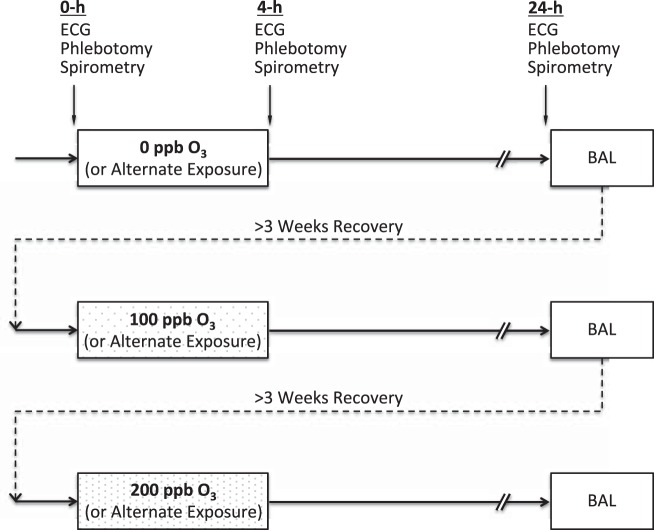
This study had a repeated measure design in which subjects were exposed to either 0 ppb (clean filtered air), 100 ppb (medium dose), or 200 ppb (high dose) ozone for 4 h in a climate-controlled chamber followed by bronchoscopy with bronchoalveolar lavage (BAL) on the following day (Fig. 1). Cardiovascular, pulmonary, and hemostatic endpoints were measured immediately before (0 h), immediately after (4 h), and 20 h after the end of exposure (24 h). Each subject completed all three types of exposure with a minimum of 3 wk in between exposure sessions to allow for recovery from any inflammation or injury sustained during the prior session. Previous studies have shown this wash-out period to be adequate for controlled ozone exposure studies (7, 14).
Fig. 1.
Study design. Subjects were exposed to 0, 100, and 200 ppb ozone (O3) in random order for 4 h with intermittent exercise in a repeated measure design. Electrocardiogram recording, phlebotomy, and spirometry were obtained immediately before (0 h), immediately after (4 h), and 20 h after (24 h) each exposure. Bronchoscopy with bronchoalveolar lavage (BAL) was performed at 24-h time point. The exposures were at least 3 wk apart to allow for recovery from previous exposure.
The order of exposures was counter-balanced and randomized. The study subjects and all but one of the study personnel, who performed the exposure experiment, were blinded to the type and order of exposures. All collected data were coded using randomly assigned 4-digit ID numbers, and the codes were unblinded only after all quantifications were performed at the stage of data analysis.
Subjects.
To increase the number of participants for analysis of the cardiovascular and hemostatic endpoints, subjects were allowed to enroll in the study even if they declined to undergo bronchoscopy. Overall, 26 subjects were recruited. Twenty-three out of 26 subjects participated in all procedures including bronchoscopy with BAL, and three subjects participated in all procedures except bronchoscopy. Four subjects withdrew from the study after the first exposure and bronchoscopy (1 moved out of the San Francisco Bay area, 2 reported inability to adhere to the experimental schedule, 1 provided no reason). The other 22 subjects completed all exposure conditions. Data from all 26 subjects were included in the analyses. Due to withdrawal of the four subjects, the final order of exposure assignments in the 22 subjects who completed all the exposures was not fully counter-balanced. Nevertheless, the differences between the number of subjects in exposure-order assignments were not statistically significantly different (χ2 = 0.46).
The inclusion criteria included 1) age between 18 to 50 years; 2) ability to perform moderate-intensity exercise; 3) being healthy with no history of cardiovascular, hematologic, or pulmonary diseases other than mild asthma; 4) no history of acute infection within the past 6 wk before start of the study; 5) nonsmoker as defined by having a history of less than ½ pack-year lifetime tobacco use and no history of any tobacco use in the past 6 mo; and 6) no history of recreational drug use. The criteria for mild asthma diagnosis included self-report of physician-diagnosed asthma, airway hyperresponsiveness to inhaled methacholine [provocative concentration of methacholine resulting in a 20% decrease in FEV1 compared with baseline ≤8.0 mg/ml] verified in our laboratory according to a protocol based on the American Thoracic Society guidelines (32), a prebronchodilator FEV1 of at least 70% of the normal predicted value, no daily asthma symptoms, and less than three to four nighttime symptoms per month (23, 36). Asthmatic subjects were required to stop their asthma and allergy medications in a sequential manner based on the duration of action of each medication (inhaled corticosteroids for 2 wk, anti-histamines and leukotriene inhibitors for 3 days, long-acting bronchodilators for 2 days, and short-acting bronchodilators for 8 h). Atopic status was assessed by skin-prick testing to 10 common aeroallergens (Dermatophagoides pteronyssinus, Aspergillus fumigatus, birch mix, Chinese elm, cat, dog, mountain cedar, mugwort sage, olive tree, perennial rye), with histamine as a positive control and saline as a negative control. A positive response was defined by a skin wheal 3 mm greater than the saline control 15 min after administration. Because the primary endpoints in the study were cardiovascular and nonrespiratory, and because these outcomes have not been reported to be affected by presence of mild asthma, all subjects were pooled together for final analyses.
Climate-controlled chamber and atmospheric monitoring.
The experiment took place in a ventilated, climate-controlled chamber at 16°C and 50% relative humidity with minor adjustments allowed for subject comfort. The chamber is a stainless steel-and-glass room of 2.5 × 2.5 × 2.4m (model W00327-3R; Nor-Lake, Hudson, WI) that was custom-built and designed to maintain temperature and relative humidity within 2°C and 4% from the set points, respectively (WebCtrl Software; Automated Logic, Kennesaw, GA). Temperature and relative humidity were recorded every 30 s and displayed in real-time (LabView 6.1; National Instruments, Austin, TX). Ozone was produced with a corona-discharge ozone generator (Model T408; Polymetrics, San Jose, CA) from a gas mixture of oxygen and argon (10% oxygen, balanced argon), and its concentration was monitored with an ultraviolet light photometer (Model 1004AH; Dasibi, Glendale, CA). The ozone analyzer was calibrated biannually with an ozone transfer standard (Model 1003PC; Dasibi) by the California Air Resources Board and precision-checked on a monthly basis.
Exposure sessions.
Each exposure session was 4 h long, with subjects exercising for the first 30 min and then resting for the following 30 min of each hour in the climate-controlled chamber. Environmental levels of ozone are highest during daytime and are usually described in terms of daily maximum 8-h average. To decrease the exposure time for practical purposes, most human exposure studies use exercise to increase the minute ventilation of subjects and replicate an 8-h daily exposure. The exercise protocol in this study consisted of running on a treadmill or pedaling a cycle ergometer. Exercise intensity was adjusted for each subject to achieve a target expired minute ventilation (VE) of 20 l/min/m2 body surface area. During exercise, VE was calculated from tidal volume and breathing frequency measured using a pneumotachograph at the 10-min and 20-min intervals of each 30-min exercise period (LabView 6.1; National Instruments, Austin, TX). Subjects remained inside the chamber for the entire 4-h exposure period. The type of exposure was chosen randomly at the time of subject enrollment and was not revealed to the subjects or to study investigators who performed bronchoscopy and processed the BAL. Immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the exposure period, subjects underwent blood pressure and heart rate measurement, electrocardiographic (ECG) monitoring for HRV measurement, phlebotomy, and spirometry in that order. The systemic inflammatory, coagulatory, and cardiac autonomic responses to this moderate intensity level of exercise have been previously described (16).
Blood pressure, heart rate, and HRV measurement.
Blood pressure, heart rate, and HRV were measured at the 0-h, 4-h, 24-h time points. The subjects were placed in a supine position on a gurney for 10 min to achieve a steady state and then underwent blood pressure and heart rate measurement using an automated sphyngomanometer (Critikon Model 8100; Tampa, FL). Subsequently, the subjects underwent ECG recording for a minimum of 10 min with a minimum of 5 min of spontaneous breathing followed by a minimum of 5 min of timed breathing using a metronome (12 breaths/min). Electrocardiograms were recorded using a Holter monitor (Forest Medical, East Syracuse, NY) connected to five electrodes placed in standard positions on the subject's chest wall to allow for the recording of two ECG lead channels.
Analysis of HRV data.
