Abstract
Extracellular nucleotides acting through P2 receptors facilitate natriuresis. To define how purinergic mechanisms are involved in sodium homeostasis, we used transgenic (TG) mice that globally overexpress human CD39 (hCD39, NTPDase1), an ectonucleotidase that hydrolyzes extracellular ATP/ADP to AMP, resulting in an altered extracellular purine profile. On a high-sodium diet (HSD, 3.5% Na+), urine volume and serum sodium were significantly higher in TG mice but sodium excretion was unaltered. Furthermore, TG mice showed an attenuated fall in urine aldosterone with HSD. Western blot analysis revealed significantly lower densities (∼40%) of the β-subunit of the epithelial sodium channel (ENaC) in medulla, and the major band (85-kDa) of γ-ENaC in TG mice cortex. To evaluate aldosterone-independent differences, in a second experiment, aldosterone was clamped by osmotic minipump at 20 μg/day, and mice were fed either an HSD or a low-sodium diet (LSD, 0.03% Na+). Here, no differences in urine volume or osmolality, or serum aldosterone were found, but TG mice showed a modest, yet significant impairment in late natriuresis (days 3 and 4). Several major sodium transporters or channel subunits were differentially expressed between the genotypes. HSD caused a downregulation of Na-Cl cotransporter (NCC) in both genotypes; and had higher cortical levels of NCC, Na-K-ATPase (α-1 subunit), and α- and γ-ENaC. The Na-K-2Cl cotransporter (NKCC2) was downregulated by HSD in wild-type mice, but it increased in TG mice. In summary, our data support the concept that extracellular nucleotides facilitate natriuresis; they also reveal an aldosterone-independent downregulation of major renal sodium transporters and channel subunits by purinergic signaling.
Keywords: purinergic receptors, extracellular nucleotides, ectonucleotidases, nucleoside triphosphate diphosphohydrolase-1, aldosterone, sodium transporters
extracellular nucleotides (ATP/ADP/UTP), acting through type-2 purinergic receptors (P2), potentially regulate renal tubular transport of water and sodium, and thereby urinary concentrating ability (5, 7, 11, 18, 19, 23, 25, 26, 28, 29, 33, 39). P2 receptor signal modulation is controlled by a narrow range of extracellular concentrations of ATP and related nucleotides, which are mediated through regulated release from cells and rapidly hydrolyzed by ectonucleotidases (10). Several types of ectonucleotidases exist, such as nucleoside triphosphate diphosphohydrolases (NTPDases), ectonucleotide pyrophosphatases (E-NPPs), alkaline phosphatases, and ecto-5′-nucelotidase (CD73) (24, 34).
NTPDases are a family of membrane-bound enzymes that sequentially hydrolyze extracellular nucleotides and thus limit P2 receptor activities and desensitization. NTPDase1 is the same as CD39, a putative B-cell activation marker. NTPDase1 hydrolyzes ATP and ADP to AMP, whereas NTPDase2 (CD39-like protein-1; CD39L1) preferentially converts ATP to ADP. Because NTPDase1 is located on the cell surface, with its catalytic domain oriented to the exterior of the cells, it can effectively hydrolyze extracellular ATP or other nucleotides when they are released into the extracellular space. In many tissues, the activities of NTPDases are coupled with CD73 activity. CD73 converts AMP to adenosine (1, 12). Adenosine is a potent agonist of P1 receptors, activation of which often opposes the cellular responses elicited by the stimulation of P2 receptors (9).
We previously localized the mRNA and protein expression of NTPDase1 and NTPDase2 in murine (rat and mouse) kidneys using in situ hybridization and immunohistochemistry, respectively (6). We documented that NTPDase1 is expressed in several structures in the kidney. These structures include vascular smooth muscle cells and endothelium in the interlobular arteries, afferent glomerular arterioles, and peritubular capillaries in the cortex. Within the medulla, NTPDase1 is expressed in ascending thin limbs of Henle's loop, ducts of Bellini, and in the pelvic wall (6). More extensive immune localization of different ectonucleotidases along the rat nephron was presented by Vekaria and associates (30).
The availability of a transgenic (TG) mouse model that globally overexpresses human CD39 (hCD39) allowed us and others to define or probe the effect of heightened scavenging of extracellular ATP on P2 receptor signaling and thus organ function (2). Recently, we reported that these TG mice exhibit defective water handling by the kidney (37). Specifically, under basal conditions, TG mice have impaired urinary concentrating ability. TG mice also have impaired arginine vasopressin (AVP) release in response to water deprivation, and concentrated urine to a significantly lesser degree compared with wild-type (WT) mice. However, TG mice kidneys are responsive to exogenous AVP or desmopressin (dDVP). On the basis of these findings we inferred that heightened nucleotide scavenging by higher levels of CD39 altered the release of endogenous AVP in response to dehydration.
P2 receptors regulate both water and sodium transport in the kidney (19, 28). We previously showed that P2Y2 receptor-knockout mice have increased expression of the bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) and impaired ability to excrete sodium when aldosterone levels were clamped (model of aldosterone escape) (36). Hence, we hypothesized that overexpression of hCD39 in mice (which would putatively lead to low tissue levels of extracellular ATP, the P2 receptor agonist) might also alter the renal responses to dietary salt intake and/or aldosterone administration. Thus the primary goal of this study was to determine whether there were phenotypic differences in natriuretic responses in TG and wild-type (WT) mice under conditions that might be expected to increase sodium retention. The secondary goal was to determine whether changes in whole cell levels of major sodium reabsorptive proteins might provide insights into the differences that were observed. To address these, we evaluated the effect of a high-salt diet alone or in combination with aldosterone infusion in TG mice vs. WT mice. We assessed the effect on natriuretic parameters and the protein expression of major renal tubular sodium transporters and channel subunits in relation to aldosterone levels and dietary sodium intake. In parallel, we immunolocalized mouse and human CD39 proteins in the kidneys of WT and TG mice.
MATERIALS AND METHODS
Experimental animals.
Generation and characterization of transgenic hCD39 mice were reported previously (2). Briefly, these mice were generated using a construct containing the murine H-2Kb (major histocompatibility class; MHC) promoter to drive expression of hCD39 in C57Bl/6 mice. Two independent lines were established from the mice that strongly expressed hCD39. Dot blot analysis revealed the two lines of TG mice have 11 and 14 copies of hCD39. Immunohistochemical examination revealed widespread expression of hCD39 in the kidney and other organs. CD39 (NTPDase1) activity was also significantly higher in different organs of the TG mice (2). We established breeding colonies of hCD39-TG and WT mice (C57Bl/6) in the Veterinary Medical Unit of the U.S. Department of Veterans Affairs (VA) Salt Lake City Health Care System. The genotypes of all mice produced were confirmed by polymerase chain reaction on tail DNA using primer pairs specific for hCD39. Breeding and animal procedures described in this study were approved by the Institutional Animal Care and Use Committee of the VA Salt Lake City Health Care System.
Study protocol.
Mice aged 2–4 mo were used in this study. The altered sodium diets obtained from a commercial source (TestDiets, Richmond, IN) were in the form of dry chow pellets. During the experimental period, all mice had ad libitum access to drinking water. Two series of experiments were conducted. In the first series, we examined the effect of a high-sodium diet (HSD; 3.51% Na+, 8% NaCl) in WT and TG mice without clamping aldosterone levels. Groups of WT and TG mice were given an HSD for 13 days. In the second series of experiment, we evaluated the effect of an HSD in a background of aldosterone infusion in WT and TG mice. Briefly, under isoflurane anesthesia, groups of WT and TG mice (n = 12 mice/genotype) were subcutaneously implanted with osmotic mini pumps (Alzet model 1002; Durect, Cupertino, CA) preloaded with aldosterone (Sigma, St. Louis, MO). Aldosterone was delivered throughout the experimental period at the rate of 20 μg/day. Immediately following implantation of the minipumps, the mice were fed a low-sodium diet (LSD; 0.03% Na+, 0.06% NaCl) for 3 days. Half the mice in each genotype were then switched to the HSD (3.51% Na+) for 4 days. The remaining half stayed on the LSD for the 4 days. Daily intake of food and water, and urine output, osmolality, and sodium excretion were monitored in all the mice from the beginning to the end of the experimental period in metabolic cages. All mice were euthanized on day 8 under isoflurane anesthesia.
Sample collection and analysis.
