Abstract
The purpose of the study was to determine the influence of neuromuscular electrical stimulation (NMES) current intensity and pulse width applied to the right elbow flexors on the discharge characteristics of motor units in the left biceps brachii. Three NMES current intensities were applied for 5 s with either narrow (0.2 ms) or wide (1 ms) stimulus pulses: one at 80% of motor threshold and two that evoked contractions at either ∼10% or ∼20% of maximal voluntary contraction (MVC) force. The discharge times of 28 low-threshold (0.4–21.6% MVC force) and 16 high-threshold (31.7–56.3% MVC force) motor units in the short head of biceps brachii were determined before, during, and after NMES. NMES elicited two main effects: one involved transient deflections in the left-arm force at the onset and offset of NMES and the other consisted of nonuniform modulation of motor unit activity. The force deflections, which were influenced by NMES current intensity and pulse width, were observed only when low-threshold motor units were tracked. NMES did not significantly influence the discharge characteristics of tracked single-threshold motor units. However, a qualitative analysis indicated that there was an increase in the number of unique waveforms detected during and after NMES. The findings indicate that activity of motor units in the left elbow flexors can be modulated by NMES current and pulse width applied to right elbow flexors, but the effects are not distributed uniformly to the involved motor units.
Keywords: neuromuscular electrical stimulation, crossed effects, motor unit, biceps brachii, pulse width
application of neuromuscular electrical stimulation (NMES) over a peripheral nerve elicits afferent volleys that are distributed widely throughout the nervous system (7, 20, 28). The magnitude of this central effect, however, depends on the characteristics of the applied NMES. For example, Chipchase et al. (6) found that the change in corticomotor responsiveness as assessed with transcranial magnetic stimulation after 30 min of NMES applied over biceps brachii depended on the intensity and pattern, but not the frequency (10 vs. 100 Hz) of stimulation. They found that corticomotor responsiveness was depressed when the applied current was greater than the perceptual threshold but less than the motor threshold, whereas it was augmented with currents that were greater than the motor threshold but only for ramp increases and not constant currents.
The central effects of NMES also depend on the width of the stimulus pulse due to its relative influence on the activation of motor and sensory axons, whether the current is applied over the nerve or over the muscle (2, 3, 25, 31). The activation of large-diameter afferent axons to evoke a Hoffmann (H) reflex, for example, is more effective with wide stimulus pulses (1 ms) than with narrow pulses (0.2 ms) (31). Due to its greater engagement of sensory axons, wide-pulse NMES can elicit more substantial increases in spinal and corticospinal excitability than narrow-pulse NMES (17, 18, 19). In contrast to the findings of Chipchase et al. (6), it has been shown that the central effects may also depend on NMES frequency. For example, the amplitude of motor-evoked responses in tibialis anterior by transcranial magnetic stimulation increased progressively during 40 min of wide-pulse NMES at 100 Hz of stimulation, but there was no effect at stimulus frequencies of 10, 50, or 200 Hz (20). In contrast to its effects on sensory axons, pulse width does not influence the incidence of recruitment reversals for pairs of motor units when they are activated by NMES (11).
Similarly, the influence of NMES on the magnitude of the responses evoked in contralateral muscles also depends on the characteristics of the applied stimulation, and the crossed effects differ from those associated with voluntary contractions. For example, Hortobágyi et al. (14) found that voluntary contractions (50 and 75% of maximum) performed with the left wrist flexor muscles were associated with an increase in the amplitude of the potential evoked in the right flexor carpi radialis by transcranial magnetic stimulation, but a decrease in the amplitude of the H reflex in the right flexor carpi radialis. However, there was no modulation of either evoked response in the right flexor carpi radialis when the left wrist flexors contracted at 25% of maximum, which suggests that higher-threshold motor units had to be activated in the left wrist flexors to influence the evoked response in the relaxed flexor carpi radialis. When contractions were evoked in the left wrist flexors by NMES (5-s stimulation at 2 kHz modulated at 50 Hz) to produce a force equal to 50% of the maximal voluntary force, the amplitudes of both the motor-evoked potential and the H reflex in the right flexor carpi radialis were augmented. Unfortunately, the influence of weaker evoked contractions that would have engaged both low- and high-threshold motor units in the left wrist flexors was not examined in this study. Nonetheless, the divergent effects of voluntary and evoked (NMES) contractions on H reflex amplitude were interpreted as involving Ia presynaptic inhibition during voluntary contractions, but not during contractions evoked by NMES.
The hypothesis for the current study was that the crossed effects of NMES would increase with current amplitude and pulse duration. Rather than measuring the influence of NMES in terms of the amplitude of evoked responses in contralateral muscles, however, the approach was to examine its effect on ongoing motor unit activity. The purpose of the study was to determine the influence of NMES current and pulse width applied to the right elbow flexors on the discharge characteristics of motor units in the left biceps brachii. Some of these data have been presented in abstract form (1).
MATERIALS AND METHODS
The study enrolled 29 participants who were free from cardiovascular and neurological disorders, but due to technical challenges, the entire protocol was completed by only 11 young adults (23 ± 4 yr, range 18–31 yr, 3 women). All participants except one were right-handed, as verified by the laterality quotient (0.7 ± 0.3 of the Edinburgh Handedness Inventory). Written informed consent was obtained from all participants and the experimental procedures were approved by the Institutional Review Board at the University of Colorado (Protocol 13-0636) and were in accordance with the Declaration of Helsinki.
Experimental setup.