Each ECG recording was then downloaded and coded. Spontaneous and metronome breathing periods were separated into two ECG files. These were then edited using Trillium 3000 software (Forest Medical). A single blinded investigator (KP) reviewed every recording for poorly defined and mislabeled beats. Time domain variables were calculated using standard statistical methods described in the 1996 Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (2). Frequency domain variables were calculated on each ECG using power spectral analysis. A clear 5-min epoch was defined from each ECG for this analysis. Because the epochs used for analysis were time-defined, rather than beat-defined, discrete Fourier transformation was used for power spectral analysis with no windowing. The power spectrum was described by the total power and by three frequency components: very low frequency (VLF), low frequency (LF), and high frequency (HF). In addition, LF and HF measures were normalized with respect to the total power and the VLF using the equation LF/(total power-VLF) × 100 and HF/(total power-VLF) × 100, respectively. After normalization, the LF to HF ratio (LF/HF) was derived.
Spirometry.
Spirometry was performed at each of the 0-h, 4-h, and 24-h time points using a dry rolling-seal spirometer (PDS; nSpire Health, Longmont, CO) following American Thoracic Society performance criteria (3). The best values for FVC and FEV1 from three acceptable FVC maneuvers were used in data analysis.
Blood sampling and analysis.
A small amount of peripheral venous blood (30–40 ml) was withdrawn from the subject's arm at each of the 0-h, 4-h, and 24-h time-points. Blood was collected in appropriate sterile polypropylene tubes and processed by the UCSF clinical laboratory at San Francisco General Hospital or in our laboratory. Complete blood count with platelets was performed using a light scatter flow cell method (Siemens ADVIA 2120; Deerfield, IL). Prothombin time was measured using a one-stage test in which a thromboplastin reagent was added to citrated plasma and clotting time was recorded. Activated partial thromboplastin time was measured by activating intrinsic clotting factors using micronized silica and then adding CaCl2 to induce clot formation. Clotting time was then recorded. Fibrinogen was measured by converting to fibrin using 100 U/ml of thrombin. Fibrinogen concentration was then measured based on the rate of conversion, which is inversely proportional to the concentration of functional fibrinogen in the plasma (Siemens ADVIA 1800; Deerfield, IL). Active plasminogen activator-inhibitor (PAI-1) was determined by using a bioimmunoassay by ARUP Laboratories (Salt Lake City, UT). Serum angiotensin-converting enzyme (ACE) activity level was measured using an enzymatic activity assay by ARUP Laboratories (Salt Lake City, UT). High-sensitivity CRP was measured via a latex-enhanced immunoturbidometric method (Siemens ADVIA 1800).
Bronchoscopy and lavage procedures.
Bronchoscopies were performed 20 ± 1 h after the end of each exposure. Our laboratory's procedures of bronchoscopy and BAL have been previously discussed in detail (4, 5). Briefly, intravenous access was established, supplemental oxygen was delivered, and the upper airways were anesthetized with topical lidocaine. Moderate sedation with intravenous midazolam and fentanyl was achieved as needed for subject comfort. The bronchoscope was introduced through the mouth and vocal cords into the airways and was then directed into the right middle lobe where lavage was performed with two 50-ml aliquots of 0.9% saline warmed to 37°C. The fluid returned was designated BAL and immediately put on ice. After bronchoscopy, each subject was observed for an approximate 2-h recovery period. Total cells were counted on uncentrifuged aliquots of BAL using a hemocytometer. Differential cell counts were obtained from slides prepared using a cytocentrifuge at 25 g for 5 min, and stained with Diff-Quik as previously described (4, 5). Cells were counted by two independent and blinded observers; the average of the two counts was used in data analysis. BAL fluid was then centrifuged at 180 g for 15 min, and the supernatant was separated and recentrifuged at 1,200 g for 15 min to remove any cellular debris before freezing at −80°C.
Serum and BAL cytokine analysis.
Concentrations of serum cytokines at 0 h and 4 h and BAL fluid cytokines (24 h) were measured using the Milliplex human 14-plex cytokine assay (Millipore, St. Charles, MO) as described previously (16). For the purpose of cytokine measurements, BAL fluid samples were concentrated to 10× by volume using Amicon Ultra-15 Centrifugal Filter units with 3-kDa membrane pores (Millipore). Cytokines measured included the following: granulocyte macrophage colony-stimulating factor (GM-CSF), IL-1β, IL-5, IL-6, IL-7, IL-8, IL-12 p70, IFN-γ, monocyte chemotactic protein 1, and TNF-α. The lower limit of detection (LOD) for the serum ELISA assays for GM-CSF, IL-1β, IL-5, IL-6, IL-7, IL-12 p70, monocyte chemotactic protein 1, IFN-γ, and TNF-α was 3.2 pg/ml and for IL-8, the LOD was 16.0 pg/ml. The concentrations of serum IL-6 and IL-8 were also measured separately by human ELISA kits (Life Technologies, Grand Island, NY) (LOD of 1.7 and 4.1 pg/ml for IL-6 and IL-8, respectively). The LOD for the BAL ELISA assays for IL-1β, IL-6, IL-7, IL-12 p70, and GM-CSF was 0.13 pg/ml, for IL-5 and TNF-α, it was 0.03 pg/ml, and for IFN-γ, it was 0.64 pg/ml.
Sample size and power calculations.
Human inhalational exposure studies have reported significant changes in blood inflammatory and coagulatory biomarkers with sample sizes of 30 subjects (21, 22, 37). In addition, statistically significant changes in HRV have been observed with a sample size of five subjects with exposure to a combination of particulate matter and ozone (41). We based our sample size on the ability to detect a 10% change in concentration of the blood biomarkers of interest with presumed standard deviations twice the magnitude of the effect size. Given these parameters, sample sizes of 20 and 25 provided statistical power of 81% and 86%, respectively, to observe a minimum change of 10% with a type I error of 5%.
Data management and statistical analysis.
All data were entered into a database (Microsoft Excel 2007; Microsoft, Redmond, WA) and analyzed using STATA statistics software (STATA IE, version 12.0; StataCorp, College Station, TX).
Our study was designed to examine the question of whether short-term exposures to ambient levels of ozone cause acute adverse cardiovascular responses in a dose-dependent manner. The primary outcomes included 1) HRV (the frequency domains that are considered to be the most sensitive measures for assessment of pollutant effects on HRV, with other HRV indices analyzed as secondary endpoints); 2) systemic inflammation [CRP and inflammatory cytokines in blood (IL-6 and IL-8) with other inflammatory cytokines in blood as secondary endpoints]; 3) vascular volume and blood pressure control (angiotensin converting enzyme); and 4) biomarkers of coagulability (fibrinogen and plasminogen activator inhibitor-1 with other coagulation measures as secondary endpoints). Hemodynamic measures (heart rate and blood pressure) were also analyzed as secondary endpoints. Generalized estimating equation regression analyses were used to examine the dose-response effect of ozone exposure on outcomes of interest.
The changes in variables that were measured at 0-h, 4-h, and 24-h time points were calculated across each exposure using the 0-h time point value as the baseline (0 h to 4 h and 0 h to 24 h representing the immediate and the next day post-exposure change, respectively) and then used in the regression analyses. For variables that were only measured at the 24-h time point (bronchoscopy variables), the 24-h measurements were used in analyses. For measurements of blood and BAL cytokines, the analyte concentrations were adjusted to account for possible changes in blood volume after exposure from the corresponding 0-h measurement in accordance with previous studies (3, 15, 27, 43) as described previously (16). In addition, cytokine concentrations that were below the detection limits of the assay were recorded as equal to the LOD (lower limit of detection). The distribution of data for each variable was examined and logarithmic transformation was performed as needed to achieve the assumption of normality. All cytokine concentrations were log-transformed before analysis. Data in the descriptive tables are presented as means ± SD (or median [interquartile range] for those variables with a skewed distribution). Parameter estimates of regressions are presented as mean ± SE and represent change in the value of each variable per 100 ppb increase in ozone exposure. A P value of <0.05 was considered to be statistically significant.
The initial regression analyses were performed without adjustment for age, sex, and body mass index (BMI) (possible moderator) covariates, and then with adjustment for these covariates in the multivariable models. The general regression model equation was outcome variable = β0 + β1*predictor variable + β2*age + β3*sex + β4*BMI. BMI was examined both as a continuous and a categorical or binary variable (using BMI < or ≥25 kg/m2 to produce relatively equal numbers of subjects in each category of normal weight and overweight). When the covariates were not significantly associated with the endpoints in any preliminary models, and neither their inclusion nor exclusion substantively altered multivariable estimates for the other covariates, they were excluded from the final models.
As mentioned in introduction, a secondary hypothesis of our study was that ozone-induced systemic responses are mediated by ozone-induced local airway inflammation. To estimate a potential mediatory effect of ozone-induced airway inflammation on systemic inflammatory endpoints, we performed multivariable regression analysis followed by Sobel-Goodman mediation testing in models with serum CRP as the dependent variable, ozone as the independent variable, and BAL neutrophilia, IL-6, and IL-8 as the mediator variables. In addition, to estimate a potential mediatory effect of ozone-induced systemic inflammation on autonomic endpoints, we performed the same analysis (Sobel-Goodman mediation testing) in models with the frequency-domain of HRV as the dependent variable, ozone as the independent variable, and serum CRP as the mediator variable.