Terminal blood samples and kidneys were collected at the time of euthanasia. Cortical and whole medullary tissues from the kidneys were dissected out, flash-frozen in liquid nitrogen, and then stored at −80°C for analysis. Aliquots of urine samples were centrifuged to obtain clear supernatants. Osmolality of the clear supernatants and serum samples were determined by the vapor pressure method (Wescor, Logan, UT). Serum and urinary sodium levels were measured on an EasyLyte (Medica, Bedford, MA) analyzer. Serum and urinary aldosterone concentrations were determined by an ELISA kit (Enzo Life Sciences, Farmingdale, NY).
Western blot analysis of tissue samples.
Cortical and medullary tissue samples were processed separately as previously described (35, 36) and shipped to Georgetown University for semiquantitative immunoblot analysis. Briefly, samples were prepared by homogenizing the frozen tissues in a buffer containing protease inhibitors. After determining the protein concentrations, the homogenates were solubilized in Laemmli sample buffer. The quality of tissue sample preparation was assessed by staining loading gels with Coomassie blue (Gelcode Blue; Pierce Endogen, Rockford, IL), and then examining the sharpness of the bands. For immunoblotting, 10–30 μg of protein from each sample was loaded into individual lanes of minigels of 7, 10, or 12% polyacrylamide (precast; BioRad, Hercules, CA). After electrotransfer of size-fractionated proteins to nitrocellulose membranes, blots were probed with our own rabbit peptide-derived polyclonal antibodies against the sodium-hydrogen exchanger type 3 (NHE3); the bumetanide-sensitive NKCC2; the thiazide-sensitive Na-Cl cotransporter (NCC); the sodium phosphate cotransporter-type 2 (NaPi-2); and the alpha, beta, and gamma subunits of the epithelial sodium channel (α-ENaC, β-ENaC, and γ-ENaC, respectively), as previously described (35, 36). We used commercially available antibodies against the α-1 subunit of Na-K-ATPase (05–369; Millipore, Temecula, CA). Loading accuracy was evaluated by probing the blots with β-actin monoclonal antibody (A1978; Sigma-Aldrich) or GAPDH antibody (SAB 1605848; Sigma-Aldrich). Band densities of transporter/channel proteins were determined and normalized to the densities of the respective β-actin or GAPDH bands.
Immunoperoxidase labeling for mouse and human CD39 proteins.
Immunoperoxidase labeling was used to localize mouse and human CD39 proteins in the kidneys of WT and TG mice. Affinity-purified polyclonal mouse CD39/ENTPD1 antibody raised in sheep was purchased (AF4398; R&D Systems, Minneapolis, MN) and used in conjunction with anti-goat immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA). Affinity-purified polyclonal human anti-ENTPD1 antibody raised in rabbits was obtained from Sigma (HPA014067) and was used in conjunction with anti-rabbit IgG (Vector Laboratories). Both primary antibodies are known to work on paraffin-embedded tissue sections. Formalin-fixed and paraffin-embedded kidneys from untreated mice were processed as described previously (8) except for the following modification with respect to antigen retrieval. Deparaffinized and rehydrated sections were microwaved in citrate-based antigen retrieval solution (Antigen Unmasking Solution, H-3300; Vector Laboratories) just until boiling (2–3 min), and then cooled in deionized water. Sections were then rinsed with PBS, blocked with BSA (mouse cd39) or BSA plus goat serum (hCD39), and processed according to the previously published method (8). For proper visualization of labeling for human CD39 in certain structures of the kidney, kidneys were also processed by snap-freezing. Briefly, kidney tissues embedded in OTC Compound (Sakura Finetek, Torrance, CA) were flash-frozen in liquid nitrogen. Tissue sections (5 μm) were cut, air-dried, and fixed in a mixture of ice-cold acetone and 7% formalin for 3 min. This was followed by avidin-biotin blocking (Vector Laboratories). The sections were blocked with 7% horse serum (Vector Laboratories) for 30 min and incubated overnight at 4°C with the human CD39 antibody. Endogenous peroxidase activity was blocked by hydrogen peroxidase, and sections were incubated with biotinylated secondary antibody. After