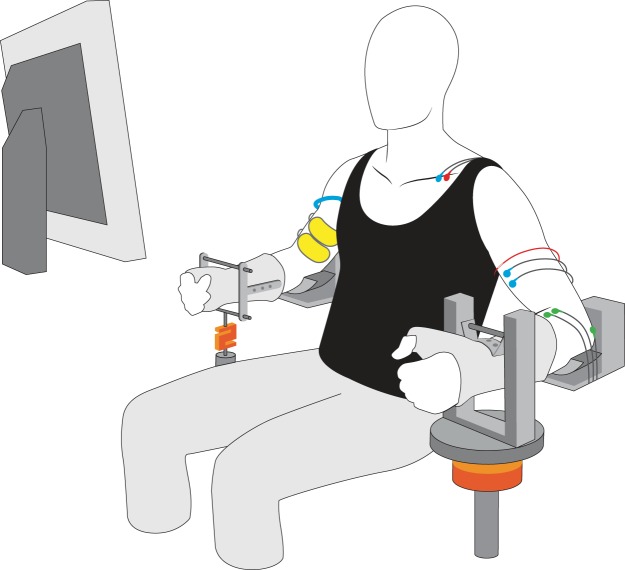
Subjects were seated comfortably with the left and right arms abducted by ∼0.79 rad from the trunk. Each elbow was flexed to 1.57 rad and the forearm was secured in a padded brace. The hand and forearm of each arm were placed in a modified wrist-hand-thumb orthosis (Orthomerica, Newport Beach, CA) and oriented in a neutral position midway between supination and pronation (Fig. 1). The upward forces exerted by the elbow flexor muscles were measured with force transducers attached to each orthosis at the level of the wrist. The force exerted by the left arm was measured with a JR3 transducer (900 N range, 182 N/V; Woodland, CA) and that for the right arm was measured with a Sealed Super Mini load cell (2,000 N range, 250 N/V; Temecula, CA). Visual feedback of elbow flexor force was provided for each arm on a computer monitor (43.2 cm) located at eye level ∼59 cm in front of the subject. Force was digitized with a Power 1401 [Cambridge Electronic Design, CED; Cambridge, UK] at 1,000 or 2,000 samples/s and stored on a computer.
Fig. 1.
The experimental setup consisted of two devices, one for each arm, to constrain the position of the forearm and hand. The upward force exerted at the wrist by the elbow flexors of each arm was measured with a force transducer attached underneath the forearm-hand orthosis. Electromyographic (EMG) signals were recorded from the left biceps brachii muscle with a subcutaneous, fine-wire electrode (red wire) and a pair of surface electrodes (blue wires). Surface EMG recordings were also obtained for brachioradialis (green wires). Neuromuscular electrical stimulation (NMES) currents were delivered to the right elbow flexors via a pair of conductive fabric electrodes (yellow pads) and a braided ground cable (blue wire) was wrapped around the proximal upper arm.
Participants were required to sustain the steady discharge of an isolated motor unit in left biceps brachii by performing a submaximal contraction before, during, and after the application of an NMES protocol to the elbow flexors of the right arm. A constant-current stimulator (DS7A Digitimer, Welwyn Garden City, UK) was used to provide an NMES current of rectangular pulses that were delivered through conductive fabric electrodes (5 × 9 cm; model 895250; Axelgaard Manufacturing, Lystrup, Denmark) placed transversally over the proximal (cathode) and distal regions of the right biceps brachii. To minimize the conduction of a stimulus artifact from the right arm to the left arm, a stainless steel braided cable (1.5 cm width) was wrapped around each participant's upper arm proximal to the stimulating electrodes and attached to a ground source (Fig. 1). The influence of six NMES protocols [three currents (in milliamperes, mA) and two pulse widths (in milliseconds, ms)] on the discharge characteristics of each isolated motor unit was compared.
Electromyographic recordings.
Single motor unit potentials were recorded from the short head of biceps brachii of the left arm using Formvar-insulated, stainless steel wires (50 μm diameter; California Fine Wire, Grover Beach, CA). Each electrode consisted of two insulated wires that were glued together with three ∼1 mm regions of the insulation removed; two regions were on one wire separated by 3 mm, and a single region was on the other wire positioned between the two regions of the same wire (9, 23). After identifying the intermarginal septum that distinguishes the short and long heads of biceps brachii, the skin over the belly of the short head was pinched tightly by one investigator while another investigator inserted the wires subcutaneously across the muscle belly with a 25-gauge needle. The needle did not penetrate the muscle fascia and was oriented perpendicular to the presumed direction of the muscle fibers. The needle was removed before the electrode was used to record motor unit action potentials. A reference surface electrocardiographic (ECG) electrode was placed over the clavicle or olecranon process (30 × 24 mm; model H124SG; Kendall). The electrical signal was amplified (×5,000), band-pass filtered (0.3–8.5 kHz; S-series; Coulbourn Instruments, Allentown, PA), sampled at 20,000 samples/s (Power 1401, CED), and stored on a computer (Spike2, v.5.20; CED). Single motor unit potentials were identified online with a dual-window discriminator (Coulbourn, V21-10) and displayed on a monitor.
The surface electromyography (EMG) of left biceps brachii (short and long heads) and brachioradialis was recorded with a bipolar arrangement (interelectrode distance of ∼20 mm) of ECG electrodes (30 × 24 mm, model H124SG; Kendall). The surface electrodes for biceps brachii were placed on the distal side of the subcutaneous electrode. Reference surface ECG electrodes were placed over the left clavicle. The EMG signals were amplified (×1,000), band-pass filtered (13-1,000 Hz; S-series, Coulbourn), sampled at 2,000 samples/s (Power 1401; CED), and stored on the computer (Spike2, v.5.20; CED).
Protocol.
After attaching the electrodes and placing the participant in the apparatus, the protocol began with several brief submaximal contractions with the left elbow flexors to determine whether the signal contained action potentials for a motor unit that could be discriminated in the short head of biceps brachii. The participant then performed several maximal voluntary contractions (MVCs) to determine the peak upward force exerted by the left elbow flexors and the surface EMG amplitude for biceps brachii and brachioradialis associated with the MVC force. The task involved increasing elbow flexor torque from baseline to maximum over 3 s and sustaining the maximal force for ∼3 s. Verbal encouragement was provided during the task and visual feedback of the upward force was provided on a monitor. The MVC force was determined from a minimum of two trials. If the peak forces from the two trials were not within 5% of each other, additional trials were performed until this criterion was met. Subjects rested for ∼60 s between each MVC trial.
Participants then performed an isometric contraction with the left elbow flexors and were instructed to gradually increase the upward force exerted by the wrist up to a target displayed on the monitor (60% MVC force). As each participant performed this ramp contraction, the investigators observed the signal recorded with the subcutaneous electrode to estimate the recruitment threshold force of a candidate motor unit and other motor units that might be discriminated. A target force slightly above the recruitment threshold of a motor unit was then displayed on the monitor and the participant performed a voluntary contraction with the left elbow flexors to match the target force. The target force was adjusted over several brief contractions to ensure that the motor unit sustained a low, regular discharge rate.
Once a motor unit and its target force had been identified, the right elbow flexors were prepared for the delivery of the NMES protocols. With the same procedure used for the left arm, participants performed several trials of the MVC task with the right elbow flexors until the MVC force was determined. Based on the MVC force, target forces equal to ∼10% and ∼20% were calculated for subsequent display on the monitor. The greater force was constrained to produce no more than moderate discomfort for the participants.