RESULTS
Subject characteristics.
The characteristics of the subjects are shown in Table 1. Ten out of the 26 subjects had mild asthma. The subjects with asthma had slightly lower FEV1 and FEV1-to-FVC ratio; however, their minute ventilation during exercise with different exposures was not significantly different from those of nonasthmatic subjects. All asthmatic subjects used short-acting β-agonist (albuterol) metered dose inhaler on an as needed basis. Three of the asthmatic subjects also used inhaled corticosteroid (2 fluticasone and 1 mometasone) and long-acting β-agonist (2 salmeterol and 1 formoterol) in a combination format (Adviar and Symbicort). None of the asthmatic subjects were on anticholinergic antimuscurinic agents, leukotriene inhibitors, or systemic steroids. All asthmatic subjects were able to successfully wean off their asthma medications, as described above in methods, before each exposure. The characteristics of the 22 subjects who underwent bronchoscopy with BAL were similar to the entire 26 subjects (data not shown).
Table 1.
Subject characteristics
| Subject Characteristics | All Subjects | Nonasthmatic | Asthmatic | P Value |
|---|---|---|---|---|
| Number | 26 | 16 | 10 | — |
| Age, years | 31.8 ± 7.6 | 30.8 ± 6.9 | 33.5 ± 8.8 | 0.38 |
| Sex, female, N [%] | 13 [50] | 7 [44] | 6 [60] | 0.44 |
| Height, cm | 169 ± 10 | 167 ± 10 | 175 ± 8 | 0.06 |
| Body mass index, kg/m2 | 24.8 ± 5.0 | 24.0 ± 3.4 | 26.5 ± 7.3 | 0.28 |
| FEV1, l | 3.6 ± 0.9 | 3.7 ± 0.9 | 3.3 ± 0.7 | 0.23 |
| FEV1, % predicted | 97.5 ± 15.1 | 102.3 ± 15.5 | 89.9 ± 11.5 | 0.03* |
| FVC, l | 4.5 ± 1.1 | 4.5 ± 1.2 | 4.6 ± 1.0 | 0.85 |
| FVC, % predicted | 99.6 ± 13.7 | 101.8 ± 15.3 | 96.1 ± 10.4 | 0.30 |
| FEV1-to-FVC ratio | 0.79 ± 0.11 | 0.84 ± 0.06 | 0.69 ± 0.11 | 0.0006* |
| Atopy, % | 21 [81] | 11 [69] | 10 [100] | 0.05 |
| VE, l/min/m2 BSA | ||||
| With 0 ppb O3 | 21.7 ± 2.5 | 22.0 ± 2.7 | 21.2 ± 2.3 | 0.50 |
| With 100 ppb O3 | 21.9 ± 3.4 | 21.4 ± 3.2 | 22.9 ± 3.9 | 0.35 |
| With 200 ppb O3 | 21.9 ± 3.1 | 22.4 ± 2.6 | 20.8 ± 4.1 | 0.26 |
Values are means ± SD. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; VE, average minute ventilation during exercise; BSA, body surface area; O3, ozone. P value is for comparison between asthmatic and nonasthmatic subjects by Student t-test.
Significant comparisons.
Climate-controlled chamber conditions.
The overall average temperature and relative humidity in the climate-controlled chamber were (means ± SD) 16.4 ± 1.9°C and 53.3 ± 10.7%, respectively (Table 2). There was a significant but small lower relative humidity with the high (200 ppb) ozone exposure. The targeted minute ventilation and ozone concentrations were achieved (Table 2). Targeted minute ventilation was similar between the exposures (Table 2) and between asthmatic and nonasthmatic subjects across different exposures (Table 1).
Table 2.
Exposure conditions
| Subject Characteristics | 0 ppb O3 | 100 ppb O3 | 200 ppb O3 |
|---|---|---|---|
| Temperature, °C | 16.4 ± 1.9 | 16.4 ± 2.1 | 16.5 ± 1.7 |
| Relative humidity, % | 54.6 ± 10.3 | 56.4 ± 11.9 | 49.0 ± 10.8 |
| O3 concentration, ppb | 9 ± 5 | 102 ± 5 | 200 ± 7 |
| VE, l/min/m2 BSA | 21.7 ± 2.5 | 21.9 ± 3.4 | 21.9 ± 3.1 |
Values are means ± SD.
Ozone-induced changes in lung function.
The degree of decline in FEV1, FVC, and FEV1/FVC ratio from 0 h to 4 h was linearly associated with the level of ozone exposure (parameter estimate ± SE for FEV1 = −0.10 ± 0.02 l [P < 0.0001], for FVC = −0.08 ± 0.02 l [P = 0.001], and for FEV1/FVC ratio = −1.05 ± 0.23 [P < 0.0001] per 100 ppb ozone). The lung function from 0 h to 24 h did not significantly change with different levels of ozone exposure (Table 3).
Table 3.
Lung function with ozone exposure
| 0 ppb O3 |
100 ppb O3 |
200 ppb O3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung Function | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h |
| FEV1, l | 3.44 ± 0.78 | 3.42 ± 0.80 | 3.33 ± 0.80 | 3.32 ± 0.79 | 3.32 ± 0.83 | 3.27 ± 0.79 | 3.39 ± 0.80 | 3.22 ± 0.88 | 3.29 ± 0.77 |
| FVC, l | 4.42 ± 1.10 | 4.39 ± 1.10 | 4.34 ± 1.09 | 4.34 ± 1.08 | 4.31 ± 1.10 | 4.31 ± 1.10 | 4.39 ± 1.10 | 4.24 ± 1.15 | 4.32 ± 1.09 |
| FEV1-to-FVC ratio | 0.79 ± 0.11 | 0.79 ± 0.10 | 0.78 ± 0.10 | 0.78 ± 0.11 | 0.78 ± 0.91 | 0.77 ± 0.11 | 0.78 ± 0.10 | 0.77 ± 0.10 | 0.77 ± 0.11 |
Values are means ± SD. The time points include immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the 4-h-long ozone exposure.
Ozone-induced changes in BAL inflammatory indexes.
Bronchoalveolar lavage data are shown in Table 4. BAL neutrophil and eosinophil concentrations increased significantly with the increasing level of ozone exposure (parameter estimate ± SE for BAL neutrophils and eosinophils of 4.8 ± 2.0 and 1.1 ± 0.5 × 103 cells/ml per 100 ppb ozone, respectively [P = 0.01 and P = 0.02]). The association between the level of ozone exposure and BAL eosinophil concentration remained significant (P < 0.03) after adjustment for presence of asthma or atopy. Of note, 18 of the 22 subjects who underwent bronchoscopy (all 10 asthmatic and 8 of the 12 nonasthmatic subjects) had atopy. Similar trends were observed when neutrophil and eosinophil percentages of total BAL leukocytes were used in regression modeling. Macrophage and lymphocyte concentrations in BAL did not show any significant change with the level of ozone exposure.
Table 4.
