Abstract
Aging men are susceptible to developing lower urinary tract symptoms, but the underlying etiology is unknown and the influence of dietary and environmental factors on them is unclear. We tested whether a folic acid-enriched diet changed urinary tract physiology and biology in control male mice and male mice with urinary dysfunction induced by exogenous testosterone and estradiol (T+E2), which mimics changing hormone levels in aging humans. T+E2 treatment increased mouse urine output, time between voiding events, and bladder capacity and compliance. Consumption of a folic acid-enriched diet moderated these changes without decreasing prostate wet weight or threshold voiding pressure. One potential mechanism for these changes involves water balance. T+E2 treatment increases plasma concentrations of anti-diuretic hormone, which is offset at least in part by a folic acid-enriched diet. Another potential mechanism involves neural control of micturition. The folic acid-enriched diet, fed to T+E2-treated mice, increased voiding frequency in response to intravesicular capsaicin infusion and increased mRNA abundance of the capsaicin-sensitive cation channel transient receptor potential vanilloid subfamily member 1 (Trpv1) in L6 and S1 dorsal root ganglia (DRG) neurons. T+E2 treatment and a folic acid-enriched diet also modified DNA methylation, which is capable of altering gene expression. We found the enriched diet increased global DNA methylation in dorsal and ventral prostate and L6 and S1 DRG. Our results are consistent with folic acid acting to slow or reverse T+E2-mediated alteration in urinary function in part by normalizing water balance and enhancing or preserving afferent neuronal function.
Keywords: cystometry, mouse model, voiding, folic acid, epigenetics
benign prostatic hyperplasia (BPH) is characterized by benign enlargement of the prostate. BPH is often associated with lower urinary tract symptoms (LUTS) that can include increased urinary frequency and urgency, increased nighttime urination, pain, weak stream, hesitancy, dribbling, incomplete emptying, and incontinence (25). Approximately 70% of men over age 70 experience BPH and/or LUTS (5).
Aging-related changes in plasma hormone concentrations are potential driving factors in the onset and progression of BPH and LUTS. Plasma testosterone concentration declines as men age, while estradiol concentration remains the same or even rises (13). Rodents treated with testosterone and estradiol (T+E2) to mimic this aging-associated hormonal milieu develop enlarged prostates, narrow prostatic urethras, and retain urine in a manner consistent with bladder outlet obstruction (4, 27).
Our group and others have been elucidating proliferative growth pathways in developing prostates with the goal of examining whether these pathways are activated inappropriately in aging men to drive BPH and LUTS (24). We recently showed that DNA methylation of the androgen receptor (Ar) controls the developing mouse prostate's response to androgens, timing of ductal initiation, and quantity of ducts formed (17). Since androgenic signaling and new prostatic ductal growth are implicated in BPH, and since DNA methylation mediates changes in transcript abundance in the aging prostate and elsewhere (10, 14, 35, 37), we considered that aberrant DNA methylation patterns may contribute to BPH and LUTS.
DNA methylation is malleable and influenced by many environmental factors, including diet. This is relevant because dietary factors have been associated with BPH symptoms and bladder function (12). Folic acid is among a handful of dietary constituents that function as methyl donors for DNA methylation (38) and can increase or decrease DNA methylation in a gene- and cell type-specific fashion (3, 8). Furthermore, folic acid deficiency is associated with nocturnal bed wetting in children (1). It is added to most one-a-day and prenatal vitamins, occurs naturally in some foods, and is supplemented in others. Serum folate concentrations have risen ∼2.5-fold in the US population since cereal grain fortification was initiated in 1998 (29). In addition, >40% of American men over age 60 take dietary supplements containing folic acid. This age group is a population at risk for developing BPH/LUTS, but it is unclear whether supplemental folic acid impacts urinary function in these men and whether dietary folic acid may be used as a tool to modify urinary function.
We characterized how dietary folic acid enrichment and T+E2 treatment influenced DNA methylation in mouse prostate, bladder, and L6 and S1 dorsal root ganglia (DRG) of neurons projecting to the bladder, prostate, and elsewhere. We also determined how these factors influence specific aspects of mouse urinary function and test the hypothesis that a folic acid-enriched diet delays or decreases severity of urinary dysfunction in T+E2-treated mice. Our results reveal that a folic acid-enriched diet is capable of modifying several potential contributors to urinary dysfunction in T+E2-treated mice. The diet protects against T+E2-mediated disruption of water balance, increases in bladder capacity and compliance, and sensitizes the voiding response to nociceptive stimulation. Folic acid may mediate the latter action by augmenting afferent Trpv1 mRNA abundance in DRGs associated with afferent neurons that project to the bladder, prostate, and elsewhere. Together, these results indicate that dietary folic acid modifies urinary tract function in mice with obstructive voiding behavior.
MATERIALS AND METHODS
Mice.
Sexually mature C57BL/6J nulliparous female mice were housed in polysulfone cages containing corn cob bedding and maintained on a 12:12-h light-dark cycle at 25 ± 5°C and 20–50% relative humidity. At least 2 wk before their first mating, female mice were placed on a base diet (control diet, Harlan Diet 2019, Harlan Teklad, Madison, WI) containing 4 mg/kg folic acid or fed the same base diet supplemented with folic acid to achieve a final concentration of 24 mg/kg folic acid (folic acid-enriched diet, Harlan Diet 120256). The folic acid-enriched diet provided 10 times more folic acid than the recommended daily requirement for mice (30). This 2-wk loading period was previously shown to be sufficient to increase maternal serum folic acid concentrations (28a) and induce changes in DNA methylation in offspring (38). Food and water were available ad libitum. Food and water bottles were weighed weekly to determine consumption. Dams were maintained on diets throughout lactation, and their offspring received the same diets after weaning and for the remainder of the experiment. Six-week-old male mice underwent sham surgery or surgery to subcutaneously implant testosterone (25 mg) and estradiol (2.5 mg; T+E2) pellets. Two months later, mice either underwent voiding analysis or were euthanized for tissue collection. Urinary tract wet weights (analytic balance) and bladder volume measurements (digital calipers) were determined from mice not undergoing cystometry. All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Serum and urine measurements.
Blood glucose concentrations were determined from tail vein blood collected after a 4-h fasting period with the AlphaTRAK 2 veterinary monitoring system (canine setting, Abbott Laboratories, Abbott Park, IL). Blood was collected by cardiac puncture posteuthanasia, allowed to clot for 30 min, and centrifuged at 6,000 g × 10 min to clear the serum. Serum homocysteine concentrations were determined using a Mouse Homocysteine (Hcy) ELISA Kit (MBS260152, MyBioSource, San Diego, CA). Serum anti-diuretic hormone (ADH) concentration was determined using an arginine vasopressin EIA kit (583951, Cayman Chemical, Ann Arbor, MI). Serum creatinine concentration was determined using a serum creatinine colorimetric assay kit (700460, Cayman Chemical). Free-catch urine was centrifuged at 8,000 g × 8 min, and the supernatant was collected for specific gravity and protein analysis. Urine specific gravity was determined by refractometry (Vet360-Check digital refractometer, feline setting, Reichert Technologies, Depew, NY). Urine protein was quantified using a Pierce BCA protein assay kit (Thermo Fisher Scientific, Madison WI).
DNA methylation determination.
DNA was isolated using a Qiagen DNeasy Blood and Tissue kit (69506, Qiagen, Frederick, MD). Global DNA methylation levels were determined using a colorimetric MethylFlash Methylated DNA Quantification kit (p-1034-96, Epigentek, Farmingdale, NY).
Spontaneous void spot assay.