The NMES protocols applied to the right elbow flexors involved combinations of three currents and two pulse widths. The current was set at either below or above motor threshold, which was identified as a single 0.2-ms pulse of current sufficient to cause the minimal palpable displacement of the distal biceps brachii tendon. One of the NMES currents was set at 80% of motor threshold, which was less than the perception threshold for all participants. The other two currents were those required to evoke elbow flexor contractions that matched the target forces of ∼10% and ∼20% MVC force for the two rectangular pulse widths (0.2 ms and 1 ms) at 40 Hz. Participants were asked about the discomfort associated with each NMES protocol with 0 denoting no discomfort and 10 corresponding to extreme discomfort. All participants reported values ≤5 for discomfort with values ranging from 2 to 5. There was no association between the level of right-arm discomfort and the influence of NMES on motor unit activity in the short head of the left biceps brachii.
In a randomized order for current and then pulse width, the NMES protocols were applied for 5 s to the right elbow flexors in the middle of a 15- to 30-s contraction performed with the left elbow flexors (Fig. 2). Participants matched a target force displayed on a monitor and one of the investigators provided verbal feedback to guide subjects on how much force to exert so that the isolated motor unit continued to regularly discharge. Once the influence of the 40-Hz protocols (three currents and two pulse widths) on the discharge characteristics of the isolated motor unit had been recorded, if the participant was willing, the subcutaneous electrode was moved to identify another motor unit.
Fig. 2.
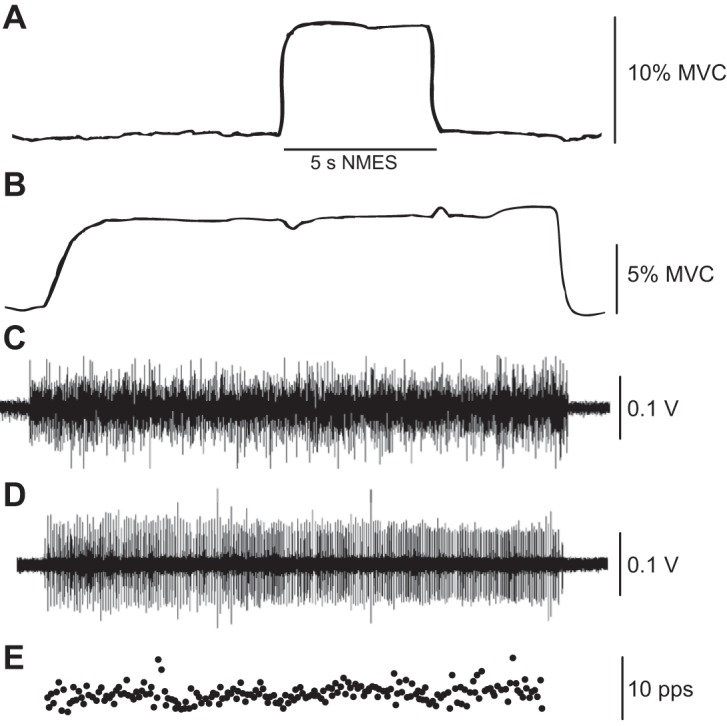
Representative data depicting the upward force evoked in the right elbow flexors (A) by an NMES protocol [∼10% maximal voluntary contraction (MVC) force, 40 Hz, and pulse width of 0.2 ms], force exerted by the left elbow flexors during a voluntary contraction (B), the interference EMG signal for biceps brachii (C), the isolated motor unit from the short head of biceps brachii (D), and its instantaneous discharge rate with superimposed traces of its action potential (E). The motor unit had a threshold force of 8.1% MVC, an average interspike interval of 52.3 ± 3.8 ms, and a coefficient of variation for interspike interval of 35.8 ± 1.0%.
Data analysis.
The force deflections were identified by visual inspection of a high-gain display of the left-arm force by the second and third authors. A number of trials were evaluated at least twice to ensure reliability of detecting the beginning and end of each deflection. Some of our previous work had demonstrated that the visual inspection method provides an accurate measure of signal onset (15).
Motor units were distinguished on the basis of waveform shape and target force, referred to as the threshold force, at which they sustained a low, regular discharge rate (30). Motor units with threshold forces ≤25% MVC force were arbitrarily identified as low-threshold units (5). Such a categorization was necessary because previous studies have reported that activation of cutaneous receptors with electrical stimulation has differential effects on the discharge characteristics of low- and high-threshold motor units (12, 16).
Discrimination of single motor unit action potentials was performed offline with an automatic decomposition algorithm (EMGLab), which has been demonstrated to achieve an accuracy of at least 90% for single-channel intramuscular recordings (21, 22). Interspike intervals >250 ms (<4 pulses/s) or <20 ms (>50 pulses/s) were excluded from the calculations of discharge rate (26). Once discriminated, the discharge times over periods of ∼5 s were determined before, during, and after the application of NMES to the right elbow flexors. Within each period, discharge times were converted into interspike intervals and the means and coefficients of variation (CVs) were calculated.
The influence of NMES on the means and CVs for interspike interval of the isolated motor units was examined with three-factor ANOVAs with repeated measures on time (before, during, and after NMES), NMES current, and pulse width. The analysis examined low-threshold units, high-threshold units, and both groups of motor units together. Other outcomes were compared with one-way ANOVAs, two-tailed t-tests, and Pearson correlation coefficients. An alpha level of P <0.05 was used to identify significant differences and statistical analyses were performed using SPSS (version 16.0, Chicago, IL). Post hoc analyses led to a Bonferroni adjustment. Data are presented in the text as means ± SD.
RESULTS
It was technically challenging to isolate and maintain the regular discharge of a single motor unit for the duration of the six NMES protocols (three currents and two pulse widths). However, from the 11 individuals who completed the demanding protocol, it was possible to track a mean of 3.45 motor units in each experimental session. No participant withdrew from the study due to discomfort associated with NMES.
Evoked and voluntary forces.
The NMES current applied to the right elbow flexors increased across the three levels of activation (Table 1). By design, the current used for the 80% motor threshold condition was insufficient to evoke a sensory or motor response in the right elbow flexors. The force evoked with the greatest NMES current (∼20% MVC force) was 2.2 and 2.5 times greater than the intermediate NMES current (∼10% MVC force) for the narrow and wide pulses, respectively. The forces evoked in the right elbow flexors during the 5 s of NMES (Fig. 2A) were reasonably constant for both pulse widths.
Table 1.
