Abstract
Diet-induced obesity deteriorates the recovery of cardiac function after ischemia-reperfusion (I/R) injury. While mechanistic target of rapamycin (mTOR) is a key mediator of energy metabolism, the effects of cardiac mTOR in ischemic injury under metabolic syndrome remains undefined. Using cardiac-specific transgenic mice overexpressing mTOR (mTOR-Tg mice), we studied the effect of mTOR on cardiac function in both ex vivo and in vivo models of I/R injury in high-fat diet (HFD)-induced obese mice. mTOR-Tg and wild-type (WT) mice were fed a HFD (60% fat by calories) for 12 wk. Glucose intolerance and insulin resistance induced by the HFD were comparable between WT HFD-fed and mTOR-Tg HFD-fed mice. Functional recovery after I/R in the ex vivo Langendorff perfusion model was significantly lower in HFD-fed mice than normal chow diet-fed mice. mTOR-Tg mice demonstrated better cardiac function recovery and had less of the necrotic markers creatine kinase and lactate dehydrogenase in both feeding conditions. Additionally, mTOR overexpression suppressed expression of proinflammatory cytokines, including IL-6 and TNF-α, in both feeding conditions after I/R injury. In vivo I/R models showed that at 1 wk after I/R, HFD-fed mice exhibited worse cardiac function and larger myocardial scarring along myofibers compared with normal chow diet-fed mice. In both feeding conditions, mTOR overexpression preserved cardiac function and prevented myocardial scarring. These findings suggest that cardiac mTOR overexpression is sufficient to prevent the detrimental effects of diet-induced obesity on the heart after I/R, by reducing cardiac dysfunction and myocardial scarring.
Keywords: myocardial infarction, diet-induced obesity, transgenic mice, heart failure, metabolic syndrome, mammalian target of rapamycin
obesity is a major factor in metabolic syndrome, which increases the risk of type 2 diabetes mellitus and cardiovascular disease (2, 17). Metabolic syndrome is described as the presence of multiple metabolic risk factors, including obesity, dyslipidemia, raised blood pressure, and insulin resistance (17). While the definition and criteria of metabolic syndrome differ in each organization (9), obesity and abnormal insulin resistance/glucose intolerance are widely accepted as key risk factors for diabetes mellitus and cardiovascular disease (2). Although some clinical studies have shown that higher body mass index is associated with lower mortality in heart failure (HF) and acute myocardial infarction (MI), also known as the “obesity paradox” (10), insulin resistance, which is present in the majority of people with metabolic syndrome, is strongly associated with heart failure (19). In fact, high-fat diet (HFD)-induced obese mouse models exhibit insulin resistance that is accompanied by cardiac dysfunction (38). Studying the effects of metabolic syndrome on heart failure is an increasingly important and relevant public health issue, as rising rates of obesity and physical inactivity are increasing the prevalence of metabolic syndrome worldwide (2). Therefore, finding potential therapeutic targets for preventing cardiovascular disease in metabolic syndrome is essential to reduce the increased risk of HF morbidity linked with metabolic syndrome.
Mechanistic target of rapamycin (mTOR), a serine/threonine protein kinase, is a key downstream effector of IGF-1 signaling and serves as a critical regulator of cell growth, metabolism, and cell survival (28, 44). mTOR forms two functionally and structurally distinct complexes, mTORC1 and mTORC2 (7, 44). mTORC1 is rapamycin sensitive and phosphorylates p70S6K and 4E-binding protein 1. In contrast, mTORC2 is the kinase responsible for the phosphorylation and activation of Akt (44). Our previous study (5), which used transgenic mice (Tg) with cardiac-specific overexpression of mTOR (mTOR-Tg mice), demonstrated that cardiac mTOR is sufficient to protect the heart against ischemia-reperfusion (I/R) injury in both in vivo and ex vivo models (5). We confirmed that both mTORC1 and mTORC2 are almost equally activated in mTOR-Tg mice (45).
Previous reports have shown that mTOR inhibition by everolimus (a derivative of rapamycin) protected the heart in an in vivo MI model (without reperfusion) (11) and ex vivo I/R model (24). On a related note, more reports have indicated that mTORC1 signaling through p70S6K negatively regulates Akt by inhibiting insulin-receptor substrate-1 (18). This negative feedback loop accounts for the observed activation of Akt and inhibition of mTORC1 by either rapamycin treatment (28) or overexpression of proline-rich Akt substrate of 40 kDa (PRAS40), which binds to and inhibits mTORC1 (49). A recent study (48), which overexpressed PRAS40 in the heart using adeno-associated viral gene transfer, showed that PRAS40 overexpression activates mTORC2 and protects the heart in HFD-induced obese mice. The activity of mTOR and its protective effects in models of obesity could be due to its known role as a key mediator of energy metabolism in many organs, including the heart. However, the cardioprotective role of mTOR itself has not been studied in the context of obesity.
In the present study, we examine the effect of mTOR overexpression on ex vivo cardiac function and in vivo I/R injury in the context of HFD-induced obese mice. Our results demonstrate that cardiac mTOR preserves cardiac function after I/R in both obese mice and lean mice and that this cardioprotection is accompanied by attenuated necrosis and inflammatory response caused by I/R injury.
MATERIALS AND METHODS
Animal models.
Animal experiments in this study were approved by the Institution Animal Care and Use Committees of the University of Hawaii (Honolulu, HI). This investigation conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). Tg mice expressing hemagglutinin-tagged wild-type (WT) rat mTOR under the direction of the murine α-myosin heavy chain promoter have been previously described in detail (45). Line 4 male (mTOR-Tg) mice, in which mTOR expression was about threefold higher than littermate controls (WT mice) (45), were used for these experiments. Male WT and mTOR-Tg mice at 6 wk of age were fed a HFD for 12 wk and weighed once a week until they reached 18 wk of age. At 6 wk of age, mice were placed on pellets of either a normal chow diet (NCD) consisting of 24.7% energy from protein, 63.4% carbohydrate, and 4.6% fat (PicoLab rodent diet 5053, 339.5 kcal/100 g, TestDiet, Richmond, IN) or a HFD consisting of 14.9% energy from protein, 26.0% carbohydrate, and 59.0% fat (S3282, 549.0 kcal/100 g, Bio-Serv, Frenchtown, NJ) for 12 wk. Previous studies have demonstrated that a 60% fat diet induces insulin resistance as well as obesity and creates a reasonable model for studying pathophysiological features of the cardiovascular system in diet-induced obesity (8, 25, 41). To evaluate HFD-induced glucose intolerance and insulin resistance, we performed glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) after 12 wk of NCD or HFD feeding as previously demonstrated (4, 36). Mice were fasted for 16 h before receiving an intraperitoneal administration of 1.5 g glucose/kg body wt in saline. Blood samples were collected from the tail vein at 0, 10, 30, 60, and 120 min in heparinized microcapillary tubes, and plasma glucose and insulin levels were determined from those samples. An ITT was performed on mice after 1 h of fasting. Animals were intraperitoneally injected with 1.0 U insulin/kg body wt in saline. Blood samples were drawn from the tail vein at 0, 10, 30, 60, and 120 min for the measurement of plasma glucose levels. Plasma glucose levels in blood samples collected from tail veins were determined using a commercially available glucose meter (OneTouch Ultra blood glucose meter, LifeScan, Milpitas, CA). After the isolation of plasma from blood samples, insulin levels were determined by ELISA (Mercodia, Winston Salem, NC). To evaluate the degree of insulin resistance, values were calculated using the following homeostatic model of assessment of insulin resistance (HOMA-IR) formula: fasting glucose (in mg/dl) × fasting insulin (in μU/ml)/405.
Ex vivo I/R in Langendorff perfused hearts.