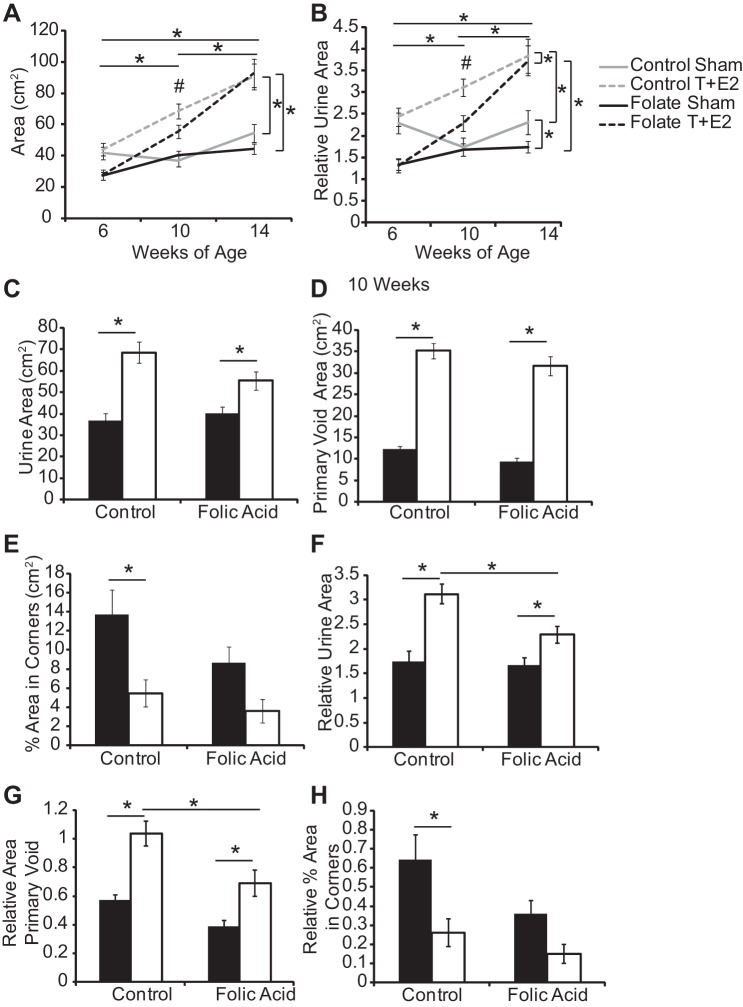
Void spot assays were conducted at 6 (before T+E2 implant), 10, and 14 wk of age and analyzed as described previously (18, 39). Mice were placed in a clean empty cage lined with 3MM Whatman filter paper (057163W, Fisher Scientific). Assays were conducted over 4 h with access to food but not water. Assays were conducted in the same quiet location and time of day unless indicated otherwise. Filter papers were imaged using UV light on a transilluminator and analyzed using Image J Software (Version 1.46r) as described previously (18). Spot number, total urine area, spot size, area within the primary (largest) void, % total urine area in primary void, % total area in the corner of the paper, and % total area in the center of the paper were analyzed as described previously (18, 39). The specific variables of urine spot number, urine area, primary void area, and % area in corners were shown previously to correlate with body mass (18) and were normalized accordingly.
Bladder bath assay.
In vitro bladder bath studies were conducted essentially as described previously (34). Excised bladders were divided longitudinally from apex to bladder neck, and each half was weighed. Sutures (4-0) were used to secure the bladder neck of each hemisection to the specimen arm and the bladder apex to a force displacement transducer (FT-03, Grass Instruments, Quincy, MA). Bladder hemisections were suspended in a 37°C water-jacketed tissue chamber filled with Krebs solution (133 mM NaCl, 16 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 1.4 mM NaH2PO4, 2.5 mM CaCl2 2H2O, 7.8 mM d-glucose, pH 7.2) aerated with 95% O2-5% CO2. Tissues were maintained at a tension of 1 g for 75 min before experimentation, with Krebs solution changed every 15 min. Tension was recorded using the AxoScope Application of pCLAMP Software (Molecular Devices, Sunnyvale, CA). Increasing concentrations (0–10 μM) of the cholinergic agonist carbachol were added stepwise thereafter. Tissues were then washed with carbachol-free Krebs solution to return the tension to baseline, and a maximal contractile response was generated by adding 60 mM KCl to the baths. The maximal response to carbachol was expressed as % maximal response to KCl, and this value was normalized to bladder mass.
Cystometry.
Mice were anesthetized with urethane (1.25 g/kg ip). A midline incision was made in the abdominal wall to expose the bladder. A purse string suture using 6-0 thread was inserted near the dome of the bladder. Catheters were created from PE-50 tubing, cut 2 mm shorter than the length of a 26-G syringe needle, and one end of the tubing was melted to create a cuff. The needle was run through the tubing and inserted through the apex and across the bladder wall. The needle was then removed and the purse string suture tightened, followed by two circumferential sutures to fix the catheter in place. The body wall and skin were sutured, and the animal was allowed to recover for 45 min before saline infusion. During experimentation, the animal was maintained in a supine position, and the cystometry catheter was connected to a three-way stopcock connected to an infusion pump and pressure transducer. Sterile saline was infused at a rate of 1.6 ml/h, and voiding was recorded for at least 1 h for each animal until a stable pattern was achieved. In some mice, capsaicin (30 μM diluted in saline) was instilled at a rate of 1.6 ml/h after mice reached steady-state voiding in response to saline infusion. Measurements were analyzed from at least six sustained voiding events per animal once a consistent pattern was achieved. Voiding events were subdivided into sustained voids, defined here as events where three or more drops of urine were produced, and drip voids, where only one or two drops of urine were produced. Parameters analyzed include intercontractile interval, defined as the length of time between sustained voids; nonvoiding contractions, defined as a change in pressure greater than 5 mmHg not associated with expression of urine; maximal intravesicular pressure associated with each voiding or nonvoiding contraction, defined as the peak to baseline pressure; void duration, defined as the time between maximal intravesicular pressure and return to baseline pressure; threshold pressure, defined as pressure to elicit a voiding contraction and voiding event; capacity, defined as the intervoid interval multiplied by the saline infusion rate; compliance, defined as capacity divided by the change in pressure between voids; and leak point pressure, defined as pressure required to induce a void by applying pressure to the bladder filled to half-capacity as described previously (22).
Real-time quantitative PCR.
Quantitative PCR (QPCR) was conducted as described previously (19) on bladder or DRGs from five mice per experimental treatment group using gene-specific primers for Trpv1, 5′-TCCTGAAGTGCATGAGGAAGG-3′ and 5′-CCACGTTGGTGTTCCAGGTAG-3′. Relative mRNA abundance was determined by the ΔΔCt method as described previously (23) and normalized to peptidyl prolyl isomerase a (Ppia) abundance.
Statistics.
Results are reported as means ± SE. Statistical analysis was performed using R version 2.13.1. Homogeneity of variance was determined using Levene's test. For parametric data with two treatment groups, Student's t-test was used to identify significant differences (P ≤ 0.05) between groups. For parametric data with more than two treatment groups, one-way ANOVA, followed by Tukey's honest significant difference (HSD), least significant difference (LSD), or a repeated measures ANOVA followed by pairwise t-tests using Holm correction were used to identify significant differences (P ≤ 0.05) between or among treatment groups. For nonparametric data with more than two treatment groups, the Kruskal-Wallis test was conducted. Contingency tables were analyzed using Fisher's exact test.
RESULTS
Here, we characterize how dietary folic acid enrichment and T+E2 treatment influence prostate and urinary function. Increased dietary folic acid levels can be encountered throughout life. We modeled this exposure paradigm by enriching folic acid levels in male mice starting at conception and continuing through adulthood. Male mice were maintained on diets for 8 wk following T+E2 pellet implantation surgery to create four experimental groups: control diet sham, control diet T+E2, folic acid diet sham, and folic acid diet T+E2 (Fig. 1).