washing, sections were treated with Avidin-Biotin Complex-HRP (Vector Laboratories) for 30 min, and signals were visualized with ImmPACK DAB (Vector Laboratories). Slides were counterstained with Gill II hematoxylin and mounted with Cytoseal 60 (Richard-Allan Scientific/Thermo-Fisher). We evaluated hCD39 antibodies from two other sources and found the one from the Sigma-Aldrich appeared to give optimal labeling under our experimental conditions. We further validated the hCD39 antibody from Sigma-Aldrich using human skin sections. Normal human skin sections were kindly provided by Dr. A. Malhotra, and were obtained by informed consent for an institutional review board-approved study (3).
Statistical analysis.
Quantitative data are expressed as means ± SE. Differences due to the main factors (genotype and dietary sodium) and their interactions were determined by two-way ANOVA using Sigma Stat Software (Chicago, IL) or GraphPad Instat Software (La Jolla, CA). Differences between individual pairs of means were determined by a multiple comparisons test (Holm-Sidak) following a significant (P < 0.05) one-way ANOVA. Kruskal-Wallis one-way ANOVA on ranks followed by Tukey's multiple comparisons test was used when data were not normally distributed or variances were not equal. Where applicable, unpaired t-tests were used to directly compare the means of two groups.
RESULTS
Localization of mouse or human CD39 in WT and TG mice.
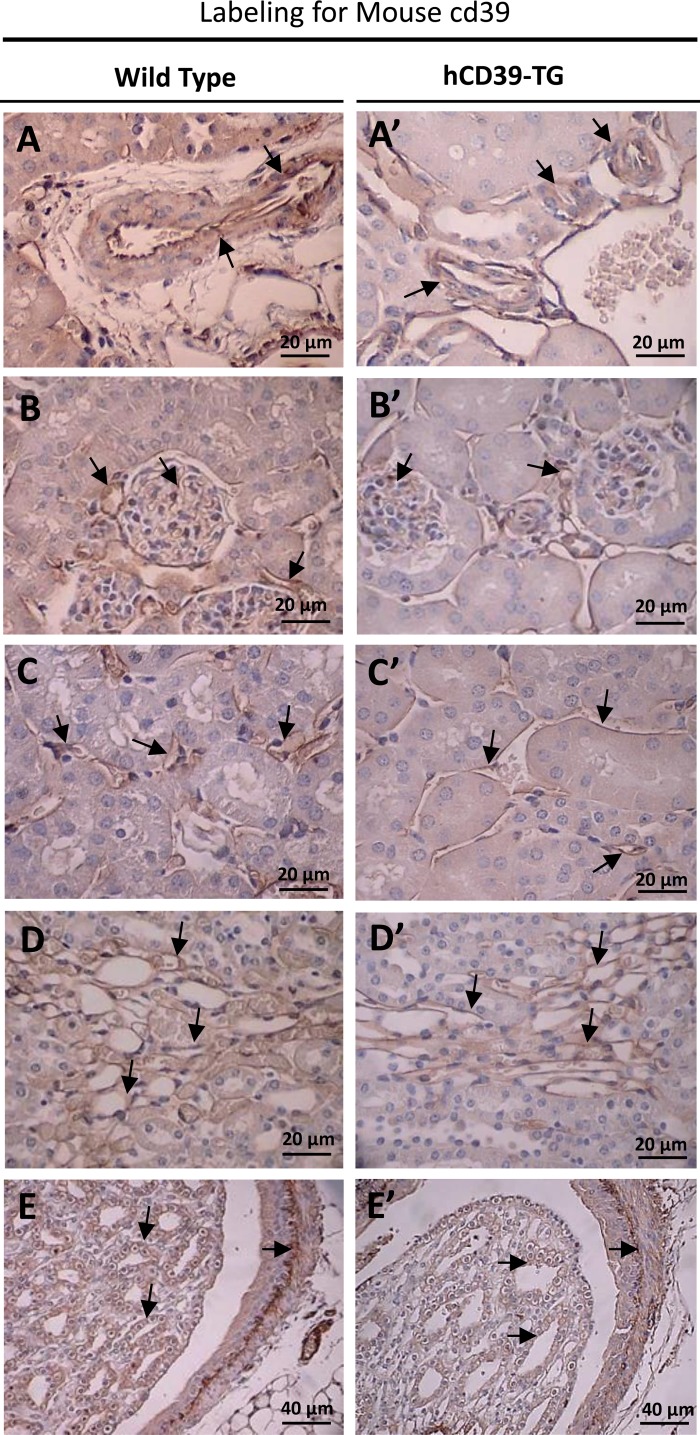
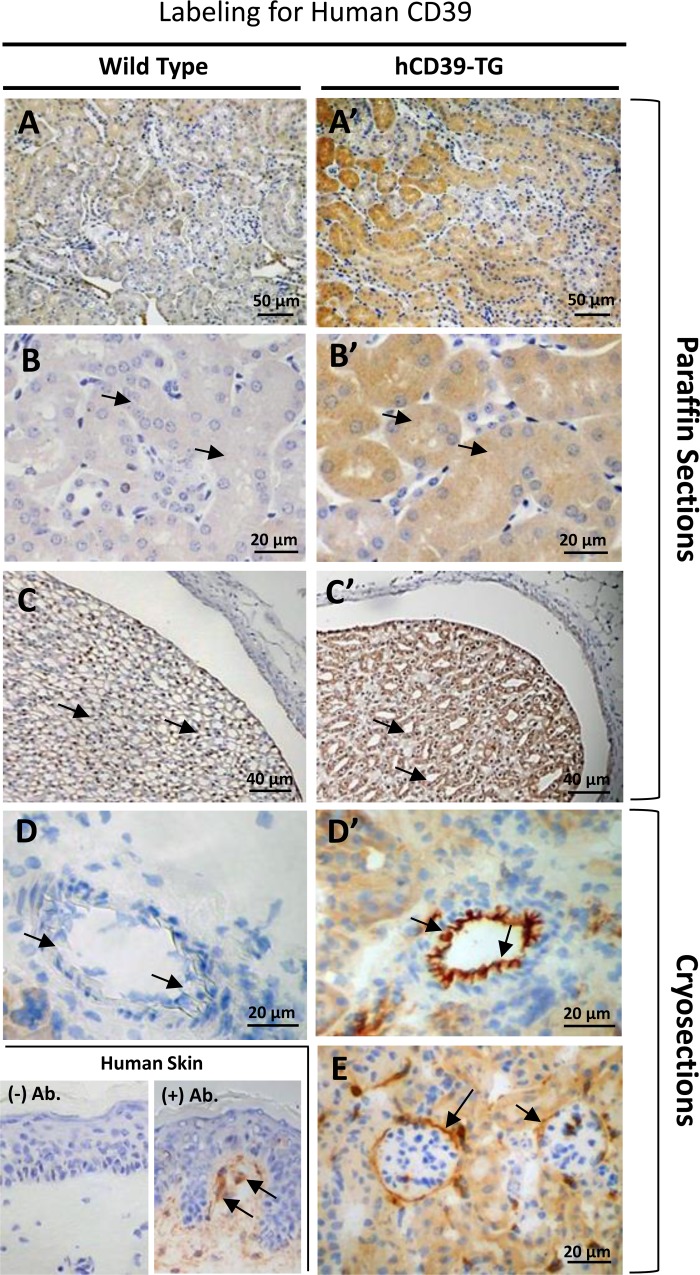
Figure 1 shows immunoperoxidase labeling for mouse cd39 protein in the kidneys of WT and TG mice. As we previously documented (6), expression of mouse protein cd39 was observed in selected structures in the kidney. These structures are blood vessels (Fig. 1, A and A′), glomeruli and glomerular blood vessels (Fig. 1, B and B′), peritubular blood vessels and capillaries (Fig. 1, C and C′), vascular bundles in the corticomedullary region (Fig. 1, D and D′), and papillary tip and the pelvic wall (Fig. 1, E and E′). There were no differences in the pattern of expression of mouse cd39 between the kidneys of WT and TG mice. In contrast, labeling for human CD39 protein appeared predominantly in TG mouse kidneys, although there was some low level background in the WT mouse kidneys probably due to cross-reactivity of the human CD39 antibody (Fig. 2). In paraffin-embedded sections, labeling for hCD39 was observed throughout TG mouse kidneys (Fig. 2, A′ and B′), including the IMCD cells of the papillary tip (Fig. 2C′), whereas the corresponding antibody labeling in WT mouse kidney was very weak (Fig. 2, A, B, and C). Labeling in blood vessels and glomeruli was not easily observed in TG kidneys processed by formalin fixation and paraffin embedding, apparently due to a lack of proper antigen retrieval from these structures. However, blood vessels and glomeruli were clearly labeled with the hCD39 antibody when cryosections were used (Fig. 2, D′ and E). Finally, shown in Fig. 2, bottom, are hCD39 antibody-labeled dendritic cells (Langerhans cells) in the human skin, which are known to express CD39 (16).
Fig. 1.
Immunoperoxidase labeling for mouse cd39 protein in the kidneys of wild-type (WT, left) and transgenic (hCD39-TG, right) mice. Labeling for mouse cd39 was performed on paraffin-embedded kidney sections. Magnification is indicated in each panel. A and A′: blood vessels; B and B′: glomeruli and glomerular blood vessels; C and C′: perivascular blood vessels and capillaries; D and D′: vascular bundles at the corticomedullary region; E and E′: IMCD cells in papilla and on pelvic wall.
Fig. 2.
Immunoperoxidase labeling for human CD39 protein in the kidneys of WT (left) and TG (hCD39-TG, right) mice. Labeling for hCD39 was performed on both paraffin-embedded and flash-frozen kidney sections. Magnification is indicated in each panel. A and A′: panoramic view of the cortical region of the kidney; B and B′: higher magnification of tubular structures in the cortex; C and C′: IMCD cells in the papilla and pelvic wall; D and D′: blood vessel; E: glomeruli. Lower left: hCD39 protein labeling in dendritic cells in human skin sections; (−) Ab., without primary antibody; (+) Ab., with primary antibody.
Effect of HSD.
