Abstract
The mammalian kidney is an organ composed of numerous functional units or nephrons. Beyond the filtering glomerulus of each nephron, various tubular segments with distinct populations of epithelial cells sequentially span the kidney from cortex to medulla. The highly organized folding of the tubules results in a spatial distribution that allows intimate contact between various tubular subsegments. This unique arrangement can promote a newly recognized type of horizontal epithelial-to-epithelial cross talk. In this review, we discuss the importance of this tubular cross talk in shaping the response of the kidney to acute injury in a sense and sensibility model. We propose that injury-resistant tubules such as S1 proximal segments and thick ascending limbs (TAL) can act as “sensors” and thus modulate the responsiveness or “sensibility” of the S2-S3 proximal segments to injury. We also discuss new findings that highlight the importance of tubular cross talk in regulating homeostasis and inflammation not only in the kidney, but also systemically.
Keywords: acute kidney injury, sepsis, Tamm-Horsfall protein, tubular cross talk, uromodulin
the kidney is a highly complex organ formed by heterogeneous populations of cells (5, 30). It is composed of functional filtering units called nephrons. Each nephron is composed of various types of tubular epithelial cells, often divided into segments, based on morphology and localization within the kidney (proximal tubules, thin descending and ascending loops of Henle, thick ascending limb of Henle, distal tubule, and collecting ducts) (5, 30). Major advances in understanding the physiological role of each tubular segment within the nephron have occurred in the last few decades (5). However, our understanding of the complex cell-to-cell interactions in the heterogeneous kidney milieu in vivo remains at a very early stage. Furthermore, under injurious conditions, as occurs in ischemic or septic acute kidney injury (AKI), various tubular segments react differentially and exhibit a wide spectrum of responses and pathology (4, 61). As we will discuss, the response and fate of a specific cell type during AKI can depend significantly on signaling from other tubular segments (16, 18, 22, 25, 31, 57). Therefore, the collective response of the kidney to specific stimuli in health and disease may depend on the role of each type of cells in the context of a complex network of interactions. Fortunately, newer advances in technology, such as two-photon live imaging and laser microdissection are enabling researchers to observe and dissect events at the cellular level in vivo (12, 14, 32, 45). In this review, we will “zoom-in” on the kidney to understand events at the cellular level and uncover how signaling between neighboring tubules can shape the response of the whole kidney and possibly impact distant organs (Fig. 1A). While the models discussed below are based on published data by the authors and other groups, some generalizations do represent the opinion of the authors.
Fig. 1.
Spatial relationships between tubules that allow cross talk. The cartoon in A underscores the importance of investigating events at the cellular level to advance our understanding of the global response of the kidney to various stimuli. The cartoon in B depicts the various segments of a nephron and highlights the specific regional distribution of each segment within distinct renal areas. PT, proximal tubule; TDC, thin descending limb; Thin AL, thin ascending limb; mTAL and cTAL, medullary and cortical thick ascending limbs, respectively; DCT, distal convoluted tubule; CCD, cortical collecting duct; mCD, medullary collecting duct; ImCD, inner medullary collecting duct. The image in C is an electron micrograph of the kidney outer medulla (×3,000) showing TAL segments contiguous to S2-S3 proximal segments (arrows), the latter recognized by their typical brush border (BB). Inset: ×50,000 magnification showing an area where the basement membranes of TAL and S3 segments are in direct contact.
Definition and Types of Cross Talk in the Kidney
Cross talk can be defined as a regulatory interaction between specific cell types that typically involves mediators such as cytokines, chemokines, growth factors, or hormone-like substances (22, 46, 57). Various types of cross talk potentially exist in the kidney. For the tubular epithelium, cross talk can occur between various tubular segments (16, 31, 37) or could engage other types of cells such as endothelial (6, 74) or even immune resident cells (33, 35, 64, 73). We will focus in this review on epithelium-to-epithelium tubular cross talk, but urge the reader to interpret the data in the context of the complex functioning renal milieu and the wide spectrum of pathological pathways that are activated in kidney injury (4, 43).