NMES current and force evoked in right elbow flexors at two pulse widths and three levels of activation
| 0.2 Ms |
1.0 Ms |
|||
|---|---|---|---|---|
| Current, mA | Force, % MVC | Current, mA | Force, % MVC | |
| Motor threshold, 80% | 9.2 ± 3.6 | 2.3 ± 1.0 | ||
| MVC force, ∼10% | 30.4 ± 9.0 | 7.6 ± 1.5 | 9.8 ± 4.5 | 7.6 ± 2.3 |
| MVC force, ∼20% | 59.2 ± 19.0 | 16.4 ± 3.3 | 26.9 ± 14.8 | 19.2 ± 4.3 |
Values are means ± SD.
Ms, millisecond; NMES, neuromuscular electrical stimulation; MVC, maximum voluntary contraction.
The target force ranged from 0.4 to 21.5% MVC force when tracking low-threshold motor units and from 31.7 to 56.3% MVC force when following high-threshold motor units. The NMES current or pulse width applied to the right arm did not influence the average force exerted by the left arm for either group of motor units, either during or after NMES. For example, the force for the left arm when tracking low-threshold motor units (means ± SD) was 8.3 ± 5.8, 8.6 ± 6.2, and 8.0 ± 5.2% MVC force for, respectively, before, during, and after right-arm NMES with narrow pulses that evoked the ∼10% MVC force. Moreover, there was no statistically significant influence of pulse width on the CV for the left-arm force during NMES. For example, the values during NMES were 2.69 ± 2.25% for the narrow pulse and 2.46 ± 1.91% for the wide pulse (P = 0.57) for the condition of ∼10% MVC force.
However, application of NMES to the right arm did cause transient deflections in the force exerted by the left elbow flexors in some conditions (Fig. 2B). The deflections coincided with the onset and offset of the NMES current applied to the right elbow flexors. The deflections were rarely observed when tracking high-threshold motor units and were not present for the 80% motor threshold current, but were present in 16 of 24 trials when evoking the ∼10% MVC force and in 21 of 24 trials when evoking the ∼20% MVC force in the right elbow flexors during the trials for low-threshold motor units. The trials in which there were no force deflections were limited to 3 of the 11 participants; for these individuals, deflections were observed in 4 of 16, 5 of 12, and 2 of 8 of those trials. The deflections were typically downward at the start of the 5-s NMES train (34 of 37 trials) and always upward at the end of the NMES train (37 of 37 trials).
Aside from the difference in the direction of the force deflection at the onset and offset of the right-arm NMES, there were several statistically significant differences in the latency, amplitude, and duration of the deflection due to NMES current or pulse width (Table 2). The latency of the response (onset and offset) was briefer with wide-pulse stimulation at 10% MVC force (P ≤ 0.05), but not at 20% MVC force. Offset latency, but not onset latency, was briefer at 20% MVC force than at 10% MVC force for narrow-pulse stimulation (P < 0.05), but briefer at 10% MVC force than at 20% MVC force for wide-pulse stimulation (P < 0.05). The duration of both deflections (onset and offset) was less for the stronger NMES current (20% MVC force) than that used to evoke 10% MVC force for both pulse widths (P < 0.001). There was no influence of NMES current (10% vs. 20%) or pulse width (narrow vs. wide) on deflection amplitude, except that the amplitude of the offset deflection elicited by the stronger NMES current (20% MVC force) with the wide pulse was greater than that associated with the weaker NMES current (10% MVC force, P < 0.02).
Table 2.
Latency, peak-to-peak amplitude, and duration of transient deflections in left-arm force when tracking low-threshold motor units at the onset and offset of NMES current applied to the right elbow flexors at two pulse widths and two levels of activation
| 0.2 Ms |
1.0 Ms |
|||||
|---|---|---|---|---|---|---|
| Latency, ms | Amplitude, % MVC | Duration, s | Latency, ms | Amplitude, % MVC | Duration, s | |
| MVC force, 10% | ||||||
| Onset | 69 ± 32 | −1.02 ± 0.54 | 0.93 ± 0.39 | 53 ± 15a | 1.02 ± 0.54 | 0.94 ± 0.55 |
| Offset | 133 ± 93 | −1.62 ± 1.96 | 0.77 ± 0.28 | 77 ± 21a | 0.90 ± 0.48 | 0.86 ± 0.36 |
| MVC force, 20% | ||||||
| Onset | 62 ± 19 | −1.34 ± 0.97 | 0.58 ± 0.29d | 62 ± 25 | 1.33 ± 0.83 | 0.52 ± 0.27d |
| Offset | 91 ± 24b | −1.43 ± 0.86 | 0.43 ± 0.11d | 88 ± 17c | 1.64 ± 1.07e | 0.54 ± 0.20d |
Values are means ± SD. Negative amplitude indicates a downward deflection.
P ≤ 0.05 compared with 0.2 ms,
P < 0.05 compared with 10% MVC force,
P < 0.05 compared with 10% MVC force,
P < 0.001 compared with 10% MVC force,
P < 0.05 compared with 10% MVC force.
Moreover, there was an effect of pulse width on associations between characteristics of the onset and offset deflections. The correlation coefficient for the amplitudes of the two deflections was statistically significant (all P < 0.01) for narrow- and wide-pulse stimulation for both the 10% (r = 0.59 and 0.56, respectively) and 20% (r = 0.84 and 0.50, respectively) NMES currents. The correlations were similar for deflection duration at the 10% (narrow r = 0.54, P < 0.01; wide r = 0.57, P < 0.01), but weaker at 20% (narrow r = 0.19, P = 0.34; wide r = 0.40, P < 0.05) NMES currents.
Although the force deflections must have been caused by transient adjustments in motor unit activity, this was not evident in the surface EMG recordings during most trials (Fig. 2C) or in the instantaneous discharge rates of isolated motor units (Fig. 2E). However, it was possible to detect slight modulation of motor unit activity and surface EMG activity when the NMES caused large-amplitude force deflections (Fig. 3).
Fig. 3.