WT and mTOR-Tg mice were subjected to an ex vivo Langendorff perfusion model as previously described (5, 35). After retrograde perfusion was established at a constant pressure (80 mmHg), hearts were perfused with modified Krebs-Henseleit buffer (11 mM glucose, 118 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, and 0.5 mM EDTA) equilibrated with 95% O2-5% CO2 at 37°C to yield a pH of 7.4. A water-filled balloon catheter was introduced into the left ventricle (LV) to record LV pressure (PowerLab, AD Instruments, Denver, CO). We measured a volume of the coronary sinus effluent in the collected perfusate to determine the coronary flow rate. For the ex vivo I/R model, hearts were perfused for 15 min, and the flow was then eliminated for 20 min followed by reperfusion for 40 min. The peak ischemic contracture during the 20-min ischemia period was determined by the alteration of LV end-diastolic pressure, as previously reported (39).
In vivo I/R.
Mice were subjected in vivo I/R as previously described (5). Mice were anesthetized by isoflurane. After mice were intubated and ventilated, a left thoracotomy was performed. The left anterior descending coronary artery was ligated with 7-0 silk sutures. After 30 min of occlusion (ischemia), the ligature around the left anterior descending coronary artery was released, and reperfusion was visually confirmed. We evaluated cardiac function in conscious mice with transthoracic echocardiography (Vevo 2100 Imaging system, Visual Sonics, Toronto, ON, Canada) using a MS400 transducer (18–38 MHz). M-mode images used for measurements were taken at the papillary muscle level (45). To determine fibrotic changes after I/R, paraffin-embedded heart sections were stained with Masson's trichrome (American MasterTech, Lodi, CA). The fibrotic area was determined by ImageJ software (5).
Western blot analysis.
Hearts were harvested, snap frozen, and crushed in liquid nitrogen. Tissue was homogenized in cold lysis buffer (Cell Signaling, Danvers, MA) as previously described (5, 45). Protein concentrations were measured by the Bradford method (Bio-Rad, Hercules, CA). SDS-PAGE was performed under reducing conditions on 4–20% gradient gels (Bio-Rad). Proteins were transferred to a nitrocellulose membrane (Bio-Rad). Blots were incubated with primary antibodies for 18–20 h at 4°C. Blots were then incubated with horseradish peroxidase-conjugated secondary antibody, and signals were detected using enhanced chemiluminescence (Cell Signaling). Primary antibodies to hemagglutinin (12CA5, Roche, Indianapolis, IN), S6 (Cell Signaling), phospho-S6 (Ser235/236, Cell Signaling), Akt (Cell Signaling), phospho-Akt (Ser473, Cell Signaling), mTOR (Cell Signaling), growth differentiation factor 15 (GDF15; Abcam, Cambridge, MA), and light chain 3 (LC3; Novus, Littleton, CO) were used for immunoblot analysis.
Biological analysis in ex vivo perfused hearts.
Enzyme activities of creatine kinase (CK) and lactate dehydrogenase (LDH) were determined in the effluent collected at baseline and 40 min of reperfusion by enzyme activity kits as previously described (CK: BioAssay Systems, Hayward, CA; LDH: Cayman Chemical, Ann Arbor, MI) (5).
Quantitative real-time PCR.
Accumulation of PCR products was monitored in real time, and cycle threshold (Ct) values were determined with a 7900HT Fast Real-Time System (Applied Biosystems, Foster city, CA). Relative changes in gene expression were determined using the ΔΔCt method with normalization to β-actin. Quantitative real-time PCR was performed with the following sets of primers: IL-6, forward 5′-AGAAGGAGTGGCTAAGGACCAA-3′ and reverse 5′-GCATAACGCACTAGGTTTGCC-3′; IL-1β, forward 5′-CCTTCCAGGATGAGGACATGAG-3′ and reverse 5′-CGTCACACACCAGCAGGTTATC-3′; TNF-α, forward 5′-AGCAAACCACCAAGTGGAGGA-3′ and reverse 5′-GCTGGCACCACTAGTTGGTTGT-3′; monocyte chemotactic protein-1, forward 5′-ATCCCAATGAGTAGGCTGGAGAGC-3′ and reverse 5′-CAGAAGTGCTTGAGGTGGTTGTG-3′; macrophage inflammatory protein-1α, forward 5′-ACCTGCTCAACATCATGAAGG-3′ and reverse 5′-AGATGGAGCTATGCAGGTGG-3′; GDF15, forward 5′-AGCTGGAACTGCGCTTACGGG-3′ and reverse 5′- TCCAGCCCAAGTCTTCAAGAG-3′; and β-actin, forward 5′- TGTTACCAACTGGGACGACA-3′ and reverse 5′-GGGGTGTTGAAGGTCTCAAA-3′.
Statistical analysis.
Data are presented as means ± SE. Group differences were analyzed by a two-tailed Student's or Welch's t-test. For multiple comparisons, two-way ANOVA was used. For all analyses, P values of <0.05 were considered significant.
RESULTS
Effects of HFD on body weight gain, glucose intolerance, and insulin resistance in WT and mTOR-Tg mice.
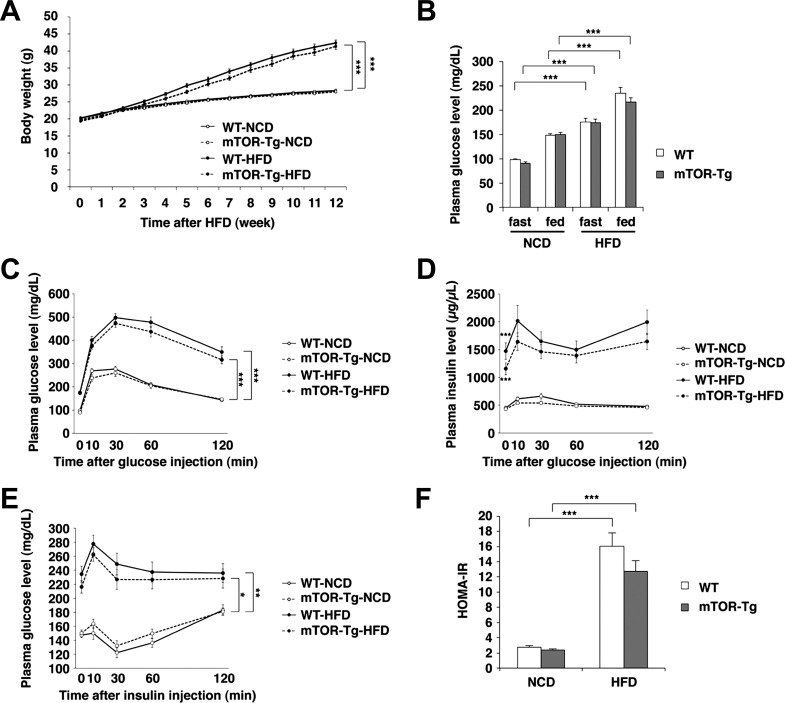
Both WT and mTOR-Tg mice fed a HFD for 12 wk exhibited an ∼60% increase in body weight compared with mice fed a NCD (P < 0.001 for both groups; Fig. 1A). Fasting and fed plasma glucose levels were higher in HFD-fed mice than in NCD-fed mice (Fig. 1B). GTTs and ITTs clearly demonstrated HFD-induced glucose intolerance and insulin resistance in both WT and mTOR-Tg mice after 12 wk of HFD feeding (GTT: P < 0.001 for both groups; ITT: P < 0.01 for WT mice and P < 0.05 for mTOR-Tg mice; Fig. 1, C and E). Plasma insulin levels were significantly higher in the HFD-fed group compared with the NCD-fed group and were accompanied by maintenance of high plasma insulin levels during GTTs in both HFD-fed strains (baseline plasma insulin level: 447.12 ± 33.43 μg/μl in WT NCD-fed mice vs. 1,472.72 ± 146.77 μg/μl in WT HFD-fed mice, P < 0.001, and 429.74 ± 17.48 μg/μl in mTOR-Tg NCD-fed mice vs. 1,155.88 ± 108.2 μg/μl in mTOR-Tg HFD-fed mice, P < 0.001; Fig. 1D). HOMA-IR was also significantly higher in the HFD-fed group compared with the NCD-fed group in both strains (P < 0.001; Fig. 1F). There were no differences in mean body weight, plasma glucose and insulin concentrations, glucose intolerance, insulin resistance, or HOMA-IR between WT and mTOR-Tg mice on the same diet. These findings clearly indicate that HFD feeding yielded effects on obesity and metabolic dysfunction like those observed in metabolic syndrome and that the extent of these effects was comparable between WT and mTOR-Tg mice.