Spatial Relationships Between Tubules That Allow Cross Talk
The nephron is composed of the glomerulus followed by the proximal convoluted tubule. This latter segment, also known as pars convoluta, resides primarily in the cortex. The pars convoluta is frequently divided into an early region called S1 and a more distal part called S2. The proximal convoluted tubule is followed by a straight descending segment, the pars recta, which enters the outer stripe of the outer medulla. It is composed sequentially of late S2 and the S3 segment. This is followed by the loop of Henle, formed by the thin descending and ascending limbs, and the thick ascending limb (TAL) (5, 30). The TAL segment spans all the way from the outer border of the inner medulla up to the cortex near the macula densa area (28). In that area, the epithelium transforms into the distal convoluted tubule, connecting segment, and cortical collecting duct. Collecting ducts from various nephrons coalesce into descending collecting ducts that span again the kidney from cortex to the papillae.
It is important to notice that the regional distribution of these various elements marks specific zones within the kidney, as summarized in Fig. 1B (17). For example, the cortex harbors S1-S2 segments, TAL, distal tubules, and collecting ducts. The outer stripe of the outer medulla, an area exquisitely sensitive to hypoxic injury, contains S2-S3 segments, TAL, and collecting ducts (17). This precise regional distribution of tubules allows for specific cross talk between various neighboring segments under both health and disease conditions. Importantly, this cross talk can in itself determine the location of pathology following a specific type of injury.
We next discuss the orientation or path of the cross talk between tubules (47). When we envision a form of communication between tubules, we intuitively think of the luminal filtrate flowing within the tubules as the most likely vehicle for vectorial transport of information. This is exemplified by the juxtaglomerular apparatus, which senses changes in the tonicity of the filtrate distally and reacts accordingly (58). This route of “vertical” information exchange is probably very important, especially in maintaining fluid and electrolyte balance. However, there is also mounting evidence to support a “horizontal” direction of cross talk that occurs between the basolateral domains of contiguous tubules (16, 18, 31, 37, 47). Indeed, the proximity of the basolateral domains of some tubules does allow for such cross talk (5). For example, electron microscopy of the outer medulla reveals close juxtaposition of the basement membranes of contiguous TAL and S2-S3 segments with separating distances of <500 nm (Fig. 1C). This intimate proximity between tubules can allow bidirectional horizontal flow of information to promote a regulatory cross talk.
In the following sections, we will focus on two forms of acute kidney injury, sepsis and ischemia-reperfusion, where tubular cross talk appears to be a key factor in the pathophysiology and response of the kidney to injury. We will explore common aspects of cross talk in these different models of injury and propose a unifying model of the overall role of tubular cross talk in maintaining homeostasis within the kidney in health and disease.
Tubular Cross Talk in Sepsis
Kidney injury is frequently seen in sepsis, especially with Gram-negative bacteria (13, 53, 59, 72). The histology of the kidney in septic patients reveals a wide spectrum of abnormalities, ranging from minimal changes to widespread cortical foci of apoptosis, necrosis, and inflammation (65). These cortical lesions are also observed in various animal models of sepsis and are a function of the duration and severity of a particular model (7, 9, 13, 70). The underlying pathophysiological mechanisms are likely multifactorial, but widespread endothelial and epithelial oxidative stress seems to play a key role in renal injury (13, 23, 39).
The driving factors behind renal oxidative stress are traditionally ascribed to systemic proinflammatory cytokines released by innate immune cells upon interaction with bacteria or endotoxin (20, 59). However, therapies targeting systemic inflammatory cytokines have failed to improve the outcome of sepsis or reduce the incidence of AKI (20). Importantly, we have recently shown that endotoxin-induced renal oxidative stress occurs even in chimeric mice lacking hematopoietic Toll-like receptor 4 (TLR4) (31). This suggested that renal injury in sepsis can occur through direct interactions between endotoxin and a specific populations of renal cells (14, 24–26).
