Abstract
Inhalation of hypertonic saline (HS) acutely enhances mucociliary clearance (MC) in both health and disease. In patients with cystic fibrosis (CF), repeated use of HS causes a sustained improvement in MC as well as clinical benefit. The pharmacodynamic duration of activity on MC may be an important determinant of its therapeutic potential in other airways diseases. Before moving toward testing the clinical benefits of HS for non-CF indications, we sought to assess the duration of pharmacodynamic effects of HS in healthy subjects by performing radiotracer clearance studies at baseline, 30-min post-HS administration, and 4-h post-HS administration. Indeed, acceleration of MC was observed when measured 30 min after HS inhalation. This acceleration was most pronounced in the first 30 min after inhaling the radiotracer in the central lung region (mean Ave30Clr = 15.5 vs. 8.6% for 30-min post-HS treatment vs. mean baseline, respectively, P < 0.005), suggesting that acute HS effects were greatest in the larger bronchial airways. In contrast, when MC was measured 4 h after HS administration, all indices of central lung region MC were slower than at baseline: Ave30Clr = 5.9% vs. 8.6% (P = 0.10); Ave90Clr = 12.4% vs. 16.8% (P < 0.05); clearance through 3 h = 29.4 vs. 43.7% (P < 0.002); and clearance through 6 h = 39.4 vs. 50.2% (P < 0.02). This apparent slowing of MC in healthy subjects 4-h post-HS administration may reflect depletion of airway mucus following acute HS administration.
Keywords: mucus clearance, hypertonic saline, airway secretions
mucociliary clearance (MC) in the lung is a well-coordinated system consisting of airway secretory cells and submucosal glands that produce a mucin-rich fluid layer on the airway surface and ciliated cells that hydrate and propel mucus out of the lung toward the mouth (24, 40). Rates of MC are known to depend on ciliary beat frequency, the volume of airway surface liquid (i.e., hydration), and the rheologic properties of mucus (40). In vitro studies have demonstrated that hypertonic saline (HS), through an osmotic effect on airway surfaces, increases the volume of airway surface liquid, improves mucus rheologic properties, and accelerates mucus transport rates. Inhaled HS has also been shown to produce an acute stimulation of MC in both healthy and diseased subjects in vivo (1, 11, 12, 31–33, 35). Based upon these results, HS was tested as a therapeutic agent for patients with cystic fibrosis (CF). In these studies, repeated administration of HS to adult CF subjects produced a surprisingly sustained (>8 h) improvement in MC (12) and yielded both improved lung function and a marked reduction in pulmonary exacerbations (13). We hypothesized that the surprisingly long-lasting effect of HS on MC in CF was necessary to achieve the observed clinical effects, and that a similar pharmacodynamic profile would be necessary to achieve comparable clinical results in non-CF disease populations. We, therefore, sought to determine the durability of HS effects on MC using normal subjects as surrogates for diseased populations expressing normal CFTR function, e.g., non-CF bronchiectasis or chronic bronchitis. The current study was designed to determine whether the effects of HS in normal lungs were brief or sustained, i.e., lasting hours rather than minutes.
In vitro studies have improved our understanding of the relationship between airway surface liquid (ASL) volume and mucus transport in normal vs. CF subjects. Matsui et al. (25) showed that radial velocities of mucus transport were dramatically depressed in CF vs. healthy well-differentiated epithelial cells. In the same cell cultures, they found a greater decrease in the height of the ASL over time in the CF cells. While a direct relationship between the mucus transport rates and the depth of ASL was not established in this study, these data are consistent with recent models of the role of hydration on MC (9). The effect and duration of a hypertonic salt stimulus on ASL volume in normal and CF airway epithelial cultures has yielded conflicting results (both between normal and CF, and within normal cell cultures). For example, we showed previously that in vitro increases in ASL depth after treatment with HS were significantly larger and longer in CF vs. non-CF epithelia (12). In normal airway epithelia Tarran et al. (38) showed the addition of powdered NaCl to the thin film (10 μm) of ASL overlying the epithelia cell cultures caused ASL height to increase 3- to 4-fold within 2 min, but ASL height returned to basal levels within 12-min postsalt administration (38). However, when they added 50 μl to the cells before the NaCl challenge, they found that the ASL volume was significantly increased at 3-h postchallenge (38). Thus the duration of HS response in normal epithelia, at least with regards to ASL volume, appeared to depend on the initial volume lining the airway surface.
To determine the durability of HS in vivo in the normal lung, we measured the effect of a single dose of HS (2.8% NaCl) on MC (measured by gamma scintigraphy) at both 30 min and 4-h post-HS inhalation for comparison to baseline MC in healthy nonsmoking adult subjects. The 4-h interval was selected as the measure of durability for two reasons. First, it significantly extended the time over which HS actions have been explored in healthy subjects. Second, inhaled epithelial sodium channel (ENaC) blockers have been developed to enhance MC from the airways of both CF and non-CF patients (17). Preclinical sheep studies suggest that a 4-h effect of ENaC blockers without and with HS on MC can be achieved in animal models (18, 19). To characterize the relative role of ENaC blockers vs. HS on MC in human subjects, characterization of HS effects alone on MC at 4 h is necessary before proceeding with pharmacodynamic studies of ENaC blocker/HS combination therapy. The 2.8% NaCl used in the current study was selected as a well-tolerated concentration that could serve as a delivery vehicle in human studies of ENaC blockers (30).
METHODS
Study design and subjects.
We conducted a randomized, open label cross-over study to measure the acute MC response to aerosolized HS administration and the durability of this effect 4-h postadministration in healthy adult [age 20–43, mean = 28+/−6 (SD)] subjects. Twelve (12) nonsmoking adults (7 male, 5 female) with normal lung function [>80% forced expiratory volume in 1 s (FEV1) predicted and FEV1/forced vital capacity ratio >70%], who were free of any acute upper or lower respiratory illness within 30 days of screening, were enrolled in the study. MC scans (described below) were performed at two separate baseline visits (no treatment). Two additional scans were then performed to measure MC 30 min after HS administration to characterize the acute HS effect, and 4-h post-HS administration to quantitate the durability of the response. The two paired baseline measures were performed to reduce, and provide formal estimates of, MC measurement variability. The order of performing the subsequent MC scans 30 min and 4-h post-HS treatment was randomized. The HS (2.8% NaCl, 4 ml) stimulus was delivered with an investigational Pari eFlow nebulizer (PARI Respiratory, Midlothian, VA) at the designated interval prior to the MC measurement. Each post-HS MC scan was performed 2 to 14 days after the preceding scan. To determine whether subjects experienced significant bronchoconstriction after HS administration, spirometry was performed immediately after each dose. A >15% drop in FEV1 was included as a predefined stopping criteria that would have excluded a subject from further study; no subjects were excluded for this reason.
