Abstract
Hypertension (HTN) resulting from subcutaneous infusion of ANG II and dietary high salt (HS) intake involves sympathoexcitation. Recently, we reported reduced small-conductance Ca2+-activated K+ (SK) current and increased excitability of presympathetic neurons in the paraventricular nucleus (PVN) in ANG II-salt HTN. Here, we hypothesized that ANG II-salt HTN would be accompanied by altered PVN SK channel activity, which may contribute to sympathoexcitation in vivo. In anesthetized rats with normal salt (NS) intake, bilateral PVN microinjection of apamin (12.5 pmol/50 nl each), the SK channel blocker, remarkably elevated splanchnic sympathetic nerve activity (SSNA), renal sympathetic nerve activity (RSNA), and mean arterial pressure (MAP). In contrast, rats with ANG II-salt HTN demonstrated significantly attenuated SSNA, RSNA, and MAP (P < 0.05) responses to PVN-injected apamin compared with NS control rats. Next, we sought to examine the individual contributions of HS and subcutaneous infusion of ANG II on PVN SK channel function. SSNA, RSNA, and MAP responses to PVN-injected apamin in rats with HS alone were significantly attenuated compared with NS-fed rats. In contrast, sympathetic nerve activity responses to PVN-injected apamin in ANG II-treated rats were slightly attenuated with SSNA, demonstrating no statistical difference compared with NS-fed rats, whereas MAP responses to PVN-injected apamin were similar to NS-fed rats. Finally, Western blot analysis showed no statistical difference in SK1–SK3 expression in the PVN between NS and ANG II-salt HTN. We conclude that reduced SK channel function in the PVN is involved in the sympathoexcitation associated with ANG II-salt HTN. Dietary HS may play a dominant role in reducing SK channel function, thus contributing to sympathoexcitation in ANG II-salt HTN.
Keywords: angiotensin II, hypertension, paraventricular nucleus, sympathetic nerve activity, small-conductance Ca2+-activated K+ channels
studies have suggested that the central nervous system substantially contributes to ANG II-salt hypertension (HTN) through elevations in sympathetic nervous system (SNS) activity (6, 26, 27, 56). Prominent forebrain circumventricular organs, including the organum vasculosum lamina terminalis and subfornical organ, lack a complete blood-brain barrier and therefore have the ability to detect alterations in plasma osmolality and ANG II levels (13, 18, 21, 48, 50) and transmit efferent activity to the hypothalamic paraventricular nucleus (PVN) (36, 47). PVN neurons, in turn, have axon projections to the spinal intermediolateral (IML) cell column and rostral ventrolateral medulla (RVLM) (10, 44), the main excitatory centers that play an important role in regulating sympathetic nerve activity (SNA) (46).
The PVN is a key regulator of SNA given its prominent position between the sensory and effector components of the SNS, and enhanced PVN activity has been demonstrated in a variety of cardiovascular diseases (4, 37, 41). Evidence demonstrates that enhanced neuronal activity within the PVN is required for the maintenance of several models of neurogenic HTN, including ANG II-salt (4, 6, 20, 55). A number of studies have demonstrated an upregulation of excitatory glutamatergic and angiotensinergic signaling within the PVN in hypertensive rats (15, 16, 30, 32). Furthermore, evidence demonstrates the role of GABA inhibition on PVN neurons (11, 40) and that GABA disinhibition contributes to the augmented SNA in HTN (31, 34). To date, little information is available regarding alterations of intrinsic membrane properties of PVN neurons in HTN. One previous study by Sonner et al. (51) demonstrated a reduction in K+ A-current in PVN-RVLM neurons in renal-vascular HTN that contributed to augmented in vitro spontaneous neuronal discharge.
Small-conductance Ca2+-activated K+ (SK) channels act as negative feedback regulators of neuronal excitability through their influence on the medium afterhyperpolarization potential (mAHP) (3, 12, 28, 43, 52, 53). Previous studies have demonstrated that SK channels greatly influence excitability in PVN neurons with axon projections to the RVLM (PVN-RVLM) (9) and that reduced SK channel current significantly enhanced the excitability of PVN-RVLM neurons in rats with ANG II-salt HTN (8). Very recently, Pachuau et al. (39) reported that diminished SK current contributed to the increased excitability of spinal IML-projecting PVN (PVN-IML) neurons in spontaneous hypertensive rats. Furthermore, our laboratory (17) has recently reported that blockade of SK channels in the PVN in normotensive rats substantially augments SNA and arterial blood pressure (ABP) in vivo. The present study was performed to determine if SK channel dysfunction is present in the PVN of anesthetized rats with ANG II-salt HTN. In addition, recent evidence suggests that the neurogenic phase of ANG II-salt HTN is more dependent on the level of dietary salt (38). Therefore, we examined the individual contributions of high salt (HS) diet and ANG II alone to PVN SK channel dysfunction.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (n = 48, 200–300 g) purchased from Charles River Labs (Wilmington, MA) were housed in a temperature-controlled room (22–23°C) with a 12:12-h light-dark cycle. Chow and tap water were available ad libitum unless otherwise noted. All experimental and surgical procedures were carried out under guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals with the approval of the Institutional Animal Care and Use Committee of Michigan Technological University.
Protocols of the animal experimental model.
Rats in the control group consumed a normal salt (NS; 0.4% NaCl) diet, whereas rats in the HS group were placed on a HS (2% NaCl) diet for 4 wk. The ANG II-salt HTN group was fed a HS diet for 4 wk and received a systemic infusion of ANG II (150 ng·kg−1·min−1) subcutaneously during the last 2 wk on the HS diet. The ANG II-only group was fed a NS diet for 4 wk and ANG II was infused subcutaneously for the last 2 wk. For the ANG II-salt HTN and ANG II groups, animals were anesthetized with isoflurane (3% in O2) and an osmotic minipump (2ML2, Alzet) was implanted subcutaneously to deliver ANG II (150 ng·kg−1·min−1) for 2 wk before experimentation.