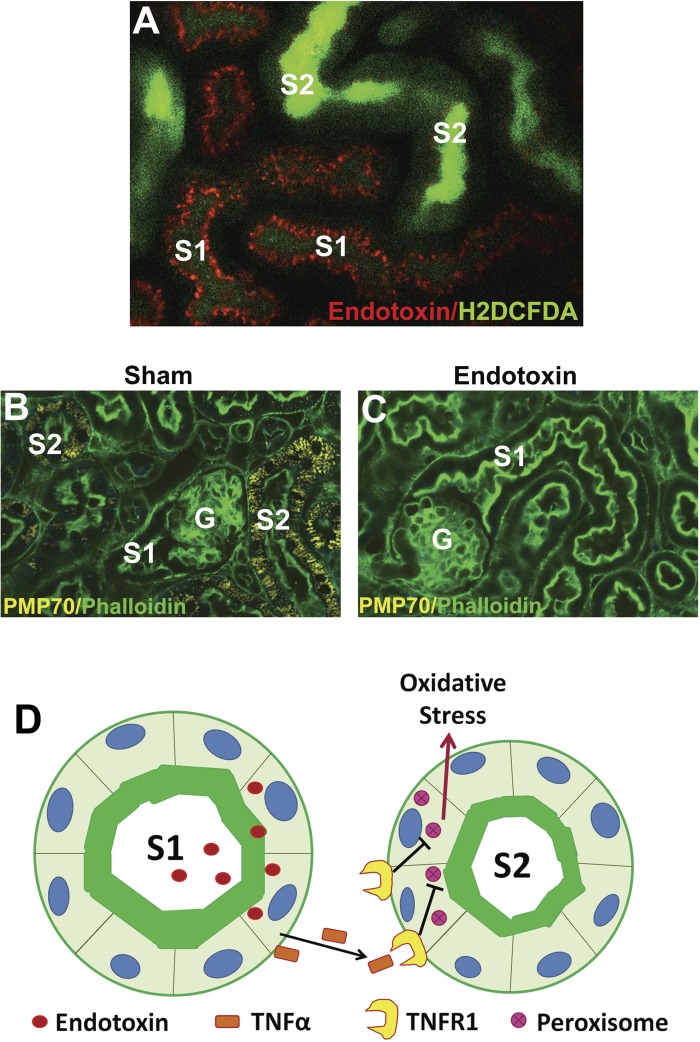
Using intravital microscopy, we determined that S1 tubules are the primary sensors of endotoxin in the kidney (Fig. 2A). Indeed, we found that systemically administered endotoxin is freely filtered and taken up nearly exclusively by S1 tubules through TLR4-dependent endocytosis (14, 26, 31). Unexpectedly, S1 tubules showed no oxidative stress despite a heavy uptake of endotoxin. Instead, severe oxidative stress was localized exclusively to neighboring S2 and S3 proximal tubular segments, which exhibited no TLR4-dependent endotoxin uptake (26, 31).
Fig. 2.
Tubular cross talk in sepsis-induced acute kidney injury (AKI). In A, intravital 2-photon microscopy reveals that systemically administered fluorescent endotoxin (red) accumulates in S1 tubules via Toll-like receptor 4 (TLR4)-mediated endocytosis. Neighboring S2 segments (but not S1) exhibit severe oxidative stress (H2DCFDA, green). In B, fluorescence microscopy of fixed kidneys shows abundant peroxisomes (PMP70, yellow) in S2 segments (but not S1) under sham conditions. G denotes glomeruli, and green is FITC-phalloidin staining of actin. In C, S2 segments show reduced peroxisomal staining 4 h after endotoxin injury. The cartoon in D depicts S1 as a sensor of danger molecules such as endotoxin in the filtrate. S1 then signals S2 through cytokines such as TNF-α which act on cognate receptors such as TNF-α receptor 1 (TNFR1) present only on S2. In cases of severe stress, this signaling causes early peroxisomal damage and oxidative stress.
These studies suggested a model in which S1 acts as sensor for “danger” entities in the filtrate. Indeed, occupying the most upstream position in the nephron, S1 is ideally suited to monitor the entering filtrate and communicate information to downstream and neighboring segments (23–25). To perform this crucial sentinel function, S1 autoprotects itself from oxidant injury similar to a professional macrophage (31). As such, it acts as an epithelial macrophage, or “epiphage.” This is accomplished in part by robust upregulation of antioxidant molecules such as heme oxygenase 1 and sirtuin 1 (31). Remarkably, S1 segments also lack peroxisomes. This has been shown in mice, rats, and humans (30, 31, 68). These important organelles play a major role in fatty acid oxidation and can be a significant source of reactive species under physiological and pathological conditions (31, 69).
In contrast to S1, S2 and S3 segments are rich in peroxisomes and these organelles are damaged early during sepsis (Fig. 2, B and C). In fact, we have shown that peroxisomes are damaged hours before any mitochondrial dysfunction occurs and are therefore a likely source of the observed oxidative stress (31). Furthermore, S2 and S3 segments (but not S1) express TNF-α receptor-1 (TNFR1) (31). This makes S2 and S3 segments very susceptible to TNF-α-mediated peroxisomal injury and oxidative stress. Because this model is operational in chimeric mice that lack hematopoietic TLR4 (26, 31), TNF-α (and other signaling molecules) likely originate from S1 following endotoxin uptake (Fig. 2D).