Informed written consent was obtained from all subjects prior to their participation. The study was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill and listed on clinicaltrials.gov (Study NCT01617369).
MC methods by gamma scintigraphy.
Prior to each MC measurement, a Co57 transmission scan was performed to define lung regions required for analyses of regional deposition and clearance (27, 46). A rectangular phantom containing Co57 (< 25 mCi) was placed 5 cm in front of the subject, who sat with their back to the gamma camera for 30 s. Two fiducial markers containing Americium241 [0.9 microcurie (uCi) each, gamma 66 KeV] were placed at anatomically defined locations outside of the lung fields on the upper and lower back of each subject before any scanning procedures. With dual isotope imaging, these markers allowed alignment of serial images for determination of regional retention.
To enhance airway deposition for MC measurements, radiolabeled Tc99m-sulfur colloid (SC) tracer was delivered by a “slow-inhalation, large particle” method (47). The submicron 99mTc-SC particles (mean 0.22 μm, geometric standard deviation 1.75 with fewer than 10% particles less than 100 nm) (5, 10) are insoluble and suspended in a normal saline solution for delivery by a jet nebulizer. A Pari LL nebulizer (PARI, Starnberg, Germany) was modified for 99mTc-SC tracer delivery by removing the lower third of the internal baffle to allow generation of large aerosol particles (MMAD 9.5 μm) (47). A closed delivery system was used to produce a very slow inspiratory airflow (80 ml/s), determined by the compressor flow rate to the nebulizer. Subjects performed single inhalations, each lasting ∼10 s from the delivery system and then exhaled at 500 ml/s using visual feedback from a flow meter in the breathing circuit. Subjects were allowed to breathe normally (off the nebulizer) between each inspiratory maneuver. Each subject practiced these maneuvers prior to the actual radioaerosol inhalation to guarantee his/her proficiency. Five to ten inhalations were required to deposit an adequate isotope dose in the lung, which was monitored in real time with a NaI single crystal detector. Approximately 40 uCi was deposited in the lung for each MC scan. Total time to deliver the isotope was less than 5 min in all cases. Immediately following isotope inhalation, the subject gargled and drank water to clear from the esophagus any activity swallowed from the oropharynx. Within a minute of the final inhalation maneuver, the subject was seated in front of a large-field-of-view gamma camera to acquire particle retention images. Serial 2-min gamma images (128X128 pixels) were captured continuously for the first 94 min after isotope delivery. Additional 15-min gamma images were obtained at 3 and 6 h after isotope inhalation. Subjects returned the following day for a 30-min image to assess retention 24 h (+/− 4 h) after initial deposition.
Regional deposition and clearance analysis of gamma camera images.
The Co57 transmission scan was used to identify and outline the lung field and to create defined central (C) and peripheral (P) regions of interest (ROI). The C/P ratio of lung activity was determined for both the initial deposition image and retention images throughout the period of MC measurement on each study day. Regional deposition and MC were measured from the right lung to avoid the confounding effects of stomach activity on the left lung. To assess C vs. P deposition/retention, two rectangular ROI were created on the right Co57 lung image (46): 1) a rectangular region encompassing the entire right lung and 2) a C ROI that was aligned and centered on the interior boundary of the whole lung region, which included ∼25% of the area of the whole lung ROI (46). The P region was the area lying between the central and whole lung outline.
We then calculated the ratio of central to peripheral counts from the deposition/retention scans, (C/P)Tc, and normalized this ratio by dividing by the C/P for the transmission scan, i.e., (C/P)trans: (C/P)Tc/(C/P)trans = C/P.
This normalization was performed to account for the differences in relative lung areas and thickness between the C and P regions (46). The C/P ratio provides an index of relative deposition/retention between the two regions that can be related to MC, i.e., as a covariate in MC analyses. A C/P of 1.0 reflects equal deposition/retention in each region. Because both the C and P region outlines both airways and lung parenchyma surrounding these airways, a C/P of ∼1 primarily reflects deposition/retention in the intermediate/smaller bronchial airways and alveoli. But because the C region also contains very large airways that the P region does not, C/P values >1 reflect a relative increase in larger airway deposition (46).
By computer analysis the ROIs of the right lung were used to determine whole, central, and peripheral lung retention (Rt) (decay and background corrected) as a fraction of the initial counts (0–4 min) over the initial 94-min imaging period. Retention at designated 10-min intervals throughout the 94-min period was determined by the sum of two 2-min images that followed each 10 min interval. The 3-, 6-, and 24-h retentions were obtained from 15-min (for 3- and 6-h time points) and 30-min (for 24-h time point) images acquired at these time points. To describe each retention vs. time data set (e.g., mean data shown in figures), the average % clearance [or 100 × (1 − Rt)] over the first 30-, 60-, and 90-min period of observation were computed (i.e., average of the 10-min clearance values from 10 to 30, 60, or 90 min). These computed values are signified as Ave30Clr, Ave60Clr, and Ave90Clr, respectively. Each calculated AveClr value utilizes all 10-min data points, rather than simply reporting a clearance value at a single time, and represents the average clearance at the midpoint of each period of retention vs. time observation (5). The total percent clearance at the 3-, 6-, and 24-h time points were also determined.
Statistical methods.
The mean clearance and regional deposition indices at each post-HS visit were compared with the average of the two baseline visits by the Student's paired t-test. Our primary hypothesis was that MC at 30-min post-HS would be faster than the baseline rate, but this difference would wane by 4 h. An overall significance level of P ≤ 0.05 was considered statistically significant, but P values <0.10 are also given where appropriate to indicate trends in the data. All values are expressed as the mean (+/− standard deviation). The significance of relationships between individual variables was tested by nonparametric Spearman's rank correlation analysis (Stata 11.0 for MacIntosh). The variability of MC baseline measurements was described by calculating the standard deviation of intrasubject differences in paired MC values obtained at the two baseline visits.