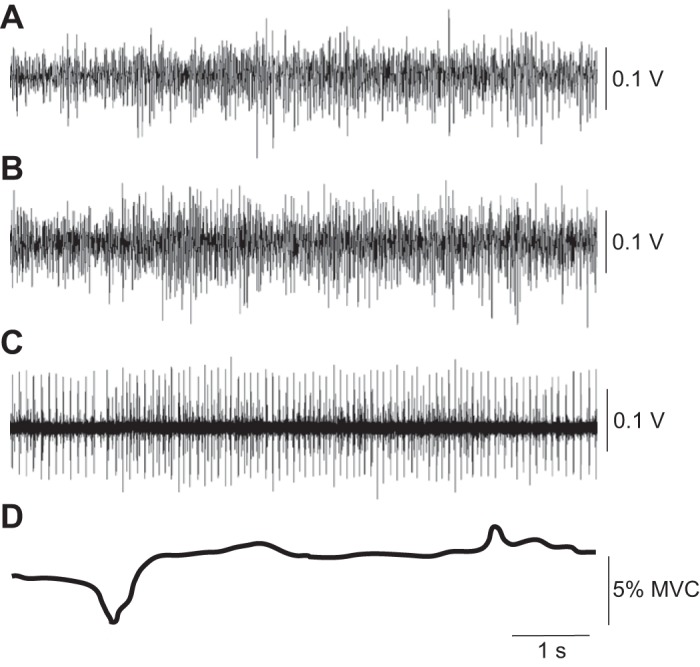
Surface recordings of interference EMG signals for brachioradialis (A) and biceps brachii (B) and fine-wire recording of motor unit activity (C) during a trial with a large-amplitude force deflection at the onset of NMES (D). The amplitude of the force deflections was 3.3% at onset and 1.3% at offset of NMES (10% MVC force, wide pulse). The threshold for the motor units was 5.6% MVC force. Note the slight modulation in the motor unit activity and biceps brachii interference EMG that coincided with the onset (downward) force deflection and the increase in the number of active motor units (C) between the two force deflections.
Discharge characteristics.
Recordings were obtained from 28 low-threshold (target force 8.7 ± 6.0% MVC force) and 16 high-threshold (target force 38.8 ± 6.3% MVC force) motor units in the short head of the left biceps brachii. The discharge times of single motor units were determined at 4- to 5-s intervals from before, during, and after an NMES current was applied to the right elbow flexors. Most motor units were discriminated for all six NMES conditions (Table 3). The discharge characteristics of the motor units were quantified as the mean and CV of interspike interval for before, during, and after the application of NMES. ANOVA indicated that none of the NMES conditions had any statistically significant (P > 0.05) influence on either the mean or CV for interspike interval of the single motor units that were tracked across the protocol (before, during, and after NMES). Consequently, the discharge characteristics of the low- and high-threshold motor units were averaged separately across the three phases (before, during, and after NMES) for each NMES protocol (Table 3).
Table 3.
Interspike intervals and coefficients of variation of motor units in left biceps brachii averaged before, during, and after NMES protocols at three currents and two pulse widths to the right elbow flexors
| 80% Motor Threshold |
10% MVC Force |
20% MVC Force |
||||
|---|---|---|---|---|---|---|
| 0.2 Ms | 1 Ms | 0.2 Ms | 1 Ms | 0.2 Ms | 1 Ms | |
| Low thresholda | ||||||
| Mean ISI | 99.7 ± 19.0 | 93.0 ± 17.1 | 95.1 ± 19.4 | 91.8 ± 15.4 | 92.8 ± 18.8 | 95.1 ± 19.4 |
| CV ISI | 16.6 ± 8.7 | 17.1 ± 7.6 | 18.1 ± 10.0 | 16.6 ± 8.0 | 17.7 ± 8.6 | 18.8 ± 10.0 |
| High thresholdb | ||||||
| Mean ISI | 80.8 ± 16.0 | 77.6 ± 13.2 | 79.9 ± 18.0 | 81.0 ± 22.1 | 82.1 ± 15.6 | 76.1 ± 14.4 |
| CV ISI | 22.8 ± 11.2 | 23.9 ± 12.6 | 24.3 ± 12.1 | 23.4 ± 10.0 | 26.3 ± 13.2 | 22.1 ± 10.1 |
Values are means ± SD.
n = 28,
n = 16.
ISI, interspike interval; CV, coefficient of variation.
Although most experiments yielded only one motor unit, the discharge characteristics of 14 motor units (7 low- and 7 high-threshold) were recorded after the protocol had been completed for one motor unit and the subcutaneous electrode was moved. Due to the possibility, albeit slight, that the second recording may have been from the same motor unit, ANOVAs were repeated on a smaller dataset in which the 14 motor units had been removed. Consistent with the analysis on the 44 motor units, ANOVA indicated that none of the NMES conditions had any statistically significant (P > 0.05) influence on either the mean or CV for interspike interval of the 30 single motor units that were tracked across the protocol (before, during, and after NMES).
Due to the emergence of force deflections at the onset and offset of NMES when tracking low-threshold motor units, the analysis also examined the association between the change in discharge characteristics from before to after each deflection and the amplitude of the force deflection. Neither the change in mean interspike interval nor the change in CV for interspike interval was significantly correlated with the amplitude of the force deflections, with one exception (Table 4). The exception was for the amplitude of the offset deflection with wide-pulse stimulation at the 10% NMES current, which was negatively correlated with the change in the CV for interspike interval (P < 0.05); thus a reduction in the variability of discharge times was associated with a greater offset deflection under these conditions.
Table 4.
Pearson correlation coefficients between the amplitude of force deflection at NMES onset and offset with changes in the means and coefficients of variation for interspike interval from before to after each deflection at two currents and two pulse widths
| 10% MVC Force |
20% MVC Force |
|||
|---|---|---|---|---|
| 0.2 Ms | 1 Ms | 0.2 Ms | 1 Ms | |
| Onset deflection | ||||
| Mean ISI | 0.01 | 0.09 | 0.14 | 0.04 |
| CV ISI | −0.31 | 0.27 | −0.02 | −0.05 |
| Offset deflection | ||||
| Mean ISI | −0.17 | 0.24 | −0.07 | 0.07 |
| CV ISI | 0.00 | −0.47a | 0.06 | −0.17 |
P < 0.05.