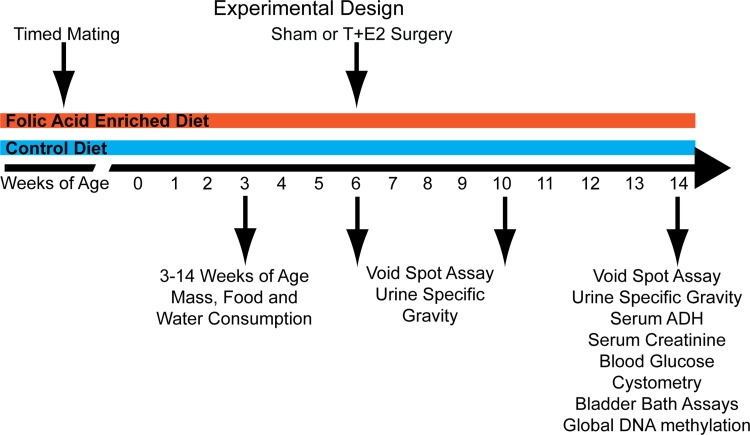
Fig. 1.
Experimental design. C57BL/6J dams were maintained on a control (4 mg/kg folic acid) or control diet supplemented with folic acid (24 mg/kg folic acid) for 2 wk before mating. Timed matings were performed, and the resulting male offspring were maintained on maternal diets through lactation, weaning, and adulthood. Body mass and food and water consumption were measured at weekly intervals starting at 3 wk of age and continuing through the end of the experiment at 14 wk of age. Six-week-old adult male mice underwent subcutaneous implantation of testosterone and estradiol (T+E2) pellets. Physiology and voiding function were determined 1 or 2 mo later at 10 and 14 wk of age, respectively.
Influence of diet and T+E2 treatment on serum homocysteine concentration, mouse body mass, and food consumption over time.
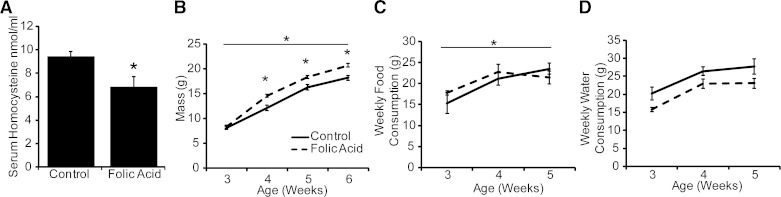
We tested whether a folic acid-enriched diet was sufficient to decrease plasma homocysteine concentration, a stable biomarker inversely related to plasma folic acid concentration. Serum homocysteine concentrations were significantly lower in 14-wk-old adult mice exposed to a folic acid-enriched diet in utero and through adulthood compared with mice receiving a control diet (Fig. 2A). T+E2 treatment alone did not impact homocysteine levels (results not shown). Therefore, the folic acid-enriched diet is sufficient to cause changes in methyl donor levels and may be responsible for observed changes in physiology.
Fig. 2.
A folic acid-enriched diet decreases serum homocysteine concentration. A: male mice were maintained on a control or folic acid-enriched diet in utero and through lactation and adulthood. Serum homocysteine concentration was quantified by ELISA. Values are means ± SE. *Significant difference, P ≤ 0.05; n = 4. Male mice were maintained on the maternal control or folic acid-enriched diet through lactation and weaning. Quantification of body mass (B), weekly food consumption (C), and weekly water consumption (D) from 3 to 6 wk of age is shown. Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥10/group.
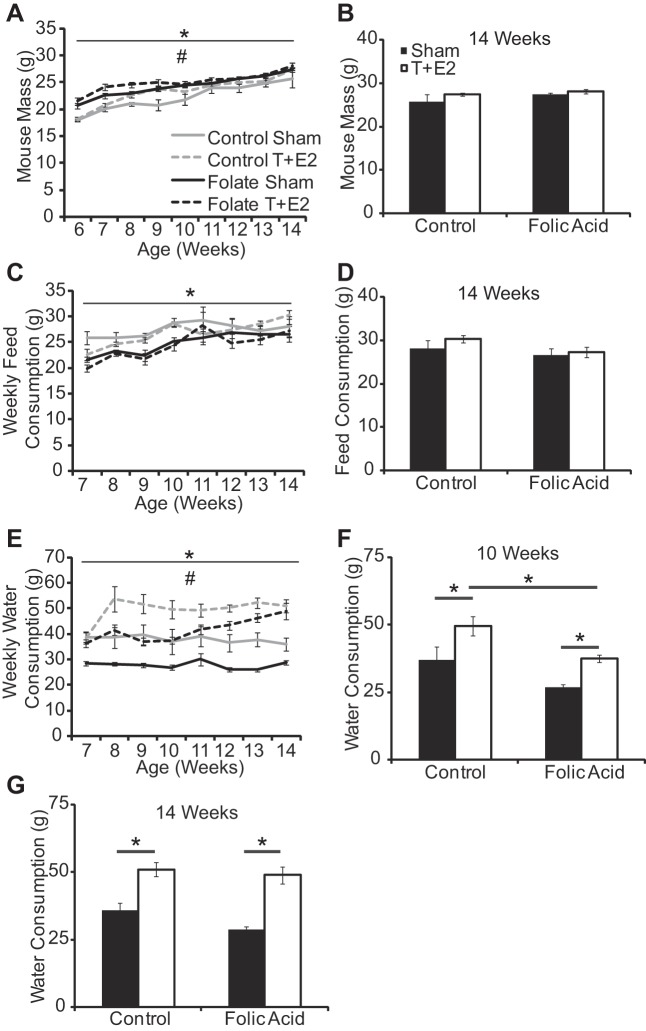
Folic acid enrichment of the standard mouse diet has the potential to influence food palatability and therefore consumption by pregnant dams as well as their offspring. This could impact several modifiers of urinary function, including water consumption, body mass, and development of prostate, bladder, and other urinary tract tissues. To address these issues, we examined the number of prostatic ductal precursors (buds) formed in male mouse fetuses and found they were not affected by the maternal diet (20). We also found that the folic acid-enriched diet increased male mouse body mass at 4–6 wk of age (Fig. 2B). There was a significant main effect of time, treatment group, and an interaction of these variables on body mass after T+E2 implantation or sham surgery (Fig. 3A, Supplemental Table 2; all supplemental material for this article is accessible on the journal website). The folic acid-enriched diet and T+E2 treatment increased body mass at select stages of early adulthood, but these differences are not present at 14 wk of age when urinary function and prostate and urethral biology were assessed (Fig. 3, A and B, Supplemental Table 1).
Fig. 3.
A folic acid-enriched diet slows a T+E2-induced increase in water consumption. Adult male mice were treated as described in Fig. 1. Mouse body mass (A and B), weekly food consumption (C and D), and weekly water consumption (E–G) were quantified from 0 to 8 wk postsurgery. Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥5/group. #Significant interaction between time and treatment group.
Food consumption increased equally over time in both diet groups at 3–5 wk of age (Fig. 2C). Following T+E2 implantation or sham surgery, there was a significant main effect of time and treatment group on food consumption (Fig. 3C, Supplemental Table 2). The folic acid-enriched diet decreased food consumption at 7–8 wk of age (Fig. 3C, Supplemental Table 1), but food consumption did not differ between diet groups from 11 to 14 wk of age, when most physiological measurements were made (Fig. 3D). Differences in body mass and food consumption diminished as mice aged; as a result, these factors do not likely account for all differences observed at later time points when most physiological measurements were made.
We did not observe a difference in water consumption between diet groups at 3–5 wk of age (Fig. 2D). There was a significant main effect of time, treatment group, and an interaction of these variables on water consumption after surgery (Fig. 3E, Supplemental Table 2). T+E2 treatment increased water intake at select stages in both diet groups (Fig. 3E, Supplemental Table 1). The folic acid-enriched diet decreased water intake at select stages in sham mice and T+E2-treated mice (Fig. 3E, Supplemental Table 1). Most importantly, the folic acid-enriched diet decreased the absolute magnitude of water intake in T+E2-treated mice until 10 wk of age (Fig. 3, F and G). These results indicate that T+E2 treatment rapidly (within 2 wk) increased fluid intake and that the folic acid-enriched diet moderated the magnitude of this response.
A folic acid-enriched diet and T+E2 treatment change water balance in mice.