Sample-size/power calculations were based on paired MC data obtained in healthy subjects using a similar MC methodology (5). With this technique, 12 subjects would be required to demonstrate an absolute increase of 6.75% in Ave60Clr from the baseline (mean baseline Ave60Clr = 9%) (i.e., an improvement of 75%) with a two-sided paired analysis where α = 0.05 and β = 0.80. Previously, we observed a 100% increase in MC (i.e., a doubling of MC) in healthy subjects immediately after inhaling 7% NaCl (35). The slow-inhalation radioaerosol delivery method used in this study has provided a greater MC signal (Ave60Clr = 15%) (47) than our prior methodology (Ave60Clr = 9%) (5), but its intrasubject repeatability was unknown. Thus we chose to perform repeat baseline MC measures to allow a post hoc determination of our ability to assess differences in MC associated with the HS challenge. The two baseline MC measurements were averaged to obtain a single baseline for each individual.
RESULTS
Whole lung clearance.
Figure 1 illustrates the mean whole lung retention vs. time curve for the mean baseline, 30-min (acute), and 4-h (durable) post-HS administration studies. Table 1 summarizes the whole lung MC kinetics displayed in Fig. 1. There were no differences in initial C/P ratios between the three study conditions.
Fig. 1.
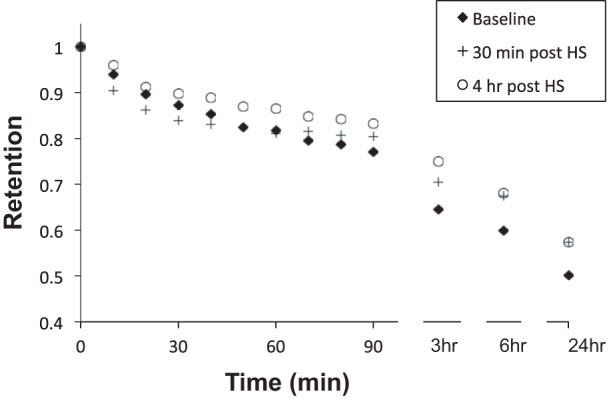
Whole lung retention vs. time. Individual baselines are the mean of two measurements in each subject.
Table 1.
Percent whole lung clearance
| C/P | Ave30Clr | Ave60Clr | Ave90Clr | 3-h Clr | 6-h Clr | 24-h Clr | |
|---|---|---|---|---|---|---|---|
| Mean baseline | 2.17 | 9.7 | 13.3 | 16.1 | 35.5 | 40.1 | 49.8 |
| 30-min post-HS (acute) | 2.16 | 13.1 | 15.5 | 16.7 | 29.5 | 32.5 | 42.6 |
| 4-h post-HS (durable) | 2.30 | 7.7 | 10.1 | 12.0 | 25.0 | 31.9 | 42.7 |
| P for base vs. 30-min post-HS | NS | 0.067 | NS | NS | 0.004 | 0.009 | 0.044 |
| P for base vs. 4-h post-HS | NS | NS | 0.053 | 0.030 | 0.002 | 0.016 | 0.009 |
Comparisons with baseline made by paired t-test.
C/P, central/peripheral; HS, hypertonic saline; NS, not significant.
MC measurements made 30 min after HS administration revealed a trend toward acceleration of whole lung clearance through the initial 30 min of the measurement (i.e., Ave30Clr). Despite this trend, clearance through 3 and 6 h was significantly slowed compared with baseline. In contrast, when measured 4 h after HS administration, there was no indication of an initial acceleration of MC, and the indices of clearance that reflect the first 60 to 90 min of tracer clearance were slowed compared with baseline. The slowing of whole lung clearance when measured 4 h after HS was even more evident at the 3- and 6-h MC time points compared with baseline. Whole lung clearance through 24 h measured after HS administration (30 min and 4 h) was also reduced compared with baseline.
Central lung clearance.
Figure 2 illustrates the mean central lung retention vs. time curves for the mean baseline, 30-min (acute), and 4-h (durable) post-HS administration studies. Table 2 summarizes the central lung MC kinetics displayed in Fig. 2. While the same general effects observed in the whole lung were evident in the central lung region, the acute HS-induced acceleration of MC was more pronounced in this region; e.g., Ave30Clr was statistically significant for the acute HS treatment vs. baseline (Table 2). However, even in the central lung region, this acute HS enhancement of MC had waned by 90 min (Ave90Clr) and was not different from baseline at 3 h. As observed for the whole lung region, the slowing of clearance at 3 and 6 h after acute HS administration was significant, but not maintained at 24 h. Like whole lung clearance, the slowing of MC measured 4-h post-HS administration was evident for observations beyond 30 min and was most dramatic 3 and 6 h after initiation of MC measurements.
Fig. 2.
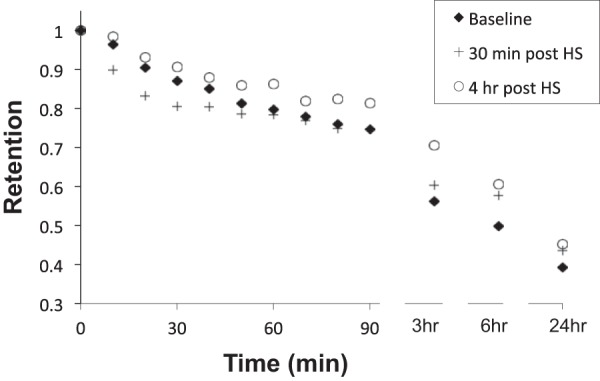
Central lung retention vs. time. Individual baselines are the mean of two measurements in each subject.
Table 2.
Percent central lung clearance
| Ave30Clr | Ave60Clr | Ave90Clr | 3-h Clr | 6-h Clr | 24-h Clr | |
|---|---|---|---|---|---|---|
| Mean baseline | 8.6 | 13.3 | 16.8 | 43.7 | 50.2 | 60.7 |
| 30-min post-HS (acute) | 15.5 | 18.1 | 20.3 | 39.7 | 42.3 | 56.5 |
| 4-h post-HS (durable) | 5.9 | 9.6 | 12.4 | 29.4 | 39.4 | 54.8 |
| P for base vs. 30-min post-HS | 0.004 | 0.056 | NS | NS | 0.005 | NS |
| P for base vs. 4-h post-HS | NS | 0.054 | 0.043 | 0.001 | 0.015 | 0.035 |
Comparisons with baseline made by paired t-test.
