Abstract
Void spot assays (VSA) and cystometry are two of the most common tests performed in mice to assess lower urinary tract function. Assay protocols and methodology vary greatly among laboratories, and little is known about reproducibility of results generated by different laboratories. We performed VSA in four mouse strains, comparing males with females and comparing results between two independent laboratories. Unique aspects of the current study include direct comparison of results of VSA performed in a similar manner in two locations and comparison of cystometry performed using two different rates of infusion in these two laboratories. Both assays were performed in male and female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice, and cystometry was performed under urethane anesthesia (10/group). Assays were performed and results analyzed as previously described. Results obtained in female mice were compared with previously reported values. Results of lower urinary tract function testing in mice vary in a consistent manner with strain and sex. Variables in husbandry, testing techniques, and analysis of results can significantly affect conclusions, particularly those obtained by cystometry. Although VSA results were remarkably similar between the two laboratories, consistent methods for performing lower urinary tract function testing in mice are required to compare results among studies with confidence.
Keywords: mice, micturition, cystometry, sex, genetic
abnormalities in urine storage and micturition, generally referred to as lower urinary tract symptoms (LUTS), are relatively common, and prevalence increases with age. LUTS in men and women have been divided into storage (irritative) symptoms (urinary frequency, urgency, and nocturia), voiding (obstructive) symptoms (straining, weak stream, intermittent stream, and incomplete emptying), or postmicturition symptoms (postmicturition dribbling), as well as pain that may occur during any phase of the micturition cycle (1, 7, 18, 19). Lower urinary tract function is commonly assessed in patients by urodynamic testing, recently defined as “an interactive diagnostic study of the lower urinary tract composed of a number of tests that can be used to obtain functional information about bladder filling, urine storage and emptying” (30).
The importance of standardization of protocols for performance of human urodynamic testing between and among studies has recently been emphasized (5, 20), but it is also recognized that variation among urodynamic testing protocols makes it extremely difficult to compare studies performed over time in the same patient, complicates comparisons of results between patients, and raises questions about how results of urodynamic testing relate to normal and aberrant physiology of micturition (26). However, when methodology utilized to perform urodynamic studies was tightly controlled in a prospective manner, results appeared to be highly reproducible and consistent within individuals and among testing sites (20 total sites) (16).
Rodents have been used extensively to investigate mechanisms underlying LUTS in humans (4, 9). Rodents provide the opportunity for more invasive investigation of the pathophysiology underlying LUTS than studies in patients, but differences in anatomy and basic physiology must be kept in mind, as well as differences between spontaneous and induced disorders resulting in LUTS. It should also be recognized that the methods used to assess lower urinary tract function in rodents are similar, but not identical, to urodynamic testing performed in humans (2). The purpose of the current study was to compare results obtained in separate laboratories from two techniques commonly used to evaluate lower urinary tract function in mice: void spot assay (VSA) and cystometry under anesthesia. Similar to urodynamic testing in patients, methods and protocols used to test lower urinary tract function in rodents vary widely among laboratories, significantly weakening the validity of direct comparisons between studies performed at different laboratories. It is also unclear whether there is a consistent relationship between results obtained from VSA and anesthetized cystometry. Further complicating the use of mouse models to study LUTS is variation among strains and between sexes. Recent publications report differences in results of lower urinary tract function testing that were attributed to genetic strain or sex differences among mice (6, 31). The age of mice used for urinary function testing also appears to have a profound impact on results (31).
Lack of standardization in rodent urinary function testing impairs our ability to collect granular phenotypic data and recognize patterns in these data, and this hampers our ability to understand relationships among various molecular and genetic factors relative to urological end points. Standardized approaches in urinary function testing in rodents would overcome some of these problems. The current study was performed to prospectively control as many variables as possible to determine the reproducibility between laboratories of results of lower urinary tract function testing in mice of the same genetic strains, to compare results of lower urinary function testing between male and female mice of the same genetic strains, and to compare results from two different infusion rates for cystometry. There are currently no recommended “best practices” for performance and comparison of results of urinary function testing in rodents. The vast majority of reports have utilized female, as opposed to male mice, leaving the question of reliability and reproducibility of lower urinary tract function testing in males unresolved. It was not the intent of this study to propose best practice protocols but rather to investigate assay reproducibility using males and females of four strains of mice, previously shown to exhibit variations in baseline voiding function. One of these is a noninvasive, inexpensive method of testing that involves allowing mice to urinate on filter paper and then quantitating spot numbers and volumes (VSA), while the other is a more invasive and terminal method which measures intrabladder pressure under conditions of continuous filling (cystometry) Interestingly, the results of this study indicate that there was less variability in results of VSA testing between laboratories than that observed in between-laboratory comparison of results of cystometry. These results further support the need for continued refinement of techniques used to assess lower urinary function testing in rodents to achieve greater consistency within and among studies.