Airway inflammation with ozone exposure
| BAL Inflammatory Cells and Cytokines | 0 ppb O3 | 100 ppb O3 | 200 ppb O3 |
|---|---|---|---|
| BAL, ×104 cells/ml | |||
| Total leukocytes | 20.6 ± 11.5 | 21.5 ± 10.6 | 22.6 ± 8.8 |
| Macrophages | 17.3 ± 10.0 | 17.6 ± 10.0 | 18.1 ± 6.9 |
| Neutrophils | 0.55 ± 0.56* | 0.94 ± 0.91* | 1.51 ± 1.79* |
| Lymphocytes | 2.8 ± 2.0 | 2.7 ± 1.5 | 2.6 ± 2.1 |
| Eosinophils | 0.14 ± 0.17* | 0.23 ± 0.26* | 0.37 ± 0.49* |
| BAL, % | |||
| Macrophages | 83.1 ± 6.8 | 80.9 ± 8.8 | 80.2 ± 10.3 |
| Neutrophils | 2.6 ± 2.3* | 4.5 ± 4.1* | 6.4 ± 5.9* |
| Lymphocytes | 13.6 ± 6.9 | 13.6 ± 7.9 | 11.8 ± 5.5 |
| Eosinophils | 0.7 ± 0.8* | 1.1 ± 1.0* | 1.5 ± 1.6* |
| Cells, ×104 cells/ml | |||
| Epithelial | 0.98 ± 1.21 | 0.74 ± 0.93 | 0.88 ± 1.06 |
| Squamous | 0.33 ± 0.26 | 0.18 ± 0.18 | 0.26 ± 0.28 |
| Red blood | 10.3 ± 10.0 | 8.0 ± 8.2 | 19.7 ± 39.8 |
| Total protein, μg/ml | 69.0 [38.0, 86.0]* | 69.6 [42.3, 96.9]* | 100.4 [70.4, 157.8]* |
| IL, pg/ml | |||
| IL-1b | 0.13 [0.13, 0.13] | 0.13 [0.13, 0.13] | 0.13 [0.13, 0.13] |
| IL-5 | 0.04 [0.03, 0.13] | 0.08 [0.03, 0.30] | 0.12 [0.03, 0.23] |
| IL-6 | 1.45 [0.40, 2.08]* | 2.47 [0.27, 5.25]* | 3.66 [0.91, 8.04]* |
| IL-7 | 0.39 [0.13, 0.57] | 0.81 [0.26, 1.14] | 0.57 [0.43, 1.54] |
| IL-8 | 5.75 [0.94, 9.33]* | 10.11 [7.81, 21.12]* | 11.35 [8.10, 17.76]* |
| IL-12 | 0.13 [0.13, 0.13] | 0.13 [0.13, 0.13] | 0.13 [0.13, 0.13] |
| IFN-γ, pg/ml | 0.64 [0.64, 0.64] | 0.64 [0.64, 0.64] | 0.64 [0.64, 0.64] |
| Granulocyte macrophage colony-stimulating factor, pg/ml | 1.16 [0.81, 2.95] | 1.86 [0.93, 3.2] | 3.04 [1.74, 4.09] |
| TNF-α, pg/ml | 0.16 [0.07, 0.37] | 0.19 [0.07, 0.58] | 0.37 [0.14, 0.74] |
Values are means ± SD and median [interquartile range]. BAL, bronchoalveolar lavage.
Values represent changes that were significant with increasing level of ozone exposure by regression analysis.
Exposure to ozone was associated with airway injury as evidenced by increasing BAL total protein with increased ozone exposure (log-transformed regression parameter estimate ± SE = 0.09 ± 0.03 μg/ml per 100 ppb ozone, P = 0.001). The BAL concentrations of IL-6 and IL-8 were linearly associated with the level of ozone exposure (log-transformed regression parameter estimate ± SE of 0.20 ± 0.10 and 0.22 ± 0.08 pg/ml per 100 ppb ozone for IL-6 and IL-8, respectively [P = 0.02 and P = 0.008]), showing increased ozone-induced expression of these cytokines in BAL. The association between the BAL concentration of GM-CSF and the level of ozone exposure did not reach statistical significance (log-transformed regression parameter estimate ± SE = 0.16 ± 0.07 pg/ml per 100 ppb ozone; P = 0.05). No significant associations were observed for BAL IL-1β, IL-5, IL-7, IL-12, IFN-γ, or TNF-α. Adjustment for total protein or inclusion of age, sex, and BMI in the models did not significantly affect the association of airway inflammatory responses with ozone exposure.
Ozone-induced changes in peripheral blood leukocytes.
The concentrations of leukocyte subtypes at each time point are shown in Table 5. There was no significant association between the level of ozone exposure and the change in total leukocyte, monocyte, neutrophil, or lymphocyte concentrations across exposures from 0 h to 4 h or from 0 h to 24 h (Table 6). However, there was a cumulative decline in the concentration of eosinophils from 0 h to 4 h with increasing levels of ozone exposure (parameter estimate ± SE = −1.8 ± 0.6 × 104 cells/ml per 100 ppb ozone; P = 0.004). These changes were present regardless of whether the subject had asthma. The change in concentration of eosinophil from 0-h to 24-h time points was not significant.
Table 5.
Systemic inflammation with ozone exposure
| 0 ppb O3 |
100 ppb O3 |
200 ppb O3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood Inflammatory Indexes | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h |
| Leukocytes, ×106 cells/ml | 5.53 ± 1.23 | 8.69 ± 2.60 | 6.47 ± 1.74 | 5.93 ± 1.28 | 9.22 ± 2.16 | 6.45 ± 1.32 | 5.75 ± 1.25 | 8.95 ± 2.69 | 6.49 ± 1.36 |
| Neutrophils, ×106 cells/ml | 3.29 ± 1.10 | 6.09 ± 2.41 | 3.47 ± 1.32 | 3.60 ± 1.00 | 6.51 ± 1.83 | 3.48 ± 0.96 | 3.39 ± 0.95 | 6.41 ± 2.58 | 3.51 ± 1.21 |
| Lymphocytes, ×106 cells/ml | 1.59 ± 0.44 | 1.86 ± 0.47 | 2.21 ± 0.54 | 1.63 ± 0.49 | 1.95 ± 0.61 | 2.07 ± 0.59 | 1.68 ± 0.40 | 1.82 ± 0.54 | 2.17 ± 0.52 |
| Monocytes, ×106 cells/ml | 0.30 ± 0.08 | 0.41 ± 0.12 | 0.39 ± 0.15 | 0.34 ± 0.11 | 0.44 ± 0.14 | 0.38 ± 0.08 | 0.32 ± 0.11 | 0.41 ± 0.12 | 0.37 ± 0.13 |
| Eosinophils, ×106 cells/ml | 0.20 ± 0.15 | 0.18 ± 0.14 | 0.22 ± 0.14 | 0.21 ± 0.15 | 0.17 ± 0.12 | 0.24 ± 0.14 | 0.21 ± 0.14 | 0.15 ± 0.11 | 0.23 ± 0.16 |
| CRP, mg/l | 0.6 [0.4, 2.4] | 0.6 [0.3, 2.5] | 1.8 [0.7, 4.0] | 0.6 [0.3, 2.8] | 0.4 [0.2, 2.8] | 2.5 [0.6, 6.1] | 0.6 [0.3, 2.4] | 0.7 [0.3, 2.4] | 2.6 [1.2, 7.0] |
| ACE, U/l | 37.5 [28.0, 50.0] | 39.0 [32.5, 46.4] | 38.8 [27.1, 49.1] | 32.5 [26.5, 52.0] | 35.2 [29.1, 53.9] | 33.8 [28.1, 55.3] | 37.5 [28.5, 50.0] | 37.8 [29.8, 49.8] | 38.5 [30.9, 48.9] |
| IL-6, pg/ml | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] | 1.7 [1.7, 1.7] |
| IL-8, pg/ml | 6.0 [4.6, 12.0] | 5.0 [4.6, 8.6] | 6.6 [5.4, 8.8] | 4.6 [4.6, 7.4] | 4.8 [4.6, 8.4] | 8.7 [4.6, 10.0] | 4.6 [4.6, 7.6] | 6.3 [4.6, 7.8] | 6.5 [4.6, 10.4] |
Values are means ± SD or median [interquartile range]. Concentrations of peripheral blood leukocytes, C-reactive protein (CRP), angiotensin-converting enzyme activity (ACE), IL-6, and IL-8 immediately before (0 h), immediately after (4 h), and 20 h after (24 h) ozone exposure are shown.
Table 6.