Experimental preparation.
Rats were anesthetized with an intraperitoneal injection of a mixture containing α-chloralose (80 mg/kg) and urethane (800 mg/kg). An adequate depth of anesthesia was determined by the absence of corneal or pedal reflexes. A water-circulating pad maintained body temperature. A left femoral artery was catheterized for the measurement of ABP, and a left femoral vein was catheterized for the administration of drugs. Heart rate (HR) was obtained from the R-wave of the electrocardiogram (lead I). After tracheal cannulation, rats were ventilated with O2-rich room air. End-tidal Pco2 (Capstar-100, Cwe) was continuously monitored to ensure it was within normal limits (35–40 mmHg). Rats were paralyzed with a continuous infusion of gallamine trethiodide. An adequate depth of anesthesia after paralysis was determined by the lack of pressor responses to a noxious foot pinch. Supplemental doses of anesthesia equal to 10% of the initial dose were given as needed. All animals were allowed to stabilize for at least 2 h after surgery to ensure the stability of recorded variables.
Recording of SNA.
A left flank incision was used to view and isolate a left renal nerve and a postganglionic splanchnic sympathetic nerve from the surrounding tissue (54). Nerve bundles were mounted on separate stainless steel wire electrodes (0.127-mm outer diameter, A-M Systems) and covered with silicon-based impression material (Coltene, Light Body). The signal was directed to an alternating current amplifier (P511, Grass Technologies) equipped with half-amplitude filters (band pass, 100-1,000 Hz) and a 60-Hz notch filter. The processed signal was rectified, integrated (10-ms time constant), and digitized at a frequency of 5,000 Hz using a 1401 Micro3 analog-to-digital converter and Spike 2 software (version 7.04, Cambridge Electronic Design, Cambridge, UK).
PVN microinjection.
Animals were placed in a stereotaxic head frame with the skull level between the bregma and lambda. The dura was exposed by removing a small piece of the skull, and a single-barreled glass microinjector pipette was lowered vertically into the PVN. The stereotaxic coordinates used were as follows: 1.2 to 1.6 mm caudal to the bregma, 0.5 mm lateral to the midline, and 7.0 to 7.2 mm ventral to the dura. The SK channel blocker apamin (Sigma) was dissolved in saline, and pH was adjusted to 7.2. The SK channel activator CyPPA (Tocris) was dissolved in DMSO. Apamin (12.5 pmol) or CyPPA (5 nmol) were administered via bilateral PVN microinjection in a volume of 50 nl per side with a pneumatic pump (WPI). The micropipette was withdrawn between bilateral microinjections, and the approximate interval between bilateral injections was ∼2 min. After each experiment, Chicago blue dye (2% in saline, 50 nl) was injected into the PVN to mark the injection sites and provide an estimate of drug diffusion area. Brains were removed, postfixed in 4% paraformaldehyde, and then transferred to 30% sucrose-PBS. The hypothalamus, including the PVN area, was sliced in coronal sections, and microinjection sites were visualized under bright-field microscopy.
Punched brain tissues from rats.
Separate groups of NS control and ANG II-salt HTN rats were deeply anesthetized with 5% isoflurane and then decapitated. Brains were quickly removed and put in the rat brain matrix prefrozen with dry ice. Hypothalamic PVNs were punched out with a 12-gauge needle (1.5-mm inner diameter). To identify hypothalamic PVN tissue, the optic tract was identified, and a 1-mm-thick brain section was taken from the rostral end point of the optic tract. Samples were frozen in liquid nitrogen and stored at −80°C for Western blot analysis of SK channel protein.
Western blot analysis of SK channel protein.
Frozen brain tissues were homogenized in buffer (50 mM Tris base, 1.0 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, and 1 mM PMSF, pH 7.4, Sigma) complete with protease inhibitors (0.1 mM leupeptin and 0.3 mM PMSF, Sigma) and stirred for 30 min at 4°C. Centrifugation at 12,000 rpm at 4°C was performed for 5 min. The supernatant was collected, and protein concentration was determined in triplicate by a Bio-Rad DC protein assay kit at an absorbance of 750 nm using BSA as a standard. Aliquots were stored at −80°C until analyzed, at which time 75 μg protein of each sample was separated using SDS-PAGE (10%) followed by the electrophoretic transfer of proteins from the gel to a nitrocellulose membrane (Bio-Rad). Membranes were blocked with 5% nonfat milk for 1 h at room temperature, washed three times, and probed with primary rabbit KCa2.1, KCa2.2, and KCa2.3 antibodies (Alomone, 1:500) or mouse anti-β-actin (Santa Cruz Biotechnology) overnight at 4°C. Membranes were incubated with goat anti-rabbit IgG-horseradish peroxidase secondary antibodies or goat anti-mouse IgG-horseradish peroxidase secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature and then detected with enhanced chemiluminescence.
Data analysis.
Summary data are expressed as means ± SE. Splanchnic SNA (SSNA) and renal SNA (RSNA) were determined as an average of the rectified, integrated signal. Baseline values of all variables were obtained by averaging a 10-min segment of data immediately preceding PVN microinjection in anesthetized rats. SSNA, RSNA, mean arterial pressure (MAP), and HR responses to apamin or CyPPA were obtained by averaging a 2-min period centered on the maximal response. Data are presented as percent changes from baseline after subtraction of background noise determined with bolus injection of the ganglionic blocker hexamethonium (30 mg/kg). Data were analyzed by one-way ANOVA. Post hoc analysis was performed with Newman-Keuls multiple-comparison test. Differences were considered statistically significant at a critical value of P < 0.05.
RESULTS
ANG II-salt HTN.