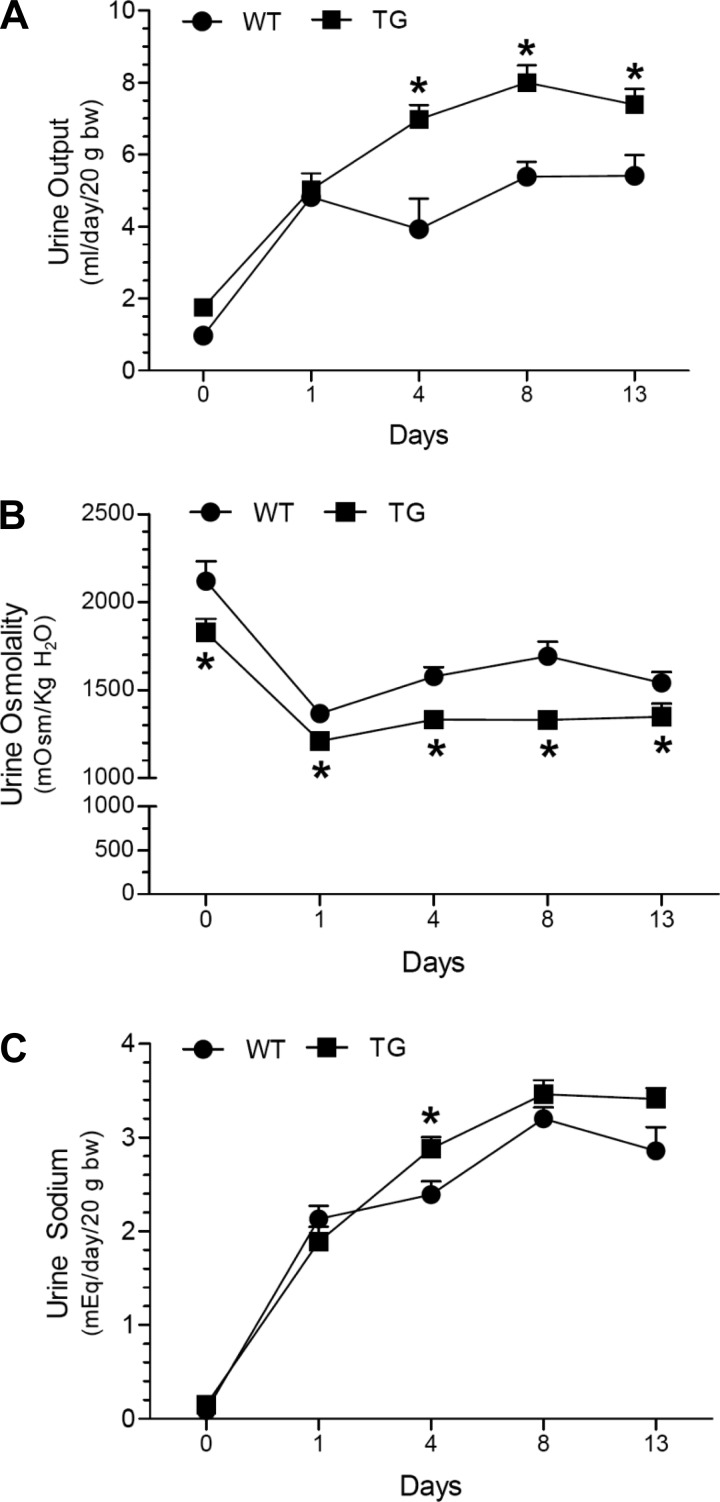
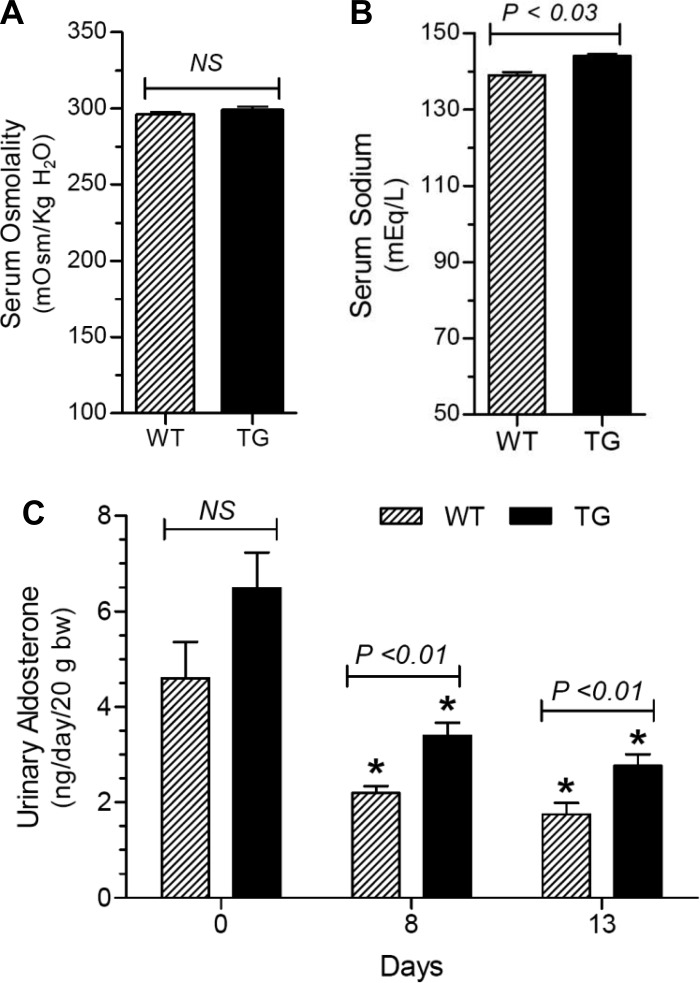
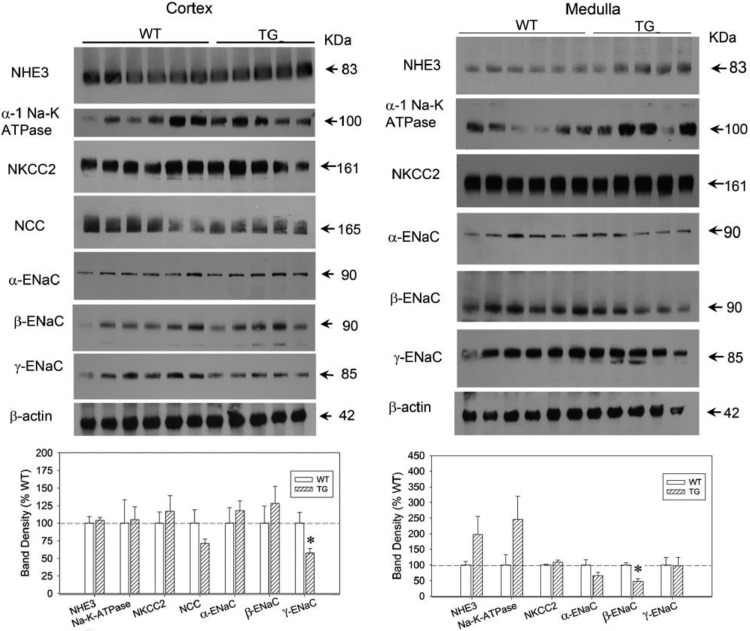
In the first series of experiments, we examined the effect of HSD on WT and TG mice for 13 days with free access to drinking water. As shown in Fig. 3, HSD caused increased urine output and decreased urine osmolality in both genotypes, starting from day 1 and reaching a plateau by day 8. At several time points, TG mice had significantly higher urine volumes and lower urine osmolalities vs. the WT controls. In contrast, urinary sodium output over the time was similar between the genotypes, except on day 4, when it was modestly higher in the TG mice. As shown in Fig. 4A, the terminal serum osmolalities were not significantly different between the genotypes. However, terminal serum sodium concentrations were significantly higher in the TG mice (Fig. 4B). Urinary aldosterone excretion was reduced by HSD in both genotypes, but was significantly different (lower in WT) after 8 and 13 days of HSD (Fig. 4C). Western blot analysis of whole tissue homogenates of cortex and medulla did not reveal a large number of significant differences between WT and TG mice with respect to sodium transporter/channel protein abundance; the only exceptions being β-ENaC and γ-ENaC in cortex and medulla, respectively, which were significantly lower in TG mice than in WT mice (Fig. 5).
Fig. 3.
Effect of a high-sodium diet (HSD) on urinary parameters in WT and TG mice. Groups of mice (n = 6 mice/genotype) were given an HSD (3.51% Na) for 13 days. To monitor 24-h urinary parameters mice were placed in metabolic cages (one mouse/cage). Means ± SE values obtained on days 0, 1, 4, 8, and 13 of the experimental period were presented for urine output (A), urine osmolality (B), and urine sodium (C). To compensate for differences in body weights of individual mice, the values for urine output and urine sodium were adjusted to 20 g body wt. *P <0.05 compared with the corresponding time point in the WT mice by unpaired t-test.
Fig. 4.
Effect of HSD on terminal serum osmolality (A) and sodium (B), and urine aldosterone levels (C) in WT and TG mice. Data are derived from the same groups of mice shown in Fig 3. Results are means ± SE (n = 6 mice/genotype). Statistical significance between the two groups was determined by unpaired t-test and us shown above the bars (NS, not significant). *P <0.05 compared with the corresponding day 0 value by unpaired t-test.
Fig. 5.
Effect of HSD on sodium transporter/channel/exchanger protein abundance in WT and TG mice. Data are derived from the same mice and experiment shown in Fig 3. Top: immunoblots for abundance of protein transporters/exchangers in the renal cortex (left) and medulla (right). Bottom: bar graph summary of the densitometric analysis of the immunoblots shown in top. Bars show percent change in band densities of different proteins in TG mice compared with the corresponding values in WT mice (100%). Results are means ± SE (n = 5/6 mice/genotype).
Effect of LSD or HSD during infusion of aldosterone.
To test which of these effects were independent of changes in aldosterone, we conducted a second series of experiments in which WT and TG mice were infused with aldosterone while eating the LSD and then switching to the HSD or remaining on the LSD (model of aldosterone escape). Irrespective of the genotype, there was a modest increase in urine output (volume) when all mice were fed the LSD (days −2 through 0, Fig. 6A); i.e., urine output increased 3.5- to 4.5-fold from baseline. This was associated with an initial steep fall in urine osmolality followed by a slower decrease (Fig. 6B). By the end of the entire experiment (through day +4) urine osmolalities in mice on the LSD reached about 50% of their baseline values, irrespective of the genotype. In contrast, when switched to the HSD on day 0, mice showed a rapid and sustained increase in urine output in both genotypes, which reached threefold to eightfold higher than the values observed in mice that remained on the LSD. This increase in urine output was matched with a corresponding 64 to 72% decrease in urine osmolality (Fig. 6B).