MATERIALS AND METHODS
Mice
129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). These strains were specifically selected because prior results of lower urinary tract function testing by performance of VSA and cystometry provided results that appeared to be uniquely associated with individual strains (31). Ten males and ten females of each strain were acquired at 8–10 wk of age to ensure that testing would be completed while the mice were 10–12 wk of age. Mice were allowed to acclimate to the vivarium for at least 7 days before any testing. All mice underwent VSA testing before cystometry, and all mice were euthanized upon completion of cystometry. VSA was typically performed the week before cystometry. Experimental protocols were approved by the University of Wisconsin Animal Care and Use Committee and the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center. All protocols conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
VSA
Standardized procedures were followed to mimic those used in a previous study (31) and to minimize the impact of husbandry on results (15). Mice were allowed free access to water until placed in cages for testing. On the day of testing, mice were placed individually in standard polycarbonate mouse cages with 16 × 26-cm filter papers (grade 540 acid-hardened filter papers, 30-cm diameter; catalog no. 1540-320, Whatman) taped to the floor. Mice remained in these enclosures for a 4-h period between 9 AM and 2 PM and during that time were given access to their regular food but not water. Water was removed to avoid water droplets leaking onto the paper, which could result in dilution and spreading of artifacts. Room lights were on while mice were in the cages, but the door to the room remained closed, no equipment was operating, and no one entered until completion of the 4-h test period. Mice were returned to standard housing afterward, and filter papers were collected for imaging after urine had dried. Filter papers were viewed under incident ultraviolet light (AutoChemi UVP Bioimaging System, Upland, CA) to reveal urine spots, and images of the papers were captured electronically.
Image Analysis
Images were retained as grayscale TIFF files, and images were analyzed with the Fiji version of ImageJ software (http://fiji.sc/Fiji) at Beth Israel Medical Center using a slight modification of the previously described technique (27). Tiny bright spots or “particles” <6.6 mm2 were excluded. This spot size corresponds to 0.5 μl of urine, and this dimension was chosen as the lower limit to eliminate particles arising from claw or tooth marks, footprints, or that resulted from tail dragging. Results of this analysis were collected and saved as number, size, and location of spots on the filter paper. This method of analysis does not eliminate artifacts or variations in data that may arise due to overlapping of urine spots or the occurrence of multiple voids in the same area, and void volumes determined by spot size should be considered semiquantitative. Voided volumes were calculated using a conversion of spot area to urine volume as previously described (31). Data were initially analyzed to identify voiding events in the corners of the cages, but it became apparent that many mice voided along the sides of the cages in addition to other areas. Void locations were therefore analyzed as occurring in the center (40% of the total area) or periphery of the cage (60% of the total area, including corners). VSA parameters were urine spot number, total volume, percentage of void in the primary (largest) area, and percentage volume in the center.
Cystometry
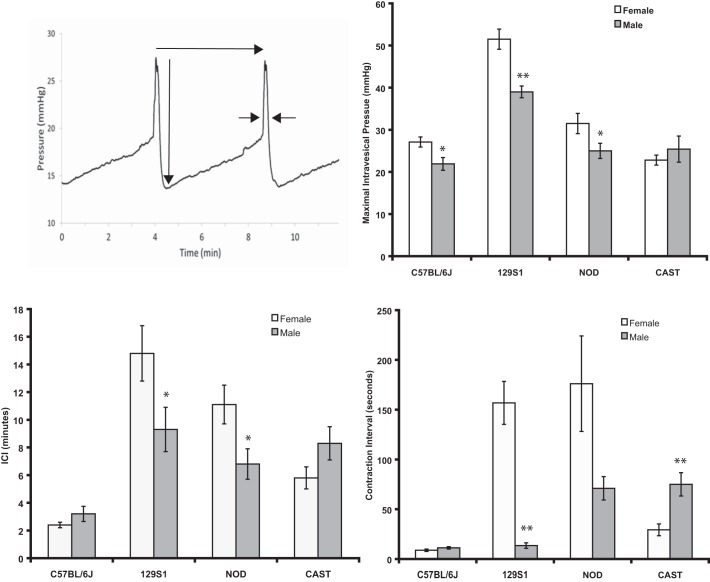
Mice were anesthetized with urethane (1.2 g/kg ip), an incision was made in the caudal aspect of the ventral surface of the abdomen to expose the bladder, a purse-string suture was placed in the dome of the bladder using 6-0 silk, and PE-50 tubing was inserted into the bladder through the center of the purse-string suture. The suture was tightened, tied, and used to secure the tubing in place. The abdominal wound was closed with a 4-0 suture in a continuous pattern. The cystometry tube was connected to a three-way stopcock, and the other two arms of the stopcock were connected to an infusion pump (Harvard Apparatus, Holliston, MA) and pressure transducer (BD Biosciences, San Jose, CA). Intravesical pressure was recorded using WinDaq software (Dataq Instruments, Akron, OH) in a PC. Sterile saline (0.9%) was infused into the bladder at the rate of 0.8 ml/h. Mice were placed in ventral recumbency on a heating pad, and intravesical pressure was recorded for at least 90 min for each mouse. Mice were euthanized by a pentobarbital sodium overdose (75 mg/kg ip) at the conclusion of data collection. Data were analyzed from traces of no less than four consecutive voiding episodes obtained after a consistent voiding pattern had been achieved. The parameters determined from cystometry recordings included the intercontractile interval (ICI; defined as the length of time between attainment of maximal intravesical pressure) and maximal intravesical pressure associated with each void. The contraction interval was defined as the duration in seconds during which intravesical pressure exceeded 50% of pressure in the descending voiding trace (see Fig. 4) (31).
Fig. 4.
Results of cystometry. The technique used to obtain measurements from cystometrogram tracings is illustrated. Long horizontal arrow indicates intercontractile interval (ICI). Long vertical arrow indicates maximal intravesical pressure. Shorter horizontal arrows indicate measurement of contraction interval (27). Shown are data on contraction interval, ICI, and maximal intravesical pressure in male and female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice. Values are means ± SE. *P < 0.05; **P < 0.01.