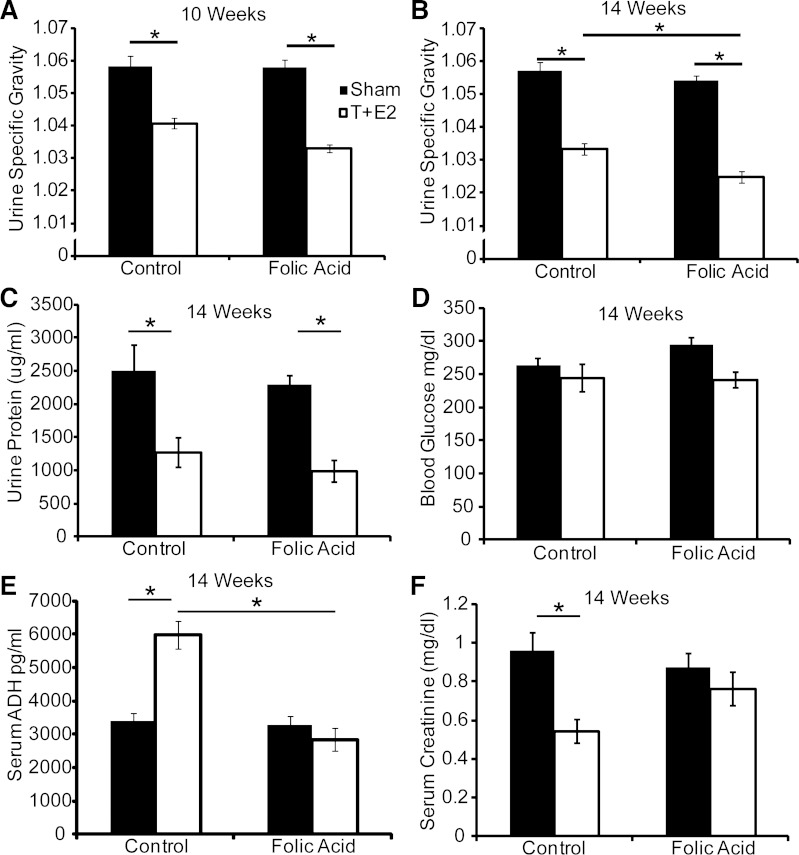
The fact that T+E2 increased fluid intake prompted us to examine whether urine concentration was also affected. Urine specific gravity and protein concentration were both decreased by T+E2 treatment (Fig. 4, A–C). Changes in water consumption are often associated with aberrant endocrine regulation. Diabetes mellitus, which is associated with diuresis and excessive thirst, is an unlikely contributor since fasting blood glucose concentrations were not different between groups (Fig. 4D). However, we found that T+E2 treatment in control diet mice increased serum ADH and decreased serum creatinine concentrations (Fig. 4, E and F) and that the folic acid-enriched diet protected against these changes (Fig. 4, E and F). Therefore, the folic acid-enriched diet may influence urinary function in T+E2-treated mice by normalizing water balance.
Fig. 4.
A folic acid-enriched diet protects against T+E2-induced increases in serum anti-diuretic hormone (ADH) concentration. Adult male mice were treated as described in Fig. 1. A and B: urine specific gravity was measured at 1 and 2 mo postsurgery; n ≥10/group. Other measurements included urine protein concentration (C), blood glucose concentration (D), serum anti-ADH concentration (E), and serum creatinine concentration (F) at 2 mo postsurgery. Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥4/group.
A folic acid-enriched diet reduced T+E2-induced abnormalities in voiding function.
T+E2-treated mice exhibit abnormal voiding function (26, 27). We conducted the spontaneous void spot assay at defined intervals (baseline, 1 and 2 mo postsurgery) to test whether the time course of T+E2-induced changes in voiding function was further impacted by diet. There was a significant main effect of time and group and an interaction of these variables on total urine area, relative total urine area and relative area of primary void (Fig. 5, A and B, Supplemental Table 4). T+E2 treatment 1 mo postsurgery increased total urine area, primary void area, and decreased the percentage of urine area within corners in control-fed mice (Fig. 5, Supplemental Table 3); when normalized to body mass, the folic acid-enriched diet partially moderated these changes (Fig. 5, F–H). The only changes which persisted until 14 wk of age were those observed in urine area (Supplemental Table 3).
Fig. 5.
A folic acid-enriched diet protects against some T+E2-induced changes in void spot assay parameters. Adult male mice were treated as described in Fig. 1. Void spot assays were conducted at baseline, 1 mo, and 2 mo postsurgery. Shown are changes in urine area (A) and relative urine area (B) over time and quantification of urine area (C), primary void area (D), and percent area in corners (E) at 1 mo postsurgery. Void spot assay parameters were normalized to mouse body mass and include relative urine area (F), relative area in primary void (G), and relative percent area in corners (H). Values are means ± SE. *Significant difference, P ≤ 0.05. For repeated measures analysis, vertical bars indicate differences between treatment groups, and horizontal bars indicate differences over time. #Significant interaction between time and treatment group, P ≤ 0.05; n ≥5/group.
Changes in prostate DNA methylation accompany folic acid-enriched diet and T+E2 treatment.
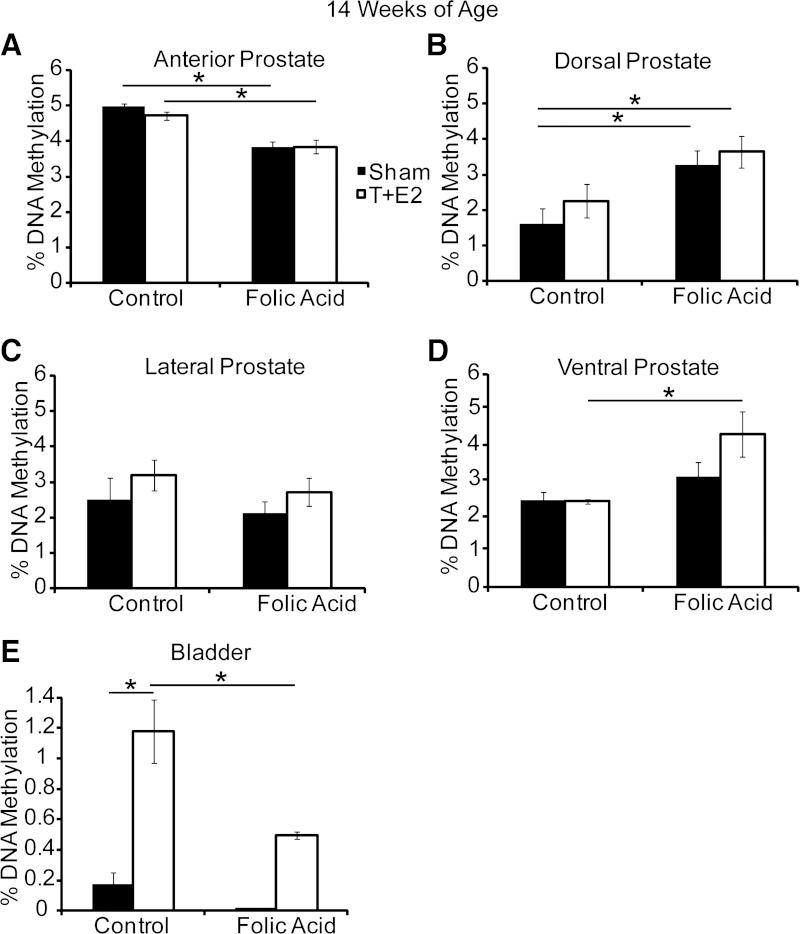
Changes in prostate and/or bladder growth and physiology could underlie the protective effects of a folic acid-enriched diet on specific aspects of urinary dysfunction in T+E2-treated mice. We first examined the prostate, which grows larger with T+E2 treatment (27). Since folic acid can influence DNA methylation, and since DNA methylation regulates prostatic growth in the mouse fetus (17), we also tested whether T+E2 treatment or the folic acid-enriched diet changes prostate or bladder global DNA methylation and prostatic wet weight. Prostate global DNA methylation was affected by the folic acid-enriched diet, but the observed effects were different among prostate lobes: DNA methylation was decreased by the folic acid-enriched diet in the anterior prostate, increased in the dorsal prostate, and unchanged in the lateral prostate (Fig. 6, A–C). While these effects of the folic acid-enriched diet were observed in both control and hormone-treated mice, the diet uniquely increased ventral prostate global DNA methylation in T+E2-treated mice (Fig. 6D). T+E2 treatment increased global DNA methylation in the bladder, and the folic acid-enriched diet protected against this increase (Fig. 6E).