The model above, while described in the setting of septic injury, is likely also functional under normal physiological conditions. That is, S1 communication with other tubular segments can serve to alert neighboring segments about filtrate content and composition, allowing them to adjust metabolic and transport functions as required (23). In turn, basal production of reactive species in S2 and S3 segments could partake of important signaling functions (21). As such, this epithelial-to-epithelial regulatory cross talk could play a major homeostatic function similar to the well-described tubuloglomerular feedback and glomerulotubular balance.
Tubular Cross Talk in Ischemia-Reperfusion
Ischemia-reperfusion injury (IRI) is a common cause of AKI (61). In many experimental models, IRI affects predominantly the outer medulla, as shown in Fig. 3A (4, 61). This is traditionally ascribed to the relative hypoxia in this area. The major tubules of interest in this region are S3 segments and TAL (Fig. 3B) (36). It is frequently observed that S3 segments are very sensitive to injury, whereas TAL cells are more resistant, probably through upregulation of cytoprotective mechanisms (11, 22, 36). Some investigators have previously proposed that signaling from TAL may be important for repair of neighboring S3 after injury (Fig. 3B) (22). Several mediators have been proposed for this reparative signaling but without definitive experimental support (22). Recent data from our laboratory strongly support an important role for Tamm-Horsfall protein (THP) as a mediator of tubular cross talk in AKI (15, 16, 18, 19).
Fig. 3.
Tubular cross talk in acute kidney injury due to ischemia-reperfusion. A: low-power magnification (×4 objective) images of kidney sections stained with periodic acid-Schiff (PAS) from mice that underwent sham (left) or renal ischemia-reperfusion injury (IRI; right). Necrotic proximal tubules can be clearly seen after injury, especially in the outer stripe of the outer medulla. B: 2 tubular types (TAL and S3) that are involved in tubular cross talk in the outer medulla. Cross talk between TAL and S3 is probably essential in maintaining kidney homeostasis (health). TAL segments are more resistant to injury compared with S3 segments and can be the source of regenerative factors important for the reparative process in neighboring injured S3 segments.
THP, also known as uromodulin (44, 49), is expressed only in the kidney, and exclusively by cells of TAL segments (18, 52). The primary structure of THP comprises three EGF-like domains, a central domain rich in cysteine residues, a zona pellucida domain, and a glycosylphosphatidylinositol (GPI)- anchoring site (52, 60). THP expression spans from the inner stripe of the outer medulla across to the macula densa area and early distal tubule in the cortex (28, 60). It is frequently used as a marker for TAL (14). Within the TAL cells, THP is predominantly sorted toward the apical domain through its GPI anchor signal, and subsequently cleaved into the urine as one of the most abundant proteins (52, 60). However, THP can also be sorted to the basolateral domain, through a yet undetermined mechanism (15, 18, 66). In the rat, Bachman and colleagues (3) previously showed that the ratio of apical to basolateral distribution is about 2:1. We observed similar findings in the mouse, where THP can also be localized to the interstitial space and around the basolateral domain of proximal S3 segments (15). In fact, beyond the interstitium between TAL and S3, THP can also be detected in the serum (8, 51, 54, 66). Seminal data from Thornley et al. (66), recently validated by others (54), showed that both urinary and serum THP are proportionally correlated with level of kidney function.
THP and early AKI.
THP mRNA and protein are significantly reduced at the peak of injury in experimental models of IRI (Fig. 4A). This has been verified independently by our laboratory in mice (15, 27) and by others in rats (55–56, 71). This decrease in THP in IRI is regulated and does not represent injury-induced synthetic failure. In fact, we and others have shown a simultaneous increase in other proteins, such as monocyte chemotactic protein-1 (MCP-1) and TNF-α, in TAL segments (27, 56). The limited data available in human AKI also suggest downregulation of THP during early injury (2, 10, 40). During recovery, THP is significantly upregulated and overexpressed in the mouse kidney compared with baseline expression (Fig. 4A) (15). To understand the relevance and impact of THP “deficiency” on AKI, we performed IRI on kidneys from THP−/− and THP+/+ mice (16, 19). Remarkably, THP deficiency aggravated AKI and resulted in excessive neutrophil infiltration after injury. Interestingly, the site of tubular damage, predominantly cell necrosis, was the S3 segments and not THP-deficient TAL cells, suggesting that THP deficiency in TAL is sensitizing neighboring S3 segments to AKI (16). To understand the underlying mechanism of the increased neutrophil infiltration in THP−/− kidneys after AKI, we studied the expression CXCL2, a potent neutrophil chemoattractant chemokine also known as macrophage inflammatory protein-2 (MIP-2) (42). In THP deficiency, S3 segments specifically overexpressed MIP-2. Neutralization of this chemokine in vivo using a specific antibody reduced neutrophil infiltration and injury in S3 segments and improved kidney function (16). Taken together, these studies suggest that THP is an important regulator of MIP-2 expression in S3 segments.