Baseline hemodynamic variables from anesthetized animals (n = 34) are shown in Table 1. ABP was significantly elevated in the ANG II-salt HTN group compared with the normotensive control group of rats on a NS diet. Subcutaneous infusion of ANG II alone elevated ABP, but the increase did not reach statistical significance, whereas HS alone had no effect on ABP. HR was not significantly different among any of the groups.
Table 1.
Baseline MAP and HR in anesthetized rats
| Group | n | MAP, mmHg | HR, beats/min |
|---|---|---|---|
| NS control | 8 | 102 ± 6 | 366 ± 17 |
| ANG II-salt hypertension | 12 | 123 ± 3* | 399 ± 10 |
| HS | 7 | 106 ± 6 | 352 ± 7 |
| ANG II | 7 | 112 ± 5 | 370 ± 17 |
Values are means ± SE; n, number of rats. MAP, mean arterial pressure; HR, heart rate; NS, normal salt (0.4% NaCl) diet; ANG II-salt hypertension, subcutaneous ANG II infusion and high-salt (HS; 2% NaCl) diet; HS, HS (2% NaCl) diet alone; ANG II, subcutaneous infusion of ANG II alone.
P < 0.05 vs. the NS control group.
Effects of PVN-injected SK channel blocker on SSNA, RSNA, MAP, and HR.
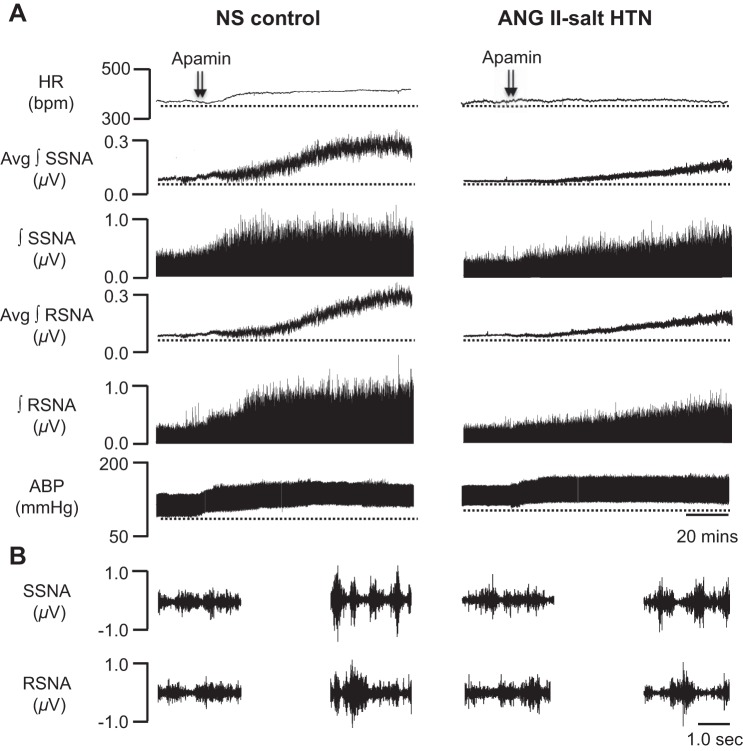
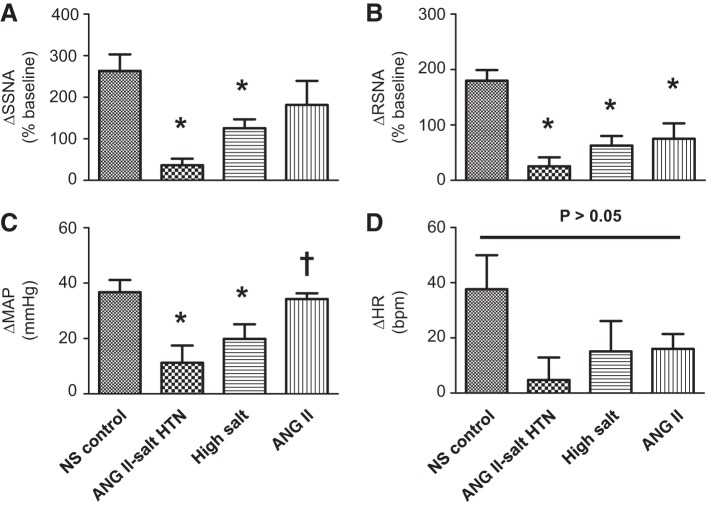
Consistent with our previously published data (17), bilateral microinjection of the SK channel blocker apamin into the PVN markedly increased SSNA, RSNA, MAP, and HR in control rats fed a NS diet. Figure 1 shows representative raw tracings from a rat on a NS diet (left) before and after bilateral microinjection of apamin (12.5 pmol, 50 nl) into the PVN. This dose of apamin has previously been shown to be the minimum dose to elicit a maximum ABP and SNA response in the PVN (17). Collectively (n = 8), PVN-injected apamin increased SSNA (+263.5 ± 39.7%), RSNA (+179.8 ± 19.3%), MAP (+36.7 ± 4.4 mmHg), and HR (+37.7 ± 12.3 beats/min), as shown in the summary data in Fig. 2.
Fig. 1.
Representative traces showing heart rate [HR; in beats/min (bpm)], splanchnic sympathetic nerve activity (SSNA), renal sympathetic nerve activity (RSNA), and arterial blood pressure (ABP) responses to bilateral paraventricular nucleus (PVN) microinjection of the small-conductance Ca2+-activated K+ (SK) channel blocker apamin (12.5 pmol) in a rat on a normal salt (NS) control diet (left) and a rat with ANG II-salt hypertension (HTN; right). A: bilateral PVN injection (50 nl each) of apamin (arrowheads) markedly increased HR, SSNA, RSNA, and ABP in a NS-fed control rat, whereas responses were obviously attenuated in a ANG II-salt HTN rat compared with the NS-fed control rat. Avg, average; ∫, integrated. B, left: 5-s trace of SSNA (top) and RSNA (bottom) before injection of apamin into the PVN. Right, 5-s trace of SSNA (top) and RSNA (bottom) after injection of apamin into the PVN.