Fig. 6.
A folic acid-enriched diet and T+E2 induce lobe-specific changes in prostate and bladder global DNA methylation. Adult male mice were treated as described in Fig. 1. Percent global DNA methylation was measured in anterior (A), dorsal (B), lateral (C), and ventral prostate (D), and the bladder (E) at 14 wk of age. Values are means ± SE. *Significant difference, P ≤ 0.05; n = 5/group.
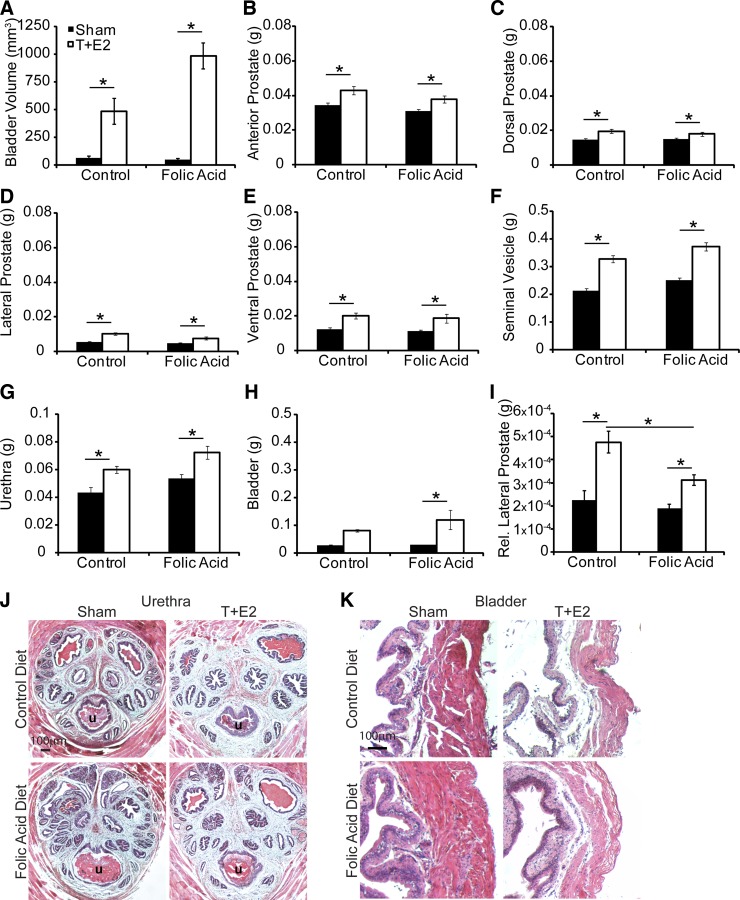
T+E2 treatment increased bladder volume and wet weight of the prostate, seminal vesicle, and urethra in both diet groups at time of euthanasia (Fig. 7). Bladder wet weight increased in T+E2-treated mice fed the folic acid-enriched diet (Fig. 7). Normalization of prostate wet weight to body mass did not change relative differences between groups, except for relative lateral prostate weight. Relative lateral prostate weight was increased by T+E2 treatment in both diet groups, but among T+E2-treated mice relative lateral prostate mass was reduced with the folic acid-enriched diet compared with control diet group (Fig. 7I). We also confirmed that a folic acid-enriched diet does not cause overt changes in lower urinary tract histology (Fig. 7, J and K). These results provide evidence that changes in prostate mass or lower urinary tract histology are unlikely to mediate the protective actions of folic acid on T+E2-induced voiding dysfunction.
Fig. 7.
A folic acid-enriched diet does not protect against T+E2 increases in bladder volume and prostate wet weight. Adult male mice were treated as described in Fig. 1. The following parameters were quantified at time of euthanasia in animals not undergoing cystometry: bladder volume (A), wet weights of anterior prostate (B), dorsal prostate (C), lateral prostate (D), ventral prostate (E), seminal vesicle (F), urethra (G), bladder (H), and relative lateral prostate (I). Lower urinary tract histology was examined in hematoxolin- and eosin-stained cross sections of urethra (J) and bladder (K). Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥10/group.
A folic acid-enriched diet and T+E2 treatment alter bladder physiology.
Since we observed changes in bladder global DNA methylation (Fig. 6E), we postulated that the folic acid-enriched diet could also impact bladder function in T+E2-treated mice. We conducted in vitro bladder bath studies and evaluated contractile responses to carbachol and potassium chloride. Responses were normalized to bladder strip wet weight to account for differences in bladder size between sham and T+E2 mice. T+E2 treatment in both diet groups decreased the maximal contractile response elicited by carbachol, but the carbachol potency (EC50) was the same across groups (Fig. 8).
Fig. 8.
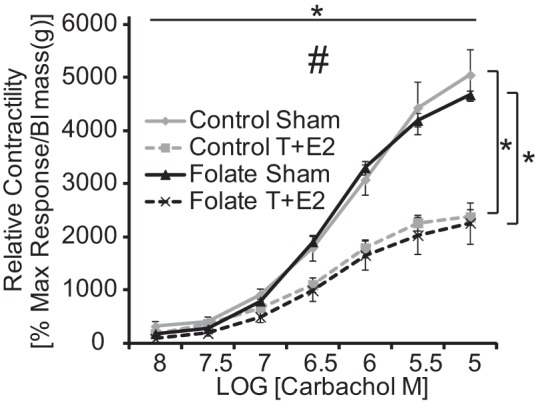
T+E2 reduces bladder contractility. Adult male mice were treated as described in Fig. 1. Relative bladder contractility (percent maximum response to KCl normalized to bladder mass) was determined in isolated bladder strips exposed to graded concentrations of the cholinergic agonist carbachol. Values are means ± SE. *Significant difference, P ≤ 0.05. Vertical bars indicate differences between treatment groups, and horizontal bars indicate differences over time. #Significant interaction between change in carbachol concentration and treatment group, P ≤ 0.05; n = 5/group.
