Abstract
Both transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1) receptors are abundantly expressed in bronchopulmonary C-fiber sensory nerves and can be activated by a number of endogenous inflammatory mediators. A recent study has reported a synergistic effect of simultaneous TRPA1 and TRPV1 activations in vagal pulmonary C-fiber afferents in anesthetized rats, but its underlying mechanism was not known. This study aimed to characterize a possible interaction between these two TRP channels and to investigate the potential role of Ca2+ as a mediator of this interaction in isolated rat vagal pulmonary sensory neurons. Using the perforated patch-clamp recording technique, our study demonstrated a distinct positive interaction occurring abruptly between TRPA1 and TRPV1 when they were activated simultaneously by their respective agonists, capsaicin (Cap) and allyl isothiocyanate (AITC), at near-threshold concentrations in these neurons. AITC at this low concentration evoked only minimal or undetectable responses, but it markedly amplified the Cap-evoked current in the same neurons. This potentiating effect was eliminated when either AITC or Cap was replaced by non-TRPA1 and non-TRPV1 chemical activators of these neurons, demonstrating the selectivity of the interaction between these two TRP channels. Furthermore, when Ca2+ was removed from the extracellular solution, the synergistic effect of Cap and AITC on pulmonary sensory neurons was completely abrogated, clearly indicating a critical role of Ca2+ in mediating the action. These results suggest that this TRPA1-TRPV1 interaction may play a part in regulating the sensitivity of pulmonary sensory neurons during airway inflammatory reaction.
Keywords: airway, sensory nerve, inflammation, transient receptor potential ankyrin 1, transient receptor potential vanilloid 1
both transient receptor potential ankyrin 1 (TRPA1) and vanilloid 1 (TRPV1) receptors are members of the superfamily of TRP channels and ligand-gated nonselective cation channels expressed in various organs. Both are well recognized as the nociceptor transducers involved in the hyperalgesia generated by tissue damage and inflammation (12, 29). In the respiratory system, they are abundantly and selectively expressed in small-diameter, slowly conducting unmyelinated (C-fiber) neurons that represent >75% of afferent fibers in the vagus nerves innervating the entire tracheobronchial tree (17, 25). These C-fiber afferents play an important role in regulating pulmonary defense reflex responses (9). Hypersensitivity of these afferents is believed to be involved in the manifestation of various symptoms associated with airway inflammation (6, 20).
Both TRPA1 and TRPV1 can be activated by a number of inflammatory mediators that are known to be released endogenously in the airways of patients during inflammatory reactions, for example, TRPV1 by proton, certain lipooxygenase metabolites, etc. (7, 13, 15, 24, 29), and TRPA1 by nitric oxide, bradykinin, 4-oxononenal, etc. (3, 5, 24, 28, 37). It is therefore highly probable that both these channels are activated during airway inflammation. Because they are colocalized in the same C-fiber neurons (25), an interaction between TRPA1 and TRPV1 may play an important role in regulating the activity and sensitivity of these neurons. Indeed, certain functional properties of TRPA1 in native nociceptive neurons are not present in heterologously expressed TRPA1, but they can be restored when both TRPA1 and TRPV1 channels are coexpressed (31, 33). Furthermore, previous investigators have suggested that the TRPA1-TRPV1 interaction in dorsal root ganglion nociceptor neurons is involved in the inflammation-induced hyperalgesia in peripheral tissues (3, 4, 23).
A recent study conducted in our laboratory using the single-fiber recording technique has reported that a simultaneous activation of both TRPA1 and TRPV1 receptors generated a pronounced synergistic effect on vagal pulmonary C-fiber sensory nerves in anesthetized Sprague-Dawley rats (22), but the underlying mechanisms are not well understood. In addition, it is well documented that TRPA1 is also expressed in the nonneuronal cells, such as airway epithelial cells, lymphocytes, etc. (11, 26). Therefore, in the in vivo study (22), we could not rule out the possibility that activation of TRPA1 may have triggered the release of intermediate mediator(s) from other target cells in the airways and pulmonary vessels, which could in turn sensitize C-fiber afferents. Although limited experimental data have also illustrated this potentiating effect in isolated pulmonary sensory neurons using the Ca2+-imaging technique, whether the currents conducted through these two TRP channels were augmented and the mechanisms involved could not be determined in that study (22).