Fig. 2.
Summary data showing the changes in SSNA (A), RSNA (B), MAP (C), and HR (D) in response to bilateral microinjection of apamin into the PVN in NS (normal salt) control, ANG II-salt HTN, high-salt (HS) diet alone, and systemic ANG II infusion alone groups. *P < 0.05 vs. the NS control group; †P < 0.05 vs. the ANG II-salt HTN group (by one-way ANOVA and Newman-Keuls multiple-comparison test).
The main goal of the present study was to determine in vivo whether SK channel function is altered in the PVN in rats with ANG II-salt HTN. Figure 1, right, shows a representative response to bilateral PVN microinjection of apamin (12.5 pmol, 50 nl) in a rat with ANG II-salt HTN. In contrast to normotensive control rats on a NS diet, the summary data (n = 7) shown in Fig. 2 demonstrate that maximum increases in SSNA, RSNA, and MAP elicited by PVN-injected apamin were significantly attenuated in ANG II-salt HTN rats (+36.4 ± 15.7%, P < 0.05 vs. NS-fed rats for SSNA; +25.3 ± 16.2%, P < 0.05 vs. NS-fed rats for RSNA; +11.3 ± 6.2 mmHg, P < 0.05 vs. NS-fed rats for MAP). Whereas the increase in HR evoked by PVN-injected apamin was blunted in rats with ANG II-salt HTN compared with NS-fed control rats, it did not reach statistical significance (+4.8 ± 8.1 beats/min, P > 0.05 vs. NS-fed rats).
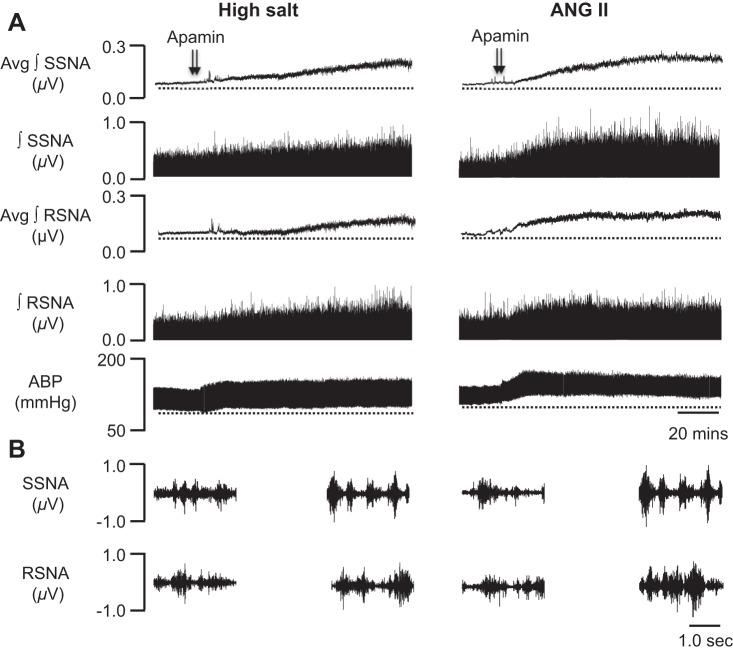
A secondary goal of the present study was to examine the relative contributions of a HS diet only or subcutaneous infusion of ANG II (150 ng·kg−1·min−1) alone to PVN SK channel dysfunction. Figure 3, left, shows a representative response to bilateral PVN microinjection of apamin (12.5 pmol, 50 nl) in a rat on a HS diet. The summary data (n = 7) shown in Fig. 2 demonstrate that maximum increases in SSNA, RSNA, and MAP elicited by PVN-injected apamin were significantly attenuated in rats on a HS diet (+125.5 ± 21.5%, P < 0.05 vs. NS-fed rats for SSNA; +62.9 ± 17.3%, P < 0.05 vs. NS-fed rats for RSNA; and +19.9 ± 5.3 mmHg, P < 0.05 vs. NS-fed rats for MAP). Similarly, the increase in HR evoked by PVN-injected apamin was blunted in rats on a HS diet but did not reach statistical significance (+15.1 ± 10.9 beats/min, P > 0.05 vs. NS-fed rats). While there was no statistically significant difference, sympathoexcitatory and pressor responses elicited by PVN-injected apamin in the HS group were less blunted compared with those in the ANG II-salt HTN group.
Fig. 3.
Representative traces showing SSNA, RSNA, and ABP responses to bilateral PVN microinjection of the SK channel blocker apamin (12.5 pmol) in a rat on a HS diet alone (left) or systemic ANG II infusion alone (right). A: bilateral PVN injection (50 nl each) of apamin (arrowheads) in a rat with HS intake increased SSNA, RSNA, and ABP in a similar manner to a rat with ANG II-salt HTN (Fig. 1, right). Bilateral injection (50 nl each) of apamin (arrowheads) in a rat with systemic ANG II infusion increased ABP in a similar manner to a rat on NS control diet, whereas SSNA and RSNA responses were obviously attenuated compared with the NS-fed control rat (Fig. 1, left). B, left: 5-s trace of SSNA (top) and RSNA (bottom) before injection of apamin into the PVN. Right, 5-s trace of SSNA (top) and RSNA (bottom) after injection of apamin into the PVN.