Peripheral lung clearance.
Figure 3 illustrates the mean peripheral lung retention vs. time curve for the mean baseline, 30 min (acute), and 4 h (durable) post-HS administration studies. Table 3 summarizes the peripheral lung MC kinetics shown in Fig. 3. Unlike central lung clearance, there was no acute acceleration of MC from the peripheral lung region following HS administration. However, slowing of MC compared with baseline, whether measured 30 min or 4 h after HS administration, was generally seen at most time points 3 to 24 h after initiation of the MC measurement.
Fig. 3.
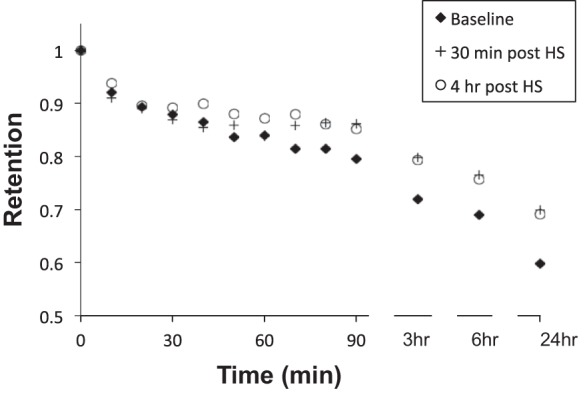
Peripheral lung retention vs. time. Individual baselines are the mean of two measurements in each subject.
Table 3.
Percent peripheral lung clearance
| Ave30Clr | Ave60Clr | Ave90Clr | 3-h Clr | 6-h Clr | 24-h Clr | |
|---|---|---|---|---|---|---|
| Mean baseline | 10.2 | 12.8 | 14.9 | 28.0 | 31.0 | 40.2 |
| 30-min post-HS (acute) | 11.0 | 13.0 | 13.3 | 20.2 | 23.5 | 30.1 |
| 4-h post-HS (durable) | 9.1 | 10.4 | 11.4 | 20.6 | 24.3 | 30.9 |
| P for base vs. 30-min post-HS | NS | NS | NS | 0.003 | 0.030 | 0.041 |
| P for base vs. 4-h post-HS | NS | NS | NS | 0.046 | NS | 0.043 |
Comparisons with baseline made by paired t-test.
Change in C/P over time.
To assess the relative changes in central vs. peripheral clearance over time, the C/P at all times during the MC measurements were plotted (Fig. 4). For scans performed at baseline or 30 min after HS, C/P decreased over the entire imaging period, and there was no difference in the C/P ratios between scans at any time point. When assessed 4-h post-HS administration, C/P was not different from baseline through 90 min. However, from 90 min through 6 h, the C/P for the 4-h post-HS challenge measurement group was significantly elevated relative to baseline, suggesting a relative slowing of particle clearance from the central vs. peripheral region that caused accumulation of tracer in the central compartment. At the 24 h time point, no differences in C/P ratio were observed, suggesting that the slowing from the central region observed 4-h post-HS treatment was able to recover, thus rebalancing the distribution of isotope in the central airways and peripheral airways within 24 h.
Fig. 4.
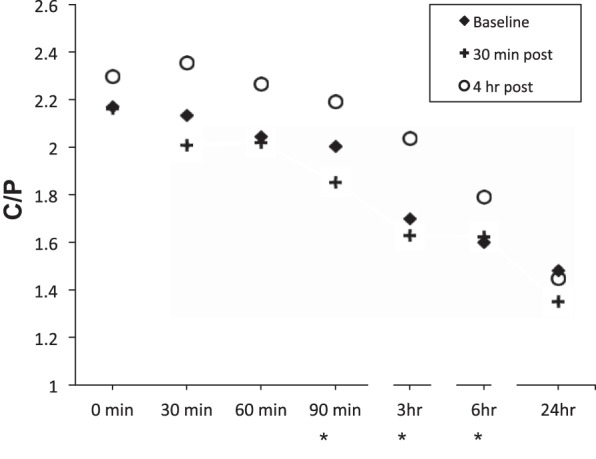
Central to Peripheral (C/P) ratio as a function of time postradiolabel deposition. Baseline is the mean of the two baseline measures. *P < 0.05 for baseline vs. 4-h post-HS treatment.
Repeatability of baseline MC measures.
The repeat measures of baseline MC in this study allowed us to compare repeatability of the data obtained with this slow-inhalation/large particle technique with those from previous studies where we used a different inhalation methodology (5). Table 4 lists the mean and standard deviation of MC data for each of the two baseline group measures, the mean delta (i.e., baseline 1 and baseline 2), and standard deviation for the current study. There was no significant difference for any paired comparisons of the two baseline measures. While there appeared to be a trend toward slower initial clearance for baseline 2 vs. 1, this observation was primarily driven by a single subject who had poor repeatability. Figure 5 illustrates the repeat data for AveClr90 with one clear outlier far from the line of identity. Excluding this subject's data, the mean delta baseline in Ave90Clr is reduced to 1.5 (+/−4.4)%.
Table 4.
Percent repeat baseline measures of mucociliary clearance kinetics
| Mean (SD) Baseline 1 | Mean (SD) Baseline 2 | Mean Delta (SD) | |
|---|---|---|---|
| AveClr60 | 14.8 (7.4) | 11.8 (6.2) | 3.0 (6.6) |
| AveClr90 | 17.7 (7.5) | 14.5 (5.2) | 3.1 (7.0) |
| 3 h | 37.6 (7.2) | 33.4 (8.8) | 4.1 (10.2) |
| 6 h | 39.4 (9.4) | 40.7 (7.8) | −1.3 (9.3) |
| 24 h | 49.7 (7.3) | 50.0 (9.3) | −0.3 (9.4) |
Fig. 5.