Ozone-induced changes in peripheral blood leukocytes, CRP, and ACE
| Change Across Exposure, per 100 ppb O3 | 0 h to 4 h, PE ± SE | P Value | 0 h to 24 h, PE ± SE | P Value |
|---|---|---|---|---|
| Blood, ×104 cells/ml | ||||
| Leukocytes | 3.9 ± 22.0 | 0.85 | −12.3 ± 13.0 | 0.34 |
| Neutrophils | 13.3 ± 20.5 | 0.51 | −4.4 ± 13.2 | 0.74 |
| Lymphocytes | −6.6 ± 4.1 | 0.10 | −6.6 ± 3.9 | 0.09 |
| Monocytes | −0.6 ± 1.3 | 0.60 | −1.9 ± 1.4 | 0.20 |
| Eosinophils | −1.8 ± 0.6* | 0.004* | 0.3 ± 0.8 | 0.69 |
| Basophils | 0.30 ± 0.34 | 0.37 | 0.09 ± 0.27 | 0.74 |
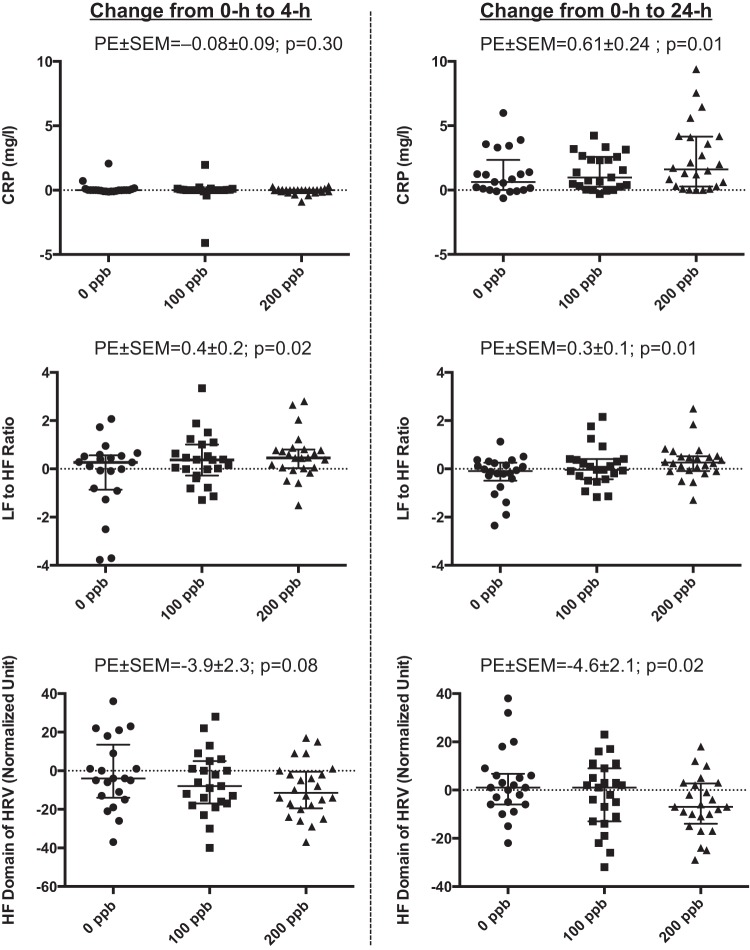
| Serum CRP, mg/l | −0.08 ± 0.09 | 0.30 | 0.61 ± 0.24* | 0.01* |
| Serum ACE, U/l | −1.39 ± 0.73 | 0.05 | −0.35 ± 0.67 | 0.60 |
Values are PE ± SE from regression analyses for changes in each outcome versus 100 ppb increase in ozone (O3) exposure.
Significant changes. The time points include immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the 4-h-long ozone exposure.
Ozone-induced changes in peripheral blood CRP, ACE, and cytokines.
The serum CRP concentrations and ACE activity levels at each time point are shown in Table 5. In linear regression analyses, the increase in the serum CRP concentration from 0 h to 24 h was significantly associated with increasing levels of exposure to ozone (parameter estimate ± SE = 0.61 ± 0.24 mg/l per 100 ppb ozone [P = 0.01]) (Table 6 and Fig. 2). The change in serum CRP at 4 h was not associated with ozone level. Changes in ACE activity (Table 6) or in measured serum cytokine levels were not significantly associated with the level of ozone exposure (cytokine data not shown). Inclusion of age, sex, and BMI did not significantly affect the observed association.
Fig. 2.
Change in systemic inflammatory and autonomic responses across ozone exposures. Graphs represent individual data as well as median and interquartile range of changes in inflammatory and autonomic variables across exposure (0 h to 4 h and 0 h to 24 h) to ozone at 0, 100, and 200 ppb levels. PE ± SE, parameter estimate ± SE from generalized estimating equation models of change in dependent variables for 100 ppb increase in ozone exposure; CRP, C-reactive protein; LF and HF, low and high frequency domains of heart rate variability; HRV, heart rate variability.
Ozone-induced changes in peripheral blood coagulation indexes.
No association between the change in fibrinogen, PAI-1, prothombin time, partial thromboplastin time, or platelets across exposure and the level of ozone exposure was observed (Table 7; regression data not shown).
Table 7.
Coagulatory and fibrinolytic indexes with ozone exposure
| 0 ppb O3 |
100 ppb O3 |
200 ppb O3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coagulatory indexes | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h |
| Fibrinogen, mg/dl | 314.4 ± 93.3 | 295.4 ± 48.6 | 304.4 ± 54.1 | 297.8 ± 53.5 | 299.9 ± 46.3 | 318.2 ± 60.3 | 312.8 ± 54.3 | 292.2 ± 52.6 | 317.2 ± 53.8 |
| PAI-1, IU/ml | 13.9 ± 22.0 | 3.6 ± 3.9 | 17.6 ± 31.3 | 12.7 ± 23.9 | 5.3 ± 11.2 | 19.9 ± 32.5 | 11.2 ± 18.8 | 9.5 ± 29.6 | 13.9 ± 20.9 |
| Platelets, ×106 cells/ml | 290.6 ± 62.7 | 299.4 ± 64.0 | 268.2 ± 56.7 | 274.0 ± 55.7 | 283.8 ± 61.1 | 261.1 ± 65.6 | 285.0 ± 56.0 | 290.4 ± 53.5 | 267.0 ± 56.4 |
| PT, s | 13.1 ± 0.6 | 13.2 ± 0.7 | 13.3 ± 0.6 | 13.0 ± 0.6 | 13.2 ± 0.6 | 13.2 ± 0.6 | 13.1 ± 0.5 | 13.1 ± 0.5 | 13.3 ± 0.5 |
| PTT, s | 30.0 ± 3.5 | 28.5 ± 3.9 | 30.6 ± 3.6 | 29.6 ± 2.9 | 28.3 ± 2.4 | 29.7 ± 2.8 | 29.7 ± 3.6 | 29.0 ± 3.2 | 30.1 ± 2.8 |
Values are means ± SD.
PAI-1, plasminogen activator inhibitor-1; PT, prothrombin time; PTT, partial thromboplastin time. The time points include immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the 4-h-long ozone exposure.
Ozone-induced changes in blood pressure, heart rate, and HRV frequency-domain indexes.
Diastolic blood pressure decreased and heart rate increased from 0 h to 4 h after all three exposures (0, 100, and 200 ppb). Heart rate also decreased from 0 h to 24 h across all three exposures. However, there were no significant trends between the changes in blood pressure (systolic or diastolic) and heart rate with level of ozone exposure from 0 h to either 4 h or 24 h.
Linear regression analysis showed a significant association between the LF-to-HF ratio and the level of ozone exposure (parameter estimate ± SE of 0.4 ± 0.2 and 0.3 ± 0.1 per 100 ppb ozone at 4-h and 24-h time points, respectively [P = 0.02 and P = 0.01]). In addition, the change in normalized HF (as well as the corresponding change in normalized LF) at 24-h time points were associated with level of ozone exposure (parameter estimate ± SE of −4.6 ± 2.1 per 100 ppb ozone [P = 0.02]) (Fig. 2 and Table 8). The association between the change in normalized HF (and the corresponding change in normalized LF) and level of ozone exposure at 4-h time point did not reach statistical significance; however, the direction of the association was consistent with those observed at the 24-h time point (Table 9). Similarly, ozone did not cause any significant change in frequency-domain HRV indexes when subjects underwent timed breathing; however, the direction of changes in these parameters was consistent with the values observed for spontaneous breathing (Table 8). No significant associations between the changes (0 h to 4 h or 0 h to 24 h) in the time-domain variables and level of ozone exposure were observed for spontaneous or timed breathing (Table 8).
Table 8.