Representative raw tracings of bilateral PVN microinjection of apamin (12.5 pmol, 50 nl) in a rat with subcutaneous infusion of ANG II alone are shown in Fig. 3, right. Interestingly, although RSNA responses to PVN-injected apamin (+80.4 ± 35.2%, P < 0.05 vs. NS-fed rats) in rats with subcutaneous infusion of ANG II alone were significantly attenuated compared with control rats on a NS diet, SSNA (+208.7 ± 66.11%), MAP (+35.3 ± 2.1 mmHg), and HR (+16 ± 5.4 beats/min) responses to PVN-injected apamin in rats with ANG II treatment alone (n = 7) shown in Fig. 2 were not statistically different (P > 0.05) from those in rats on a NS diet.
Effects of PVN-injected SK channel activator on SSNA, RSNA, MAP, and HR.
Initial experiments demonstrated a loss of PVN SK channel function in ANG II-salt HTN. In an effort to further determine the role of SK channels in regulating sympathetic outflow in this disease model, we performed bilateral microinjections of the SK channel activator CyPPA (5.0 nmol, 50 nl/each) into the PVN. This dose of CyPPA has been reported to effectively activate SK channels in vivo (17). CyPPA microinjection (n = 5) into the PVN did not significantly alter SSNA (+14.2 ± 4%), RSNA (+7.0 ± 8%), MAP (−5.2 ± 3.1 mmHg), and HR (0.2 ± 7.0 beats/min) compared with baseline (P > 0.05 vs. baseline).
Histological analysis.
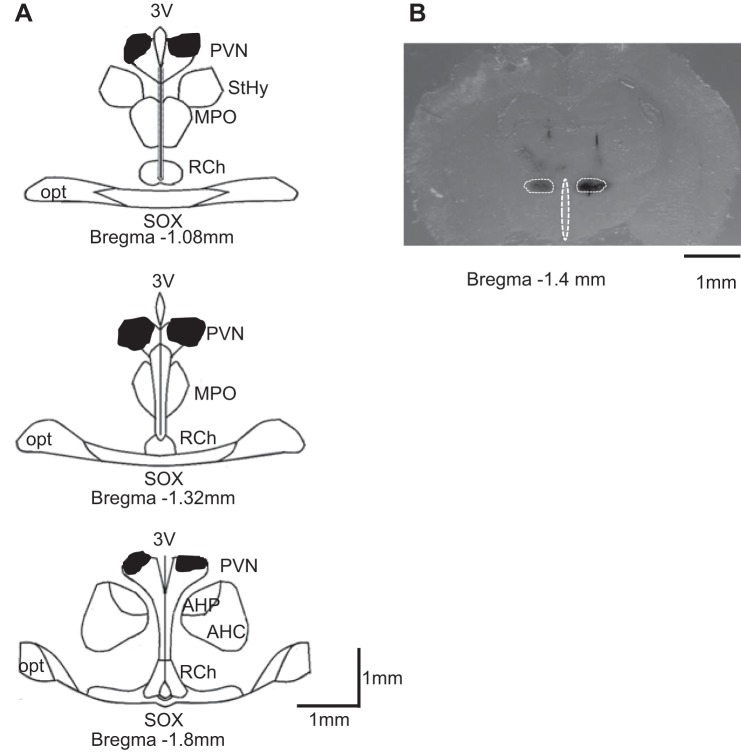
Histological examinations of coronal brain slices from similar rostral-caudal positions were performed to estimate drug diffusion area within the hypothalamus. Figure 4A shows the composite dye diffusion area made by overlying slices from several different brains to show the outermost distribution of dye. Figure 4B shows a representative of a single injection tracing within the PVN. Dye distribution was largely contained within the area encompassing the PVN. Note that the composite dye diffusion area shown in Fig. 4A was larger than any single tracing from an individual brain but represents the widest possible distribution of injected dye for the entire group.
Fig. 4.
Schematic representation of coronal sections throughout the rat hypothalamus. A: shaded areas indicate brain regions exposed to dye used to mark the injection sites bilaterally. The shape of each area was determined by overlaying tracings of the outermost diffusion area of injected dye (50 nl) observed on each section through the PVN. B: representative single bilateral injection of dye within the PVN (50 nl of 2% Chicago blue dye each). 3V, third cerebral ventricle; StHy, striohypothalamic nucleus; MPO, medial preoptic nucleus; RCh, retrochiasmatic area; opt, optic tract; SOX, supraopticdecussation; AHP/AHC, anterior hypothalamic area.
Comparison of SK channel protein expression.
We compared the protein expression (Western blot analysis) of SK1–SK3 channels in the hypothalamic PVN between normotensive control rats on a NS diet (n = 7) and ANG II-salt HTN rats (n = 7). No statistically significant difference in PVN SK1–SK3 expression was detected between NS control and ANG II-salt HTN animals (Fig. 5, A and B).
Fig. 5.
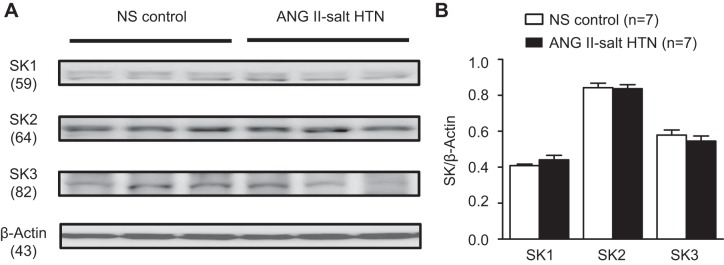
Expression of SK1–SK3 channels in the hypothalamic PVN. A: Western blot analysis showing protein expression of SK1–SK3 channels between rats in the NS control and ANG II-salt HTN groups. B: summary data showing no significant statistical difference in protein expression of SK1–SK3 channels in the punched PVN between the NS control group (n = 7) and ANG II-salt HTN group (n = 7). All values were normalized to β-actin.