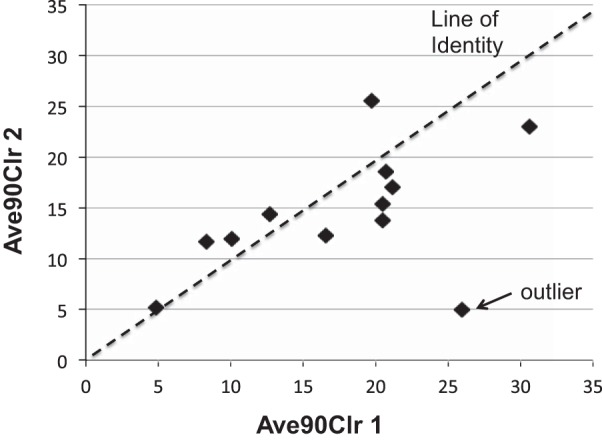
AveClr90 for two baseline measures in each individual. Line of identity is shown for perfect repeatability.
DISCUSSION
Inhaled HS, via its colloid osmotic effects, acutely increases the volume of ASL and the rate of MC from the lung in both healthy and CF subjects (1, 11, 12, 31–33, 35, 38, 41). This acceleration of MC appears to translate into therapeutic benefits for CF subjects (12, 13). However, the literature does not provide in vivo data that describes the duration of the HS effect on MC following a single dose in either the healthy or diseased lung. Previous in vivo, gamma scintigraphy studies have only assessed the effect of HS on MC when inhaled HS was administered either immediately before (within 15 min) or 15 min after radiolabel particle inhalation that defines the start of the MC measurement (11, 31–33, 35). A sustained acceleration of MC after repeated HS dosing in CF was also previously reported, but this study could not determine whether the observed effect related to a prolonged pharmacodynamic action or secondary changes in the CF airway that resulted from repeated treatments during the 2-wk treatment interval (12).
In the current study, we observed an acute enhancement of MC when radiotracer was delivered 30-min post-HS administration. However, the effect was statistically significant only over the initial 30 min of the MC measurement interval and only in the central lung region (Fig. 2 and Table 2). These data suggest that acute HS effects are more pronounced in the larger bronchial airways of the lung, perhaps as a function of the greater quantity of HS delivered to these regions on a volume/surface area basis. The relatively modest acceleration of MC observed acutely after HS administration in this study compared with previous studies in healthy subjects (11, 35) may reflect both the lower tonicity of HS used here and the longer interval between the end of the HS administration and inhalation of the radiolabel for MC measurements (30 min). The latter explanation would suggest that the HS effect on MC in the normal lung is acute, waning in less than an hour.
HS administration also unexpectedly resulted in a slowing of MC at later time points, which was evident in both protocols (i.e., radiotracer delivery 30 min and 4-h post-HS administration). When assessing the acute effect of HS on MC, a biphasic response was observed: an initial acceleration and delayed slowing. In the protocol designed to define the sustained effects of HS on MC, i.e., radiotracer delivery 4 h after inhalation, MC slowing was pronounced at all time points. This slowing was noted particularly in the central lung compartment and at the later time points of MC measurement, e.g., beyond 3 h in the whole lung (Fig. 1 and 2, and Table 1 and 2). The rise in C/P over time (Fig. 4) also suggested that there was a slowing of particle clearance from the central lung region over these later intervals. The difference in 24-h clearance from baseline was greatest and most evident in the peripheral lung region, suggesting this region also exhibited slowed particle clearance. Thus the observations from the central and peripheral lung regions with respect to clearance kinetics, from both 30 min and 4-h post-HS administration protocols, suggest that MC was slowed within a few hours after HS administration.
The mechanism(s) responsible for producing the delayed post-HS slowing of MC are not clear. As noted previously, MC rates are thought to be a function of ciliary beat, mucus biophysical properties (largely dependent on hydration), and mucus volume. Therefore, we considered each possibility.
With regards to a role for ciliary beat frequency (CBF) in the delayed response, hyperosmolar agents applied to in vitro cell cultures have been shown to acutely slow ciliary beat frequency (8, 26, 44). However, this effect has been shown to be short-lived and would be more likely seen in the acute assessment of MC post-HS challenge rather than as a delayed response. While we cannot rule out a delayed slowing of CBF throughout the bronchial tree, a delayed CBF seems unlikely based on available evidence.
The recent “gel-on-brush” model of the ASL compartment has emphasized the role of mucus hydration in determining MC rates (9). The increased MC rates observed acutely after HS administration likely reflect HS-induced hydration of the mucus layer. An HS-mediated decrease in the mucus layer osmotic modulus, coupled with a reduction in mucus layer–periciliary liquid (PCL) brush frictional interactions, is predicted to accelerate MC. However, there are no data suggesting that HS induces a paradoxical reduction of airway surface hydration 4 h after delivery of HS, making mucus layer dehydration an unlikely explanation for the observed delayed slowing of MC.
We speculate that the most likely explanation is a depletion of resident mucus from the airways caused by the immediate, accelerated clearance associated with HS administration. The basis of this speculation rests on an unresolved issue in airways biology, i.e., whether the normal lower airway is covered by a continuous mucus blanket or, rather, a discontinuous layer of mucus “flakes” or “islands” (39). Early studies by Iravani and Van As (20) in the rat lung suggested that mucus was transported by MC as mucus flakes or islands. Mucus droplets less than 4 μm in diameter were observed throughout the airways, but more prevalent in the proximal airways. Aggregation of these droplets into flakes ranging in size from 10 to 70 μm was also observed in the larger airways, with occasional larger plaques or islands forming from further agglomeration. In contrast, other early studies reported a “blanket” of mucus covering the airway surface (28, 37). Many of the “mucus blanket” observations emanated primarily from data describing the nasal airways (23) where there is a greater density of mucus secreting glands and cells. However, the mucus blanket notion for the lower airways was also supported by rapid freeze scanning electron microscopy studies (37).
The discontinuous mucus layer scenario is depicted in Fig. 6. In this paradigm, particle transport associated with mucus “islands” are transported relatively rapidly, whereas particles that deposit between these islands, onto the PCL, are transported much more slowly. For example, in well-differentiated human tracheobronchial epithelial cell cultures exhibiting spontaneous, radial mucociliary transport, Matsui et al. (24) showed that particles immersed in the PCL moved 80% slower when mucus was absent vs. being present in the culture. Consequently, if the density of mucus islands in the airway surface is reduced by a preceding, potent stimulus for clearance (e.g., HS), then the aggregate rate of MC will be reduced during the period of mucus island depletion. Note, it is not clear how the tracer particles (in our case sulfur colloid particles) and these mucus islands interact to result in particle egress from the airways. Further in vitro studies will be needed to assess the nature of these interactions.