Fig. 1.
High-fat diet (HFD)-induced obesity, glucose intolerance, and insulin resistance are comparable between wild-type (WT) and mechanistic target of rapamycin (mTOR)-overexpressing transgenic (mTOR-Tg) mice. A: body weight changes during HFD feeding (n = 36–40 mice/group). B: 16-h fasting or fed plasma glucose levels [n = 24 WT mice fed a normal chow diet (WT-NCD mice), 24 mTOR-Tg fed a NCD (mTOR-Tg-NCD mice), 22 WT mice fed a HFD (WT-HFD mice), and 22 mTOR-Tg mice fed a HFD (mTOR-Tg-HFD mice)]. C and D: plasma glucose and insulin levels during glucose tolerance tests. E: plasma glucose levels during insulin tolerance tests. F: homeostatic model of assessment of insulin resistance (HOMA-IR) calculated with the formula described in materials and methods. *P < 0.05, **P < 0.01, and ***P < 0.001, HFD vs. NCD (by two-way ANOVA or Student's t-test).
HFD feeding enhances baseline cardiac function in WT and mTOR-Tg hearts.
To examine the effect of mTOR overexpression against I/R injury in cardiomyocytes, we subjected hearts isolated from mice in four groups (WT NCD-fed, mTOR-Tg NCD-fed, WT HFD-fed, and mTOR-Tg HFD-fed mice) to an ex vivo Langendorff perfusion model as previously described (5, 35). There were no differences in baseline functional parameters between WT and mTOR-Tg hearts in NCD-fed groups, including size of the hearts (Table 1), which is consistent with our previous report (5). Mice fed the HFD for 12 wk had increased contractile activity in both WT and mTOR-Tg hearts, as previously reported (47). LV systolic pressure, LV developed pressure (LVDP), LV dP/dtmax, and LV dP/dtmin were significantly higher in the HFD-fed group than in the NCD-fed group in both strains, accompanied by increases in coronary flow and heart weight (Table 1). These findings suggest that the HFD feeding increased contractile function. Whereas there was no difference in cardiac function between WT and mTOR-Tg mice on the same diet, in the HFD-fed group, the average heart weight in WT mice was higher than that of mTOR-Tg mice. Taken together, the effect of HFD on baseline cardiac function was comparable between WT and mTOR-Tg mice.
Table 1.
Baseline cardiac function in ex vivo perfused hearts isolated from NCD-fed or HFD-fed WT and mTOR-Tg mice
| WT NCD-Fed Group | mTOR-Tg NCD-Fed Group | WT HFD-Fed Group | mTOR-Tg HFD-Fed Group | |
|---|---|---|---|---|
| Number of mice/group | 24 | 28 | 24 | 26 |
| LV systolic pressure, mmHg | 133.6 ± 6.3 | 134.8 ± 4.2 | 163.7 ± 7.7** | 160.0 ± 7.0** |
| LV end-diastolic pressure, mmHg | 3.60 ± 0.43 | 4.83 ± 0.48 | 4.92 ± 0.43 | 4.94 ± 0.52 |
| LV developed pressure, mmHg | 130.0 ± 6.2 | 130.0 ± 4.2 | 158.8 ± 7.8** | 154.9 ± 7.1** |
| LV dP/dtmax, mmHg/s | 5,356 ± 314 | 5,476 ± 239 | 7,183 ± 325*** | 6,836 ± 380** |
| LV dP/dtmin, mmHg/s | −2,937 ± 141 | −2,959 ± 107 | −3,822 ± 107*** | −3,621 ± 162** |
| Heart rate, beats/min | 308.1 ± 8.1 | 299.5 ± 9.4 | 285.8 ± 14.7 | 277.5 ± 11.8 |
| Coronary flow, ml/min | 3.14 ± 0.22 | 3.19 ± 0.25 | 4.23 ± 0.18*** | 3.98 ± 0.20* |
| Heart weight, mg | 139.67 ± 5.4 | 134.00 ± 3.9 | 161.92 ± 3.86** | 150.65 ± 3.36**† |
| Heart weight/tibia length, mg/mm | 6.23 ± 0.24 | 5.95 ± 0.16 | 7.23 ± 0.16** | 6.78 ± 0.15***† |
Values are means ± SE. WT, wild-type mice; NCD, normal chow diet; mTOR-Tg, mechanistic target of rapamycin-overexpressing transgenic mice; HFD, high-fat diet.
P < 0.05,
P < 0.01, and
P < 0.001, NCD vs. HFD;
P < 0.05, WT vs. mTOR-Tg groups (by Student’s t-test).
Overexpression of mTOR prevents cardiac dysfunction after ex vivo I/R in hearts from HFD-fed mice.
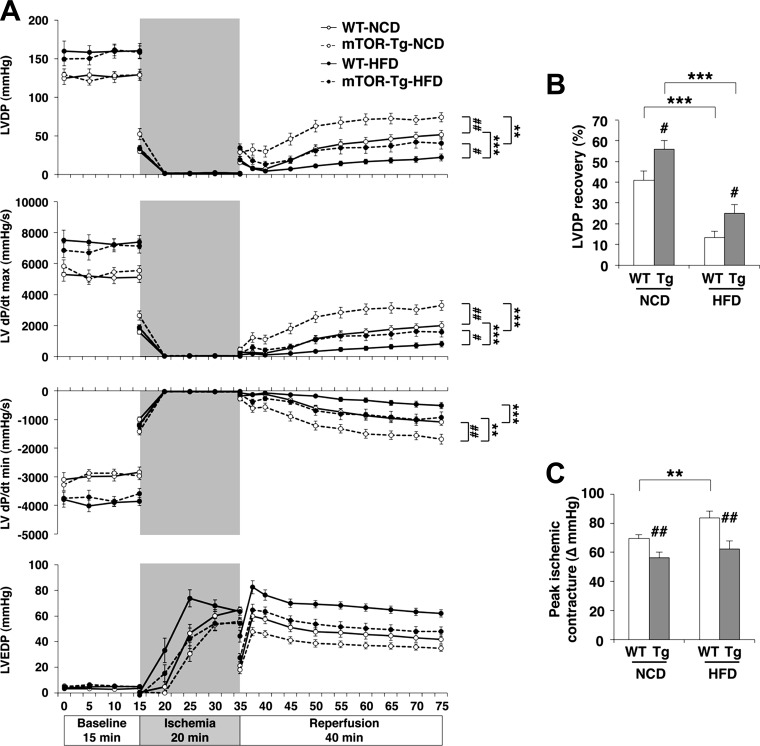
We examined cardiac functional recovery in all four mouse groups using ex vivo I/R models. In a manner consistent with our previous report (5), LVDP in mTOR-Tg NCD-fed hearts was significantly higher at all reperfusion time points compared with WT NCD-fed hearts (P < 0.01; Fig. 2A). End-point LVDP recovery in mTOR-Tg NCD-fed hearts was ∼30% higher compared with WT NCD-fed hearts (P < 0.05; Fig. 2B). LVDP during reperfusion and end-point LVDP recovery were strikingly reduced by HFD feeding compared with NCD feeding in WT hearts (LVDP during reperfusion: WT NCD-fed vs. WT HFD-fed groups, P < 0.001; LVDP recovery: 41.90 ± 3.85% in the WT NCD-fed group vs. 14.50 ± 3.10% in the WT HCD-fed group, P < 0.001; Fig. 2, A, top, and B). mTOR-Tg HFD-fed hearts showed significantly higher functional recovery compared with WT HFD-fed hearts (P < 0.05; Fig. 2, A, top, and B). LV dP/dtmax and LV dP/dtmin during I/R experiments showed results consistent to the LVDP results (Fig. 2A, middle). These data indicate that the overexpression of cardiac mTOR prevents HFD-induced deterioration of cardiac function during ex vivo I/R.