DISCUSSION
The PVN is a key site for the integration of SNA, and increased PVN neuronal activity is essential to maintain the elevated levels of ABP in ANG II-salt HTN (6). Here, we demonstrate a downregulation of SK channel function in the PVN, which may underlie the central neuronal mechanisms of increased sympathetic outflow in rats with ANG II-salt HTN. We report three novel findings. First, SSNA, RSNA, and MAP responses to SK channel blockade in the PVN were attenuated in ANG II-salt HTN compared with normotensive rats on a NS diet, indicating a loss of PVN SK channel function in animals with treatment of systemic infusion of ANG II in combination with a HS (2% NaCl) diet. Second, SSNA, RSNA, and MAP responses to PVN SK channel blockade in rats on a HS diet alone were attenuated compared with control rats with NS intake in a similar manner to ANG II-salt HTN rats, indicating that perhaps HS intake is a larger contributor to SK channel dysfunction in the PVN in animals with ANG II-salt HTN. Finally, whereas SNA responses to PVN-injected apamin trended toward an attenuated response, with only RSNA demonstrating a significant difference in rats with systemic ANG II infusion only, MAP responses were similar to rats on a NS diet. This evidence indicates that HS intake, rather than the systemic infusion of ANG II, may play an important role in downregulating SK channel function in the PVN contributing to the elevated sympathetic outflow in ANG II-salt HTN.
Previous studies have demonstrated that chronic ANG II-salt treatment induces neurogenic HTN (6, 26, 29); however, little is known about the cellular mechanisms that contribute to the elevated sympathetic outflow in this hypertensive model. The present study is the first to demonstrate impairment in SK channel function in the PVN of rats with ANG II-salt HTN in vivo. In line with our previous work (17), SK channel blockade in the PVN in normal rats elicited a significant augmentation in SSNA and RSNA, displaying the contribution of SK channel inhibition to PVN neuronal excitability and its influence on sympathoexcitation. In the present study, SSNA, RSNA, and ABP responses to SK channel blockade in the PVN were substantially attenuated in rats with ANG II-salt HTN compared with control rats with NS intake, indicating a large degree of SK channel dysfunction. SK channel function in ANG II-salt HTN rats has previously been studied using an in vitro whole cell patch-clamp design under brain slice preparation to examine presympathetic PVN neurons (8). Similar to the present study, it was demonstrated that blockade of SK channels of presympathetic PVN neurons significantly increased neuronal discharge in control rats with NS intake but had less effect in ANG II-salt HTN rats, indicating reduced SK channel activity. Loss of SK channel function diminishes the mAHP and can lead to the loss of spike frequency adaptation in presympathetic PVN neurons that help govern SNA. PVN-RVLM neurons from the ANG II-salt group had a greater subthreshold depolarizing input resistance in response to ramp current injection and demonstrated an afterdepolarizing potential as opposed to the mAHP noted in control neurons. Both the increased depolarizing input resistance and revealed afterdepolarizing potential can potentially lead to increased excitability of PVN-RVLM neurons (8). The mechanism(s) whereby PVN SK channel dysfunction translates into elevated SNA remain to be fully determined. PVN neurons have axon projections to several autonomic control centers including the RVLM, the nucleus tractus solitarii in the dorsal brain stem, and the spinal IML (9, 10, 35). Augmented excitability of the above presympathetic PVN neurons has the potential to increase SNA through multiple pathways within the SNS.
The present study sought to determine the contribution of SK channels among PVN neurons to the sympathetic outflow in rats with ANG II-salt HTN. The ANG II-salt HTN model depends on the interaction of circulating ANG II and dietary salt (37). Studies examining depressor responses to ganglionic blockade and whole body norepinephrine spillover have demonstrated that the neurogenic component of ANG II-salt HTN depends on the level of dietary salt (26, 27, 29). A novel aspect of this study was our examination of the separate components of the ANG II-salt model on PVN SK channel function. Our data demonstrate that SSNA, RSNA, and ABP responses to PVN-injected apamin were not significantly different between rats with ANG II-salt HTN and rats on a HS diet only, although HS intake alone failed to increase ABP (Table 1). These data suggest that perhaps dietary HS intake is a larger contributor to loss of SK channel function than circulating ANG II. The attenuated SNA and MAP responses to PVN SK channel blockade in rats that consumed only a HS diet indicate that SK channel dysfunction may be a primary contributor to the elevated sympathetic outflow in rats with ANG II-salt HTN since we demonstrated that SK channel dysfunction in the PVN is independent of the systemic infusion of ANG II.
Despite a large body of epidemiological studies indicating that excess dietary salt is a primary contributor to essential HTN (7, 33, 45), the effects of a HS diet alone on neuronal excitability in the PVN remains largely undetermined. HS intake alone is generally not sufficient to produce HTN in rats; however, it has been demonstrated that excess dietary salt has the ability to sensitize neural circuitry (pathways) within the SNS (1, 2, 23, 42). Microinjection of apamin into the PVN of rats fed a HS diet elicited attenuated ABP and SNA responses compared with control rats fed a NS diet (Fig. 3). This loss of SK channel function in the PVN of HS-fed rats potentially primes the SNS through loss of spike frequency adaptation within presympathetic PVN neurons. Our data are of considerable interest when taken into account with previous studies demonstrating augmentations in excitability of the SNS after HS treatment, including one very recent study (49) indicating that a HS diet alone contributes to exaggerated SNA and ABP responses to various excitatory stimuli. Similar to evidence that HS intake can augment the excitability of brain stem SNS circuitry, the present study shows that a HS diet alone has the ability sensitize the neural circuitry in the PVN. The loss of SK channel function in animals with HS intake provides a possible mechanism whereby a HS diet potentially alters membrane properties to influence the excitability of presympathetic PVN neurons. The sensitization of the PVN in the present study, and the RVLM in previous studies, occurs in the absence of HTN and provides a potential mechanism that can cause exaggerated SNS responses that contribute to neurogenic HTN. The effects of a HS diet on neuronal membrane properties and excitability in cardiovascular control areas of the brain are of great interest and will require considerable attention in future studies.