Fig. 6.
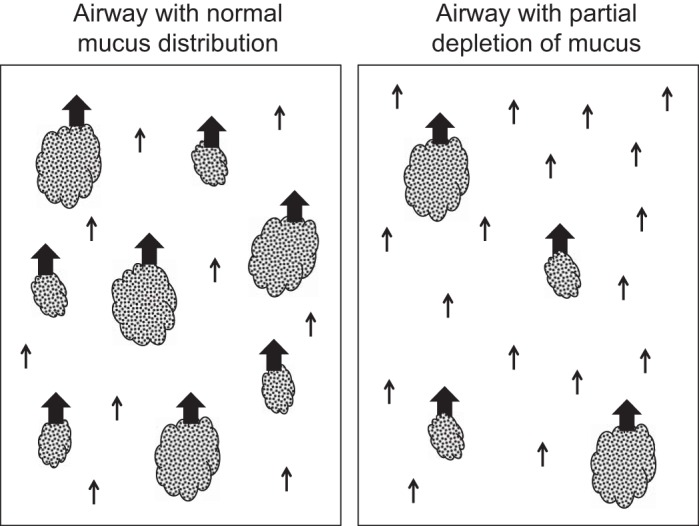
A schematic representation of the distribution of mucus “islands” on an airway surface. The velocity of these mucus islands is faster (indicated by the size of the arrows) than the airway fluid devoid of these mucus islands (and the associated particle tracers residing within these regions).
In vivo observations of particle transport in the trachea indicate heterogeneity of mucus transport that is consistent with a heterogeneous surface consisting of fast moving mucus “droplets” or “flakes” and slow moving PCL regions (15, 34, 45). Evidence also suggests that MC transport heterogeneity does not reflect variations in CBF (22, 43). For example, Liote et al. (22) showed that nasal mucociliary transport in healthy subjects was not correlated with the beat frequency of ciliated nasal cells, but rather to the volume/hydration of human nasal mucus deposited on mucus-depleted frog palates. In contrast, a recent study by Bermbach et al. (7) suggests that particles can be transported via ciliary activity in the absence of mucus when studied using ex vivo intrapulmonary human airways. However, mucus transport rates were not compared both in the absence and presence of mucus in these preparations, so it is difficult to ascertain the degree to which the absence of mucus affected overall particle transport rates. Further, there were no data to indicate whether their measured transport rates were correlated with CBF, as their conclusions would suggest. Finally, the data of Bermbach et al. (7) contrast with data from both the mucus-depleted frog palate (36) and bovine trachea (41, 42) where mucus had to be present for particle transport to occur. In these latter studies, particles impacting a mucus-depleted epithelium did not move, whereas replacement of previously collected endogenous mucus onto the mucus-depleted epithelium restored full ciliary activity and normal particle transport. Thus, on balance, current experimental data are consistent with our hypothesis that HS depletes mucus “droplets” and “flakes,” resulting in an apparent decrease in MC.
Alternatively, it might be argued that an excess of less clearable mucus accumulating in the central airways post-HS stimulation can explain our data. However, for this scenario to occur, the initial HS stimulus would have had to acutely induce an excess of mucus secretion onto the airway surface that the MC apparatus was not able to adequately clear. It is unlikely that HS, especially at a tonicity of 2.8%, induces copious secretions of mucus from glands and goblet cells. Experience with other known mucus secretagogues, e.g., uridine 5’-triphosphate (UTP), in the healthy lung suggests that the MC apparatus responds to a rapid increase in mucus secretion by increasing, rather than decreasing, the rate of mucus clearance from the whole lung (29). In addition, excess mucus in the large airways, at either the 30 min or 4-h post-HS challenge MC assessment, would likely have resulted in an increased central airway deposition of inhaled radiolabeled particles compared with baseline MC measurements (6, 21). However, there was no difference in initial C/P values between the three study days to suggest this scenario occurred (Table 1). Finally, there is also no evidence from tracheal mucus transport studies that acute hypersecretion from mucus glands leads to a slowing of clearance in healthy airways (2). Thus it seems unlikely that an excess of nonclearable mucus in the airways would first cause an acute speeding followed by a delayed slowing of MC.
There have been very few studies that have addressed the delayed response of MC to an inhaled challenge. We recently assessed the effect of inhaled endotoxin 4-h postchallenge (3) and observed a slowing of MC in healthy nonsmokers, similar to that seen here with HS. Unfortunately, we did not assess the acute, immediate effect of endotoxin challenge in the same individuals, so it is not known whether this delay followed an acute stimulation of MC. In that study, we chose the 4-h time point to correspond to the peak time observed for airway inflammation associated with endotoxin challenge. In a recent study of dust mite-sensitive asthmatics, we also found a slowing of MC 4-h postinhaled dust mite challenge compared with a baseline MC, but only in those patients who also had a late-phase bronchoconstriction associated with the challenge. In this case, the radiolabeled particle deposition was also enhanced in the central airways, i.e., C/P of these asthmatics was increased compared with their baseline, no challenge condition. We, therefore, concluded that in these patients the 4-h delayed clearance was more likely due to copious, viscous (dehydrated) secretions in the central bronchial airways associated with the asthmatic late-phase reaction to the antigen challenge. Finally, in our cross-over study on the effects of UTP vs. vehicle on MC in healthy adults, we found a significant order effect for the two treatments (29). As discussed above, UTP stimulated MC compared with vehicle in these studies, but surprisingly the subjects who received UTP first had a much slower MC associated with the subsequent vehicle treatment (on average 2 to 3 days later) than MC for the vehicle treatment for those who first received vehicle. These data would suggest that an acute depletion of mucus from the airway surface and cells might cause a subsequent delay in basal MC that lasts for days.