Fig. 2.
Overexpression of cardiac mTOR prevents cardiac dysfunction after transient ischemia in HFD hearts. A: left ventricular (LV) developed pressure (LVDP), LV dP/dtmax, LV dP/dtmin, and LV end-diastolic pressure (LVEDP) during I/R in WT-NCD, mTOR-Tg-NCD, WT-HFD, and mTOR-Tg-NCD hearts. B: maximum LVDP recovery (percentage of baseline) measured at 40 min of reperfusion. C: ischemic contracture during the 20-min ischemia period as determined by peak ischemic contracture (ΔLVEDP from 0 min of ischemia). n = 24 WT-NCD mice, 28 mTOR-Tg-NCD mice, 24 WT-HFD mice, and 26 mTOR-Tg-HFD mice. *P < 0.05, **P < 0.01, and ***P < 0.001, NCD vs. HFD; #P < 0.05 and ##P < 0.01, WT vs. mTOR-Tg mice (by two-way ANOVA or Student's t-test).
Ischemic contracture after ischemia is a crucial determinant of I/R and is caused by cytosolic Ca2+ overload and ATP depletion (26, 46). In WT hearts, HFD feeding significantly increased ischemic contracture during 20-min ischemia compared with NCD feeding (peak ischemic contracture: 69.47 ± 2.65 mmHg in the WT NCD-fed group vs. 83.9 ± 4.50 mmHg in the WT HFD-fed group, P < 0.01; Fig. 2, A, bottom, and C). mTOR-Tg hearts exhibited less ischemic contracture than WT hearts in both NCD-fed and HFD-fed groups (WT NCD-fed vs. mTOR-Tg NCD-fed groups, P < 0.01; WT HFD-fed vs. mTOR-Tg HFD-fed groups, P < 0.01; Fig. 2, A, bottom, and C). These results suggest that overexpression of mTOR suppresses ischemic contracture.
Cardiac mTOR suppresses necrosis in ex vivo I/R with HFD-fed hearts.
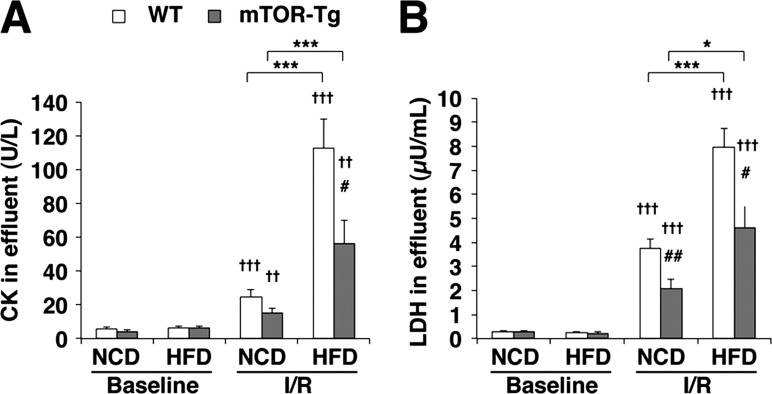
Since necrosis is a key factor in the pathogenesis of I/R injury (51), we measured CK and LDH concentrations in the effluent during reperfusion as markers of cardiomyocyte necrosis after I/R. As shown in our previous report (5), overexpression of mTOR attenuated both CK and LDH release after I/R in NCD-fed groups (Fig. 3, A and B). The administration of a HFD exacerbated I/R-induced CK and LDH release in both strains (CK: WT NCD-fed vs. WT HFD-fed groups, P < 0.001, and mTOR-Tg NCD-fed vs. mTOR-Tg HFD-fed groups, P < 0.001; LDH: WT NCD-fed vs. WT HFD-fed groups, P < 0.001, and mTOR-Tg NCD-fed vs. mTOR-Tg HFD-fed groups, P < 0.05; Fig. 3, A and B). As we expected, both CK and LDH concentrations were significantly lower in effluent samples from mTOR-Tg HFD-fed hearts compared with those of WT HFD-fed hearts after I/R (CK: WT HFD-fed vs. mTOR-Tg HFD-fed groups, P < 0.05; LDH: WT HFD-fed vs. mTOR-Tg HFD-fed groups, P < 0.05; Fig. 3, A and B). These data indicate that overexpression of mTOR attenuates the deteriorative effect of a HFD on the necrosis that follows I/R.
Fig. 3.
Overexpression of cardiac mTOR prevents cardiac injury after ex vivo transient ischemia in HFD hearts. A and B: activities of creatine kinase (CK; A) and lactate dehydrogenase (LDH; B) in the effluent collected during the reperfusion period. To determine enzyme activities immediately after ex vivo I/R injury, effluents from hearts exposed to either 20 or 40 min of global ischemia were collected at control perfusion (baseline) and after 40-min reperfusion (I/R). n = 14 WT-NCD mice, 5 mTOR-Tg-NCD mice, 13 WT-HFD mice, 15 mTOR-Tg-HFD mice. *P < 0.05 and ***P < 0.001, NCD vs. HFD; #P < 0.05 and ##P < 0.01, WT vs. mTOR-Tg mice; ††P < 0.01 and †††P < 0.001, baseline vs. I/R (by Student's t-test).
Effects of HFD on the mTOR signaling pathway in WT and mTOR-Tg mice.
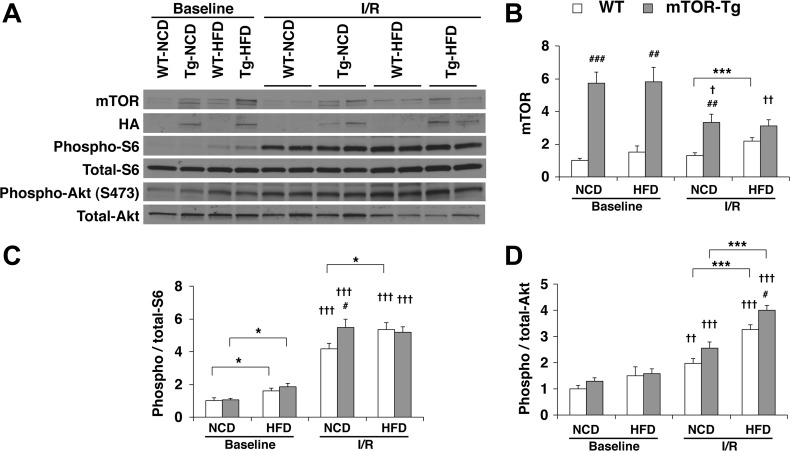
The HFD increased baseline phosphorylation of S6 in both WT and mTOR-Tg mice (Fig. 4). Phosphorylation of Akt also increased in HFD-fed mice, although it was not statistically significant (Fig. 4). I/R increased phosphorylation of both S6 and Akt compared with the baseline phosphorylation in all genotype and diet conditions, whereas I/R decreased the level of mTOR expression in mTOR-Tg hearts compared with baseline (Fig. 4). In I/R hearts, phosphorylation levels of both S6 and Akt were significantly increased in all groups compared with baseline conditions. In HFD conditions, Akt phosphorylation was higher after I/R in mTOR-Tg mice than in WT mice, whereas no difference was observed in S6 phosphorylation between WT HFD-fed and mTOR-Tg HFD-fed mice after I/R (Fig. 4). However, since phosphorylation levels of both S6 and Akt in hearts after I/R were high, especially in HFD conditions, it was difficult to identify whether mTOR overexpression made a difference in activation between mTORC1 and mTORC2 after I/R.