Data Analysis
Data were calculated for each group as arithmetic means ± SE. Data generated in males and females of the same strain were compared by Student's t-test. One-way ANOVA followed by a post hoc Tukey's HSD (honest significant difference) test was performed to compare data among strains within males or females (GraphPad Prism, San Diego, CA). A P value <0.05 was considered significant.
RESULTS
Mice
Mice were weighed before performance of VSA testing. Male CAST/EiJ mice weighed significantly less than males of other strains, and all females weighed less than males of the same strain (Table 1).
Table 1.
Body weight
| Male | Female | |
|---|---|---|
| 129S1/SvImJ | 27.1 ± 0.8 | 21.8 ± 0.8† |
| C57BL/6J | 27.6 ± 0.5 | 19.2 ± 0.2† |
| NOD/ShiLtJ | 29.1 ± 1.3 | 24.5 ± 0.4† |
| CAST/EiJ | 16.6 ± 0.3* | 13.4 ± 0.6† |
Values are means ± SE expressed in grams. Male CAST/EiJ mice weighed less than males of other strains (
P < 0.05). All females weighed less than males of the same strain (
P < 0.05).
VSA
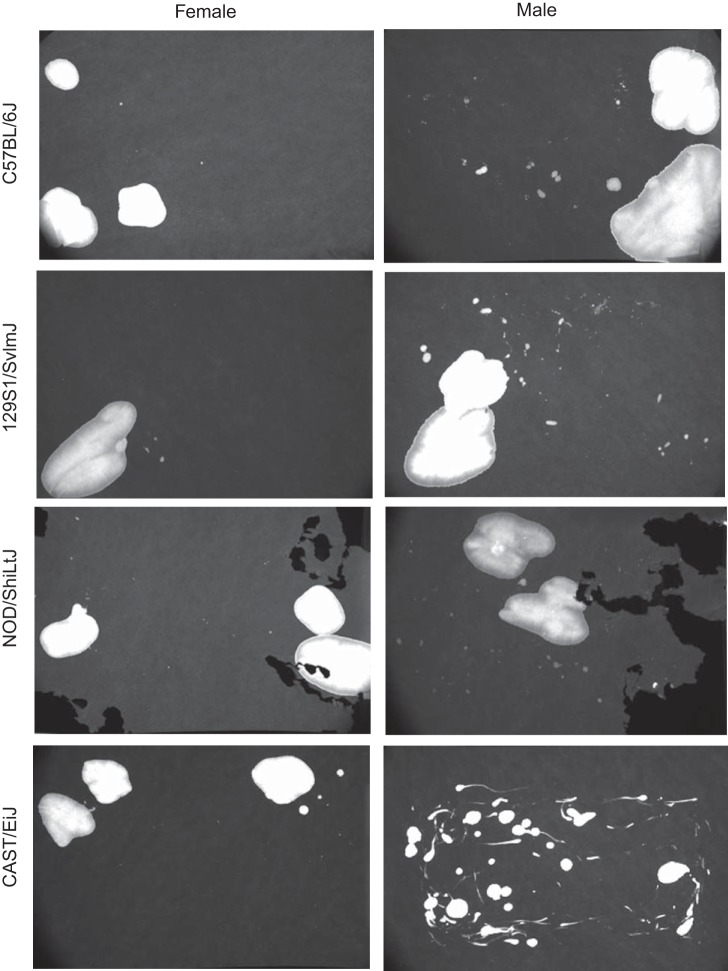
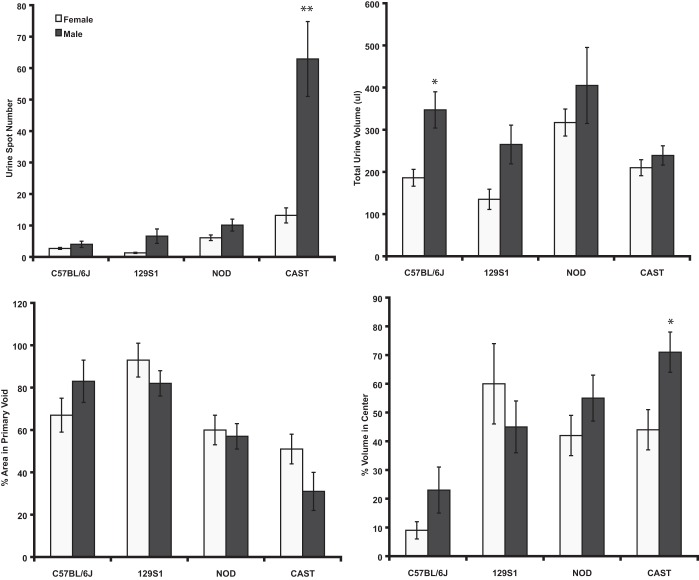
Figure 1 illustrates typical voiding patterns for the four strains of both sexes. It is clear that results of VSA for male CAST/EiJ were significantly different compared with all other males and even to females of the same strain. The “comet-like” shape on many of the spots could be due to anatomy or behavior, indicating genital dragging or possibly a unique way of marking of territory. Void papers from 10 mice of each sex for the four strains were photographed, and four parameters were quantified from each: spot number, total urine volume, % area in the primary (largest) void, and % of urine located in the center 40% of the paper (Fig. 2; data are presented as means ± SE). In general, there were no significant differences among any of the parameters between sexes; however, the strains consistently differed from each other. Only 3 of 16 comparisons of VSA data varied significantly between sexes within a given strain. There was a significant sex difference in void spot number (13 ± 2, females vs. 63 ± 12, males) and % center void (44 ± 7% females vs. 71 ± 9%, males) in CAST/EiJ mice, and the total urine volume deposited by males was greater in C57BL/6J mice (186 ± 20 vs. 347 ± 43 μl). Overall, there was a substantially greater number of differences between strains than between sexes of the same strain (Table 2).