Fig. 6.
Effect of low- or high-sodium feeding in a background of aldosterone infusion on urinary parameters in WT and TG mice. Groups of mice (n = 12 mice/genotype) were infused with aldosterone (20 μg/day sc) starting on day −3 and then fed a low-sodium diet (LS; 0.03% Na) for 3 days. On day 0, half the number of mice in WT and TG groups (n = 6/genotype) were switched to a high-sodium (HS) (3.51% Na) diet and maintained on it for 4 more days. The other half of mice in WT and TG groups (n = 6/genotype) continued to eat a low-sodium diet. All mice had ad libitum access to drinking water throughout the experimental period. All mice were euthanized on day +4. Thus at the time of euthanasia, four groups of mice [two groups eating a low-sodium (WT-LS and TG-LS) diet and two groups eating a high-sodium (WT-HS and TG-HS) diet]. A: urine output; B: urine osmolality; C: urinary sodium excretion. *P < 0.05 compared with the corresponding value in the WT-HS group by unpaired t-test. D: differences (Δ) in daily urinary excretion of sodium between the WT and TG mice fed HS (WT-HS and TG-HS) by subtracting the mean values obtained in WT mice from the individual values obtained in the TG mice and then computing the means ± SE. *Significantly different (P < 0.04 and 0.02) from the day +2 value by unpaired t-test.
As expected, urinary sodium excretion remained low and close to baseline values in both the genotypes when the mice were fed the LSD (Fig. 6C). Urine sodium excretion was robust in both genotypes up to +2 days on the HSD. On days +3 and +4 on the HSD, TG mice began to reach a plateau and a significant impairment in natriuresis was found on day +4. Figure 6D plots urine sodium differences between TG mice and the mean of the WT mice on each day in the HSD groups. Surprisingly, values were slightly positive for TG mice on the LSD diet and on days +1 and +2 of the HSD (indicating greater natriuresis in TG), but they were relatively impaired in the later course of natriuresis (days +3 and +4).
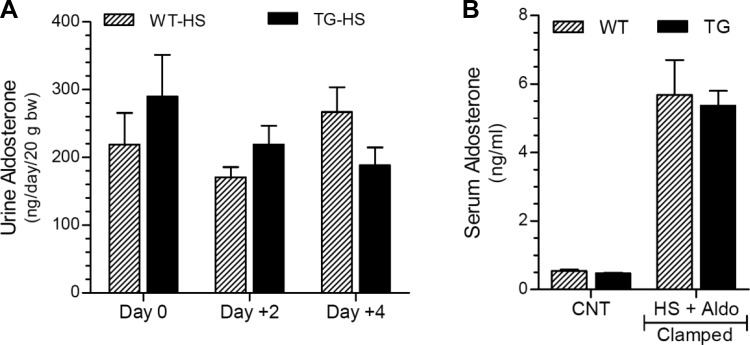
To confirm the efficiency of aldosterone clamping in the above experiment, we determined urine aldosterone excretion on days 0, +2, and +4 in WT and TG mice given the HSD. Terminal serum aldosterone levels were also determined in WT and TG mice given the HSD and compared with untreated WT and TG control mice. As shown in Fig. 7A, there were no significant differences in mean urinary aldosterone excretion between the genotypes on days 0, +2, and +4; and these values are severalfold higher than the values observed in untreated WT and TG mice (Fig. 4C). Similarly, terminal serum aldosterone levels in the WT and TG mice fed the HSD were more than 10-fold higher compared with the corresponding values in untreated WT and TG control mice (Fig. 7B).
Fig. 7.
Efficiency of clamping aldosterone levels in WT and TG mice given a high-sodium (HS) diet. A: urine aldosterone levels on days 0, +2, and +4; B: terminal serum aldosterone levels (day +4) compared with untreated control mice (CNT). Data presented here are derived from the same mice and experiment depicted in Fig. 6.
Western blot analysis of sodium transporter/channel exchangers.
Whole tissue homogenates of renal cortex and medulla were subjected to semiquantitative immunoblotting to determine the protein abundance of sodium transporter/channel exchangers (top in Figs. 8–11). Values of digitized band densities were statistically processed using two-way ANOVA to assess the contributions of each variable per se, such as genotype (WT vs. TG) and diet (LSD vs. HSD), followed by the interaction of these two variables for the observed alterations in the protein abundance (bottom in Figs. 8–11). The corresponding statistical significance values are shown below the bottom panels (Figs. 8–11). P values shown on the first line in each panel refer to the contribution due to genotype. P values shown on the second line correspond to the effect of diet. P values in the third line indicate the interaction of genotype and diet.
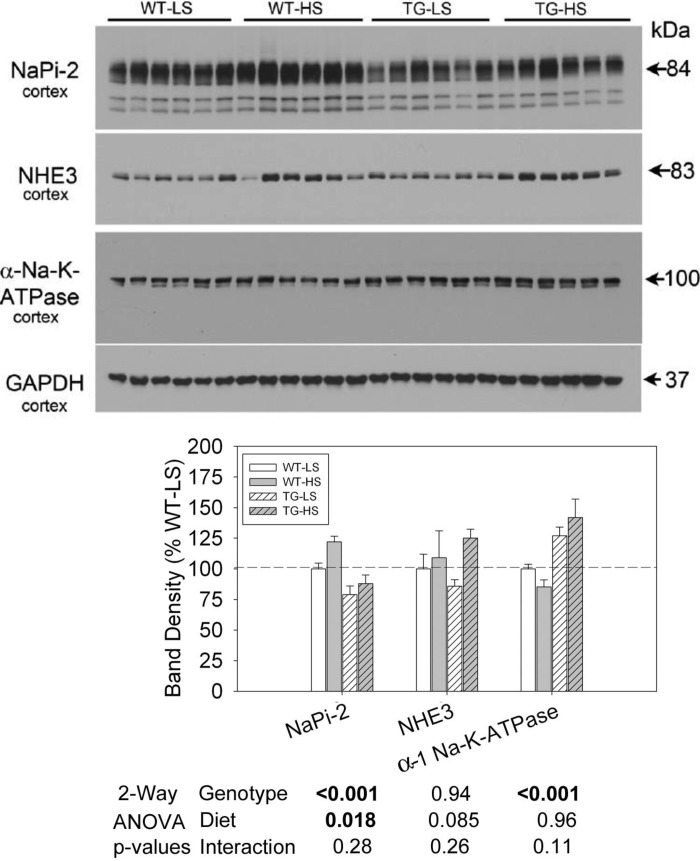
Fig. 8.
Effect of LS or HS diet on a background of aldosterone infusion on protein abundance of proximal tubular sodium transporters in WT and TG mice. Data presented here are derived from the same mice and experiment depicted in Fig. 6. Top: immunoblots of various proximal tubule sodium transporters and exchangers. Bottom: bar graph summary of the densitometric analysis of the immunoblots shown at top. Bars show percent change in band densities of different proteins in TG mice compared with the corresponding values in WT mice (100%). Results are means ± SE (n = 6 mice/genotype). Statistical significance, determined by two-way ANOVA, is shown at the bottom of the bar graphs.
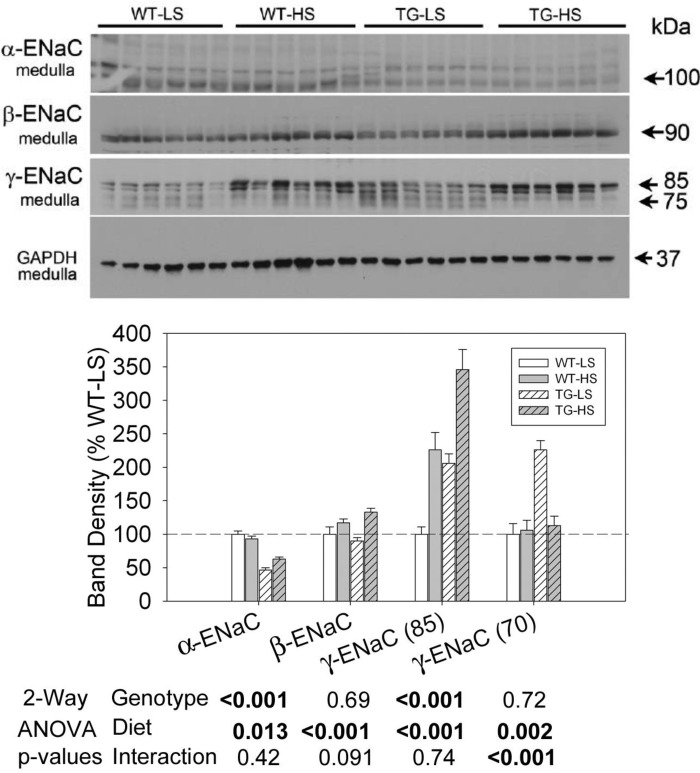
Fig. 11.