To determine the role ANG II may have in altering PVN SK channel function, we examined responses to PVN-injected apamin in subcutaneous ANG II-infused rats on a NS diet. Both SSNA and RSNA responses to PVN SK channel blockade were attenuated compared with control rats on a NS diet, although SSNA did not demonstrate statistical significance compared with NS-fed control rats. In contrast, MAP responses to apamin were similar between the NS and ANG II groups (Fig. 2). ANG II alone appears to have less of a role in SK channel dysfunction than dietary HS intake, and PVN-injected apamin elicited a differential response between SSNA and RSNA. This is of particular interest considering evidence indicating that a HS diet plays a larger role in the neurogenic component of ANG II-salt HTN (25, 38). Loss of SK channel function due to a HS diet likely plays a dominant role in the development of the neurogenic phase of ANG II-salt HTN. The mechanisms responsible for the different effect of PVN-injected apamin on SSNA and RSNA in ANG II-infused rats are not clear. The augmented sensory outflow from the circumventricular organs to the PVN due to systemic ANG II infusion may potentially target specific subpopulations of neurons leading to a differential SNA response to PVN SK channel blockade. Future studies are needed to investigate the mechanism for the differential between SNA and MAP responses to PVN-injected apamin in rats with systemic administration of ANG II.
We have demonstrated a loss of SK channel function in the PVN in ANG II-salt HTN using both in vivo (Fig. 2) and in vitro (8) approaches; however, our data also indicate that SK1–SK3 channel protein is not different between NS control and ANG II-salt HTN groups (Fig. 5). This potentially suggests that downregulation of SK channels in ANG II-salt HTN are due to loss of function as opposed to a difference in the number of channels expressed within presympathetic PVN neurons. We are cautious in this interpretation because punched PVN tissue contains both parvocellular and magnocellular neurons with a variety of functions and projections to noncardiovascular control regions of the brain. In addition, studies that examined other regions of the central nervous system have demonstrated SK channel expression in astrocytes (5) and microglia (19, 24), and punched PVN tissue would not differentiate between cell types. One recent study (39) has also demonstrated no difference in SK channel expression level between Wistar-Kyoto and spontaneously hypertensive rats, supporting the notion that protein expression of SK channels is not altered in hypertensive rats. This study (39) also showed that PVN SK channel dysfunction in spontaneously hypertensive rats involves altered casein kinase II activity that disrupts the Ca2+-calmodulin complex, thereby reducing SK channel activity. Interestingly, the present study demonstrates that PVN microinjection of the SK channel activator CyPPA had no influence on SNA and ABP in ANG II-salt HTN. CyPPA is known to significantly increase the Ca2+ sensitivity of SK channels (22), and our data indicate that disruption of intracellular Ca2+ dynamics less likely contribute to the downregulation of SK channel activity in the PVN in this chronic disease model of ANG II-salt HTN. The mechanism(s) responsible for the reduced activity of SK channels in the PVN in rats with ANG II-salt HTN requires further attention in future studies.
This is the first study to demonstrate PVN SK channel dysfunction in rats with ANG II-salt HTN using an in vivo whole animal design. While in vivo experimental designs preserve the substantial number of synaptic and reflex systems, potentially lost using in vitro preparations, there still remain limitations that must be recognized. The present study required the use of anesthesia, which has the ability to influence cardiovascular responses (14). The effects of anesthesia on hemodynamic balance may potentially be reflected by the lack of HR differences in responses to PVN-injected apamin among our treatment groups. Furthermore, there is a possibility that microinjected drugs spread outside the area of the PVN. To exclude this possibility, we examined the diffusion of Chicago blue dye microinjected at the end of the experiment and presented a composite demonstrating the largest diffusion area. Our present data and a previously published report from our group (17) indicate that the estimated diffusion area is confined close to the PVN.
In summary, the present study suggests that reduced SK channel activity in the PVN contributes to ANG II-salt HTN. Interestingly, reduced function of SK channels was present in the HS-fed group in the absence of HTN. This indicates that SK channel dysfunction is more than likely not secondary to the development of HTN. Finally, in examining the individual contributions of systemic ANG II treatment and dietary HS intake to reduce PVN SK channel activity, we demonstrated that dietary HS intake plays a larger role in PVN SK channel dysfunction compared with circulating ANG II. These data support available evidence suggesting that the neurogenic component of ANG II-salt HTN is primarily dependent on the level of dietary salt.
Perspectives
HTN is one of the main risk factors for the development of cardiovascular disease. As such, determination of the mechanisms responsible for the development and maintenance of HTN is of great importance in identifying targets for possible interventions in disease progression. SK channels are unique, in that blockade within the PVN elicits robust increases in SNA (approximately +250–300%) and ABP (approximately +30 mmHg), demonstrating their significant involvement in inhibiting the presympathetic PVN neuronal excitability/activity (17). The present study supports the notion that intrinsic cellular mechanisms may play a significant role in ANG II-salt HTN. Restoration of SK channel function potentially provides a significant target for attenuating the activation of the SNS in neurogenic HTN.
GRANTS
This work was funded by American Heart Association Grants 11SDG7420029 (to Z. Shan) and 10SDG2640130 (to Q.-H. Chen) and National Heart, Lung, and Blood Institute Grant 1-R15-HL-122952 (to Q.-H. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A.L., L.G., M.J.H., and J.Z. performed experiments; R.A.L., L.G., M.J.H., and Q.-H.C. analyzed data; R.A.L., A.D.C., L.P.L., Z.S., and Q.-H.C. interpreted results of experiments; R.A.L., L.G., J.Z., and Q.-H.C. prepared figures; R.A.L. and L.G. drafted manuscript; R.A.L., L.P.L., and Q.-H.C. edited and revised manuscript; R.A.L., L.G., M.J.H., A.D.C., J.Z., L.P.L., Z.S., and Q.-H.C. approved final version of manuscript; Z.S. and Q.-H.C. conception and design of research.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Mingjun Gu for excellent technical assistance.