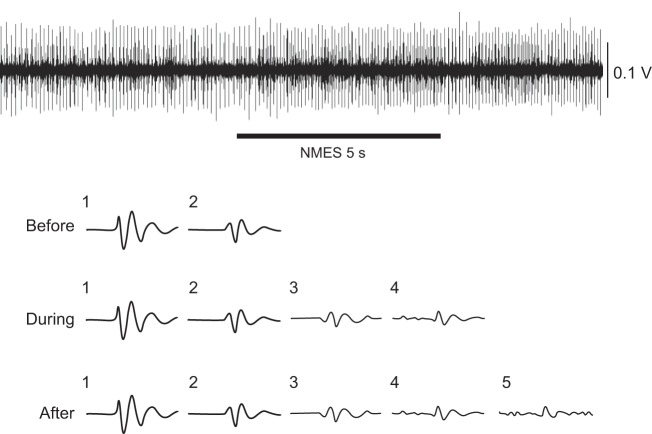
Despite the minimal influence of the NMES currents applied to the right elbow flexors on the discharge characteristics of the tracked motor units in the left arm, NMES did alter the activity of at least some motor units in the short head of the left biceps brachii that were not tracked across the protocol. The influence of NMES on the activity of other motor units was most obvious in the many trials in which we were unable to track an isolated motor unit once the NMES current was applied. To indicate the magnitude of this effect, the number of unique waveforms identified by the automatic decomposition algorithm (EMGLab) was counted during each of the three phases (before, during, and after NMES) for motor unit recordings that were successfully tracked across the protocol (Figs. 3 and 4). Although not all of these waveforms would have represented a different motor unit, a change in the number of waveforms across the three phases provides an estimate of changes in the amount of motor unit activity. As indicated in Table 5, the average number of waveforms detected by the algorithm increased during and after NMES. The increase in number of waveforms was statistically significant when the NMES current evoked a contraction (10 and 20% MVC), but not when the current was set at 80% motor threshold. Changes in the number of waveforms were not related either to NMES current or pulse width (Table 5). Across the two pulse widths (0.2 and 1.0 ms) and the two NMES currents (10 and 20% MVC), the net number of identified waveforms went from 3.3 ± 2.0 before NMES to 5.1 ± 3.2 during and 5.0 ± 3.6 after NMES (P < 0.001).
Fig. 4.
Representative trial showing the waveforms detected in a fine-wire recording (top trace) by the automatic decomposition algorithm before, during, and after NMES. The discharge times of motor unit 1 (threshold = 5.6% MVC force) were tracked during this trial.
Table 5.
Waveforms detected by the algorithm in subcutaneous electrode recording from left biceps brachii before, during, and after application of NMES at two currents and pulse widths to right elbow flexors
| 80% Motor Threshold |
10% MVC Force |
20% MVC Force |
||||
|---|---|---|---|---|---|---|
| 0.2 Ms | 1 Ms | 0.2 Ms | 1 Ms | 0.2 Ms | 1 Ms | |
| Low thresholda | ||||||
| Before | 2.7 ± 1.5 | 2.7 ± 1.4 | 2.6 ± 1.5 | 2.9 ± 1.6 | 3.1 ± 2.2 | 2.8 ± 1.7 |
| During | 3.6 ± 2.4 | 3.4 ± 1.5 | 4.0 ± 2.9 | 3.1 ± 1.2 | 3.7 ± 1.7 | 3.9 ± 3.0 |
| After | 3.9 ± 2.9 | 3.9 ± 1.8 | 4.4 ± 3.1 | 3.3 ± 1.6 | 4.1 ± 2.4 | 4.0 ± 2.1 |
| High thresholdb | ||||||
| Before | 4.1 ± 2.9 | 5.0 ± 1.7 | 4.2 ± 1.9 | 3.8 ± 1.8 | 5.1 ± 2.7 | 4.2 ± 1.9 |
| During | 7.3 ± 4.3 | 8.0 ± 3.1 | 8.7 ± 2.4 | 7.6 ± 1.1 | 9.1 ± 3.4 | 9.1 ± 3.2 |
| After | 9.9 ± 6.7 | 10.1 ± 5.3 | 8.4 ± 2.3 | 9.0 ± 4.2 | 8.4 ± 4.2 | 12.3 ± 6.3 |
Values are means ± SD.
n = 28;
n = 16.
The net number of waveforms detected during and after NMES included some that were recruited and others that were derecruited. Those waveforms derecruited from before to during NMES did not differ with either pulse width or NMES current, and were relatively few in number with only 14 waveforms missing from the 462 that were identified before NMES. The loss of waveforms was greater after NMES with 103 missing from the 637 that were detected during NMES, but again the change did not differ with either pulse width or NMES current. The derecruitment of waveforms, therefore, was greater after NMES compared with during NMES.
DISCUSSION
The results were not consistent with the hypothesis that the influence of NMES applied to the right elbow flexors on motor unit activity in the short head of the left biceps brachii would increase with the amplitude of applied current and width of the stimulus pulse. The main finding was that the crossed effects were distributed nonuniformly across the involved motor units and were largely unrelated to NMES stimulus intensity and pulse width. The two main effects associated with NMES consisted of a transient deflection in the force exerted by the left elbow flexors, but only when tracking low-threshold motor units, and a change in the activity of some, but not all, motor units in the short head of biceps brachii that was evident at both NMES currents and pulse widths.
Force deflections during voluntary contractions.
The transient deflections in the left-arm force were not due to a stimulus artifact associated with the application of NMES to the right arm. When an artifact was present, which occurred occasionally, it was observed in the single motor unit recording (fine wire, subcutaneous electrode) as one artifact for each stimulus pulse and not just at the beginning and end of the NMES train. The presence of artifacts in the recordings obtained from the left arm were attenuated by the stainless steel braided cable that was wrapped around the right upper arm of each participant (Fig. 1). Moreover, the transient deflections in the left-arm force were present only when tracking low-threshold motor units and not high-threshold motor units. The force deflections were also not attributable to the unexpected application of NMES because one investigator counted down (3-2-1) to indicate the onset and offset of the NMES train in all trials for both groups of motor units.
The force deflection associated with NMES onset was downward, which meant a brief reduction but not cessation, of motor unit activity that contributed to the left-arm force. The brief reduction in ongoing motor unit activity that coincided with NMES onset did not involve all the active motor units because those that were tracked throughout the protocol typically did not exhibit any modulation of activity at either the onset or offset of NMES. When the amplitude of the force deflection was large, however, it was possible to detect slight modulation of motor unit activity in both the fine wire and surface EMG recordings (Fig. 3). Moreover, in the condition wide-pulse stimulation at 10% MVC force, the change in CV for interspike interval from before to after the deflection was significantly correlated with the amplitude of the offset deflection. However, the deflection-associated adjustments in motor unit activity were usually not observed in the surface EMG recordings of either biceps brachii or brachioradialis (Figs. 2 and 3). One reason for the apparent absence of an effect is that interference EMG signals are relatively insensitive to modest adjustments in the discharge characteristics of low-threshold motor units, especially recordings obtained with bipolar electrodes (10). Another possible reason for the lack of modulation in the surface EMG recordings from biceps brachii and brachioradialis is that motor units in other synergist muscles may have caused the force deflections. Although we did not record the EMG activity in triceps brachii, the force deflections are unlikely to have involved antagonist muscles because they were present only when low-threshold motor units were being tracked.