Effect of LS or HS diet on a background of aldosterone infusion on protein abundances of ENaC subunits in medullary collecting ducts of WT and TG mice. Data presented here are derived from the same mice and experiment depicted in Fig. 6. Top: immunoblots for protein abundance of various ENaC subunits. Bottom: bar graph summary of the densitometric analysis of the immunoblots shown at top. Bars show percent change in band densities of different proteins in TG mice compared with corresponding values in WT mice (100%). Results are means ± SE (n = 6 mice/genotype). Statistical significance, determined by two-way ANOVA, is shown at the bottom of the bar graphs.
Proximal tubule sodium transporters and exchangers.
Figure 8 shows representative Western blots for cortex homogenates in the second experiment, and the densitometry summary. Two-way ANOVA demonstrated that TG mice had significantly lower expression of NaPi-2 and significantly higher expression of α-1 Na-K-ATPase (regardless of diet). Furthermore, NaPi-2 was significantly increased by the HSD in both genotypes. There were no significant effects of diet or genotype on NHE3 band densities.
Thick ascending limb (TAL) and distal convoluted tubule (DCT) sodium transporters and exchangers.
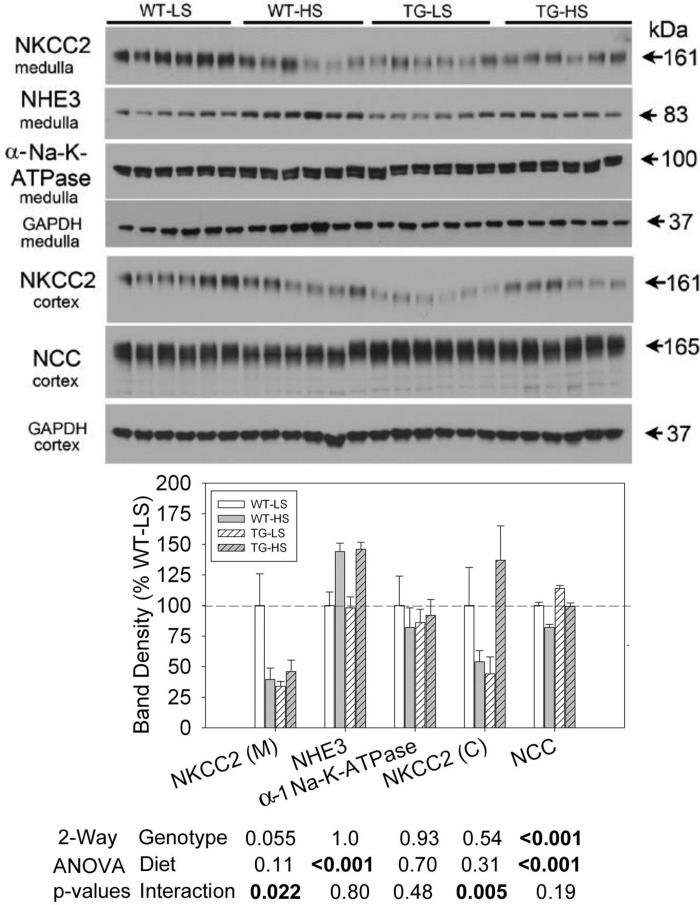
Figure 9 displays representative blots and densitometry summary of medulla and cortex (as indicated) from the second experiment. NKCC2 in medulla (medullary TAL) was reduced by HSD, but only in the WT mice (Holm-Sidak adjusted P = 0.00846, WT-HSD vs. WT-LSD). TG mice had significantly lower medullary NKCC2 (than WT) under the LSD (P = 0.00456) and did not change when given the HSD. Cortical NKCC2 responded similarly in the WT mice with a strong (around 50%) decrease in band density with the HSD. Furthermore, similar to the medulla, TG mice had lower NKCC2 levels under the LSD (as compared with WT), however, the difference was not significant (P = 0.0922). The switch to the HSD led to an upregulation of NKCC2 in TG mice (P = 0.00791, TG-HSD vs. TG-LSD). HSD also led to a significant (about 40%) upregulation of medullary (TAL) NHE3 abundance with no genotype differences in this response. There were no significant effects of diet or genotype on α-1 Na-K-ATPase abundance in the medulla (primarily medullary TAL). In the DCT, NCC abundance was higher in TG mice relative to WT mice, and was reduced by the HSD [as has been shown previously for aldosterone escape (32)]. There was no significant interactive term indicating that the reduction in NCC was not relatively impaired in the TG mice.
Fig. 9.
Effect of LS or HS diet and on a background of aldosterone infusion on protein abundances of thick ascending limb (TAL) and distal convoluted tubule (DCT) sodium transporters in WT and TG mice. The data presented here are derived from the same mice and experiment depicted in Fig. 6. Top: immunoblots for protein abundance of various sodium transporters and exchangers of TAL and DCT. Bottom: bar graph summary of the densitometric analysis of the immunoblots shown at top. Bars show percent change in band densities of different proteins in TG mice compared with corresponding values in WT mice (100%). Results are means ± SE (n = 6 mice/genotype). Statistical significance, determined by two-way ANOVA, is shown at the bottom of the bar graphs.
The epithelial sodium channel.
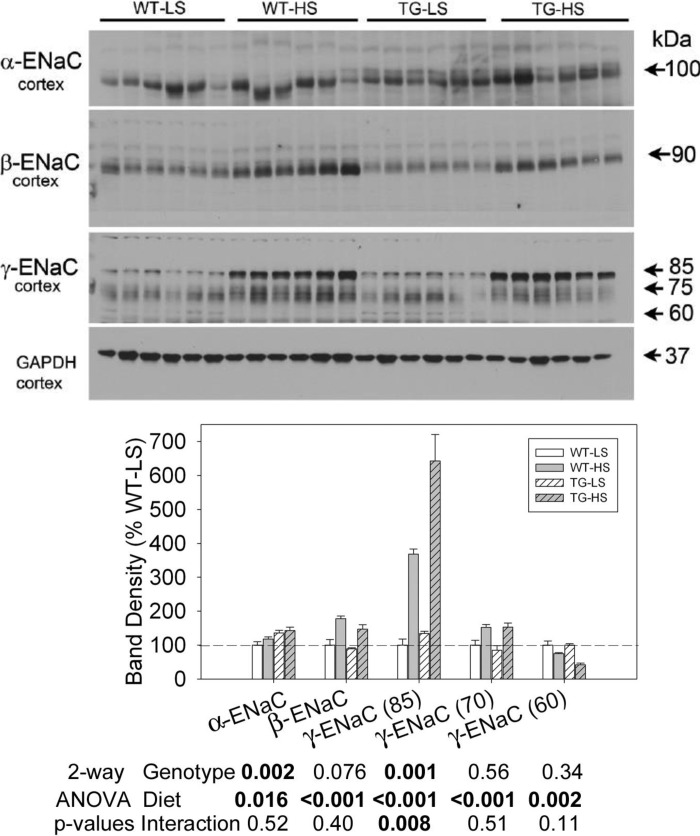
Representative blots for the ENaC subunits and densitometry summaries are shown in Fig. 10 (cortex) and Fig. 11 (medulla). In cortex, α-ENaC was significantly higher in the TG mice and was also elevated slightly but significantly by the HSD. β-ENaC and the major (85-kDa) and minor (75-kDa) bands of γ-ENaC were similarly regulated. All were increased by HSD. There was a significant interactive term for γ-ENaC (85-kDa) in that the increase was more pronounced in TG mice with densities more than sixfold higher in TG mice on the HSD relative to the TG mice on the LSD. A small band at 60 kDa for γ-ENaC was decreased by the HSD, but it was not different between the genotypes.
Fig. 10.