Fig. 1.
Images typical of those obtained from male and female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice. Note that NOD/ShiLtJ mice tend to eat the paper due to the high concentration of glucose in their urine.
Fig. 2.
Void spot assay (VSA) data for male and female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice. Values are means ± SE. *P < 0.05; **P < 0.01.
Table 2.
Comparison of void spot assay results between strains among males and females
| Spot Number | Total Volume, μl | % Primary Void | % Center Void | |
|---|---|---|---|---|
| Females | ||||
| 129S1/SvlmJ | 1.3 ± 0.5a | 134.8 ± 24.4a | 92.4 ± 7.7a | 60.5 ± 14.7a |
| C57BL/6J | 2.7 ± 0.3a | 185.7 ± 19.5a | 67 ± 7.5a,b | 9.1 ± 10.5b |
| NOD/ShiLtJ | 6.1 ± 1a | 316.7 ± 32.4b | 60.4 ± 7.1b | 42.4 ± 6.9a |
| CAST/EiJ | 13.2 ± 7.6b | 209.5 ± 18.8a | 51.2 ± 28.1b | 44.4 ± 7.2a |
| Males | ||||
| 129S1/SvlmJ | 6.6 ± 2.3a | 264.6 ± 46.2a | 81.5 ± 5.7a | 45.3 ± 9.3a,b |
| C57BL/6J | 4 ± 1a | 346.7 ± 42.9a | 82.9 ± 8.4a | 23.2 ± 8.3a |
| NOD/ShiLtJ | 10.1 ± 2a | 404.5 ± 90.3a | 56.8 ± 6.3a,b | 55.5 ± 8.1b |
| CAST/EiJ | 63 ± 12b | 239.1 ± 23.2a | 30.8 ± 9.4b | 71.3 ± 7.3b |
Values are means ± SE. Data were compared for each strain among males or females. Different letters indicate significant differences (P < 0.05) between strains for males or females.
Cystometry
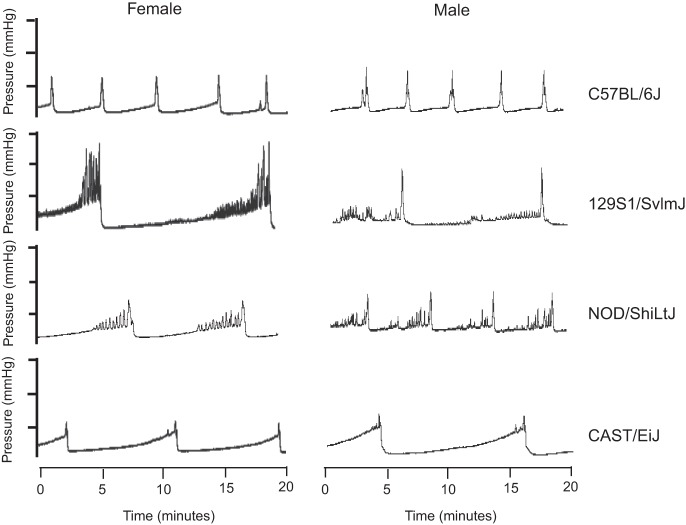
Representative cystometric tracings for males and females of each strain are shown in Fig. 3. The technique used for analysis of cystometric tracings is illustrated in Fig. 4 (data presented as means ± SE). There were some notable differences in cystometric parameters between male and female mice of several strains. The ICI was significantly longer in 129S1/SvlmJ and NOD/ShiLtJ females (887 ± 123 and 666 ± 84 s, respectively) compared with strain-matched males [556 ± 95 (P < 0.05) and 405 ± 68 s (P < 0.05), respectively; Fig. 4]. Maximal intravesical pressure was significantly greater in females than males in 129S1/SvImJ, C57BL/6J, and NOD/ShiLtJ mice [51 ± 2 vs. 39 ± 1 mmHg (P < 0.05), 27 ± 1 vs. 22 ± 2 mmHg (P < 0.05), and 32 ± 2 vs. 25 ± 2 mmHg (P < 0.05), respectively (Fig. 4)].The contraction interval was significantly longer in 129S1/SvImJ females than males (157 ± 22 vs. 14 ± 3 s, respectively) (P < 0.05) but was significantly shorter in CAST/EiJ females than males (29 ± 6 vs. 75 ± 12 s, respectively; Fig. 4). In both of these strains, there were relatively high fluctuations in pressure that resulted in prolonged contraction intervals. Multiple strain-dependent differences emerged when results of cystometry performed in females or males were compared among strains (Table 3). In general, findings in 129S1/SvImJ females or males appeared to be relatively unique compared with females and males of the three other strains. Interestingly, results of cystometry performed in female NOD/ShiLtJ were significantly different from those obtained from female CAST/EiJ mice, but there were no differences between males of these two strains.