Heart rate variability frequency and time-domain indexes with ozone exposure
| 0 ppb O3 |
100 ppb O3 |
200 ppb O3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HRV Indexes | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h | 0 h | 4 h | 24 h |
| Spontaneous breathing | |||||||||
| LF, normalized units | 54.4 ± 18.6 | 58.5 ± 13.8 | 51.5 ± 18.0 | 49.1 ± 14.8 | 55.2 ± 16.1 | 52.0 ± 15.6 | 46.6 ± 16.2 | 55.3 ± 16.1 | 51.6 ± 15.6 |
| HF, normalized units | 45.6 ± 8.6 | 41.6 ± 13.8 | 48.5 ± 18.0 | 50.9 ± 14.8 | 44.8 ± 16.1 | 48.0 ± 15.6 | 53.4 ± 16.2 | 44.7 ± 16.1 | 48.5 ± 15.6 |
| LF-to-HF ratio | 1.7 ± 1.5 | 1.7 ± 1.1 | 1.5 ± 1.2 | 1.2 ± 0.8 | 1.5 ± 1.0 | 1.3 ± 0.8 | 1.1 ± 0.7 | 1.5 ± 0.9 | 1.3 ± 0.7 |
| Timed breathing | |||||||||
| LF, normalized units | 28.4 ± 17.2 | 37.3 ± 20.3 | 31.0 ± 18.0 | 29.1 ± 14.6 | 36.0 ± 17.1 | 29.9 ± 16.9 | 29.6 ± 14.0 | 33.6 ± 16.7 | 27.7 ± 15.5 |
| HF, normalized units | 71.6 ± 17.2 | 62.7 ± 20.3 | 69.0 ± 18.0 | 71.0 ± 14.6 | 64.1 ± 17.1 | 70.2 ± 16.9 | 70.4 ± 14.0 | 66.4 ± 16.7 | 72.3 ± 15.5 |
| LF-to-HF ratio | 0.5 ± 0.5 | 0.9 ± 1.1 | 0.6 ± 0.6 | 0.5 ± 0.7 | 0.7 ± 0.6 | 0.5 ± 0.4 | 0.5 ± 0.7 | 0.6 ± 0.5 | 0.5 ± 0.4 |
| Spontaneous breathing | |||||||||
| SDNN | 56.2 ± 26.5 | 55.2 ± 37.6 | 58.6 ± 28.8 | 61.9 ± 37.3 | 61.4 ± 51.0 | 61.5 ± 32.1 | 54.3 ± 23.6 | 54.0 ± 30.8 | 62.0 ± 37.1 |
| SDANN | 0.5 ± 2.3 | 3.0 ± 9.0 | 3.9 ± 7.9 | 5.3 ± 11.3 | 8.2 ± 14.2 | 9.4 ± 23.7 | 1.9 ± 4.2 | 6.6 ± 19.6 | 0.6 ± 2.9 |
| SDNNI | 54.2 ± 24.4 | 46.6 ± 29.3 | 52.8 ± 31.6 | 54.1 ± 28.5 | 47.4 ± 34.4 | 55.4 ± 32.7 | 50.6 ± 20.7 | 46.1 ± 32.0 | 59.8 ± 36.4 |
| RMSSD | 45.8 ± 33.0 | 44.0 ± 45.4 | 46.1 ± 31.6 | 44.5 ± 25.4 | 46.0 ± 39.2 | 51.9 ± 39.6 | 42.5 ± 19.1 | 42.7 ± 34.7 | 49.4 ± 42.7 |
| Timed breathing | |||||||||
| SDNN | 58.1 ± 27.0 | 53.0 ± 32.2 | 60.3 ± 27.0 | 56.1 ± 19.4 | 61.4 ± 36.2 | 66.3 ± 33.6 | 56.0 ± 25.2 | 59.7 ± 35.4 | 63.9 ± 39.5 |
| SDANN | 40.5 ± 32.9 | 32.7 ± 30.7 | 32.1 ± 29.8 | 37.7 ± 32.3 | 36.6 ± 36.1 | 62.4 ± 53.6 | 29.5 ± 24.8 | 42.7 ± 43.3 | 39.9 ± 30.4 |
| SDNNI | 31.8 ± 15.4 | 31.4 ± 18.2 | 31.6 ± 11.6 | 28.2 ± 9.4 | 32.3 ± 21.3 | 35.1 ± 15.5 | 34.6 ± 19.7 | 32.0 ± 22.4 | 33.5 ± 20.6 |
| RMSSD | 48.0 ± 32.4 | 37.0 ± 32.9 | 49.3 ± 32.8 | 47.2 ± 24.8 | 50.7 ± 40.8 | 54.7 ± 37.1 | 45.5 ± 29.8 | 48.4 ± 44.1 | 52.7 ± 37.1 |
Values are means ± SD. HRV, heart rate variability; LF, low frequency-domain; HF, high frequency-domain. SDNN, SD of all R-R intervals; SDANN, SD of the mean of all intervals; SDNNI, mean of all R-R SDs; RMSSD, root mean square of successive differences in normal R-R intervals. The time points include immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the 4-h-long ozone exposure.
Table 9.
Ozone-induced changes in HRV frequency-domain indexes
| Change Across Exposure, per 100 ppb O3 | 0 h to 4 h, PE ± SE | P Value | 0 h to 24 h, PE ± SE | P Value |
|---|---|---|---|---|
| Spontaneous breathing | ||||
| LF, normalized units | 3.9 ± 2.3 | 0.08 | 4.6 ± 2.1* | 0.02* |
| HF, normalized units | −3.9 ± 2.3 | 0.08 | −4.6 ± 2.1* | 0.02* |
| LF-to-HF ratio | 0.21 ± 0.17* | 0.02* | 0.30 ± 0.13* | 0.01* |
Values presented as PE ± SE from regression modeling for change in HRV indexes versus 100 ppb increase in ozone (O3) exposure.
Significant changes. The time points include immediately before (0 h), immediately after (4 h), and 20 h after (24 h) the 4-h-long ozone exposure.
Association between ozone effects on airway inflammation, systemic inflammation, and HRV.
Analysis of ozone-induced changes in lung function or BAL markers of inflammation did not show any significant associations with serum CRP or HRV frequency-domain indexes across exposures. On the other hand, the changes in normalized frequency-domains of HRV (HF and LF) were significantly associated with the changes in serum CRP across exposures after adjustment for age and level of ozone (Table 10). To examine a potential mediating role for CRP in ozone-induced changes in HRV frequency-domains, we performed Sobel-Goodman mediation testing. The test showed 21.1% ± 14.9% of the total effect of ozone on HRV frequency domains to be mediated through its effect on serum CRP; however, this mediation effect did not reach statistical significance (P = 0.15).
Table 10.
Association between ozone-induced changes in HRV and systemic inflammation
| Predictors of Change in HRV | Univariate Model, PE ± SE | P Value | Multivariate Model, PE ± SE | P Value |
|---|---|---|---|---|
| Age, 10 yr | 4.0 ± 1.6* | 0.01* | 4.8 ± 1.4* | 0.001* |
| Ozone level, 100 ppb | −4.6 ± 2.1* | 0.02* | −4.1 ± 2.1* | 0.04* |
| Change in serum CRP, mg/l | −1.02 ± 0.58 | 0.07 | −1.34 ± 0.50* | 0.008* |
Values are PE ± SE from regression analyses. In multivariate models, ozone-induced change in HRV high frequency domain index was associated with that in serum CRP (e.g., 1 mg/l increase in serum CRP is associated with 1.34 normalized unit decrease in high frequency domain index of HRV after adjustment for age and level of ozone exposure).
Significant changes.
DISCUSSION
In this study, we examined the effects of inhalational exposure to increasing doses of ozone [0 ppb, “medium” (100 ppb), and “high” (200 ppb)] on endpoints relevant to cardiovascular diseases. We found that exposure to ozone induced dose-dependent 1) decrease in lung function, 2) airway injury as manifested by an increase in total BAL protein, and 3) airway inflammation as evidenced by increases in BAL neutrophils, eosinophils, and pro-inflammatory cytokines. In addition, we found evidence of a dose-dependent systemic inflammatory response to ozone at the 24-h time point as indicated by an increase in serum CRP. Furthermore, we found that exposure to ozone also induced a significant increase in LF-to-HF ratio of HRV at both 4-h and 24-h time points and a significant decrease in the normalized HF domain (and corresponding increase in LF domain) of HRV at the 24-h time point, suggestive of a relative increase in sympathetic tone. We did not find any evidence of a pro-coagulatory or vascular volume-control dysfunction response to ozone exposure.
Both the ozone-induced increase in serum CRP and adverse effects on HRV that we observed in our study are consistent with a recent report on a controlled human exposure study in which follow up (24 h post-exposure) effects on CRP and immediate (post-exposure) effects on HRV after a 2-h exposure to a higher level of ozone (300 ppb) were observed (13). Our results extend these findings by demonstrating that the adverse changes in systemic inflammatory (CRP) and autonomic responses (HRV) continue to be present at lower levels of exposure and seem to be dose-dependent in nature. In addition, our findings show that the observed changes in autonomic response (HRV), which were observed as early as 4 h into exposure, persist at least up to 20 h (24-h time point) after the end of the exposure. These findings are also consistent with other previously reported human studies.
In a controlled human exposure study of the potential for ozone to enhance the decreased HRV response to concentrated ambient particles, evidence of effect modification was observed (19). We observed a similar effect of ozone and particle co-exposures on HRV in another controlled human exposure (37). An independent reduction of HRV was also reported in a field study of traffic-related air pollution and HRV in patients with coronary artery disease in the Boston area (52). A second study in Boston showed an interactive effect of ambient ozone and high temperature to reduce HRV (42). A field study in Beijing also observed that exposure to ambient ozone was associated with a reduction in HRV (30). In a study of urban cyclists in Ottawa, Canada, there was a delayed reduction of HRV 3 h after exposure to ozone (51). Finally, in a study of traffic-related air pollution and HRV in Mexico City, ozone had the greatest effect on HRV of any pollutant studied (47). The results of these studies and ours when taken together provide support for the epidemiological evidence that chronic exposure to higher levels of ambient ozone is associated with increased risk of mortality due to ischemic heart disease (28). As noted in the introduction, not all human studies have found an effect of ozone on HRV. One recent controlled exposure study reported no effect of ozone either during or at 2 and 6 h after exposure to 300 ppb (8).