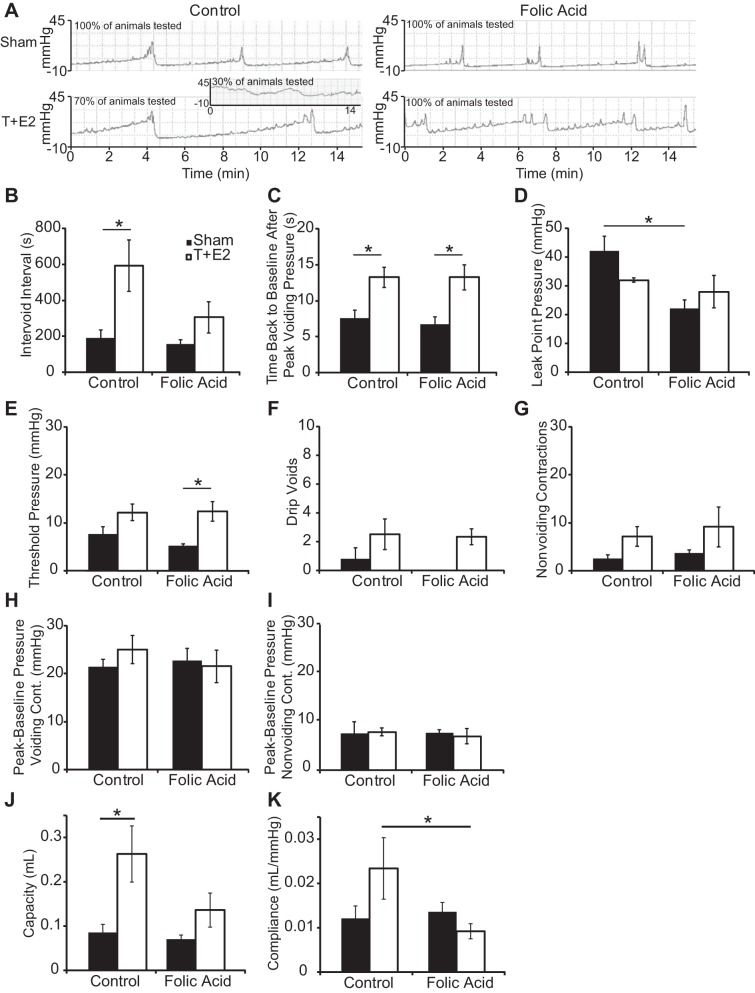
Cystometry was performed on urethane-anesthetized mice to analyze bladder response to filling and emptying, end points requiring appropriate neural function. T+E2 treatment reduced the number of control-diet fed mice that elicited a voiding contraction during the monitoring period (2 h/mouse), and the folic acid-enriched diet protected against this decrease (Table 1). In the subset of mice that voided during the monitoring period, T+E2 treatment increased intervoid interval and void duration, measured as the time to return to baseline after maximal intravesicular pressure of a voiding contraction (Fig. 9, A–C). The folic acid diet protected against the T+E2-induced increase in intervoid interval (Fig. 9B). The folic acid-enriched diet also decreased leak point pressure in sham mice (Fig. 9D) and increased threshold pressure in T+E2-treated mice (Fig. 9E). Neither the folic acid-enriched diet nor T+E2 treatment altered the following parameters over an interval of six sustained voids (3 or more drops): total number of drip voids (1–2 drops), number of nonvoiding contractions, or peak-to-baseline pressure of voiding and nonvoiding contractions (Fig. 9, F–I). Cystometry in anesthetized mice was also used to measure bladder capacity and compliance, indicators of bladder remodeling and function. T+E2 treatment increased bladder capacity, and the folic acid-enriched diet protected against this increase (Fig. 9J). The folic acid-enriched diet also maintained bladder compliance at control levels even in the presence of T+E2 (Fig. 9K). Together, these results indicate that T+E2 treatment altered voiding parameters consistent with obstruction and the folic acid-enriched diet moderated at least some of these end points.
Table 1.
Mice eliciting a voiding contraction during a 2-h cystometry monitoring period
| n | % Total Mice Able To Elicit Voiding Contraction Over 2-h Period | |
|---|---|---|
| Control sham | 9 | 100 |
| Control T+E2 | 10 | 70 |
| Folate sham | 12 | 100 |
| Folate T+E2 | 12 | 100 |
| P value | 0.01 |
See the text for definitions.
Fig. 9.
A folic acid-enriched diet protects against a T+E2-induced increase in intervoid interval and bladder capacity and compliance. Adult male mice were treated as described in Fig. 1. At 2 mo postsurgery, urethane-anesthetized mice were analyzed by cystometry. A: representative cystometrograms from animals in each treatment group which elicited voiding contractions. Inset: representative of the 30% of T+E2 mice on the control diet which did not elicit a voiding contraction during the 2-h monitoring period and instead showed a steady rise in pressure with no apparent voiding contractions. The following parameters were quantified from the remainder of mice: intervoid interval (B), time back to baseline after peak voiding pressure (C), leak point pressure (D), threshold pressure to elicit a void (E), drip voids (1–2 drops) over a 6-sustained void (3 or more drops) interval (F), number of nonvoiding contractions over a 6-sustained void interval (G), peak-to-baseline pressure of voiding contractions (H), peak-to-baseline pressure of nonvoiding contractions (I), bladder capacity (J), and bladder compliance (K). Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥5/group.
The inability of some control diet T+E2-treated mice to generate bladder contractions, and restoration of this function with a folic acid-enriched diet (Supplemental Table 1), could derive from treatment-related differences in afferent sensation. To test this hypothesis, we focused on capsaicin-sensitive TRPV1 receptor function. Capsaicin activates TRPV1 receptors, and ∼60% of bladder afferent neurons are capsaicin sensitive (16). Capsaicin was delivered by intravesicular infusion to urethane-anesthetized mice and decreased the interval between sustained voids (consisting of 3 or more drops) (Fig. 10A). We next examined the total number of drip voids (1–2 drops) within the interval of six sustained voids for each group. Diet modified the T+E2-treated mouse response to capsaicin by increasing the frequency of drip voids over a six sustained void interval (Fig. 10B). Capsaicin also ameliorated the T+E2-induced increase in time for bladder pressure to return to baseline after a void in mice from both diet groups (Fig. 10C). These results indicate that capsaicin increases voiding frequency in all mice tested and that the folic acid-enriched diet in T+E2 mice may sensitize to afferent stimulation and bladder filling.
Fig. 10.
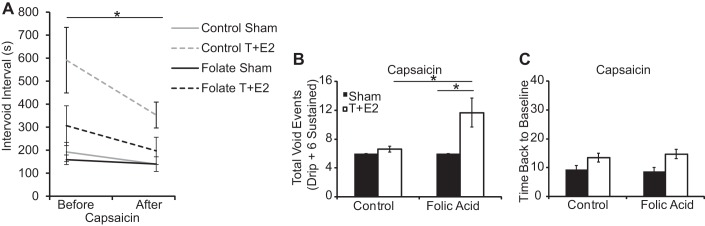
A folic acid-enriched diet enhances T+E2 bladder sensitivity to afferent stimulation. Adult male mice were treated as described in Fig. 1. At 2 mo postsurgery, capsaicin was administered by intravesicular infusion into urethane-anesthetized mice. The following parameters were determined: intervoid interval of sustained voids (3 more drops) before vs. after capsaicin infusion (A), number of voiding events defined as the number of drip voids (1–2 drops) occurring over the course of a 6-sustained void (3 or more drops) interval following capsaicin infusion (B), and time back to baseline after peak voiding pressure (C). Values are means ± SE.*Significant difference, P ≤ 0.05; n ≥3/group.
A folic acid-enriched diet increased nociceptor transcript abundance in dorsal root ganglia of T+E2-treated mice.
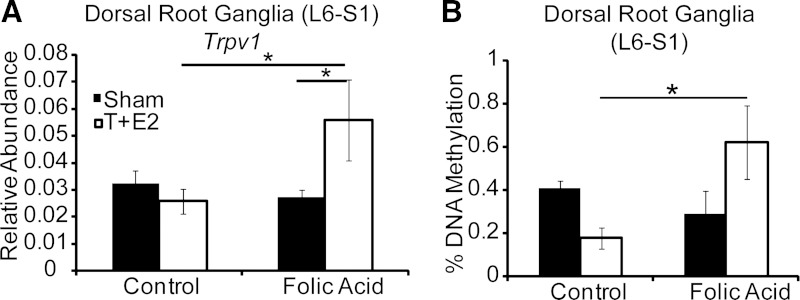
Changes in nociceptor abundance are one possible mechanism to explain the increase in voiding events observed with capsaicin. We found that mRNA abundance for the capsaicin-sensitive cation channel Trpv1 was greater in L6 and S1 DRGs from T+E2 mice receiving the folic acid-enriched diet vs. the control diet (Fig. 11A). Increased Trpv1 abundance could explain enhanced capsaicin sensitivity in folic acid-treated mice as shown in Fig. 10. Together, these results suggest increased bladder afferent stimulation may occur in T+E2 mice on the folic acid-enriched diet through increased Trpv1 expression.
Fig. 11.
A folic acid-enriched diet increases T+E2-induced Trpv1 mRNA abundance and dorsal root ganglia (DRG) global DNA methylation. Adult male mice were treated as described in Fig. 1. A: relative abundance of Trpv1 mRNA in L6 and S1 dorsal root ganglia 2 mo postsurgery. B: global DNA methylation in DRG 2 mo postsurgery. Values are means ± SE. *Significant difference, P ≤ 0.05; n ≥3/group.