Effect of LS or HS diet on a background of aldosterone infusion on protein abundances of epithelial sodium channel (ENaC) subunits in cortical collecting ducts of WT and TG mice. Data presented here are derived from the same mice and experiment depicted in Fig, 6. Top: immunoblots for protein abundance of ENaC. Bottom: bar graph summary of the densitometric analysis of the immunoblots shown at top. Bars show percent change in band densities of different proteins in TG mice compared with the corresponding values in WT mice (100%). Results are means ± SE (n = 6 mice/genotype). Statistical significance, determined by two-way ANOVA, is shown at the bottom of the bar graphs.
In the medulla (Fig. 11), TG mice had significantly decreased α-ENaC abundance, and the abundance of this protein was further decreased by HSD in both genotypes. In contrast, the major bands associated with β- and γ-ENaC (similar to cortex) were more abundant with the HSD, and TG mice had higher expression of γ-ENaC (85-kDa). Similarly, the 70-kDa band for γ-ENaC was significantly higher in the TG mice on the LSD; however, it was decreased by the HSD so that it was no longer different than levels in WT mice, leading to a significant interactive term.
DISCUSSION
A variety of reports support a role for the fine-tuning of salt and water reabsorption by extracellular purine nucleotides [e.g., ATP and ADP (reviewed in 6, 19, 29)]. To characterize and define how purinergic mechanisms are globally involved in overall body homeostasis and to explore the full extent of the impact of P2 receptor signaling on sodium handling, in this study we used a TG mouse model in which human CD39/NTPDase1 is globally overexpressed (2). CD39 is a vascular NTDase that hydrolyzes extracellular ATP and ADP to AMP, thus effectively terminating P2 receptor signaling. Human CD39 overexpression in TG mice is driven by H-2Kb (MHC) resulting in global expression involving all cells in the body. Accordingly, previous immunohistochemical examination revealed widespread expression of hCD39 with increased activity in different organs (2). Thus the cellular expression of hCD39 in the TG mouse kidney is very different from the expression pattern of cd39 in WT mouse kidney, which is restricted in nature (6, 30). In addition, these TG mice have elevated plasma adenosine and AMP levels as breakdown products of ATP (2). We immunolocalized hCD39 protein in the kidney and showed that unlike endogenous mouse cd39 protein, labeling with hCD39 antibody revealed widespread expression in a majority of renal tubules including collecting ducts and in vascular structures of the TG mouse. With this background information we will examine and interpret our findings.
The TG mice were studied under two conditions (or series). In the first series, we assessed their ability to adapt to an HSD. Normally, high sodium detected by the macula densa cells post-TAL will lead to reduced production of renin, angiotensin II, and eventually aldosterone by the adrenal cortex. In addition, extracellular ATP is involved in renal macula densa signaling, and Oppermann et al. (17) showed that mice deficient in CD39 have a markedly compromised tubuloglomerular feedback regulation of glomerular filtration rate (GFR). However, macula densa- and pressure-dependent inhibition of renin secretion was intact in CD39-null mice (17). In this first series, we found that TG mice had relatively higher urine aldosterone excretion (a surrogate for circulating levels) on days 8 and 13 of the HSD, suggesting an impaired response to HSD. Urine aldosterone was also numerically (but not significantly) higher in the basal (day 0) collection in TG mice. This inadequate suppression of aldosterone would be expected to result in impaired natriuresis. Although we found no significant differences in absolute sodium excretion at any time point, we did observe that urine volumes were higher and urine osmolality was lower in the TG mice. This might suggest that to excrete the high sodium load, TG mice needed to excrete more water. They also may have been thirstier due to the elevation in serum sodium. In any event, they appeared to have some impairment in sodium homeostasis while eating the HSD. Mechanistically, an increase in aldosterone would be expected to increase the activity of distal tubular proteins, NCC and ENaC. We found a significant reduction in the major (85-kDa) band associated with γ-ENaC in cortex, and β-ENaC in the medulla in the TG mice. These two directional changes (possibly due to proteolytic cleavage of these bands; i.e., activation) have been observed before with aldosterone infusion or low-NaCl diets (14) and might suggest higher activity of ENaC in the TG mice. Nonetheless, we cannot rule out other possibilities (e.g., increased vascular tone in these mice might reduce the GFR and sodium load to both the macula densa and distal tubule). This would also lead to compromised natriuretic capability and explain enhanced aldosterone secretion.
In the next series, in an attempt to study natriuretic regulation in the hCD39-TG mice independent of changes in aldosterone, we utilized another familiar model [i.e., the model of aldosterone escape (32)]. In this model, animals initially retain sodium due to the clamped aldosterone levels, but then manifest an escape (i.e., diuresis and natriuresis despite the continual presence of the hormone). The molecular mechanisms for escape are not completely understood. What we found in the aldosterone-clamped TG mice was diminished ability to achieve maximal sodium excretion, rather than a defect in the early aspects of the escape. On days 1 and 2 of the escape, urine sodium rose identically in the TG and WT mice, suggesting the mechanisms involved in this early alleviation of sodium retention were not attenuated. Regulation of NHE3 trafficking in the proximal tubule may play a role in early escape, especially if there is a rise in blood pressure [reviewed in (15)]. Later escape was significantly blunted in the TG mice. This may have been manifested by inefficient downregulation of TAL through collecting duct sodium transporters and channels. It may have also resulted from hemodynamic differences (e.g., reduced GFR leading to a reduction in the sodium load to the distal nephron for excretion).
With regard to sodium transporters, we believed that NKCC2 and NCC were strong candidate mediators of this natriuretic impairment in the TG mice. NCC downregulation has been shown to be quite robust across different strains and species in the aldosterone escape model (22, 32, 36), and aldosterone is known to strongly upregulate this protein (4, 31). We found higher levels of NCC in the TG mice (relative to WT, two-way ANOVA P < 0.001). HSD did reduce NCC (P < 0.001), but the reduction was somewhat blunted in the TG mice and, in fact, band density in the TG-HSD mice was about the same as it was for WT-LSD mice (99 ± 3 and 100 ± 3, respectively).
The relationship between purinergic signaling and NKCC2 vis-à-vis aldosterone appears to be a complex one. First, we and others (23, 35) observed that genetic deletion of P2Y2 receptor per se upregulates the protein abundance of NKCC2 in the medulla, and is associated with increased natriuresis in response to acute furosemide treatment. However, another group (13) using P2Y2 receptor-knockout mice with a different genetic background, did not observe upregulation of NKCC2 protein or transepithelial transport compared with WT mice. Second, although NKCC2 abundance is not customarily known to be upregulated by aldosterone, we have observed that in response to HSD plus aldosterone infusion, this protein was upregulated in the P2Y2 receptor-knockout mice, which parenthetically, also displayed impaired natriuresis (36). In the current study, NKCC2 was strongly downregulated in the WT mice by HSD (with aldosterone infusion), and in contrast, upregulated in the TG mice, leading to a robust and significant interactive term for both cortex and medulla (P < 0.001 for both). Although the higher expression of NKCC2 in the TG mice on the HSD is in agreement with our prediction that ATP reduces the abundance of this protein, it is unclear why TG mice have reduced NKCC2 under LSD conditions. However, compared with P2Y2 receptor-knockout mice, there are more complex alterations in the extracellular purine profile in hCD39-TG mice that may affect the activities of P2 and P1 receptors. Finally, we demonstrated that NKCC2 is differentially regulated during aldosterone escape in lean vs. obese Zucker rats (22). Obese Zucker rats have higher levels of NKCC2 and impaired aldosterone escape (22).
ENaC could also be considered a candidate mediator of escape (26). Although protein levels have been shown to be inconsistently regulated in this model across studies, it is possible that the activity of the whole channel is regulated in another fashion, perhaps by proteolytic cleavage or trafficking. We found a number of genotype differences in the appearance of the subunits on Western blots that might hint at differences in regulation and perhaps activity. In contrast to HSD alone, when aldosterone levels were clamped, band densities for α- (cortex) and 85-kDa γ-ENaC (both cortex and medulla) were relatively higher in the TG mice. Additional studies are needed to determine the mechanisms underlying these modifications, and their effects on channel activity.
In this context it is interesting to note that recently, Rajagopal and associates (20) working with mpkCCDc14 cells, reported that ATP stimulates calcium-activated chloride channel (CaCC)-mediated Cl− absorption under conditions of aldosterone stimulation. The direction of Cl− transport is voltage-dependent and dictated by the level of ENaC activity and extracellular ATP levels. Although extracellular ATP levels are elevated during both volume expansion and depletion, ENaC activity is elevated only during volume depletion (20). Therefore, the potential exists for ectonucleotidases, such as CD39, to fine-tune ATP effects on ENaC.