Fig. 4.
Role of Tamm-Horsfall protein (THP) in tubular cross talk during AKI. A: graph summarizing the expression of THP after ischemic injury based on published studies. AKI is a state of THP deficiency observed especially at the peak of injury. Recovery from AKI is associated with increased expression of THP, which is needed to suppress inflammatory signaling in the kidney. B: tubular cross talk model, supporting that THP is essential in suppressing proinflammatory signaling in neighboring S3 proximal tubules. THP regulates the expression of CXCL-2 and IL-23, both of which can stimulate neutrophil influx into the kidney. In addition, both cytokines are downstream of NF-κB, which suggests that THP regulates the activation of NF-κB.
THP and the recovery phase of AKI.
As discussed above, THP is overexpressed in the recovery phase in AKI (15). Importantly, we observed a significant shift of THP from the apical domain toward the basolateral domain and in the interstitium around S3 segments. This was verified with confocal microscopy and immunogold electron microscopy, and confirmed by measuring the increase in THP in the sera of mice recovering from AKI (15). Functionally, the presence of THP appears to be essential for halting the progression of the inflammatory response ensuing after AKI (15). Proinflammatory cytokines/chemokines such as MIP-2, TNF-α, and MCP-1 remained elevated in THP−/− kidneys, thereby delaying recovery (15). The source of these cytokines, as specifically shown for MCP-1, was still the S3 proximal segments. These data support an important role for THP in halting S3 inflammatory signaling and ushering in recovery (15, 18).
THP-dependent model of tubular cross talk in AKI.
Based on the data presented above, we proposed a model of tubular cross talk whereby THP produced in TAL acts as an important modulator of inflammatory signaling in neighboring S3 segments (Fig. 4B) (16, 18, 25, 41). The relative THP deficiency, seen in wild-type kidneys, may indeed be part of the pathophysiology of ischemic AKI (27). Whether THP acts directly on S3 remains to be investigated. However, a direct mode of action is suggested by the localization of THP on the basolateral domain of S3 segments (15), a site where a putative THP receptor (scavenger receptor-SRB-1) (50) is expressed and upregulated after AKI (16). Direct action is also supported by cell culture models whereby treatment with THP attenuates inflammatory signaling from proximal tubular cells induced by endotoxin or hypoxia (El-Achkar TM, unpublished observations). In particular, we found that THP inhibits hypoxia- or endotoxin-induced transcriptional activation of TNF-α and MCP-1 in human proximal tubular cells (HK-2 cells). Nevertheless, we cannot rule out completely the presence of a THP-dependent secondary mediator that in turn acts on S3 segments.
We did not include in our discussion other nephrotoxic models of injury, such as cisplatin nephrotoxicity, which is known to affect proximal tubules in the outer stripe of the OM (48). However, it is worth mentioning that Linkermann et al. (37, 57) used this model to show that S3 tubules can “strike back” at TAL. Indeed, they showed that injury to S3 segments can cause apoptosis to neighboring TAL, through a Fas ligand-dependent mechanism. They validated these observations using a co-culture model, where proximal tubules treated with cisplatin induced apoptosis of TAL, an effect that was blocked by a FAS ligand inhibitor (37).