In view of these important unanswered questions, we designed this study to characterize the interaction between TRPA1 and TRPV1 in isolated rat vagal pulmonary sensory neurons using the perforated patch-clamp recording technique. In addition, we further investigated the potential role of calcium (Ca2+) as a mediator of the TRPA1-TRPV1 interaction because of the high permeability to Ca2+ in both these channels (7, 36) and the important regulatory function of intracellular Ca2+ on the activity and sensitivity of these channels (4, 8, 10, 38, 40).
MATERIALS AND METHODS
The study protocol was approved by the University of Kentucky Institutional Animal Care and Use Committee and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Identification of Vagal Pulmonary Sensory Neurons
Vagal sensory neurons innervating the lungs and airways were identified by retrograde labeling with the fluorescent tracer, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), following a protocol described previously (19). Briefly, young male (40–100 g) Sprague-Dawley rats were anesthetized with isoflurane inhalation. DiI (0.2 mg/ml; 1% ethanol concentration) was initially dissolved and sonicated in ethanol, diluted in saline, and then instilled into the lungs (0.05 ml). Experiments were performed 7–11 days later, an interval previously determined to be sufficient for the dye to diffuse to the cell body (19).
Isolation of Nodose and Jugular Ganglion Neurons
The DiI-labeled rats were anesthetized with isoflurane inhalation and decapitated. The head was immediately immersed in ice-cold DMEM/F-12 solution. Nodose and jugular ganglia were extracted and cultured separately. Each ganglion was desheathed, cut into eight pieces, placed in a mixture of 0.04% type IV collagenase and 0.02% dispase, and incubated in a humidified chamber for 1 h in 5% CO2 in air at 37°C. The ganglion suspension was centrifuged (150 g, 5 min), and the supernatant was aspirated. The cell pellet was then resuspended in a modified-DMEM/F-12 solution [DMEM/F-12 supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM minimal essential medium nonessential amino acids] and gently titrated with a small-bore fire-polished Pasteur pipette. The dispersed cell suspension was centrifuged (500 g, 8 min) through a layer of 15% bovine serum albumin to separate the cells from the myelin debris. The pellets of nodose and jugular neurons were then plated onto dry poly-l-lysine-coated glass coverslips. Initially, small droplets of cells were incubated to promote cell adhesion at a high density. After 3 h, the coverslips were immersed in the modified-DMEM and incubated overnight (5% CO2 in air at 37°C).
Electrophysiological Recording
Cells were selected for electrophysiological recording based on the following criteria: 1) labeled with DiI fluorescence, 2) spherical shape with no neurite outgrowths, and 3) activated by capsaicin (Cap; 0.1 or 0.3 μM) or allyl isothiocyanate (AITC; 30 μM). Patch-clamp recordings were made in a whole-cell perforated-patch configuration (gramicidin 50 μg/ml) using Axopatch 200B/pCLAMP 10.2 (Axon Instruments, Union City, CA). Recording were performed at room temperature in a small-volume (0.2 ml) perfusion chamber that was continuously perfused by gravity feed (VC-6; Warner Instruments, Hamden, CT) with extracellular solution (ECS) at 1 ml/min. Borosilicate glass was pulled by using a micropipette puller and fire polished to a tip resistance of 1.5–3.0 MΩ. The series resistance was 6–10 MΩ and was not compensated. The regular ECS contained the following (in mM): 136 NaCl, 10 HEPES, 10 glucose, 5.40 KCl, 1.80 CaCl2, 1 MgCl2, and 0.33 NaH2PO4; the pH was adjusted to 7.4 with NaOH, and osmolarity was adjusted to 300 mOsm with glucose. In the Ca2+-free ECS, 5 mM of EGTA was added to buffer ambient Ca2+. The intracellular solution contained (in mM) the following: 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, and 10 HEPES; the pH was adjusted to 7.2 with KOH, and osmolarity was adjusted to 300 mosM with glucose.
Chemical solutions were applied by using a three-channel fast-stepping perfusion system (SF-77B; Warner Instruments), with its tip (width 700 μm) positioned within 500-μm distance from the cell recorded to ensure that the cell was fully within the stream of the perfusate. The duration of each chemical application was 4 s, programed and controlled by the SF-77B and computer. All experiments were performed under the voltage-clamp mode, and the resting membrane potential was held at −70 mV. Data were filtered at 10 kHz.
Experimental Protocols
Six study series were carried out.