Fig. 4.
Overexpression of cardiac mTOR induces functional activation of both mTORC1 and mTORC2 in post-I/R hearts. A: representative immunoblots of mTOR signaling molecules in hearts subjected to the ex vivo Langendorff perfusion model. Baseline hearts were harvested after 15 min of equilibration perfusion ex vivo. I/R hearts were harvested after a course of baseline conditions followed by 20-min ischemia and then 40-min reperfusion. Immunoblot analysis was performed with the indicated antibodies. Blots are representative of six independent experiments. Densitometric quantitative analyses of mTOR (B), phospho-S6 (C), and phospho-Akt (D) were normalized to baseline levels of WT-NCD hearts in each experiment. n = 6 baseline hearts and 12 I/R hearts. *P < 0.05 and ***P < 0.001, NCD vs. HFD; #P < 0.05, ##P < 0.01, and ###P < 0.001, WT vs. mTOR-Tg hearts; †P < 0.05, ††P < 0.01, and †††P < 0.001, baseline vs. I/R (by Student's t-test).
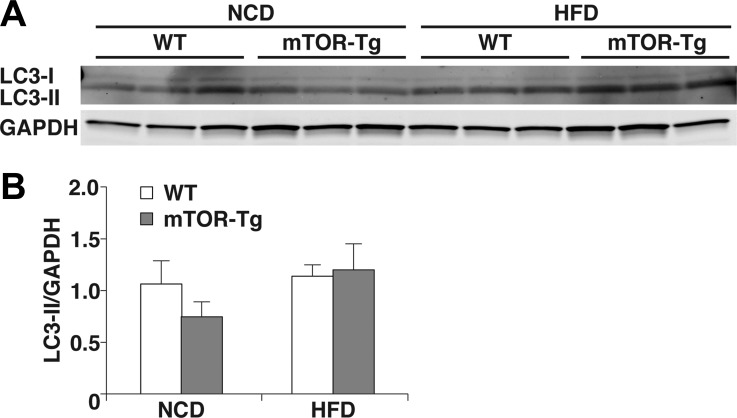
mTORC1 suppresses autophagy in the mTOR signaling pathway (14). Dysregulation of autophagic flux is known as a key pathogenic factor in cardiac diseases, including I/R injury and diet-induced obesity (29, 55). Therefore, we examined the effect of cardiac mTOR on autophagy after I/R in both NCD and HFD conditions by assessing the expression of LC3-II, an indicator of autophagosome initiation (16). We did not see significant differences in LC3-II expression between WT NCD-fed, mTOR-Tg NCD-fed, WT HFD-fed, or mTOR-Tg HFD-fed mice. The level of LC3-II in mTOR-Tg NCD-fed mice was slightly lower than other three groups, but the lack of a significant difference suggests that autophagy was not modified by mTOR under our HFD treatment conditions (Fig. 5, A and B).
Fig. 5.
Overexpression of mTOR does not affect autophagic activity in a NCD nor HFD. A: representative immunoblots of light chain 3 [LC3; LC3-I (top) and LC3-II (bottom)] and GAPDH levels in hearts subjected to the ex vivo Langendorff perfusion model. Hearts were harvested after I/R as described in Fig 4. B: densitometric analysis of LC3-II levels normalized to GAPDH. n = 6 for all groups.
Cardiac mTOR attenuates the production of proinflammatory factors in I/R injury.
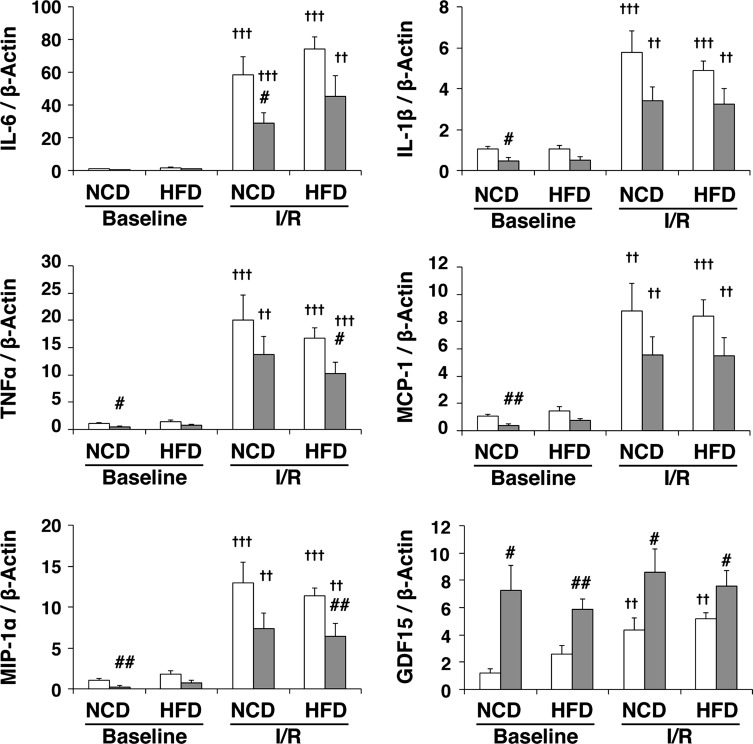
To assess the effect of mTOR in the inflammatory response after I/R, we measured levels of IL-6, IL-1β, TNF-α, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α. In a manner consistent with our previous report (5), expression levels of these proinflammatory cytokines and chemokines in NCD-fed mouse hearts were significantly increased in I/R hearts compared with baseline hearts in both strains, and mTOR overexpression suppressed the expression of these proinflammatory factors compared with WT mice (Fig. 6). Interestingly, while mTOR overexpression also suppressed cardiac expression of proinflammatory factors in response to the HFD, HFD treatment did not significantly affect them in WT and mTOR-Tg hearts under baseline or I/R conditions (Fig. 6).
Fig. 6.
Overexpression of mTOR suppresses the induction of proinflammatory cytokines and chemokines after I/R in HFD-fed hearts. mRNA expression levels of proinflammatory cytokines (IL-6, IL-1β, and TNF-α), chemokines [monocyte chemotactic protein (MCP)-1 and macrophage inflammatory protein (MIP)-1α], and growth differentiation factor (GDF)15 in hearts subjected to ex vivo I/R are shown. mRNA was measured by quantitative real-time PCR. n = 6 for each group of baseline hearts; n = 12 WT-NCD hearts, 12 mTOR-Tg-NCD hearts; 10 WT-HFD hearts, and 14 mTOR-Tg-HFD hearts for I/R. #P < 0.05 and ##P < 0.01, WT vs. mTOR-Tg hearts; ††P < 0.01 and †††P < 0.001, baseline vs. I/R (by Student's t-test).