THP-Dependent Cross Talk in Neutrophil Homeostasis Beyond the Kidney
Tubular cross talk in the outer medulla may also have systemic implications. In fact, it was previously observed that mice deficient in THP exhibit a systemic inflammatory phenotype, marked by increased levels of several cytokines and chemokines in the blood (38). We observed recently that THP−/− mice have systemic neutrophilia caused by increased granulopoiesis in the bone marrow (41). This was caused by activation of the IL-23/IL-17 axis in the THP−/− kidneys. The IL-23/IL-17 axis is a proinflammatory signaling cascade (1, 29) that starts with the production of IL-23, typically in immune cells (67), which acts on specialized type of T cells (1, 62, 67) or neutrophils (34) to produce IL-17. IL-17 is a potent stimulator of other inflammatory cytokines and can induce systemic granulopoiesis through the production of granulocyte colony-stimulating growth factor (1, 29, 62–63). To determine the source of IL-23 in THP−/− kidneys, we used real-time PCR on RNA extracted from various cell types in the kidney (epithelial and immune cells) using laser microdissection and FACS (41). Surprisingly, we found that S3 tubular segments, and not renal immune cells, were the source of IL-23. This suggested that THP regulated the synthesis of IL-23 in proximal tubules, thereby affecting the IL-23/IL-17 axis and systemic granulopoiesis. These findings underscore the importance of tubular cross talk in the outer medulla as a modulator of systemic granulopoiesis and neutrophil homeostasis.
Unifying Model of Tubular Cross Talk: Sense and Sensibility
Based on the data from the sepsis and IRI models of AKI, we propose that specific tubules, such as S1 in sepsis and TAL in ischemic injury, function to sense the injury. In contrast, the S2-S3 segment appears to be sensitized by S1 or TAL (the sensors) to injury. Hence, we propose the model of sense (S1 and TAL) and sensibility (S2-S3 segments) for tubular cross talk (Fig. 5). In this model, S1 senses systemic danger as conveyed by information in the filtrate (31), whereas TAL is a sensor of endogenous kidney stress. This TAL function is facilitated by it spanning from the cortex to the medulla and regulating the level of THP (15, 27, 41). These sensor segments then activate the S2-S3 segments, which in turn shape the overall response of the kidney through the production of cytokines or chemokines (16, 41). However, this process itself will also sensitize S2-S3 to injury (26, 31). Our recent data also suggest that the response of S2-S3 will not only influence the kidney's adaptability to injury but can also have widespread systemic effects (41).
Fig. 5.
Sense and sensibility model of tubular cross talk. S1 and TAL segments sense kidney stress and are resistant to injury. S1 is a sensor of systemic stress through monitoring of the filtrate. TAL segments are sensors of endogenous kidney stress. Both sensors signal to the S2-S3 segments. The S2-S3 segments release various cytokines that can shape the response of the kidney to injury, but can also affect distant organs through an effect on granulopoiesis by activating the IL-23/IL-17 axis. Under physiological stress, this system maintains homeostasis. However, with severe injury, the S2-S3 segments are particularly susceptible to damage, probably due to failure of quenching the increased load of oxidative stress.
Conclusions
We discussed the importance of tubular cross talk between various tubular epithelial segments in the kidney during AKI. The available data from models of sepsis and ischemia suggest that tubular cross talk shapes not only the response of the kidney but also of the whole organism to acute kidney injury in a sense and sensibility model; injury-resistant tubules such as S1 proximal segments and TAL can act as “sensors,” and thus modulate the responsiveness or “sensibility” of the S2-S3 proximal segments to injury. We propose that this form of horizontal cross talk between tubules is important in the adaptation of the kidney not only in states of injury but also is likely operational under normal physiological conditions. A detailed understanding of the molecular mechanisms involved in this cross talk has great potential to increase our ability to modulate kidney function in health and disease.
GRANTS
This work was supported by a Veterans Affairs merit award (T. M. El-Achkar), National Institutes of Health (NIH) Grant R01-DK080067 (P. C. Dagher), NIH O'Brien Center Grant P30-DK079312 (P. C. Dagher), and Dialysis Clinics, Inc. (P. C. Dagher).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.M.E.-A. and P.C.D. prepared figures; T.M.E.-A. and P.C.D. drafted manuscript; T.M.E.-A. and P.C.D. edited and revised manuscript; T.M.E.-A. and P.C.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge present and past members of the El-Achkar Laboratory (St. Louis University and Indiana University) and the Dagher Laboratory (Indiana University) who contributed significantly to many studies cited in this review.