Finally, we believe that it is not likely that increased levels of adenosine in the TG mice are responsible for the observed impairment of natriuretic response. Rajagopal and Pao (21) showed in mIMCD-K2 cells that adenosine stimulates chloride secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) by activating apical A2b receptors and signaling through cAMP/protein kinase A. Based on this observation Rajagopal and Pao proposed that the adenosine receptor pathway may provide one mechanism for enhanced urinary NaCl excretion in the setting of high dietary NaCl intake. Our findings of impaired natriuresis suggest either that this mechanism is not active in vivo or that it is masked by the reduction in ATP in the TG mice.
In summary, our findings support a primary role for extracellular nucleotides in the manifestation of natriuresis under HSD. Genotypic differences in sodium transporter expression, vascular activity, and hemodynamics all may play a role in these differences. Moreover, both aldosterone-dependent and -independent mechanisms are apparent.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-081041 to B. K. Kishore and S. C. Robson, and DK-082507 to C. M. Ecelbarger, a Marriott Cardiovascular Fellowship to C. M. Ecelbarger, and the resources and facilities at the VA Salt Lake City Health Care System. Additional funding sources include an Established Investigator Award from the American Heart Association to C. M. Ecelbarger and a National Kidney Foundation of Utah and Idaho grant to B. K. Kishore.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.Z., S.C.R., B.K.K., and C.M.E. conception and design of research; Y.Z., K.L.M., K.M.H., B.K.K., and C.M.E. performed experiments; Y.Z., K.L.M., K.M.H., B.K.K., and C.M.E. analyzed data; Y.Z., S.C.R., K.M.D., B.K.K., and C.M.E. interpreted results of experiments; Y.Z., B.K.K., and C.M.E. prepared figures; B.K.K. and C.M.E. drafted manuscript; Y.Z., S.C.R., K.M.D., B.K.K., and C.M.E. edited and revised manuscript; Y.Z., S.C.R., K.L.M., K.M.H., K.M.D., B.K.K., and C.M.E. approved final version of manuscript.
ACKNOWLEDGMENT
We thank E. Csizmadia for technical assistance in immunoperoxidase labeling, and Dr. A. Malhotra for providing human skin samples.
Parts of this work were presented at Kidney Week 2012 of the American Society of Nephrology, October–November 2012, San Diego, CA, and appeared as a printed abstract in the proceedings of that meeting (38).
REFERENCES
- 1.Deaglio S, Robson SC. Ectonucleotidases as regulators of purinergic signaling in thromobosis, inflammation, and immunity. Adv Pharmacol 61: 301–332, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, Fisicaro N, Mysore TB, Kaczmarek E, Cowan PJ, d'Apice AJ. Thromboregulatory manifestations in human CD transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 113: 1440–1446, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, Tecilazich F, O'Donnell CP, Ferran C, Malhotra A. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS One 8: e70559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA. The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci USA 95: 14522–14557, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishore BK, Chou CL, Knepper MA. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 269: F863–F869, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Kishore BK, Isaac J, Fausther M, Tripp SR, Shi H, Gill PS, Braun N, Zimmermann H, Sévigny J, Robson SC. Expression of NTPDase1 and NTPDase2 in murine kidney: relevance to regulation of P2 receptor signaling. Am J Physiol Renal Physiol 288: F1032–F1043, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Kishore BK, Nelson RD, Miller RL, Carlson NG, Kohan DE. P2Y2 receptors and water transport in the kidney. Purinergic Signal 5: 491–499, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishore BK, Zhang Y, Gevorgyan H, Kohan DE, Schiedel AC, Müller CE, Peti-Peterdi J. Cellular localization of adenine receptors in the rat kidney and their functional significance in the inner medullary collecting duct. Am J Physiol Renal Physiol 305: F298–F305, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kukulsko F, Lévesque SA, Sévigny J. Impact of ectoenzymes on p2 and p1 receptor signaling. Adv Pharmacol 61: 263–299, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 61: 221–261, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Longhi MS, Robson SC, Bernstein SH, Serra S, Deaglio S. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med 91: 165–172, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques RD, Prætorius HA, Leipziger J. P2Y2 receptor knock-out mice display normal NaCl absorption in medullary thick ascending limb. Front Physiol 4: 280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masilamani S, Kim GH, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol 298: R851–R861, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nature Med 8: 358–365, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann J. Tubulogelomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol 294: F965–F970, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Pochynyuk O, Rieg T, Bugaj T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem 283: 36599–36607, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prætorius H, Leipziger J. Intrarenal purinergic signaling in the control or renal tubular transport. Annu Rev Physiol 72: 377–393, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopal M, Kathapalia PP, Widdicombe JH, Pao AA. Differential effects of extracellular ATP on chloride transport in cortical collecting duct cells. Am J Physiol Renal Physiol 303: F483–F491, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopal M, Pao AC. Adenosine activates A2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension 55: 1123–1128, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riazi S, Khan O, Hu X, Ecelbarger CA. Aldosterone infusion with high-NaCl diet increases blood pressure in obese but not lean Zucker rats. Am J Physiol Renal Physiol 291: F597–F605, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Rieg T, Bundey RA, Chen Y, Deschens G, Junger W, Insel PA, Vallon V. Mice lacking P2Y2 receptors have salt-resistant hypertension and facilitated renal Na+ and water reabsorption. FASEB J 21: 3717–3726, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Schetinger MR, Morsch VM, Bonan CD, Wyse AT. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 32: 77–98, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Shirley DG, Bailey MA, Unwin RJ. In vivo stimulation of apical P2 receptors in collecting ducts: evidence for inhibition of sodium reabsorption. Am J Physiol Renal Physiol 288: F1243–F1248, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toney GM, Vallon V, Stockand JD. Intrinsic control of sodium excretion in the distal nephron by inhibitory purinergic regulation of the epithelial Na+ channel. Curr Opin Nephrol Hypertens 21: 52–60, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol 294: F10–F27, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Vallon V, Rieg T. Regulation of renal transport mechanisms. Am J Physiol Renal Physiol 301: F463–F475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vekaria RM, Shirley DG, Sévigny J, Unwin RJ. Immunolocalization of ectonucleotidases along the rat nephron. Am J Physiol Renal Physiol 290: F550–F560, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Velazquez H, Bartiss A, Bernstein P, Ellison DH. Adrenal steroids stimulate thiazide-sensitive NaCl transport by rat renal distal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 270: F211–F219, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Wang XY, Masilamani S, Nielsen J, Kwon TH, Brooks HL, Nielsen S, Knepper MA. The renal thiazide-sensitive Na-Cl cotransporter as mediator of the aldosterone-escape phenomenon. J Clin Invest 108: 215–222, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wildman SS, Marks J, Turner CM, Yew-booth L, Peppiat-Wildman CM, King BF, Shirley DG, Wang W, Unwin RJ. Sodium-dependent regulation of renal amiloride-sensitive currents by apical P2 receptors. J Am Soc Nephrol 19: 731–742, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signaling cascade. Biochim Biophys Acta 1783: 673–694, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Li L, Kohan DE, Ecelbarger CM, Kishore BK. Attenuation of lithium-induced natriuresis and kaliuresis in P2Y2 receptor knockout mice. Am J Physiol Renal Physiol 305: F407–F416, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol 300: F567–F668, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Morris KL, Sparrow SK, Dwyer KM, Enjyoji K, Robson SC, Kishore BK. Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am J Physiol Renal Physiol 303: F420–F430, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Robson SC, Morris KL, Dwyer KM, Kishore BK, Ecelbarger CM. Impaired aldosterone escape in transgenic mice over-expressing human CD39: role of altered sodium transporter regulation (Abstract). J Am Soc Nephrol 23: 498A–499A, 2012. [Google Scholar]
- 39.Zhang Y, Sands JM, Kohan DE, Nelson RD, Martin CF, Carlson NG, Kamerath CD, Ge Y, Klein JD, Kishore BK. Potential role of purinergic signaling in urinary concentration in inner medulla: insights from P2Y2 receptor gene knockout mice. Am J Physiol Renal Physiol 295: F1715–F1724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]