Fig. 3.
Representative cystometric tracings of females and males of the 4 strains tested.
Table 3.
Comparison of cystometry results between strains among males and females
| ICI | Max Pressure | Contractile Interval | |
|---|---|---|---|
| Females | |||
| 129S1/SvlmJ | 887 ± 123a | 51.5 ± 2.4a | 156.8 ± 21.6a |
| C57BL/6J | 143 ± 13.5b | 27.1 ± 1.2b,c | 8.8 ± 1.2b |
| NOD/ShiLtJ | 665.8 ± 84a | 31.5 ± 2.3b | 176.1 ± 48a |
| CAST/EiJ | 350 ± 46b | 22.8 ± 1.2c | 29.3 ± 5.9b |
| Males | |||
| 129S1/SvlmJ | 556 ± 95.4a | 39 ± 1.4a | 13.5 ± 2.8a |
| C57BL/6J | 189.1 ± 32.8b | 21.9 ± 1.5b | 11.2 ± 1.2a |
| NOD/ShiLtJ | 405 ± 67.5a,b | 25 ± 1.8b | 71 ± 11.8b |
| CAST/EiJ | 500 ± 74.4a | 25.4 ± 3.1b | 75.0 ± 11.7b |
Values are means ± SE.
ICI, intercontractile interval.
Data were compared for each strain among males or females. Different letters indicate significant differences (P < 0.05) between strains for males or females.
Comparison With Previously Published Values
Results obtained from VSA and cystometry performed in 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ females were compared with previously reported results generated using young females of the same strains (also obtained from The Jackson Laboratory) (31). Techniques used to perform VSA by the prior and current studies were nearly identical. Group size in previously reported data ranged from 3–4 mice/strain; group size in the present study was 10 mice/strain.
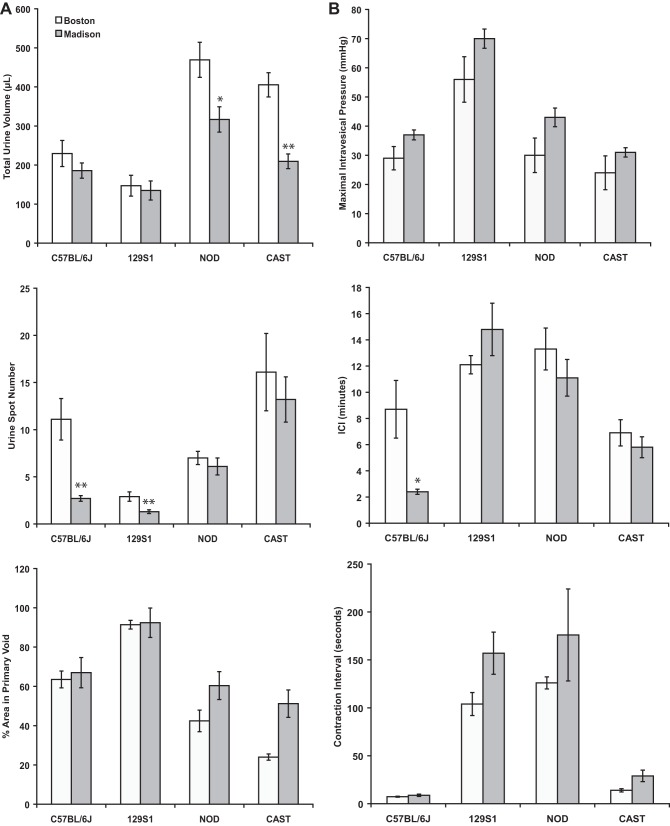
VSA data obtained by the two laboratories were remarkably similar (Fig. 5A; data are presented as means ± SE), and even when mean values were somewhat different (e.g., total volumes shown in Fig. 5A), the rank order for strains was maintained. This provides robust evidence that VSA can be used by different laboratories and different personnel to obtain meaningful and reliable information on murine voiding behavior.
Fig. 5.
A: comparison of VSA data from current study (Madison) and that reported by Yu et al. (31) (Boston) in female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice. B: comparison of cystometry data from current study and that reported by Yu et al. (31) in female 129S1/SvImJ, C57BL/6J, NOD/ShiLtJ, and CAST/EiJ mice. Values are means ± SE. *P < 0.05; **P < 0.01.
Different infusion rates were used to perform cystometry in the current and previous (27) studies (0.8 and 1.5 ml/h, respectively). This was done deliberately in an attempt to distinguish results obtained in the two studies. Despite a difference in infusion rates, the pressure amplitudes (Fig. 5B) and ICI values were remarkably similar and showed once again highly strain-specific urodynamic parameters. A significant difference was only observed in the ICI between the two studies in C57BL/6J females [8.9 ± 2.2 vs. 2.4 ± 0.2 min in the previous (31) and present studies, respectively]. However, the relatively similar ICI values in the presence of substantially different rates of infusion resulted in a significant difference in total infusion volume during ICI for females of all four strains (Table 4).
Table 4.
Volume infused during intercontractile interval
| Strain | Yu et al. (31) | Present Study |
|---|---|---|
| 129S1/SvImJ | 302.5 ± 17.5 | 196.8 ± 28* |
| C57BL/6J | 217.5 ± 55 | 32 ± 2.7* |
| NOD/ShiLtJ | 332.5 ± 40 | 148 ± 18.7* |
| CAST/EiJ | 167.5 ± 25 | 77.3 ± 10.7* |
Values are means ± SE expressed in microliters. Volume infused during ICI was significantly less in the current study for females of all strains tested (
P < 0.05) than in Yu et al. (31).