REFERENCES
- 1.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278: 1910–1914, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Aregger F, Pilop C, Uehlinger DE, Brunisholz R, Carrel TP, Frey FJ, Frey BM. Urinary proteomics before and after extracorporeal circulation in patients with and without acute kidney injury. J Thorac Cardiovasc Surg 139: 692–700, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann S, Koeppen-Hagemann I, Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry 83: 531–538, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner BM, Rector FC. Brenner & Rector's The Kidney. Philadelphia, PA: Saunders Elsevier, 2008, p. 2. [Google Scholar]
- 6.Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, Wu QQ, Hartono JR, Winterberg PD, Lu CY. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int 79: 288–299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol 168: 5817–5823, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Dawnay AB, Cattell WR. Serum Tamm-Horsfall glycoprotein levels in health and in renal disease. Clin Nephrol 15: 5–8, 1981. [PubMed] [Google Scholar]
- 9.Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM, Sher A, Star RA. Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69: 832–836, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dehne MG, Boldt J, Heise D, Sablotzki A, Hempelmann G. [Tamm-Horsfall protein, alpha-1- and beta-2-microglobulin as kidney function markers in heart surgery]. Anaesthesist 44: 545–551, 1995. [DOI] [PubMed] [Google Scholar]
- 11.di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol Renal Physiol 277: F195–F203, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol 283: C905–C916, 2002. [DOI] [PubMed] [Google Scholar]
- 13.El-Achkar TM, Hosein M, Dagher PC. Pathways of renal injury in systemic gram-negative sepsis. Eur J Clin Invest 38, Suppl 2: 39–44, 2008. [DOI] [PubMed] [Google Scholar]
- 14.El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006. [DOI] [PubMed] [Google Scholar]
- 15.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol 304: F1066–F1075, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol 300: F999–F1007, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007. [DOI] [PubMed] [Google Scholar]
- 18.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis 59: 452–461, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eskandari MK, Bolgos G, Miller C, Nguyen DT, DeForge LE, Remick DG. Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J Immunol 148: 2724–2730, 1992. [PubMed] [Google Scholar]
- 21.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hato T, Dagher PC. How the innate immune system senses trouble and causes trouble. Clin J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hato T, El-Achkar TM, Dagher PC. Sisters in arms: myeloid and tubular epithelial cells shape renal innate immunity. Am J Physiol Renal Physiol 304: F1243–F1251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hato T, Winfree S, Kalakeche R, Dube S, Kumar R, Yoshimoto M, Plotkin Z, Dagher PC. The macrophage mediates the renoprotective effects of endotoxin preconditioning. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heitmeier M, McCracken R, Micanovic R, Khan S, El-Achkar TM. The role of tumor necrosis factor alpha in regulating the expression of Tamm-Horsfall protein (uromodulin) in thick ascending limbs during kidney injury. Am J Nephrol 40: 458–467, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoyer JR, Seiler MW. Pathophysiology of Tamm-Horsfall protein. Kidney Int 16: 279–289, 1979. [DOI] [PubMed] [Google Scholar]
- 29.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest 116: 1218–1222, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jennette JC, Heptinstall RH. Heptinstall's Pathology of the Kidney. Philadelphia, PA: Lippincott Williams & Wilkins, 2007. [Google Scholar]
- 31.Kalakeche R, Hato T, Rhodes G, Dunn KW, El-Achkar TM, Plotkin Z, Sandoval RM, Dagher PC. Endotoxin uptake by S1 proximal tubular segment causes oxidative stress in the downstream S2 segment. J Am Soc Nephrol 22: 1505–1516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohda Y, Murakami H, Moe OW, Star RA. Analysis of segmental renal gene expression by laser capture microdissection. Kidney Int 57: 321–331, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22: 317–326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest 120: 331–342, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Semin Nephrol 30: 268–277, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol Renal Physiol 275: F623–F631, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Linkermann A, Himmerkus N, Rolver L, Keyser KA, Steen P, Brasen JH, Bleich M, Kunzendorf U, Krautwald S. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int 79: 169–178, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem 287: 16365–16378, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayeux PR, MacMillan-Crow LA. Pharmacological targets in the renal peritubular microenvironment: implications for therapy for sepsis-induced acute kidney injury. Pharmacol Ther 134: 139–155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaughlin PJ, Aikawa A, Davies HM, Ward RG, Bakran A, Sells RA, Johnson PM. Uromodulin levels are decreased in urine during acute tubular necrosis but not during immune rejection after renal transplantation. Clin Sci (Lond) 84: 243–246, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Micanovic R, Chitteti BR, Dagher PC, Srour EF, Khan S, Hato T, Lyle A, Tong Y, Wu XR, El-Achkar TM. Tamm-Horsfall protein regulates granulopoiesis and systemic neutrophil homeostasis. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science 229: 479–481, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Murakami H, Liotta L, Star RA. IF-LCM: laser capture microdissection of immunofluorescently defined cells for mRNA analysis rapid communication. Kidney Int 58: 1346–1353, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Neuhofer W, Beck FX. Survival in hostile environments: strategies of renal medullary cells. Physiology (Bethesda) 21: 171–180, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994–1007, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Pennica D, Kohr WJ, Kuang WJ, Glaister D, Aggarwal BB, Chen EY, Goeddel DV. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science 236: 83–88, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Pfistershammer K, Klauser C, Leitner J, Stockl J, Majdic O, Weichhart T, Sobanov Y, Bochkov V, Saemann M, Zlabinger G, Steinberger P. Identification of the scavenger receptors SREC-I, Cla-1 (SR-BI), and SR-AI as cellular receptors for Tamm-Horsfall protein. J Leukoc Biol 83: 131–138, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrol Dial Transplant 25: 1896–1903, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 80: 338–347, 2011. [DOI] [PubMed] [Google Scholar]
- 53.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest 112: 460–467, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M. The serum uromodulin level is associated with kidney function. Clin Chem Lab Med 52: 1755–1761, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395, 1994. [DOI] [PubMed] [Google Scholar]
- 56.Safirstein R, Megyesi J, Saggi SJ, Price PM, Poon M, Rollins BJ, Taubman MB. Expression of cytokine-like genes JE and KC is increased during renal ischemia. Am J Physiol Renal Fluid Electrolyte Physiol 261: F1095–F1101, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Safirstein RL. Am I my brother's keeper?: fratricide in the kidney. Kidney Int 79: 149–150, 2011. [DOI] [PubMed] [Google Scholar]
- 58.Schnermann J. Homer W. Smith Award lecture. The juxtaglomerular apparatus: from anatomical peculiarity to physiological relevance. J Soc Nephrol 14: 1681–1694, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 42: 658–676, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol 7: 189–200, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22: 285–294, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341, 2009. [DOI] [PubMed] [Google Scholar]
- 64.Tadagavadi RK, Reeves WB. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J Immunol 185: 4904–4911, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takasu O, Gaut JP, Watanabe E, To K, Fagley RE, Sato B, Jarman S, Efimov IR, Janks DL, Srivastava A, Bhayani SB, Drewry A, Swanson PE, Hotchkiss RS. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med 187: 509–517, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornley C, Dawnay A, Cattell WR. Human Tamm-Horsfall glycoprotein: urinary and plasma levels in normal subjects and patients with renal disease determined by a fully validated radioimmunoassay. Clin Sci (Lond) 68: 529–535, 1985. [DOI] [PubMed] [Google Scholar]
- 67.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, Steinmetz OM, Prinz I, Magnus T, Korn T, Stahl RA, Kurts C, Panzer U. IL-17A production by renal gammadelta T cells promotes kidney injury in crescentic GN. J Am Soc Nephrol 23: 1486–1495, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Usuda N, Yokota S, Hashimoto T, Nagata T. Immunocytochemical localization of d-amino acid oxidase in the central clear matrix of rat kidney peroxisomes. J Histochem Cytochem 34: 1709–1718, 1986. [DOI] [PubMed] [Google Scholar]
- 69.Vasko R, Ratliff BB, Bohr S, Nadel E, Chen J, Xavier S, Chander P, Goligorsky MS. Endothelial peroxisomal dysfunction and impaired pexophagy promotes oxidative damage in lipopolysaccharide-induced acute kidney injury. Antioxid Redox Signal 19: 211–230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol 292: F261–F268, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Yoshida T, Kurella M, Beato F, Min H, Ingelfinger JR, Stears RL, Swinford RD, Gullans SR, Tang SS. Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int 61: 1646–1654, 2002. [DOI] [PubMed] [Google Scholar]
- 72.Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol 22: 999–1006, 2011. [DOI] [PubMed] [Google Scholar]
- 73.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang W, Edwards A. A model of nitric oxide tubulovascular cross talk in a renal outer medullary cross section. Am J Physiol Renal Physiol 292: F711–F722, 2007. [DOI] [PubMed] [Google Scholar]