The NMES currents and pulse widths did produce some statistically significant differences in the characteristics of the two force deflections. Most of these differences were due to the influence of NMES current. The most consistent effect was a briefer duration for both the onset and offset deflections at 20% MVC force compared with 10% MVC force. Some of the effects of NMES current varied with pulse width, such as wide-pulse stimulation eliciting a briefer latency and greater amplitude for the offset deflection at 20% MVC force than at 10% MVC force. Similarly, the latency for both the onset and offset deflections was briefer at 10% MVC force for wide-pulse stimulation compared with narrow-pulse stimulation. Also, the latency for the offset deflection with narrow-pulse stimulation was longer at 10% MVC force than at 20% MVC force. These findings indicate that differences in the NMES-evoked afferent volleys that produced crossed effects were most often due to the magnitude of the current and its interaction with pulse width.
Percutaneous activation of peripheral axons with electrical stimulation, such as NMES, sends a volley of afferent feedback into the spinal cord (4) that can evoke responses in the contralateral limb (8, 24, 29). For example, electrical stimulation (five 1-ms pulses at 300 Hz) of the superficial radial nerve at the level of the hand (cutaneous afferents) evokes an interlimb reflex depression of EMG activity at a medium (75–120 ms) latency in contralateral flexor carpi radialis during an ongoing contraction (50% MVC) (32). The transient force depression at NMES onset in the current study, therefore, had a direction and latency consistent with it being mediated by a medium-latency interlimb reflex. However, it seems unlikely that a similar reflex pathway could explain the upward force deflection at NMES offset. Moreover, the interpretation is confounded by the observation that the force deflections were present only during the tracking of low-threshold motor units.
Alternatively, the central effects of NMES may simply be greater during changes in the amplitude of the applied electrical stimulation. For example, Chipchase et al. (6) found that the influence of NMES on the responsiveness of the pathway from the motor cortex to biceps and triceps brachii, as assessed with transcranial magnetic stimulation, was greater when the stimulus intensity changed compared with when it remained constant. They measured the amplitude of motor-evoked potentials before and after 30 min of either constant- or variable-amplitude NMES (0.1-ms pulses) applied to biceps brachii. Six different NMES conditions were examined in which current amplitude was varied to evoke weak and strong sensory or motor responses. The variable current condition involved intermittent contractions (4 s on, 6 s off) in which current increased and decreased linearly during each 4-s contraction. The amplitude of the motor-evoked responses in both biceps and triceps brachii increased after the variable-current NMES, but not after either the low- or high-intensity constant current NMES.
Some insight on the underlying neural mechanisms may be obtained from intracellular motor neuron recordings, which have indicated that electrical stimulation (pulse width 0.1 ms at 100–200 Hz for 0.8–1.0 s) of the peroneal nerve can elicit short-latency postsynaptic potentials in most motor neurons innervating the contralateral medial gastrocnemius muscle (13). When the response elicited in these motor neurons includes an inhibitory postsynaptic potential, it reached an early peak, decayed rapidly (20–40 ms), and then became a steady-state depolarization (400–500 ms) despite continued nerve stimulation. When the electrical stimulation ended, the postsynaptic potential slowly decayed back to the resting membrane potential. Moreover, the magnitude of the inhibitory response appeared to be greater in motor neurons with lower activation thresholds (27), which may explain why the force deflections were observed in the current study only when low-threshold motor units were being tracked. Such adjustments in the amplitude and direction of the postsynaptic potentials elicited in motor neurons by a train of stimuli applied to a contralateral nerve may underlie the force deflections observed in the current study at NMES onset and offset.
Motor unit activity.
The other primary finding of the current study was that the discharge characteristics of some but not all motor units in the left biceps brachii were influenced by NMES applied to the right elbow flexors. Careful measurements indicated that the discharge times of some low- and high-threshold motor units were not influenced by NMES at all. However, a qualitative analysis indicated that the activity of other motor units was modulated by the application of NMES. Unfortunately, the magnitude of this effect could not be quantified in the current study due to the recordings being made with fine-wire electrodes; it is necessary to use a recording system with greater spatial resolution, such as high-density surface EMG electrodes, to determine the discharge times of multiple, concurrently active motor units (10). Nonetheless, it was possible to estimate the influence of right-arm NMES on motor unit activity in left biceps brachii by counting the unique waveforms detected by an automatic decomposition algorithm (21, 22) before, during, and after NMES (Fig. 4 and Table 5). The results indicated a statistically significant increase in the number of detectable waveforms during and after NMES with no effects due to either current (10 and 20% MVC force) or pulse width. Rather, the change in the number of waveforms (new waveforms + lost waveforms) was greater after NMES compared with during NMES.
The current results also indicate that the influence of right-arm NMES on motor unit activity in the left biceps brachii is not due to the differential modulation of low- and high-threshold motor units. Percutaneous electrical stimulation can have a marked influence on the recruitment order of motor units in an underlying muscle (11) and in the recruitment threshold force of a nearby muscle (12), but the issue in the current study is the crossed effect of electrical stimulation that presumably engages interlimb reflex pathways. The right-arm NMES undoubtedly involved oligosynaptic pathways to the motor neurons innervating the left biceps brachii. Intracellular recordings from motor neurons indicate that such pathways are susceptible to several sources of modulation and that the input received by the motor neurons can vary across the population (13, 27). Despite the nonuniform effects elicited in the motor neurons innervating the left biceps brachii, however, the current findings suggest that the predominant effect was greater in low-threshold motor units due to the observation that the transient force deflections occurred only when low-threshold motor units were being tracked.
In summary, the current study found that application of NMES to right elbow flexors evoked a mixed response in the activity of motor units in the short head of the left biceps brachii. One response involved transient upward and downward deflections in the left-arm force that coincided with NMES onset and offset, respectively, but only when tracking low- and not high-threshold motor units and when NMES evoked a contraction in the right elbow flexors. The other response was the absence of an influence of NMES on the discharge characteristics of tracked low- and high-threshold motor units, but the modulation of activity for other motor units in the same muscle. Decomposition of the fine-wire recordings indicated that changes in the number of active motor units was greater immediately after NMES was turned off compared with after it was turned on. The findings are consistent with a crossed effect that was mediated by a medium-latency reflex with a predominant influence on the activity of low-threshold motor units, but which can be modulated by NMES current and pulse width.