DISCUSSION
The results of this study confirmed differences in voiding function among the strains of mice studied and between males and females of the same strain. The only differences between males and females of the same strain relative to VSA testing were observed in CAST/EiJ mice. In particular, void spot number was substantially greater in males than females in this strain. Potential explanations for this include fundamental differences in bladder physiology or behavioral factors. ICI determined by cystometry were the same for male and female CAST/EiJ, suggesting that increased void spot number observed in awake males during VSA was the result of behavioral factors. These observations also question whether VSA data can be correlated with cystometry. At least in young healthy mice, there doesn't seem to be an obvious way to draw inferences about urodynamic parameters from void spots.
It should be emphasized that VSA testing is semiquantitative, particularly when used to estimate void volumes. A number of factors (behavioral, environmental, hydration status, metabolic function, cage size, parameters used to assess the size, number, and location of spots, etc.) can affect results. Various methods used to assess lower urinary tract function in mice should be considered complementary, and results of testing should be compared among treatment groups and between methods of assessment to develop a more comprehensive overview of voiding function.
Comparison of results of cystometry between males and females of the same strain revealed multiple differences. Maximal intravesical pressure was greater in females than males in three of four strains, ICI was greater in females than males in two of four strains, and differences in ICI was different between males and females in two of four strains. The relationship of voiding to high pressure fluctuations in intravesical pressure in 129S1/SvlmJ or NOD/ShiLtJ before achieving maximal intravesical pressure was not determined in this or a previous (31) study. Cystometric studies in mice (23, 24) and rats (24–26) have attributed intraluminal pressure oscillations that accompany urine flow to opening and closing of the urethra. These reports indicate that pressure oscillations occur during voiding (23–26), but do not describe the same patterns of pressure fluctuation that we observed in 129S1/SvlmJ or NOD/ShiLtJ mice. Results of these studies strongly indicate that results of experiments in various strains of mice or results from males and females of the same or different strains should not be indiscriminately combined, and that data from appropriate strain and sex controls are essential to interpretation of studies that entail manipulations that may alter the results of cystometry.
A direct comparison of results of voiding studies in mice performed in two different laboratories using the same strains has not been previously described. Results of VSA testing between the two laboratories were remarkably similar. These data suggest that it is possible to generate comparable VSA data in mice in laboratories at different locations provided results are generated using identical strains, sexes, testing methodology, and husbandry conditions. However, as with all studies entailing live animals, technical factors can substantially affect results. We have recently reported that husbandry factors, including time of day of testing, cage changes within 24 h of testing, as well as age and body weight, significantly affected the results of VSA conducted with male C57BL/6J mice (15). However, we also observed that repeated testing of male mice over 3 consecutive days produced nearly identical results, particularly when testing was consistently performed at the same time of day (e.g., morning, afternoon, or night) (15). These observations, in conjunction with the findings of the present study, emphasize the importance of tightly controlling as many parameters as possible to allow reliable comparisons to be made among studies and support prospective implementation of processes to support fidelity of these comparisons. With appropriate control of these variables, we conclude the VSA is a legitimate and reliable semiquantitative assay of mouse micturition physiology.
Results of cystometry performed in the two laboratories were remarkably similar. However, this was somewhat unexpected in light of the different rates of infusion used to perform cystometry in the two studies. Similarities in ICI in female mice of these four strains indicate that a greater volume of fluid accumulated in bladders between voids when the higher infusion rate (1.5 ml/h, Boston vs. 0.8 ml/h, Madison) was used. It is also interesting to note that this was accompanied by nearly identical maximal intravesical pressures in studies performed in the two locations. These findings also suggest that the threshold volume associated with initiation of micturition varied significantly between the two studies. Threshold pressure (intravesical pressure at which voiding was initiated) was not specifically measured in either study. Future comparative studies should include this variable, because it would be assumed that the threshold pressure required to initiate micturition should be consistent among mice. Data were obtained from mice at 12–16 wk of age in the previous study, and the weight of female mice of various strains was similar to that observed in the current study (31). Data were collected from fewer mice per group (3–4) in the previous study (31), compared with 10 mice/group in this study. It is tempting to speculate that the rate of infusion may alter mechanosensation and thus the volume at which micturition is initiated, but subsequent studies performed at the University of Wisconsin using female C57BL/6J mice demonstrated that adjusting the infusion rate incrementally from 0.8 to 1.5 or from 1.5 to 0.8 ml/h caused a concomitant decrease or increase in ICI (data not shown). A recent study in which the infusion rate was modulated in an attempt to stimulate an average of 10 voiding cycles within 10 min in diabetic or control mice that either did or did not receive angiotensin II resulted in infusion rates that varied from 0.45 to 5.0 ml/h (8). It should be noted that bladder capacity varied among animals in that study, but these results also suggest that the mechanical response to bladder distention may vary among individual mice. Conditions of testing in the current study were controlled to duplicate the previous study (with the exception of the infusion rate); the findings in this study suggest that slight technical differences in the performance of cystometry or slight variations in size or age of mice (and therefore size of the bladder) may influence the results. The findings in the current and previous (31) study emphasize the importance of controlling as many variables as possible when performing cystometry, including strain, sex, age, size, and parameters of performance of the study (particularly infusion rate). Our results further indicate that performance of cystometry in mice should include more granular analysis of results, including determination of threshold pressure, compliance, and bladder and void volume.