Serum CRP has been shown to be a risk factor for cardiovascular disease, metabolic syndrome, and mortality even in individuals without existing cardiovascular disease (25, 26, 44, 45). Similarly, the HF component (the best reflection of parasympathetic tone), the LF component (the best reflection of sympathetic tone), and the LF-to-HF ratio of HRV (representing relative sympathetic tone) have been shown to be predictive of cardiovascular disease and mortality (38). Overall, the directional changes seen in serum CRP and in the frequency-domain variables of HRV are coherent with the epidemiological evidence of increased risk of mortality in association with ambient ozone exposure. Furthermore, these findings implicate inflammatory and autonomic modulations as potential mechanisms by which ozone exerts adverse cardiovascular effects.
In this study, although the inhalational exposure to medium and high ambient levels (100 and 200 ppb) of ozone caused local lung injury and inflammation (as demonstrated by increased total protein, neutrophils, eosinophils, and pro-inflammatory cytokines in BAL) and led to a systemic response (as indicated by changes in serum CRP), we did not find any association between the changes in local and systemic inflammatory endpoints that we measured. Nevertheless, the systemic inflammation is most likely the result of local lung inflammatory processes, and our inability to find such an association may be due to the timing of the measurements. Specifically, BAL, for assessment of local lung inflammation, was performed only at the 24-h time point, at the same time that the serum CRP response was observed. There may be a time lapse between the local and systemic inflammatory responses and thus systemic inflammation may only be correlated with local lung inflammation at an earlier time point.
We also examined the relationship between the autonomic (changes in HRV) and inflammatory (serum CRP) responses to determine whether inflammatory responses had a mediating effect on autonomic responses. We found a significant positive association between the changes in the frequency-domain of HRV and serum CRP after adjustment for ozone level. Given that the changes in serum CRP across exposures were due to the inhalation of ozone, the observed association could be suggestive of a possible contribution of the systemic inflammation to ozone-induced autonomic responses. Although an analysis of the mediating effect of serum CRP on the frequency-domain of HRV showed a contribution of about 20%, this effect did not reach statistical significance (P = 0.15). Because our study was not designed to primarily examine this possible mediation effect, it may have been underpowered for such an analysis. Overall, the mechanism underlying ozone-induced adverse changes in the frequency-domain of HRV remains to be elucidated. However, the direct irritant effects of ozone inhalation in the airways may play a role in these autonomic responses.
Ozone exposure at low and high levels appeared to have no effect on the coagulatory and fibrinolytic endpoints beyond what has been shown to be due to the exercise protocols used in our laboratory's human ozone exposure studies (16). However, Devlin et al. (13) did find evidence of a pro-coagulatory state with higher levels (300 ppb) of ozone exposure. Our study was not powered to show a lack of effect for these endpoints at the lower doses of ozone that was examined, and further research on a potential pro-thrombotic effect of ozone should be done to address this topic.
Previous human studies have reported an increase in airway eosinophils with exposure to ozone in atopic asthmatic individuals (33, 50). Animal studies have also reported increases in airway eosinophils with exposure to ozone in allergic airway disease models of asthma (34, 39, 46, 48). In our study, we found an increase in BAL eosinophils with exposure to increasing levels of ozone that did not seem to be affected by the atopic or asthmatic status of the subjects. Unlike the above-cited previous studies, which had suggested an exacerbating effect of ozone on a pre-existing eosinophilic inflammatory background, our observation suggests that inhalation of ozone leads to a dose-dependent nonspecific airway eosinophilia that may be unrelated to presence of asthma or atopy.
Our study has both strengths and limitations. The strengths include the following: an experimental study of the potential cardiovascular effects of ozone in humans, a relatively large number of subjects for a controlled human exposure study, careful assessment of potentially confounding exercise effects, use of two different concentrations of ozone that allowed an exposure-response analysis, and measurement of biomarkers of both systemic and airway inflammation. Limitations include the following. First, our study design with 0, 100, and 200 ppb exposure levels assumes a linear response to these levels of ozone. Although it is possible that the responses at lower levels of ozone are nonlinear, at the doses studied, the linear assumption did produce a relatively well-fitting regression model (for example, estimated within-subject correlation coefficient of 0.46 for serum CRP). Examination of lower levels of ozone (i.e., <100 ppb) would address this concern. Second, although ozone is a prototypic oxidant gas that can cause oxidative stress and injury to the airway cells, we did not examine an oxidative stress pathway for the effects observed in this study. However, in a previous study, we did show an ozone-induced increase in peripheral blood 8-isoprostane, a marker of lipid peroxidation and oxidative stress, at 200 ppb exposure level (11). Because ozone is known to cause lipid peroxidation through generation of reactive oxygen species in the airways, it is plausible that its adverse cardiovascular effects, even at lower levels of exposure, are mediated through oxidation of lipids. Third, although we found evidence for ozone effects on serum CRP level and autonomic pathways, we only assessed a limited set of biomarkers of systemic inflammation and coagulability. We did not measure other important biomarkers such as adhesion molecules, von Willebrand factor, or endothelial dysfunction from impairment of nitric oxide metabolism (12). However, examination of the effects of ozone on an expanded set of biomarkers would have required a much larger sample size beyond the scope of this study. Fourth, we recruited subjects that were relatively young and healthy, and it is possible that only individuals with pre-existing cardiovascular disease are at risk for ozone-induced acute effects (30). We chose to recruit young, healthy subjects for safety reasons, given that the effects of ozone inhalation on cardiovascular endpoints in elderly subjects who might be at greater risk have not been previously studied. Nevertheless, we did observe a significant dose-dependent effect on serum CRP and frequency-domain variables of HRV in the subjects studied. Fifth, the temperature and relative humidity in the chamber exceeded the preplanned set points during some of the individual exposures. This is most likely because minor changes to temperature and humidity settings were allowed as requested by subjects for their comfort during exercise sessions. These minor adjustments in the settings resulted in temperature and relative humidity outside the preplanned values. Although it is possible that these variations may have adversely affected the measured outcomes, there were no systematic differences across exposures that could have contributed to the findings of the study, and on the average, the temperature and relative humidity in the chamber remained relatively close to the targeted range.
In conclusion, we found that inhalation of ambient levels of ozone results in dose-dependent adverse systemic inflammatory and cardiac autonomic effects, even at levels as low as 100 ppb, as indicated by an increase in serum CRP and changes in frequency-domain variables of HRV. These effects may contribute to the cardiovascular morbidity associated with short-term exposure to ambient ozone. As expected from previous studies, we also found evidence of dose-dependent airway inflammation and injury as manifested by changes in molecular and cellular markers of inflammation and increased total protein in BAL fluid. Further experiments with larger sample size and greater statistical power are required to determine whether local lung inflammation after inhalation of ambient levels of ozone leads to systemic inflammation and downstream cardiac autonomic responses; however, our results are suggestive of such a mechanistic pathway.
GRANTS
This work was supported by California Air Resources Board (CARB Contract No. 04-322); National Institutes of Health Grants NIH/NHLBI K23 HL-083099, NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR-024130, and UL1 RR-024131; Northern California Institute for Research and Education; and University of California San Francisco Cardiovascular Research Institute Faculty Development Funds.
DISCLAIMER
The statements and conclusions in this publication are those of the authors and not necessarily those of the funding agencies. The mention of commercial products, their source, or their use in connection with material reported herein is not to be construed as actual or implied endorsement of such products.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The authors do not have any financial interests to disclose with regards to the work done and presented in this manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: M.A., K.P., and J.R.B. conception and design of research; M.A., H.W., A.D., J.F., S.D., W.C., K.P., and J.R.B. performed experiments; M.A., A.D., J.F., and K.P. analyzed data; M.A., A.D., K.P., and J.R.B. interpreted results of experiments; M.A. prepared figures; M.A. drafted manuscript; M.A., H.W., A.D., J.F., K.P., and J.R.B. edited and revised manuscript; M.A., H.W., A.D., J.F., K.P., and J.R.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Al Fuller from San Francisco General Hospital Bronchoscopy Services and Chav Doherty from San Francisco General Hospital Clinical Laboratories for technical assistance, Ellen Eisen from UC Berkeley School of Public Health for statistical consultation, and Deborah Drechsler from California Air Resources Board for scientific advice.