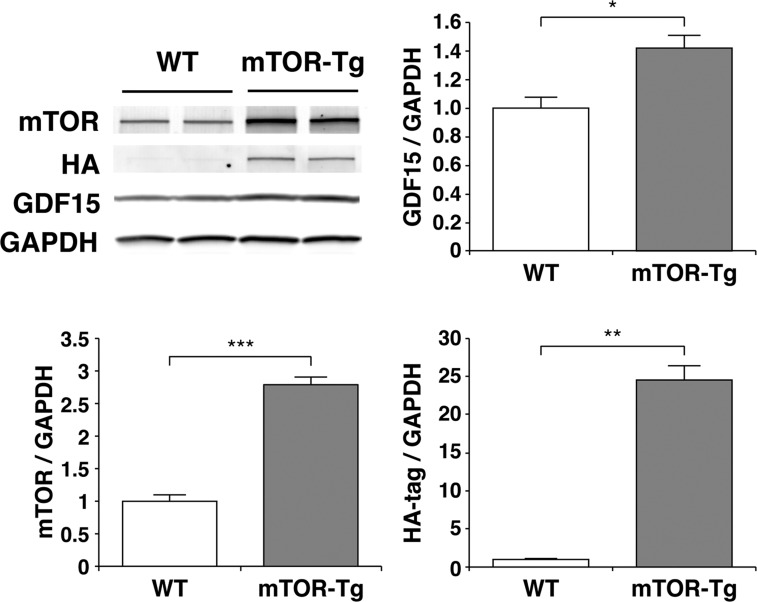
Previous reports have shown that cardiac GDF15, a member of the transforming growth factor-β superfamily, plays an important role in cardioprotection against myocardial ischemia (23) and pathological hypertrophy (52). Since mTORC2 regulates GDF15 expression in the heart (12), we examined GDF15 mRNA expression in the hearts of WT and mTOR-Tg mice fed either NCD or HFD. The expression level of GDF15 was significantly higher in mTOR-Tg hearts compared with WT hearts across all experimental groups (Fig. 6, bottom right). I/R significantly increased GDF15 expression in both WT NCD-fed and WT HFD-fed hearts compared with baseline levels (P < 0.01; Fig. 6, bottom right). To confirm our findings at the mRNA level, we measured baseline protein levels of GDF15 in the hearts of NCD-fed mice. The level of GDF15 protein in the heart was ∼40% higher in mTOR-Tg mice compared with WT mice (Fig. 7). These data suggested that mTOR overexpression increased GDF15 in both NCD and HFD conditions.
Fig. 7.
Overexpression of mTOR increases the level of GDF15 protein at baseline. Representative immunoblots compare expression of mTOR and GDF15 in hearts isolated from WT and mTOR-Tg mice without additional treatments. Immunoblots were performed with the primary antibodies described in materials and methods. HA, hemagglutinin. Blots are representative of n = 5 WT mice and 6 mTOR-Tg mice. Densitometry graphs were normalized to GAPDH and represent normalized average densities ± SE. *P < 0.05, **P < 0.01, and ***P < 0.001 (by Student's t-test).
Overexpression of mTOR rescues the detrimental effects of HFD-induced obesity on post-I/R cardiac function and LV remodeling in vivo.
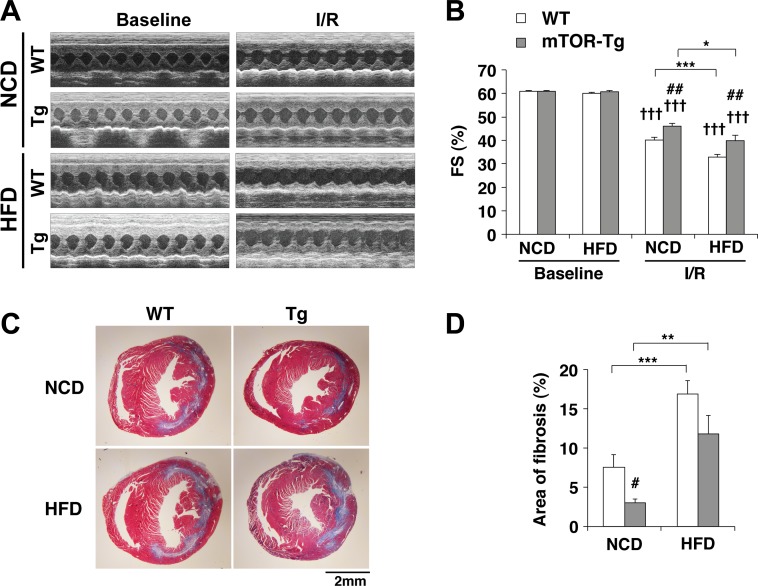
To confirm the ex vivo cardio protective effect of mTOR against I/R under conditions of HFD-induced obesity, we performed in vivo I/R on WT and mTOR-Tg mice after 12 wk of HFD feeding, as previously described (5). Echocardiography showed that systolic function in both WT and mTOR-Tg groups was reduced 1 wk after I/R (Fig. 8, A and B). Fractional shortening in the mTOR-Tg group was higher than in the WT group, suggesting again that mTOR overexpression protects hearts during the acute phase after I/R, which is consistent with our previous report (5). The HFD further exacerbated the reduction in fractional shortening caused by in vivo I/R in both WT and mTOR-Tg mice (WT groups: WT NCD-fed vs. WT HFD-fed groups, P < 0.001; and mTOR-Tg groups: mTOR-Tg NCD-fed vs. mTOR-Tg HFD-fed groups, P < 0.05; Fig. 8B). mTOR-Tg mice exhibited higher fractional shortening than WT mice after in vivo I/R, and this difference was observed in both types of dietary conditions (NCD: WT vs. mTOR-Tg mice, P < 0.01; and HFD: WT vs. mTOR-Tg mice, P < 0.01; Fig. 8B). We also evaluated myocardial fibrosis in the heart 1 wk after I/R. The result was consistent with the changes observed in cardiac function, and overexpression of mTOR reduced fibrosis in post-I/R hearts compared with WT mice in both NCD and HFD feeding conditions (Fig. 8, C and D). As previously described (5, 21, 27), myocardial scarring in the heart after in vivo I/R was located along circumferential myofibers in the midcardium and extended from the initial infarct to the remote zone (Fig. 8C). Our results confirm that HFD-induced obesity worsens the decreased cardiac function observed in vivo I/R injury and suggest that overexpression of mTOR lessened the detrimental effects of a HFD, at least in part, by suppressing adverse LV remodeling.
Fig. 8.
Overexpression of cardiac mTOR preserves cardiac function after in vivo transient ischemia in HFD hearts. A: representative M-mode images of operated WT-NCD, mTOR-Tg-NCD, WT-HFD, and mTOR-Tg-HFD mice at baseline and 1 wk after I/R surgery. B: mean scores for fractional shortening (FS; in %) at 1 wk after I/R. C: representative Masson's trichrome staining after 1 wk of surgery. D: quantitative analysis of interstitial fibrosis detected by Masson's trichrome staining. n = 12 WT-NCD hearts, 12 mTOR-Tg-NCD hearts, 15 WT-HFD hearts, and 10 mTOR-Tg-HFD hearts. *P < 0.05, **P < 0.01, and ***P < 0.001, NCD vs. HFD; #P < 0.05 and ##P < 0.01, WT vs. mTOR-Tg hearts; †††P < 0.001, baseline vs. I/R (by Student's t-test).
DISCUSSION
In the present study, we demonstrate that diet-induced obesity deteriorates post-I/R cardiac functional recovery in both ex vivo Langendorff perfused hearts and an in vivo coronary ligation model. With these models, we also show that overexpression of cardiac mTOR protects the heart against I/R injury in both obese and lean mice. We also observed that overexpression of mTOR suppresses I/R-induced inflammation and necrosis in ex vivo I/R models. Our in vivo I/R models show that in both obese and lean mice, mTOR overexpression prevents extension of myocardial scarring, which is a key pathophysiological event in adverse LV remodeling due to I/R injury (21). We propose that increased mTOR expression inhibits post-I/R myocardial scarring, thus resulting in preserved cardiac function in diet-induced obese mice.