We next examined global DNA methylation of L6 and S1 DRGs, which could potentially influence Trpv1 abundance. Although DRG global DNA methylation was affected by neither diet nor T+E2 treatment alone (Fig. 11B), it was uniquely increased by the folic acid-enriched diet in T+E2-treated mice (Fig. 11B). These results indicate the combination of a folic acid-enriched diet and hormone treatment changes DRG global DNA methylation and Trpv1 mRNA abundance.
DISCUSSION
Here, we reveal new end points of voiding dysfunction in T+E2-treated mice and show that a folic acid-enriched diet modifies voiding function in sham animals as well as in T+E2-treated mice. Consumption of a folic acid-enriched diet by control mice altered global prostate DNA methylation and decreased voiding leak point pressure. T+E2 treatment increased mouse urine output, time between voiding events, bladder capacity, and serum concentrations of ADH. Consumption of a folic acid-enriched diet moderated these T+E2 effects. The folic acid-enriched diet in T+E2-treated mice decreased urine output and time between voiding events, prevented an increase in serum ADH, bladder capacity and compliance, altered global DNA methylation in prostate, bladder, and DRG neurons, enhanced expression of Trpv1 in DRGs, and increased voiding in response to capsaicin. Overall, our results indicate that T+E2 treatment induces obstruction and urinary dysfunction and a folic acid-enriched diet is capable of moderating some of these changes. Whether the diet prevents, slows, or reverses T+E2-induced changes in obstruction and bladder decompensation is an area for future study. Our results argue against a reversal or inhibition of T+E2-induced increases in prostate wet weight as the primary mechanism of folic acid action since this end point was unaltered by diet, but reveal potential alternative mechanisms, including alterations in neural control of micturition and systemic water balance.
T+E2 treatment was shown previously to induce structural changes in the prostate and prostatic urethra that could potentially impair urinary flow and cause urinary obstruction. These structural changes were characterized by increased mouse prostate wet weight, increased ductal area within the periurethral region, and reduced urethral cross-sectional area (27). The physiological outcome of these changes with respect to mouse voiding function was not previously examined. Bladder outlet obstruction increases voiding pressure in men and rodents (2, 28, 31, 33). In the current study, a folic acid-enriched diet failed to moderate the T+E2-induced increase in prostate wet weight and threshold pressure (Figs. 7 and 9). Thus the effects of a folic acid-enriched diet in a T+E2 model of voiding dysfunction are not likely to be mediated by effects on prostate or urethral size. Whether a high folic acid diet prevents T+E2 induced urethral narrowing or fibrosis or mediates changes in myogenic and viscoelastic elements of the bladder is an area for future study.
Restoration of water balance in T+E2-treated mice consuming a folic acid-enriched diet is another potential mechanism by which the folic acid-enriched diet could moderate urinary dysfunction in T+E2-treated mice. We made the discovery that T+E2 treatment changes many aspects of systemic water balance and these changes are consistent with T+E2-induced nephrogenic diabetes insipidus. They include increased water consumption (Fig. 3, E–G) and urine production (Fig. 5A), decreased urine specific gravity (Fig. 4, A and B) and urine protein concentration (Fig. 4C), decreased serum creatinine (Fig. 4F), and increased serum ADH (Fig. 4E). Consumption of the folic acid-enriched diet protects against the T+E2-induced changes in serum creatinine and ADH (Fig. 4, E and F). Further investigation into how T+E2 treatment influences these end points is warranted, particularly if T+E2 treatment causes mice to become resistant to ADH challenge and if the folic acid-enriched diet alters the time course of this resistance. These results further indicate that T+E2 treatment may have direct effects on bladder function independently of changes in prostate mass. This is supported by the fact that T+E2 treatment in female rats, which lack a prostate, have increased bladder capacity and altered histology compared with controls (7).
It is likely that changes in T+E2-treated mouse bladder function arise due to a combination of changes in the prostate as well as a direct effect on bladder tissue. Our results reveal bladder afferent sensitization as a potential mechanism by which the folic acid-enriched diet could improve T+E2-treated mouse urinary function. Chronic T+E2 treatment strikingly reduced detrusor cross-sectional thickness, caused bladder fibrosis (27), and reduced the percentage of mice eliciting a voiding contraction in response to intravesicular saline (Table 1). Surprisingly, bladder strips from T+E2-treated mice remain capable of contracting in response to cholinergic stimulation in vitro, indicating at least some retention of functional activity (Fig. 8). These results are consistent with those observed with aging (32). We tested whether dietary folic acid enrichment retains or restores bladder sensitivity to filling in T+E2-treated mice by pharmacologically stimulating an afferent pathway mediated by TRPV1. Intravesicular capsaicin infusion into bladders of T+E2-treated mice uniquely (albeit temporarily) increased the frequency of drip voids in mice consuming a folic acid-enriched diet but not in mice consuming a control diet (Fig. 10B). These data indicate a potentially heightened afferent sensitivity in bladders of mice consuming the folic acid-enriched diet and raise questions about whether it would also augment urinary function in other mouse models of bladder outlet obstruction. It is also possible that a folic acid-enriched diet may protect against changes in lower urinary tract function induced by other insults (genetic or environmental) which converge upon bladder afferent sensitivity or detrusor function.
One interpretation of increased Trpv1 mRNA abundance in mice consuming the folic acid enriched- compared with the control diet is that it reflects differences in the stage of progression to bladder decompensation. Bladder obstruction, irritation, and hypertrophy have been shown to increase afferent sensitivity in animals and humans (6, 11, 31). However, downregulation of nicotinic acetylcholine receptors in sensory neurons occurs after bladder outlet obstruction (6). This downregulation is thought to happen as a decompensation mechanism for initial increased sensitivity. These data are important because they establish a time course: afferent hyperexcitability first, bladder decompensation second. It is therefore possible that the folic acid diet delays T+E2-induced bladder decompensation vs. the control diet. This notion is supported by the results showing that the folic acid-enriched diet moderates the T+E2-induced increase in relative urine area, intervoid interval, and bladder capacity and compliance (Figs. 5F and 9, B, J, and K) and increases the ability of T+E2 mice to elicit a voiding contraction (Table 1) and respond to capsaicin (Fig. 10).
We also identified T+E2- and folic acid diet-dependent changes in global DNA methylation of L6 and S1 DRG neurons and bladder. While we cannot speculate whether this change was specific to L6 and S1 DRG neurons, or occurred generally across all DRG neurons, these findings are significant since this is the first link between altered DNA methylation and T+E2-mediated changes in urinary function. DNA methylation and histone acetylation are known regulators of gene expression in rat nociceptive DRG neurons (15). Environmental stressors increase DNA methyltransferase 1 (Dnmt1) abundance uniquely in rat L6-S2 DRG neurons, which increases promoter DNA methylation of cannabinoid receptor 1 and decreases its expression (15). This relieves repression of TRPV1 and thereby increases TRPV1 expression and visceral pain sensitivity (15). Cannabinoid receptors and TRPV1 are important mediators of bladder afferent signaling, nociception, and voiding function (9, 36). Whether gene-specific DNA methylation and expression of cannabinoid and TRPV1 channels are affected by T+E2 and a folic acid-enriched diet and whether this is unique to L6 and S1 DRG neurons remains to be determined. Additionally, it has recently been shown that changes in chromatin structure are responsible for changes in Trpv1 expression (15), and the possibility that changes in chromatin structure are responsible for the changes in Trpv1 expression we observed is an area for future study (15). While the focus of this study was DNA methylation, folic acid on its own or through its ability to decrease serum homocysteine can also act as an antioxidant and can have beneficial actions on endothelial cell function and collagen deposition (29a). How these other end points contribute to urinary function and the timing of these events remain to be determined.