A variety of techniques for performing VSA, as well as for analyzing results, have been described. The length of time mice have been placed in cages with stationary paper has ranged from 1 (3, 25) to 5 h (11). Continuous recording of voiding for 24 h in mice has also been described (21, 22). This technique entails the use of slowly moving paper placed below a raised floor. In other studies, mesh has been placed over the filter paper (11, 12), or mice have been housed in wire enclosures suspended above the filter paper (27) to prevent the mice from damaging the paper. Other investigators changed the filter paper periodically during VSA testing (12); however, we have observed that disturbing the mice by changing paper or cages causes fluctuations in the observed voiding pattern (15). It has further been suggested that mice be gavaged with water before testing to standardize the state of hydration (27). The impact of technical modifications in techniques needs further evaluation and must be considered results among studies are compared.
Similarly, data obtained by VSA have been analyzed by a variety of methods. Relatively simple methods have entailed counting and reporting the relative number of urine spot that are less or greater than 0.2 mm in diameter (6), or identifying the number of urine spots with an area <0.2 cm2 as an indicator of frequent voiding of small volumes of urine (3, 25). Other investigators have scored voiding patterns observed on filter paper using a five-point scale based on the size and number of urine spots observed (11, 12). Although the size (diameter) of urine spots has been reported to have a high correlation with the volume of urine voided that created the spot (27, 31), this method of estimation of urine volume should be considered semiquantitative, since the volume is not measured by weight or collection in a volume-calibrated receptacle, and the size of the void spot may be altered by movement of the animal or repeated voiding in a given spot. Regardless, within the context of standardized performance of VSA, this technique appears to provide a relatively accurate estimate of void volume that provides useful data that can be compared among animals.
The technique used for analysis of results of VSA in the current study has previously been reported in detail (31), and we chose to use this method to allow direct comparison of results obtained in the current study with those previously reported (31). As previously mentioned, technical aspects of performance of VSA and differences among methods of analysis of results have the potential to significantly affect the validity of comparison of results of various studies.
The technique used to perform cystometry in anesthetized mice in the current and previous study (31) is very similar to that described in other reports (15, 29). Technical aspects that vary in performance of cystometry in mice (in addition to generating these data in anesthetized or awake mice) include the size of the catheter used, length of fluid infusion before collection of data, and interval between catheter placement and performance of cystometry. Rates of fluid infusion during performance of cystometry in mice have ranged from 0.6 to 6 ml/h (6, 14, 28). Infusion rates were adjusted to obtain 10 voiding cycles within 10 min in mice, and it was found that infusion rates required to achieve this objective varied from 0.45 to 5.0 ml/h (8). When cystometry is performed in awake mice, movement or increases in intra-abdominal pressure by muscle contraction can significantly affect intravesical pressure, and it has been recommended that intra-abdominal pressure be measured by placement of a balloon-tipped catheter within the abdominal cavity or placement of a similar catheter within the colon (13, 17). Intra-abdominal pressure is then subtracted from intravesical pressure to eliminate the influence of movement-generated fluctuations. Performance of cystometry in anesthetized mice has the advantage of reducing variability of baseline and intervoid pressure due to movement or abdominal contraction. However, the effects of anesthesia on results of cystometry in mice remain incompletely understood.
Similar to analysis of VSA data, a variety of methods for analysis of data obtained by performance of cystometry in mice has been described. The various parameters reportedly obtained from cystometry in mice have been reviewed (2) and include intravesical pressure (basal, intermicturition, threshold, and maximal), nonvoiding contraction, void frequency, void volume, bladder capacity, residual volume, and bladder compliance. However, there currently is little consensus on how various parameters, such as threshold pressure (the intravesical pressure thought to initiate a voiding contraction), are determined. For example, a nonvoiding contraction has been variously defined as an increase from 1 (10) to 6.8 (6) cmH2O not associated with voiding of urine, but there is no general agreement as to how to define nonvoiding bladder contraction (8). Again, the method of analysis of results of cystometry used in the current study was chosen to allow direct comparison with results of a previous study (31).
Conclusions
Lower urinary function testing of mice using VSA can be performed by different laboratories and produces comparable results, provided that study factors are tightly controlled. While it is still possible that conditions under which cystometry is performed can be standardized to improve reproducibility and allow valid, reliable comparisons of results among studies, this clearly will require further refinement. Differences in results of lower urinary function testing vary among strains of mice and between males and females of the same strain. The influence of variables in technical aspects of performance of testing and analysis of results require further study to allow valid comparisons among studies and improved communication regarding results of functional testing.
GRANTS
This work was supported by National Institutes of Health Grants 3P20DK097818-02S3 and 1U54DK104310.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.E.B., Z.W., C.M.V., W.A.R., K.P.K., M.Z., and W.G.H. provided conception and design of research; D.E.B., Z.W., K.P.K., W.Y., L.G., and W.G.H. performed experiments; D.E.B., Z.W., W.A.R., K.P.K., W.Y., L.G., and W.G.H. analyzed data; D.E.B., Z.W., C.M.V., W.Y., and W.G.H. interpreted results of experiments; D.E.B. and Z.W. prepared figures; D.E.B., Z.W., L.G., M.Z., and W.G.H. drafted manuscript; D.E.B., Z.W., C.M.V., W.A.R., K.P.K., W.Y., M.Z., and W.G.H. edited and revised manuscript; D.E.B., Z.W., C.M.V., W.A.R., K.P.K., W.Y., L.G., M.Z., and W.G.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Peiqing Wang in completing this work.