The question arises as to how relevant our findings may be for treatment of defective MC in patients with airways disease. We showed improved basal clearance after 2 wk of repetitive HS dosing that lasted at least 8 h after the last dose in adult CF subjects (12), suggesting no slowing of MC following treatment with HS in CF subjects. Likewise, long-term studies of HS treatment in CF show a sustained improvement in lung function and reduced disease exacerbations during 12 mo of treatment (13). The prolonged effect of HS in CF may relate to the continued presence of copious mucus secretions that persists after acute HS administration, providing a “reserve” of mucus that can mediate prolonged acceleration of particle clearance if appropriately hydrated. This hypothesis implies that a single dose of HS imparts a sustained effect on MC in CF, which requires further direct testing. If corroborated, however, our data may have implications for the efficacy of HS at different disease stages depending on the underlying quantity and quality of mucus in the airways. Similarly, HS efficacy of other airways diseases with depressed MC, e.g., COPD and non-CF bronchiectasis, may depend upon these factors.
Finally, our use of a modified isotope inhalation protocol aimed at improving airways deposition should be considered. All assessments of these data, compared with historical data in our lab, suggest that this method does indeed achieve similar or greater airways deposition than our previous methods. The repeat measures of MC performed in this study demonstrated greater MC signals and improved repeatability compared with our previous method (5). For example, our previous method (5) resulted in a mean Ave60Clr (without cough) of 9.0% (for two measures in each subject) vs. 13.3% in the current study. The corresponding standard deviation of the delta between the two measures of Ave60Clr was 8.1 and 6.6%, respectively. Given the lesser variability in repeat measures and larger mean MC signal, the new protocol should have provided sufficient power with N = 12 to detect the acute effect of HS observed previously (35).
In summary, inhaled 2.8% HS in normal subjects was associated with a short-lived acceleration of MC, predominately in the central airways. Paradoxically, MC was slowed 3- to 6-h post-HS administration. Because it is unlikely that a late slowing of CBF or accumulation of nonclearable mucus accounted for this observation, we hypothesize that mucus depletion caused by the early acceleration of clearance by HS produced this effect. These studies point to the need to rigorously define in normal subjects whether the in vivo lower airways are covered by a continuous viscoelastic mucus blanket or heterogeneous mucus “islands.” Further, these data have implications on the potential benefit of HS in disease states with varying degrees of mucus retention and/or hypersecretion.
GRANTS
The study was supported by a contract with Parion Sciences (Durham, NC) and Gilead Sciences (Seattle, WA) and National Heart, Lung, and Blood Institute tPPG Grant 1PO01HL108808. J. Balcazar was supported by National Heart, Lung, and Blood Institute Training Grant T32 HL007106.
DISCLOSURES
Karl Donn works for Parion Sciences. Thomas O'Riordan works for Gilead Sciences. Richard Boucher has a financial interest in Parion Sciences.
AUTHOR CONTRIBUTIONS
W.D.B., F.F., J.R.B., K.L.Z., K.D., T.G.O., R.C.B., and S.H.D. conception and design of research; W.D.B., J.W., F.F., J.R.B., K.L.Z., and H.D. performed experiments; W.D.B., J.W., F.F., K.L.Z., and H.D. analyzed data; W.D.B., K.L.Z., K.D., T.G.O., R.C.B., and S.H.D. interpreted results of experiments; W.D.B., J.W., and S.H.D. prepared figures; W.D.B., J.W., F.F., K.L.Z., and H.D. drafted manuscript; W.D.B., J.W., F.F., J.R.B., K.L.Z., K.D., T.G.O., R.C.B., and S.H.D. edited and revised manuscript; W.D.B., K.L.Z., K.D., T.G.O., R.C.B., and S.H.D. approved final version of manuscript.
REFERENCES
- 1.Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Resp Crit Care Med 164: 1964–1970, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol 283: L329–L335, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bennett WD, Alexis NE, Almond M, Herbst M, Zeman KL, Peden DB. Effect of inhaled endotoxin on mucociliary clearance and airway inflammation in mild smokers and nonsmokers. J Aerosol Med Pulm Drug Deliv 27: 459–465, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett WD, Herbst M, Alexis NE, Zeman KL, Wu J, Hernandez ML, Peden DB. Effect of inhaled dust-mite allergen on regional particle deposition and mucociliary clearance in allergic asthmatics. Clin Experim Allergy 41: 1719–1728, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett WD, Laube BL, Corcoran T, Zeman K, Sharpless G, Thomas K, Wu J, Mogayzel PJ Jr, Pilewski J, Donaldson S. Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv 26: 157–164, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Berger J, Albert RE, Sanborn K, Lippmann M. Effects of atropine and methacholine on deposition and clearance of inhaled particles in the donkey. J Toxicol Environ Health 4: 587–604, 1978. [DOI] [PubMed] [Google Scholar]
- 7.Bermbach S, Weinhold K, Roeder T, Petersen F, Kugler C, Goldmann T, Rupp J, König P. Mechanisms of cilia-driven transport in the airways in the absence of mucus. Am J Respir Cell Mol Biol 51: 56–67, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Boek WM, Keles N, Graamans K, Huizing EH. Physiologic and hypertonic saline solutions impair ciliary activity in vitro. Laryngoscope 109: 396–399, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 24: 937–941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis MA, Jones AG, Trindade H. A rapid and accurate method for sizing radio-colloids. J Nucl Med 15: 923, 1974. [PubMed] [Google Scholar]
- 11.Daviskas E, Anderson SD, Gonda I, Eberl S, Meikle S, Seale JP, Bautovich G. Inhalation of hypertonic saline aerosol enhances mucociliary clearance in asthmatic and healthy subjects. Eur Respir J 9: 725–732, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 354: 241–250, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 354: 229–240, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Eng PA, Morton J, Douglass JA, Riedler J, Wilson J, Robertson CF. Short-term efficacy of ultrasonically nebulized hypertonic saline in cystic fibrosis. Pediatr Pulmonol 21: 77–83, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Gerber V, Gehr P, Straub R, Frenz M, King M, Im Hof V. Mucus quality on horse tracheal epithelium: microscopic grading based on transparency. Respir Physiol 107: 67–74, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Hilding A. The physiology of drainage of nasal mucus I. The flow of the mucus currents through the drainage system of the nasal mucosa and its relation to ciliary activity. Arch Otolaryngol 15: 92–100, 1932. [Google Scholar]