A new question that has emerged from this study is whether the folic acid-enriched diet moderates specific aspects of T+E2-induced urinary dysfunction by influencing urinary tract development or by interfering with T+E2 insult later in life. Whether exposure to a high folic acid diet in utero alone can act as a preventative or in adulthood alone can act as a treatment for urinary tract dysfunction remains to be determined.
GRANTS
This work was supported by National Institutes of Health Grants DK099328, DK096074, and DK104310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.P.K., L.L.A., W.A.R., D.E.B., and C.M.V. provided conception and design of research; K.P.K., L.L.A., H.M.A., and C.M.V. performed experiments; K.P.K., H.M.A., and C.M.V. analyzed data; K.P.K., L.L.A., H.M.A., Z.W., P.W., W.A.R., D.E.B., and C.M.V. interpreted results of experiments; K.P.K. and C.M.V. prepared figures; K.P.K. and C.M.V. drafted manuscript; K.P.K., L.L.A., H.M.A., Z.W., P.W., W.A.R., D.E.B., and C.M.V. edited and revised manuscript; K.P.K., L.L.A., H.M.A., Z.W., P.W., W.A.R., D.E.B., and C.M.V. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Altunoluk B, Davutoglu M, Garipardic M, Bakan V. Decreased vitamin b(12) levels in children with nocturnal enuresis. ISRN Urol 2012: 789706, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and myosin isoform expression. J Urol 172: 1524–1528, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barua S, Chadman KK, Kuizon S, Buenaventura D, Stapley NW, Ruocco F, Begum U, Guariglia SR, Brown WT, Junaid MA. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PloS One 9: e101674, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernoulli J, Yatkin E, Konkol Y, Talvitie EM, Santti R, Streng T. Prostatic inflammation and obstructive voiding in the adult Noble rat: impact of the testosterone to estradiol ratio in serum. Prostate 68: 1296–1306, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 132: 474–479, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Bschleipfer T, Nandigama R, Moeller S, Illig C, Weidner W, Kummer W. Bladder outlet obstruction influences mRNA expression of cholinergic receptors on sensory neurons in mice. Life Sci 91: 1077–1081, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Cayan F, Tek M, Balli E, Oztuna S, Karazindiyanoglu S, Cayan S. The effect of testosterone alone and testosterone + estradiol therapy on bladder functions and smooth muscle/collagen content in surgically menopause induced rats. Maturitas 60: 248–252, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Crider KS, Quinlivan EP, Berry RJ, Hao L, Li Z, Maneval D, Yang TP, Rasmussen SA, Yang Q, Zhu JH, Hu DJ, Bailey LB. Genomic DNA methylation changes in response to folic acid supplementation in a population-based intervention study among women of reproductive age. PloS One 6: e28144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly D, Rong W, Chess-Williams R, Chapple C, Grundy D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J Physiol 583: 663–674, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damaschke NA, Yang B, Bhusari S, Svaren JP, Jarrard DF. Epigenetic susceptibility factors for prostate cancer with aging. Prostate 73: 1721–1730, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jongh R, van Koeveringe GA, van Kerrebroeck PE, Markerink-van Ittersum M, de Vente J, Gillespie JI. Damage to the bladder neck alters autonomous activity and its sensitivity to cholinergic agonists. BJU Int 100: 919–929, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Espinosa G. Nutrition and benign prostatic hyperplasia. Curr Opin Urol 23: 38–41, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Henrique R, Jeronimo C, Hoque MO, Carvalho AL, Oliveira J, Teixeira MR, Lopes C, Sidransky D. Frequent 14-3-3 sigma promoter methylation in benign and malignant prostate lesions. DNA Cell Biol 24: 264–269, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology 148: 148–157, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu-Tsai M, Winter J, Woolf CJ. Regional differences in the distribution of capsaicin-sensitive target-identified adult rat dorsal root ganglion neurons. Neurosci Lett 143: 251–254, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Keil KP, Abler LL, Laporta J, Altmann HM, Yang B, Jarrard DF, Hernandez LL, Vezina CM. Androgen receptor DNA methylation regulates the timing and androgen sensitivity of mouse prostate ductal development. Dev Biol 396: 237–245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil KP, Abler LL, Altmann HM, Bushman W, Marker PC, Li L, Ricke WA, Bjorling DE, Vezina CM. Influence of animal husbandry practices on void spot assay outcomes in C57BL/6J male mice. Neurourol Urodyn [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keil KP, Mehta V, Branam AM, Abler LL, Buresh-Stiemke RA, Joshi PS, Schmitz CT, Marker PC, Vezina CM. Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology 153: 6091–6103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keil KP, Peterson RE, Vezina CM. A maternal methyl donor-enriched diet, capable of altering fetal DNA methylation, does not prevent fetal prostate development from inhibition by TCDD. In: Organohalogen Compound Database. Dioxin; 2014: 843, 2014. [Google Scholar]
- 22.Lee SH, Lysiak JJ, Steers WD. Bladder and urethral function in a mouse model of cavernous nerve injury. Neurourol Urodyn 32: 1038–1043, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 24.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol 15: 340–345, 1978. [PubMed] [Google Scholar]
- 25.McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, Foster HE Jr, Gonzalez CM, Kaplan SA, Penson DF, Ulchaker JC, and Wei JT. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 185: 1793–1803, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson TM, Moses MA, Uchtmann KS, Keil KP, Bjorling DE, Vezina CM, Wood RW, Ricke WA. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J Urol 193: 722–729, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology 153: 5556–5565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitti VW. Pressure flow urodynamic studies: the gold standard for diagnosing bladder outlet obstruction. Rev Urol 7, Suppl 6: S14–S21, 2005. [PMC free article] [PubMed] [Google Scholar]
- 28a.Oh KH, Lee JH, Kim JN, Chang N. Effects of folic acid intake on plasma folate, vitamin B12 and homocysteine levels, hepatic SAM/SAH ratio and the expression of cerebral myelin basic protein in pregnant and lactating rats. FASEB J 23: 904–5, 2009. [Google Scholar]
- 29.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, Yetley EA, Rader JI, Sempos CT, Johnson CL. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr 142: 886–893, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Pushpakumar SB, Kundu S, Metreveli N, Sen U. Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling. PloS One 8: e83813, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roscoe B. Jackson Memorial Laboratory, Green EL. Biology of the Laboratory Mouse. New York: McGraw-Hill, 1966. [Google Scholar]
- 31.Schroder A, Uvelius B, Newgreen D, Andersson KE. Bladder overactivity in mice after 1 week of outlet obstruction. Mainly afferent dysfunction? J Urol 170: 1017–1021, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Smith PP, DeAngelis A, Kuchel GA. Detrusor expulsive strength is preserved, but responsiveness to bladder filling and urinary sensitivity is diminished in the aging mouse. Am J Physiol Regul Integr Comp Physiol 302: R577–R586, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatemichi S, Akiyama K, Kobayashi M, Yamazaki Y, Yokoyama O, Uruno T. A selective alpha1A-adrenoceptor antagonist inhibits detrusor overactivity in a rat model of benign prostatic hyperplasia. J Urol 176: 1236–1241, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Tengowski MW, Bjorling DE, Albrecht RM, Saban R. Use of gold-labeled ovalbumin to correlate antigen deposition and localization with tissue response. J Pharmacol Toxicol Methods 37: 15–21, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Teschendorff AE, West J, Beck S. Age-associated epigenetic drift: implications, and a case of epigenetic thrift? Hum Mol Genet 22: R7–R15, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang ZY, Wang P, Bjorling DE. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. J Urol 191: 1153–1158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West J, Widschwendter M, Teschendorff AE. Distinctive topology of age-associated epigenetic drift in the human interactome. Proc Natl Acad Sci USA 110: 14138–14143, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12: 949–957, 1998. [PubMed] [Google Scholar]
- 39.Yu W, Ackert-Bicknell C, Larigakis JD, MacIver B, Steers WD, Churchill GA, Hill WG, Zeidel ML. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. Am J Physiol Renal Physiol 306: F1296–F1307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.