REFERENCES
- 1.Adams JM, Madden CJ, Sved AF, Stocker SD. Increased dietary salt enhances sympathoexcitatory and sympathoinhibitory responses from the rostral ventrolateral medulla. Hypertension 50: 354–359, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension 52: 932–937, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74: 245–269, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong WE, Rubrum A, Teruyama R, Bond CT, Adelman JP. Immunocytochemical localization of small-conductance, calcium-dependent potassium channels in astrocytes of the rat supraoptic nucleus. J Comp Neurol 491: 175–185, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bardgett ME, Holbein WW, Herrera-Rosales M, Toney GM. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-α. Hypertension 63: 527–534, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 23: 531–550, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J Neurophysiol 104: 2329–2337, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QH, Toney GM. Excitability of paraventricular nucleus neurones that project to the rostral ventrolateral medulla is regulated by small-conductance Ca2+-activated K+ channels. J Physiol 587: 4235–4247, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 285: R1231–R1239, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol 24: 96–101, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Fluckiger JP, Sonnay M, Boillat N, Atkinson J. Attenuation of the baroreceptor reflex by general anesthetic agents in the normotensive rat. Eur J Pharmacol 109: 105–109, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res 1447: 28–37, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Gabor A, Leenen FH. Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension 61: 1083–1090, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Gui L, LaGrange LP, Larson RA, Gu M, Zhu J, Chen QH. Role of small conductance calcium-activated potassium channels expressed in PVN in regulating sympathetic nerve activity and arterial blood pressure in rats. Am J Physiol Regul Integr Comp Physiol 303: R301–R310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutman MB, Ciriello J, Mogenson GJ. Effects of plasma angiotensin II and hypernatremia on subfornical organ neurons. Am J Physiol Regul Integr Comp Physiol 254: R746–R754, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, Kohsaka S, Inoue K, Nakanishi H. Microglial Ca2+-activated K+ channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci 31: 17370–17382, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzig TC, Buchholz RA, Haywood JR. Effects of paraventricular nucleus lesions on chronic renal hypertension. Am J Physiol Heart Circ Physiol 261: H860–H867, 1991. [DOI] [PubMed] [Google Scholar]
- 21.Honda K, Negoro H, Dyball RE, Higuchi T, Takano S. The osmoreceptor complex in the rat: evidence for interactions between the supraoptic and other diencephalic nuclei. J Physiol 431: 225–241, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, Strobaek D, Christophersen P. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151: 655–665, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 276: R1600–R1607, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Kaushal V, Koeberle PD, Wang Y, Schlichter LC. The Ca2+-activated K+ channel KCNN4/KCa3.1contributes to microglia activation and nitric oxide-dependent neurodegeneration. J Neurosci 27: 234–244, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension 48: 927–933, 2006. [DOI] [PubMed] [Google Scholar]
- 26.King AJ, Novotny M, Swain GM, Fink GD. Whole body norepinephrine kinetics in ANG II-salt hypertension in the rat. Am J Physiol Regul Integr Comp Physiol 294: R1262–R1267, 2008. [DOI] [PubMed] [Google Scholar]
- 27.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 50: 547–556, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Kuroki MT, Guzman PA, Fink GD, Osborn JW. Time-dependent changes in autonomic control of splanchnic vascular resistance and heart rate in ANG II-salt hypertension. Am J Physiol Heart Circ Physiol 302: H763–H769, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci 32: 372–380, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 2: 1244–1247, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol 275: R1523–R1529, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miselis RR. The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 230: 1–23, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep 9: 228–235, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Osborn JW, Olson DM, Guzman P, Toney GM, Fink GD. The neurogenic phase of angiotensin II-salt hypertension is prevented by chronic intracerebroventricular administration of benzamil. Physiol Rep 2: e00245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pachuau J, Li DP, Chen SR, Lee HA, Pan HL. Protein kinase CK2 contributes to diminished small conductance Ca2+-activated K+ channel activity of hypothalamic pre-sympathetic neurons in hypertension. J Neurochem 130: 657–667, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JB, Jo JY, Zheng H, Patel KP, Stern JE. Regulation of tonic GABA inhibitory function, presympathetic neuronal activity and sympathetic outflow from the paraventricular nucleus by astroglial GABA transporters. J Physiol 587: 4645–4660, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev 5: 73–86, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Pawloski-Dahm CM, Gordon FJ. Increased dietary salt sensitizes vasomotor neurons of the rostral ventrolateral medulla. Hypertension 22: 929–933, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, Strobaek D, Stocker M. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current IAHP and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem 280: 41404–41411, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons-Morton DG, Karanja N, Lin PH, Grp DSCR. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol 529: 221–236, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol 586: 5231–5245, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol 293: R2279–R2289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmonds SS, Lay J, Stocker SD. Dietary salt intake exaggerates sympathetic reflexes and increases blood pressure variability in normotensive rats. Hypertension 64: 583–589, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simpson JB. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology 32: 248–256, 1981. [DOI] [PubMed] [Google Scholar]
- 51.Sonner PM, Filosa JA, Stern JE. Diminished A-type potassium current and altered firing properties in presympathetic PVN neurones in renovascular hypertensive rats. J Physiol 586: 1605–1622, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol 305: H1407–H1416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takeda K, Nakata T, Takesako T, Itoh H, Hirata M, Kawasaki S, Hayashi J, Oguro M, Sasaki S, Nakagawa M. Sympathetic inhibition and attenuation of spontaneous hypertension by PVN lesions in rats. Brain Res 543: 296–300, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]