- 17.Hirsh AJ, Sabater JR, Zamurs A, Smith RT, Paradiso AM, Hopkins S, Abraham WM, Boucher RC. Evaluation of second generation amiloride analogs as therapy for cystic fibrosis lung disease. Pharmacol Exp Ther 311: 929–938, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, Sabater JR, Abraham WM, Donowitz M, Cha B, Johnson KB, St George JA, Johnson MR, Boucher RC. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther 325: 77–88, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Hirsh AJ, St George J, Thelin W, Stapleton K, Montgomery B, Boucher R, Johnson MR. GS 9411: a potential aerosol pharmacotherapy for CF. Am J Resp Crit Care Med 179: A1195, 2009. [Google Scholar]
- 20.Iravani J, Van As A. Mucus transport in the tracheobronchial tree of normal and bronchitic rats. J Pathol 106: 81–93, 1972. [DOI] [PubMed] [Google Scholar]
- 21.Kim CS, Eldridge MA. Aerosol deposition in the airway model with excessive mucus secretions. J Appl Physiol 59: 1766–1772, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Lioté H, Zahm JM, Pierrot D, Puchelle E. Role of mucus and cilia in nasal mucociliary clearance in healthy subjects. Am Rev Respir Dis 140: 132–136, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Lucas AM, Douglas LC. Principles underlying ciliary activity in the respiratory tract 2. A comparison of nasal clearance in man, monkey and other mammals. Archs Otolaryngol 20: 518–541, 1934. [Google Scholar]
- 24.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest 102: 1125–1131, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Min YG, Lee KS, Yun JB, Rhee CS, Rhyoo C, Koh YY, Yi WJ, Park KS. Hypertonic saline decreases ciliary movement in human nasal epithelium in vitro. Otolaryngol Head Neck Surg 124: 313–316, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Newman S, Bennett WD, Biddiscombe M, Devadason SG, Dolovich MB, Fleming J, Haeussermann S, Kietzig C, Kuehl PJ, Laube BL, Sommerer K, Taylor G, Usmani OS, Zeman KL. Standardization of techniques for using planar (2D) imaging for aerosol deposition assessment of orally inhaled products. J Aerosol Med Pulm Drug Deliv 25, Suppl 1: S10–S28, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Nowell JA, Tyler WS. Scanning electron microscopy of the surface morphology of mammalian lungs. Am Rev Respir Dis 103: 313–328, 1971. [DOI] [PubMed] [Google Scholar]
- 29.Olivier KN, Bennett WD, Geary CA, Hohneker KW, Zeman KL, Edwards LJ, Boucher RC, Knowles MR. Acute safety and effects of mucociliary clearance of aerosolized uridine 5′-triphosphate +/− amiloride in normal human adults. Am J Resp Crit Care Med 154: 217–223, 1996. [DOI] [PubMed] [Google Scholar]
- 30.O'Riordan TG, Donn KH, Hodsman P, Ansede JH, Newcomb T, Lewis SA, Flitter WD, White VS, Johnson MR, Montgomery AB, Warnock DG, Boucher RC. Acute hyperkalemia associated with inhalation of a potent ENaC antagonist: Phase 1 Trial of GS-9411. J Aerosol Med Pulm Drug Deliv 27: 200–208, 2014. [DOI] [PubMed] [Google Scholar]
- 31.Pavia D, Thomson ML, Clarke SW. Enhanced clearance of secretions from the human lung after the administration of hypertonic saline aerosol. Am Rev Respir Dis 117: 199–203, 1978. [DOI] [PubMed] [Google Scholar]
- 32.Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, Bye PT. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med 153: 1503–1509, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M, Hemming AL, Regnis JA, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 52: 900–903, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sackner MA, Rosen MJ, Wanner A. Estimation of tracheal mucous velocity by bronchofiberscopy. J Appl Physiol 34: 495–499, 1973. [DOI] [PubMed] [Google Scholar]
- 35.Sood N, Bennett WD, Zeman K, et al. Increasing concentration of inhaled saline with or without amiloride: effect on mucociliary clearance in normal subjects. Am J Respir Crit Care Med 167: 158–163, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Spungin B, Silberberg A. Stimulation of mucus secretion, cillary activity, and transport on frog palate epithelium. Am J Physiol Cell Physiol 247: C299–C308, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Sturgess JM. The mucous lining of major bronchi in the rabbit lung. Am Rev Respir Dis 115: 819–827, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Tarran R, Grubb BR, Parsons D, Picher M, Hirsch AJ, Davis CW, Boucher RC. The CF salt controversy: In vivo observations and therapeutic approaches. Mol Cell 8: 149–158, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Van As A. Pulmonary airway clearance mechanisms: a reappraisal. Am Rev Respir Dis 115: 721–726, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Wanner A, Salathe M, O'Riordan TG. Mucociliary clearance in the airways. Am J Resp Crit Care Med 154: 1868–1902, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Wills PJ, Hall RL, Chan W, Cole PJ. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus-depleted bovine trachea. J Clin Invest 99: 9–13, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wills PJ, Garcia Suarez MJ, Rutman A, Wilson R, Cole PJ. The ciliary transportability of sputum is slow on the mucus-depleted bovine trachea. Am J Respir Crit Care Med 151: 1255–1258, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Winters SL, Daza AV, Yeates DB. Direct and indirect manipulation of mucosal Na and Cl on the mucociliary transport system in the anesthetized dog. Am Rev Respir Dis 147: 296, 1993.8430951 [Google Scholar]
- 44.Yaghi A, Zaman A, Dolovich MB. The direct effect of hyperosmolar agents on ciliary beating of human bronchial epithelial cells. J Aerosol Med Pulm Drug Deliv 25: 88–95, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Yeates DB, Aspin N, Levison H, Jones MT, Bryan AC. Mucociliary tracheal transport rates in man. J Appl Physiol 39: 487–495, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Zeman KL, Wu J, Donaldson SH, Bennett WD. Comparison of 133 xenon ventilation equilibrium scan (XV) and 99m technetium transmission (TT) scan for use in regional lung analysis by 2D Gamma scintigraphy in healthy and cystic fibrosis lungs. J Aerosol Med Pulm Drug Deliv 26: 94–100, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeman KL, Wu J, Bennett WD. Targeting aerosolized drugs to the conducting airways using very large particles and extremely slow inhalations. J Aerosol Med Pulm Drug Deliv 23: 363–369, 2010. [DOI] [PubMed] [Google Scholar]