GRANTS
Support for this study was provided by National Institute on Aging Grant T32 AG-000279-08 to Robert Schwartz (Goodstein Professor of Medicine, University of Colorado Denver), which supported D. Mani. A. Almuklass is supported by a scholarship from King Saud bin Abdulaziz University for Health Sciences in Saudi Arabia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.G.A., J.R.G., and R.M.E. conception and design of research; I.G.A., D.M., A.A., and B.M. performed experiments; I.G.A., D.M., A.A., and B.M. analyzed data; I.G.A., D.M., A.A., B.M., J.R.G., and R.M.E. interpreted results of experiments; D.M. prepared figures; R.M.E. drafted manuscript; I.G.A., D.M., A.A., B.M., and J.R.G. edited and revised manuscript; I.G.A., D.M., A.A., B.M., J.R.G., and R.M.E. approved final version of manuscripte.
ACKNOWLEDGMENTS
We thank Professor Jacques Duchateau and anonymous reviewers for comments that improved the quality of the manuscript, and Ryan Price for drawing Figure 1.
REFERENCES
- 1.Amiridis IG, Mani D, Almuklass A, Gould JR, Matkowski B, Enoka RM. Modulation of motor unit activity in biceps brachii in response to neuromuscular electrical stimulation applied to the contralateral arm. Euro Congress Sport Sci, Amsterdam, The Netherlands, July 2–5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blouin JS, Walsh LD, Nickolls P, Gandevia SC. High-frequency submaximal stimulation over muscle evokes centrally generated forces in human upper limb skeletal muscles. J Appl Physiol 106: 370–377, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Bostock H, Rothwell JC. Latent addition in motor and sensory fibres in human peripheral nerve. J Physiol 498: 277–294, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534: 903–912, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chipchase LS, Schabrun SM, Hodges PW. Corticospinal excitability is dependent on the parameters of peripheral electric stimulation: a preliminary study. Arch Phys Med Rehabil 92: 1423–1430, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Chipchase LS, Schabrun SM, Hodges PW. Peripheral electrical stimulation to induce cortical plasticity: a systematic review of stimulus parameters. Clin Neurophysiol 122: 456–463, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Dietz V, Macauda G, Schraft-Altermatt M, Wirz M, Kloter E, Michels L. Neural coupling of cooperative hand movements: a reflex and FMRI study. Cereb Cortex 25: 948–958, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Enoka RM, Robinson GA, Kossev AR. A stable, selective electrode for recording single motor-unit potentials in humans. Exp Neurol 99: 761–764, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Farina D, Holobar A, Merletti R, Enoka RM. Decoding the neural drive to muscles from the surface electromyogram. Clin Neurophysiol 121: 1616–1623, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res 114: 117–123, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Garnett R, Stephens JA. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol 311: 463–473, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heckman CJ, Miller JF, Munson M, Paul KD, Rymer WZ. Reduction in postsynaptic inhibition during maintained electrical stimulation of different nerves in the cat hindlimb. J Neurophysiol 71: 2281–2293, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Hortobágyi T, Taylor JL, Petersen NT, Russell G, Gandevia SC. Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory inputs in humans. J Neurophysiol 90: 241–2459, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Jesunathadas M, Aidoor SS, Keenan GK, Farina D, Enoka RM. Influence of amplitude cancellation on the accuracy of determining the onset of muscle activity from the surface electromyogram. J Electromyogr Kinesiol 22: 494–500, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda K, Burke RE, Walmsley B. Differential control of fast and slow twitch motor units in the decerebrate cat. Exp Brain Res 29: 57–74, 1977. [DOI] [PubMed] [Google Scholar]
- 17.Lagerquist O, Collins DF. Influence of stimulus pulse width on M-waves, H-reflexes, and torque during tetanic low-intensity neuromuscular stimulation. Muscle Nerve 42: 886–893, 2010. [DOI] [PubMed] [Google Scholar]
- 18.Mang CS, Bergquist AJ, Roshko SM, Collins DF. Loss of short-latency afferent inhibition and emergence of afferent facilitation following electrical stimulation. Neurosci Lett 529: 80–85, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Mang CS, Clair JM, Collins DF. Neuromuscular electrical stimulation has a global effect on corticospinal excitability for leg muscles and a focused effect for hand muscles. Exp Brain Res 209: 355–363, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Mang CS, Lagerquist O, Collins DF. Changes in corticospinal excitability evoked by common peroneal nerve stimulation depend on stimulation frequency. Exp Brain Res 203: 11–20, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Marateb HR, Muceli S, McGill KC, Merletti R, Farina D. Robust decomposition of single-channel intramuscular EMG signals at low force levels. J Neural Eng 8: 066015, 2011. [DOI] [PubMed] [Google Scholar]
- 22.McGill KC, Lateva ZC, Marateb JR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Mottram CA, Jakobi JM, Semmler JG, Enoka RM. Motor-unit activity differs with load type during a fatiguing contraction. J Neurophysiol 93: 1381–1392, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T, Barss T, Klamer T, Komiyama T, Zehr EP. Amplification of interlimb reflexes evoked by stimulating the hand simultaneously with conditioning from the foot during locomotion. BMC Neurosci 14: 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyroud D, Dodd D, Gondin J, Maffiuletti NA, Kayser B, Place N. Wide-pulse high-frequency neuromuscular stimulation of triceps surae induces greater muscle fatigue compared with conventional stimulation. J Appl Physiol 116: 1281–1289, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Pascoe MA, Holmes MR, Stuart DG, Enoka RM. Discharge characteristics of motor units during long-duration contractions. Exp Physiol 99: 1387–1398, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Powers RK, Binder MD. Distribution of oligosynaptic group I input to the cat medial gastrocnemius motoneuron pool. J Neurophysiol 53: 497–517, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Schabrun SM, Ridding MC, Galea MP, Hodges PW, Chipchase LS. Primary sensory and motor cortex excitability are co-modulated in response to peripheral electrical nerve stimulation. PLoS One 7: e51298, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt MW, Hinder MR, Summers JJ, Garry MI. Long-lasting contralateral motor cortex excitability is increased by unilateral hand movement that triggers electrical stimulation of opposite homologous muscles. Neurorehabil Neural Repair 25: 521–530, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel KM, Stratton J, Burke JR, Glendinning DS, Enoka RM. The influence of age on the assessment of motor unit activation in a human hand muscle. Exp Physiol 81: 805–819, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Veale JL, Mark RF, Rees S. Differential sensitivity of motor and sensory fibres in human ulnar nerve. J Neurol Neurosurg Psychiatry 36: 75–86, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zehr EP, Collins DF, Chua R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp Brain Res 140: 495–504, 2001. [DOI] [PubMed] [Google Scholar]