Previous large-scale clinical studies suggest that diabetes is a powerful risk factor for death and heart failure after an acute MI (1, 33, 34). Metabolic syndrome, which includes prediabetes and diabetes (17), is recognized as a predictor of heart failure in patients with acute MI (50). An animal study (47) using HFD-induced obese mice has shown that obesity further deteriorates cardiac function in I/R injury. Our results are consistent with those findings, as we observed that cardiac function recovery after temporary ischemia in ex vivo perfused hearts was worse in HFD-induced obese mice than in lean mice, and this worse outcome was accompanied by an increased release of necrotic markers CK and LDH in obese mice compared with lean mice. In vivo models of I/R injury have also shown that diet-induced obesity deteriorated cardiac function after I/R compared with lean mice. Previously, we reported that mTOR overexpression protects the heart against I/R injury in both in vivo and ex vivo models using the same Tg mice as in the present study (5). The preent study demonstrates that mTOR overexpression preserved cardiac function and prevented cardiac injury in both in vivo and ex vivo models of I/R injury in both obese and lean mice. This may initially seem contrary to a recent report (42) that demonstrated that mTORC1 inhibition by either rapamycin or partial mTOR deletion protected the heart against MI (without reperfusion) in diet-induced obese mice. However, these findings might be indicative of activation of phosphatidylinositol 3-kinase/Akt signaling by suppression of negative feedback inhibition of insulin-receptor substrate-1 by mTORC1 (18). In fact, another study using mTOR-binding protein PRAS40, which inhibits mTORC1, showed that PRAS40 overexpression activates mTORC2 and exhibits cardioprotection in HFD-induced obese mice. In our Tg mice, overexpression of WT mTOR activates both mTORC1 and mTORC2 signaling pathways (5, 45). In our study, I/R increased phosphorylation levels of both S6 and Akt in all groups, especially in the HFD-fed groups. This was likely affected by the hyperinsulinemia observed in both HFD-fed groups (Fig. 1), and the elevated insulin levels probably activated both kinases. Whereas no statistical difference in S6 phosphorylation was observed between WT HFD-fed and mTOR-Tg HFD-fed mice after I/R, Akt phosphorylation was higher in mTOR-Tg mice than WT mice under HFD conditions. Our results, combined with the previous reports discussed above, suggest that mTORC2 activation might be a key factor for cardioprotection against I/R injury under both obese conditions. However, since we did not selectively manipulate the mTORC2 signaling pathway in the present study, further experiments are required to demonstrate the cardioprotective role of mTORC2 under obese conditions.
Autophagy is a catabolic cellular process mediated by lysosomes and plays an important role in cellular homeostasis in many organs, including the heart (14, 29). The mTORC1 axis is a potential antiautophagic response that regulates the UNC-51-like kinase complex (14). In our study, mTOR overexpression appeared to slightly suppress LC3-II expression, but this was not statistically significant. A previous report (43) has shown that HFD-induced obesity exhibits autophagy dysregulation. In contrast, LC3-II expression in our HFD-fed mice was comparable to that of NCD-fed mice. Since mTOR overexpression did not significantly affect baseline levels of LC3-II, it is unlikely that the protective effects of mTOR are mediated through autophagic activity. Another detection system, such as LC3-green fluorescent protein mice (32), would be necessary to define the role of mTOR-regulated autophagy in metabolic syndrome.
In a recent report (21), we used three-dimensional images rendered from multiple histological sections from the heart after in vivo I/R injury to show that myocardial scarring after I/R, but not straight MI (no reperfusion), extends along myofibers rather than coronary arteries. In the present study, histological assays in in vivo I/R models showed that fibrotic scars extended along myofibers in the midcardium and were larger in HFD-induced obese mice than in lean mice and that mTOR overexpression prevented the extension of myocardial scarring along myofibers compared with WT hearts in both feeding conditions. This pattern of scarring is similar to that of contraction band necrosis, which is observed in reperfusion after ischemia (31). Proposed pathogeneses of contraction band necrosis are shared with hypercontracture, including Ca2+ overload and ATP resynthesis (40). We observed that in ex vivo I/R models, myocardial contracture during diastole in both late ischemia and reperfusion phases was higher in HFD-fed mice compared with NCD-fed mice and that mTOR overexpression suppressed the level of contracture in both feeding conditions. While prolonged and/or excess hypercontracture seems to be associated with band necrosis (40), the mechanisms of ischemic contracture and hypercontracture in I/R injury have not been characterized well. In the present study, HFD-fed mTOR-Tg mice exhibited less ischemic contracture, better cardiac recovery, and less myocardial injury compared with HFD-fed WT mice. The difference in myocardial contracture in mTOR-Tg mice may contribute to their better functional recovery after I/R, especially in obese mice.
Chronic inflammation accounts for pathophysiological features of obesity-induced insulin resistance (15, 53). It is also known that cardiomyocytes are key resources of cytokines and chemokines (6). However, our results demonstrated that there was no significant difference in expression levels of I/R-induced cytokines and chemokines between the hearts of NCD and HFD mice, and this trend held true for both WT and mTOR-Tg mice. A possible explanation for this lack of difference is that 1–3 days after the onset of MI in vivo, leukocytes are recruited and accumulated in the infarct region (13). It is likely that activation of an inflammatory response was mainly triggered by circulating leukocytes rather than the cells of the heart, including cardiac fibroblasts and cardiomyocytes. We have previously reported that overexpression of cardiac mTOR suppresses the production of proinflammatory cytokines and chemokines in both transverse aortic constriction-induced cardiac hypertrophy and I/R (5, 45). In the present study, we also observed that mTOR overexpression suppressed proinflammatory cytokine and chemokine mRNA expression in I/R injury compared with WT hearts in both feeding conditions. Cardiac mTOR-mediated downregulation of inflammatory cytokine production from cardiomyocytes might contribute to reduced LV remodeling after I/R. However, it is known that the magnitude of LV remodeling is directly proportional to the initial infarct size after acute MI (30). Further experiments are required to determine the importance of suppression of the inflammatory response during mTOR-mediated cardioprotection against I/R injury and subsequent LV remodeling in obese mice.
Previous studies using genetic mouse models demonstrated that GDF15 protects the heart against pathological hypertrophy (54) and heart failure resulting from MI (22, 23). Our data show that overexpression of cardiac mTOR increased expression levels of GDF15 mRNA and protein in baseline conditions. Expression of GDF15 is regulated by activating transcription factor 4, which is downstream of the PKR-like endoplasmic reticulum kinase-eukaryotic translation initiation factor-2α pathway (3, 20). This pathway could overlap with the results of another study (37), in which TSC deletion caused mTOR activation and increased phosphorylation of PKR-like endoplasmic reticulum kinase. Based on those results and the increased GDF15 we observed, it is likely that cardiac mTOR may regulate the expression of GDF15 and its signaling pathway. Further experiments will be needed to elucidate and define the role of cardiac mTOR in regulating GDF15.
In conclusion, we demonstrated that overexpression of cardiac mTOR prevented the detrimental effects of fat-induced obesity on cardiac dysfunction in ex vivo and in vivo I/R and that cardiac mTOR overexpression was accompanied by suppression of proinflammatory cytokine production and necrosis. The rising rate of morbidity from heart failure after acute MI is further exacerbated by the presence of metabolic syndrome, and, thus, the effective treatment of both diseases remains a critical issue in the clinic. Interestingly, recent reports have shown that several mTOR-stabilizing proteins control the level of mTOR expression. Understanding the mechanisms of mTOR protein regulation and further studying the cardioprotective effects of mTOR against I/R injury could provide an important clue for therapies to prevent HF in the midst of metabolic syndrome.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-098423 (to T. Matsui) and G12-MD-007601 (to the Histology and Imaging Core Facility, University of Hawaii) and Training Grant T32-HL-115505 (to J. K. Higa).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.A., J.K.H., and T.M. conception and design of research; T.A., J.K.H., H.A., N.Y., B.S., and T.M. performed experiments; T.A., J.K.H., H.A., and T.M. analyzed data; T.A., J.K.H., H.A., N.Y., and T.M. interpreted results of experiments; T.A., J.K.H., N.Y., B.S., and T.M. prepared figures; T.A., J.K.H., and T.M. drafted manuscript; T.A., J.K.H., N.Y., and T.M. edited and revised manuscript; T.A., J.K.H., H.A., N.Y., B.S., and T.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Kenta Hara for helpful advice.