REFERENCES
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, van Kerrebroeck P, Victor A, Wein A. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–178, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Soler R, Füllhase C. Rodent models for urodynamic investigation. Neurourol Urodyn 30: 636–646, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol Urodyn 30: 673–682, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown ET, Krlin RM, Winters JC. Urodynamics: examining the current role of UDS testing. What is the role of urodynamic testing in light of recent AUA urodynamics and overactive bladder guidelines and the VALUE study? Curr Urol Rep 14: 403–408, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Cornelissen LL, Misajet B, Brooks DP, Hicks A. Influence of genetic background and gender on bladder function in the mouse. Auton Neurosci 140: 53–58, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: results from the EPIC study. BJU Int 101: 1388–1395, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Dolber PC, Jin H, Nassar R, Coffman TM, Gurley SB, Fraser MO. The effects of Ins2(Akita) diabetes and chronic angiotensin II infusion on cystometric properties in mice. Neurourol Urodyn 34: 72–78, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fry CH, Daneshgari F, Thor K, Drake M, Eccles R, Kanai AJ, Birder LA. Animal models and their use in understanding lower urinary tract dysfunction. Neurourol Urodyn 29: 603–608, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, Vezina CA, Sarma AV, Macoska JA. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate 73: 1123–1133, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodges SJ, Zhou G, Deng FM, Aboushwareb T, Turner C, Andersson KE, Santago P, Case D, Sun TT, Christ GJ. Voiding pattern analysis as a surrogate for cystometric evaluation in uroplakin II knockout mice. J Urol 179: 2046–2051, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Jin LH, Andersson KE, Kwon YH, Park CS, Yoon SM, Lee T. Substantial detrusor overactivity in conscious spontaneously hypertensive rats with hyperactive behaviour. Scand J Urol Nephrol 43: 3–7, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Jusuf AA, Kojima S, Matsuo M, Tokuhisa T, Hatano M. Vesicourethral sphincter dysfunction in ncx deficient mice with an increased neuronal cell number in vesical ganglia. J Urol 165: 993–998, 2001. [PubMed] [Google Scholar]
- 15.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn [Epub before print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus SR, Dmochowski R, Albo ME, Xu L, Klise SR, Roehrborn CG. Urodynamic standardization in a large-scale, multicenter clinical trial examining the effects of daily tadalafil in men with lower urinary tract symptoms with or without benign prostatic obstruction. Neurourol Urodyn 29: 741–747, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats–effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn 27: 88–95, 2008. [DOI] [PubMed] [Google Scholar]
- 18.McVary KT, Roehrbohn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, Wei JT. Update on AUA guideline of the management of benign prostatic hyperplasia. J Urol 185: 1793–1803, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, N'dow J, Nordling J, de la Rosette JJ. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 64: 118–140, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Raz O, Tse V, Chan L. Urodynamic testing: physiological background, setting-up, calibration and artefacts. BJU Int 114: 22–28, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Stewart FA, Michael BD, Denekamp J. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat Res 75: 649–659, 1978. [PubMed] [Google Scholar]
- 22.Stewart FA, Lundbeck F, Oussoren Y, Luts A. Acute and late radiation damage in mouse bladder: a comparison of urination frequency and cystometry. Int J Radiat Oncol Biol Phys 21: 1211–1219, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Streng T, Li X, Lehtoranta M, Mäkelä S, Poutanen M, Talo A, Tekmal RR, Santti R. Infravesical obstruction in aromatase over expressing transgenic male mice with increased ratio of serum estrogen-to-androgen concentration. J Urol 168: 298–302, 2002. [PubMed] [Google Scholar]
- 24.Streng T, Lehtoranta M, Poutanen M, Talo A, Lammintausta R, Santti R. Developmental, estrogen induced infravesical obstruction is reversible in adult male rodents. J Urol 168: 2263–2268, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Streng T, Santti R, Talo A. Similarities and differences in female and male rat voiding. Neurourol Urodyn 21: 136–141, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Streng T, Santti R, Andersson KE, Talo A. The role of the rhabdosphincter in female rat voiding. BJU Int 94: 138–142, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Sugino Y, Kanematsu A, Hayashi Y, Haga H, Yoshimura N, Yoshimura K, Ogawa O. Voided stain on paper method for analysis of mouse urination. Neurourol Urodyn 27:5 48–552, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland RS, Kogan BA, Piechota HJ, Bredt DS. Vesicourethral function in mice with genetic disruption of neuronal nitric oxide synthase. J Urol 157: 1109–1116, 1997. [PubMed] [Google Scholar]
- 29.Wang ZY, Wang P, Merriam FV, Bjorling DE. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 139: 158–167, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Winters JC, Dmochowski RR, Goldman HB, Herndon CD, Kobashi KC, Kraus SR, Lemack GE, Nitti VW, Rovner ES, Wein AJ. Urodynamic studies in adults: AUA/SUFU guideline. J Urol 188: 2464–2472, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296–F1307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]