REFERENCES
- 1.Ahmadizad S, El-Sayed MS. The acute effects of resistance exercise on the main determinants of blood rheology. J Sports Sci 23: 243–249, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Anon. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 3.Anon. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 152: 1107–1136, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Arjomandi M, Schmidlin I, Girling P, Boylen K, Ferrando R, Balmes J. Sputum induction and bronchoscopy for assessment of ozone-induced airway inflammation in asthma. Chest 128: 416–423, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Arjomandi M, Witten A, Abbritti E, Reintjes K, Schmidlin I, Zhai W, Solomon C, Balmes J. Repeated exposure to ozone increases alveolar macrophage recruitment into asthmatic airways. Am J Respir Crit Care Med 172: 427–432, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balmes JR, Aris RM, Chen LL, Scannell C, Tager IB, Finkbeiner W, Christian D, Kelly T, Hearne PQ, Ferrando R, Welch B. Effects of ozone on normal and potentially sensitive human subjects. Part I: Airway inflammation and responsiveness to ozone in normal and asthmatic subjects. Res Rep Health Eff Inst 1–37, 1997. [PubMed] [Google Scholar]
- 8.Barath S, Langrish JP, Lundback M, Bosson JA, Goudie C, Newby DE, Sandstrom T, Mills NL, Blomberg A. Short-term exposure to ozone does not impair vascular function or affect heart rate variability in healthy young men. Toxicol Sci 135: 292–299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 115: 175–187, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Buadong D, Jinsart W, Funatagawa I, Karita K, Yano E. Association between PM10 and O3 levels and hospital visits for cardiovascular diseases in Bangkok, Thailand. J Epidemiol 19: 182–188, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Arjomandi M, Balmes J, Tager I, Holland N. Effects of chronic and acute ozone exposure on lipid peroxidation and antioxidant capacity in healthy young adults. Environ Health Perspect 115: 1732–1737, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM, Ballinger SW. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am J Physiol Lung Cell Mol Physiol 297: L209–L216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin RB, Duncan KE, Jardim M, Schmitt MT, Rappold AG, Diaz-Sanchez D. Controlled exposure of healthy young volunteers to ozone causes cardiovascular effects. Circulation 126: 104–111, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Devlin RB, McDonnell WF, Becker S, Madden MC, McGee MP, Perez R, Hatch G, House DE, Koren HS. Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4 ppm ozone for 2 hr: a comparison of mediators found in bronchoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol Appl Pharmacol 138: 176–185, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol 37: 247–248, 1974. [DOI] [PubMed] [Google Scholar]
- 16.Donde A, Wong H, Frelinger J, Power K, Balmes JR, Arjomandi M. Effects of exercise on systemic inflammatory, coagulatory, and cardiac autonomic parameters in an inhalational exposure study. J Occup Environ Med 54: 466–470, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drechsler-Parks DM. Effect of nitrogen dioxide, ozone, and peroxyacetyl nitrate on metabolic and pulmonary function. Res Rep Health Eff Inst: 1–37, 1987. [PubMed] [Google Scholar]
- 18.Enviornmental Protection Agency. National Trends in Ozone Levels (Online). http://www.epa.gov/airtrends/ozone.html. [1 November 2014]. [Google Scholar]
- 19.Fakhri AA, Ilic LM, Wellenius GA, Urch B, Silverman F, Gold DR, Mittleman MA. Autonomic effects of controlled fine particulate exposure in young healthy adults: effect modification by ozone. Environ Health Perspect 117: 1287–1292, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Z, Sun J, Wan W, Hu E, Calatayud V. Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ Pollut 193: 296–301, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Frampton MW, Balmes JR, Cox C, Krein PM, Speers DM, Tsai Y, Utell MJ. Effects of ozone on normal and potentially sensitive human subjects. Part III: Mediators of inflammation in bronchoalveolar lavage fluid from nonsmokers, smokers, and asthmatic subjects exposed to ozone: a collaborative study. Res Rep Health Eff Inst: 73–79, 1997. [PubMed] [Google Scholar]
- 22.Frampton MW, Utell MJ, Zareba W, Oberdorster G, Cox C, Huang LS, Morrow PE, Lee FE, Chalupa D, Frasier LM, Speers DM, Stewart J. Effects of exposure to ultrafine carbon particles in healthy subjects and subjects with asthma. Res Rep Health Eff Inst: 1–47, 2004. [PubMed] [Google Scholar]
- 23.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2012 (Online). http://www.ginasthma.org/. [1 November 2014]. [Google Scholar]
- 24.Gong H Jr, Wong R, Sarma RJ, Linn WS, Sullivan ED, Shamoo DA, Anderson KR, Prasad SB. Cardiovascular effects of ozone exposure in human volunteers. Am J Respir Crit Care Med 158: 538–546, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Hamer M, Chida Y, Stamatakis E. Utility of C-reactive protein for cardiovascular risk stratification across three age groups in subjects without existing cardiovascular diseases. Am J Cardiol 104: 538–542, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care 25: 2016–2021, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev 65: 149–209, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, van Donkelaar A, Hughes E, Shi Y, Gapstur SM, Thun MJ, Pope CA 3rd. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med 188: 593–599, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jerrett M, Burnett RT, Pope CA 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med 360: 1085–1095, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia X, Song X, Shima M, Tamura K, Deng F, Guo X. Acute effect of ambient ozone on heart rate variability in healthy elderly subjects. J Expo Sci Environ Epidemiol 21: 541–547, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Kanner RE, Connett JE, Altose MD, Buist AS, Lee WW, Tashkin DP, Wise RA. Gender difference in airway hyperresponsiveness in smokers with mild COPD. The Lung Health Study. Am J Respir Crit Care Med 150: 956–961, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Khatri SB, Holguin FC, Ryan PB, Mannino D, Erzurum SC, Teague WG. Association of ambient ozone exposure with airway inflammation and allergy in adults with asthma. J Asthma 46: 777–785, 2009. [PMC free article] [PubMed] [Google Scholar]
- 34.Kierstein S, Krytska K, Sharma S, Amrani Y, Salmon M, Panettieri RA Jr, Zangrilli J, Haczku A. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergen-sensitized mice. Allergy 63: 438–446, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, Schmitt MT, Case M, Devlin RB, Peden DB, Diaz-Sanchez D. Lung function and inflammatory responses in healthy young adults exposed to 006 ppm ozone for 66 hours. Am J Respir Crit Care Med 183: 1215–1221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liard R, Leynaert B, Zureik M, Beguin FX, Neukirch F. Using Global Initiative for Asthma guidelines to assess asthma severity in populations. Eur Respir J 16: 615–620, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112: 3930–3936, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, Turiel M, Baselli G, Cerutti S, Malliani A. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 59: 178–193, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Plopper CG, Joad JP, Miller LA, Schelegle ES, Fanucchi MV, Van Winkle LS, Tyler NK, Avdalovic MV, Evans MJ, Lasley WL, Buckpitt AR, Pinkerton KE, Tarkington BK, Davis S, Nishio SJ, Gershwin LJ, Wu R, Hyde DM. Lung effects of inhaled corticosteroids in a rhesus monkey model of childhood asthma. Clin Exp Allergy 42: 1104–1118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56: 709–742, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Power KL, Balmes J, Solomon C. Controlled exposure to combined particles and ozone decreases heart rate variability. J Occup Environ Med 50: 1253–1260, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Ren C, O'Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol 173: 1013–1021, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowbottom DG, Green KJ. Acute exercise effects on the immune system. Med Sci Sports Exerc 32: S396–S405, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB Sr, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 110: 380–385, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Sattar N, Gaw A, Scherbakova O, Ford I, O'Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 108: 414–419, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, Baker GL, Pantle LM, Joad JP, Pinkerton KE, Wu R, Evans MJ, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol 191: 74–85, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Shields KN, Cavallari JM, Hunt MJ, Lazo M, Molina M, Molina L, Holguin F. Traffic-related air pollution exposures and changes in heart rate variability in Mexico City: a panel study. Environ Health 12: 7, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Chung KF. Interaction of ozone exposure with airway hyperresponsiveness and inflammation induced by trimellitic anhydride in sensitized guinea pigs. J Toxicol Environ Health 51: 77–87, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Superko HR, Adams WC, Daly PW. Effects of ozone inhalation during exercise in selected patients with heart disease. Am J Med 77: 463–470, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Vagaggini B, Taccola M, Cianchetti S, Carnevali S, Bartoli ML, Bacci E, Dente FL, Di Franco A, Giannini D, Paggiaro PL. Ozone exposure increases eosinophilic airway response induced by previous allergen challenge. Am J Respir Crit Care Med 166: 1073–1077, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Weichenthal S, Kulka R, Dubeau A, Martin C, Wang D, Dales R. Traffic-related air pollution and acute changes in heart rate variability and respiratory function in urban cyclists. Environ Health Perspect 119: 1373–1378, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanobetti A, Gold DR, Stone PH, Suh HH, Schwartz J, Coull BA, Speizer FE. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environ Health Perspect 118: 324–330, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med 184: 836–841, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]