REFERENCES
- 1.Aguilar D, Solomon SD, Kober L, Rouleau JL, Skali H, McMurray JJ, Francis GS, Henis M, O'Connor CM, Diaz R, Belenkov YN, Varshavsky S, Leimberger JD, Velazquez EJ, Califf RM, Pfeffer MA. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation 110: 1572–1578, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640–1645, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol 40: 14–21, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Aoyagi T, Birumachi J, Hiroyama M, Fujiwara Y, Sanbe A, Yamauchi J, Tanoue A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 148: 2075–2084, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi T, Kusakari Y, Xiao CY, Inouye BT, Takahashi M, Scherrer-Crosbie M, Rosenzweig A, Hara K, Matsui T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303: H75–H85, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyagi T, Matsui T. The cardiomyocyte as a source of cytokines in cardiac injury. J Cell Sci Ther 2011: S5–003, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 296: E592–E602, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122: 1109–1118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaser RS, Levy P. Metabolic syndrome: a work in progress, but a useful construct. Circulation 115: 1812–1818, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Bucholz EM, Rathore SS, Reid KJ, Jones PG, Chan PS, Rich MW, Spertus JA, Krumholz HM. Body mass index and mortality in acute myocardial infarction patients. Am J Med 125: 796–803, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buss SJ, Muenz S, Riffel JH, Malekar P, Hagenmueller M, Weiss CS, Bea F, Bekeredjian R, Schinke-Braun M, Izumo S, Katus HA, Hardt SE. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J Am Coll Cardiol 54: 2435–2446, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Felkin LE, Narita T, Germack R, Shintani Y, Takahashi K, Sarathchandra P, Lopez-Olaneta MM, Gomez-Salinero JM, Suzuki K, Barton PJ, Rosenthal N, Lara-Pezzi E. Calcineurin splicing variant calcineurin Aβ1 improves cardiac function after myocardial infarction without inducing hypertrophy. Circulation 123: 2838–2847, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatica D, Chiong M, Lavandero S, Klionsky DJ. Molecular mechanisms of autophagy in the cardiovascular system. Circ Res 116: 456–467, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghanim H, Aljada A, Hofmeyer D, Syed T, Mohanty P, Dandona P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 110: 1564–1571, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb RA, Andres AM, Sin J, Taylor DP. Untangling autophagy measurements: all fluxed up. Circ Res 116: 504–514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109: 433–438, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci 30: 35–42, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA 294: 334–341, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jousse C, Deval C, Maurin AC, Parry L, Cherasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 282: 15851–15861, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Katz MY, Kusakari Y, Aoyagi H, Higa JK, Xiao CY, Abdelkarim AZ, Marh K, Aoyagi T, Rosenzweig A, Lozanoff S, Matsui T. Three-dimensional myocardial scarring along myofibers after coronary ischemia-reperfusion revealed by computerized images of histological assays. Physiol Rep 2: e12072, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-β superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 98: 351–360, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 17: 581–588, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Khan S, Salloum F, Das A, Xi L, Vetrovec GW, Kukreja RC. Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol 41: 256–264, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100: 1589–1596, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Koretsune Y, Marban E. Mechanism of ischemic contracture in ferret hearts: relative roles of [Ca2+]i elevation and ATP depletion. Am J Physiol Heart Circ Physiol 258: H9–H16, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Kusakari Y, Xiao CY, Himes N, Kinsella SD, Takahashi M, Rosenzweig A, Matsui T. Myocyte injury along myofibers in left ventricular remodeling after myocardial infarction. Interact Cardiovasc Thorac Surg 9: 951–955, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maejima Y, Chen Y, Isobe M, Gustafsson AB, Kitsis RN, Sadoshima J. Recent progress in research on molecular mechanisms of autophagy in the heart. Am J Physiol Heart Circ Physiol 308: H259–H268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKay RG, Pfeffer MA, Pasternak RC, Markis JE, Come PC, Nakao S, Alderman JD, Ferguson JJ, Safian RD, Grossman W. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation 74: 693–702, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki S, Fujiwara H, Onodera T, Kihara Y, Matsuda M, Wu DJ, Nakamura Y, Kumada T, Sasayama S, Kawai C, et al. Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation 75: 1074–1082, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15: 1101–1111, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukamal KJ, Nesto RW, Cohen MC, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Impact of diabetes on long-term survival after acute myocardial infarction: comparability of risk with prior myocardial infarction. Diabetes Care 24: 1422–1427, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Murcia AM, Hennekens CH, Lamas GA, Jimenez-Navarro M, Rouleau JL, Flaker GC, Goldman S, Skali H, Braunwald E, Pfeffer MA. Impact of diabetes on mortality in patients with myocardial infarction and left ventricular dysfunction. Arch Intern Med 164: 2273–2279, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Nagoshi T, Matsui T, Aoyama T, Leri A, Anversa P, Li L, Ogawa W, del Monte F, Gwathmey JK, Grazette L, Hemmings B, Kass DA, Champion HC, Rosenzweig A. PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115: 2128–2138, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Aoyagi T, Hiroyama M, Kusakawa S, Mizutani R, Sanbe A, Yamauchi J, Kamohara M, Momose K, Tanoue A. Both V1A and V1B vasopressin receptors deficiency result in impaired glucose tolerance. Eur J Pharmacol 613: 182–188, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell 29: 541–551, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, Dey A, Rothermel B, Kim YB, Kalinowski A, Russell KS, Kim JK. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 54: 3530–3540, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Reichelt ME, Willems L, Peart JN, Ashton KJ, Matherne GP, Blackburn MR, Headrick JP. Modulation of ischaemic contracture in mouse hearts: a ‘supraphysiological’ response to adenosine. Exp Physiol 92: 175–185, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Sinovas A, Abdallah Y, Piper HM, Garcia-Dorado D. Reperfusion injury as a therapeutic challenge in patients with acute myocardial infarction. Heart Fail Rev 12: 207–216, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Sankaralingam S, Abo Alrob O, Zhang L, Jaswal JS, Wagg CS, Fukushima A, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the obesity paradox. Diabetes. 2014. Dec 18. pii: db141050. [Epub ahead of print]. doi: 10.2337/db14-1050. [DOI] [PubMed] [Google Scholar]
- 42.Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res 114: 549–564, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125: 1134–1146, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15: 155–162, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, Hara K, Rosenzweig A, Matsui T. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol 299: C1256–C1266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steenbergen C, Murphy E, Watts JA, London RE. Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart. Circ Res 66: 135–146, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffett G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H2504–H2514, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Volkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, Herzig S, Glembotski CC, Sussman MA. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med 6: 57–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volkers M, Konstandin MH, Doroudgar S, Toko H, Quijada P, Din S, Joyo A, Ornelas L, Samse K, Thuerauf DJ, Gude N, Glembotski CC, Sussman MA. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation 128: 2132–2144, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Bibra H, St John Sutton M. Impact of diabetes on postinfarction heart failure and left ventricular remodeling. Curr Heart Fail Rep 8: 242–251, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, Nagashima M, Arita S, Hongo S, Ota H, Kobayashi Y, Miyazaki A, Hirano T. Preventive effects of heregulin-β1 on macrophage foam cell formation and atherosclerosis. Circ Res 105: 500–510, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 98: 342–350, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Xu X, Ren J. MTOR overactivation and interrupted autophagy flux in obese hearts: a dicey assembly? Autophagy 9: 939–941, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]