Series 1.
To determine the concentration-response relationship of TRPV1 agonist, inward currents were recorded in each isolated pulmonary sensory neuron when the concentration of Cap was increased by successive tripling of the concentration at 20-min intervals, starting at 0.01 μM until it reached the highest concentration of 1 μM (e.g., Fig. 1A); similarly, the concentration-response relationship of AITC was obtained in another group of neurons by successive tripling of its concentration at 20-min intervals, starting at 10 μM until it reached the highest concentration of 100 μM. In some of these neurons, the concentration-current relationships of Cap and AITC were obtained again after the cells were perfused with Ca2+-free ECS for 10 min.
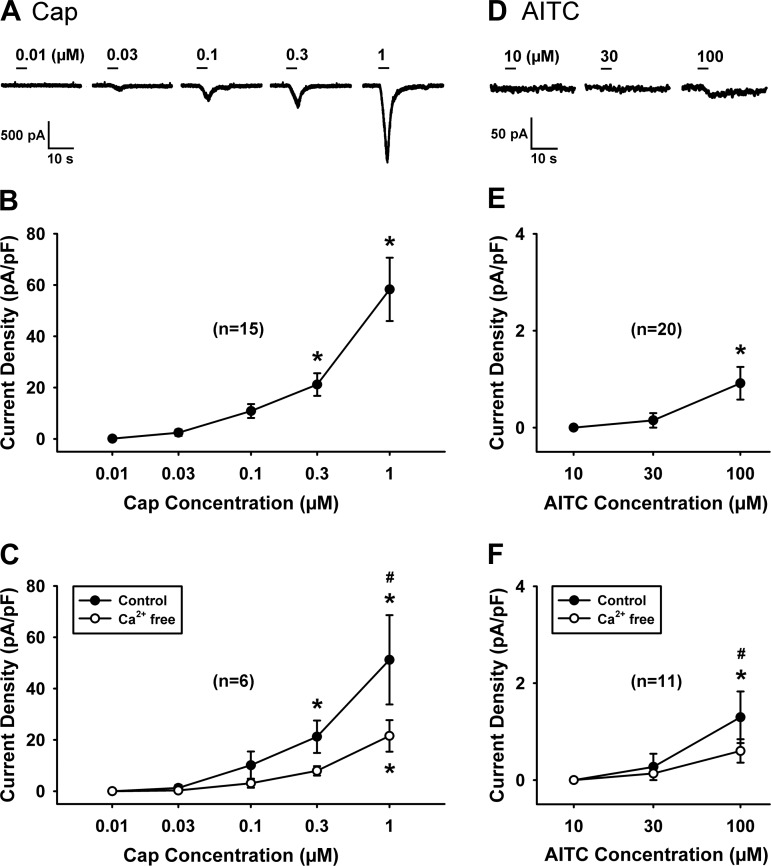
Fig. 1.
Concentration-response relationships of capsaicin (Cap) and allyl isothiocyanate (AITC) in isolated vagal pulmonary sensory neurons. A and D: experimental records illustrating the inward currents recorded when concentrations of Cap and AITC were increased by successive tripling of the concentration at 20-min intervals until it reached 1 μM and 100 μM, respectively, in 2 jugular neurons (A: 14.6 pF; D: 13.6 pF); the duration of each chemical application was 4 s. In our preliminary study, desensitization occurred in neurons after they received application of 300 μM AITC; therefore, the response to AITC was not measured beyond the concentration of 100 μM. B and E: concentration-response curves of Cap (n = 15; 8 jugular and 7 nodose neurons) and AITC (n = 20; 12 jugular and 8 nodose neurons) obtained in regular extracellular solution (ECS). C and F: concentration-response curves of Cap (n = 6; 3 jugular and 3 nodose neurons) and AITC (n = 11; 6 jugular and 5 nodose neurons) obtained in regular ECS (●, control) and after the neurons were perfused by Ca2+-free ECS for 10 min (○, Ca2+ free); the neurons studied in C and F were also included in B and E. The responses were similar between nodose and jugular neurons, and their data were pooled. *P < 0.05, significantly different from the response to lowest concentration. #P < 0.05, significantly different between control and Ca2+-free conditions.
Series 2.
To determine whether there is an interaction between simultaneous TRPA1 and TRPV1 activations, the inward current was recorded in pulmonary sensory neurons when AITC (30 μM) and Cap (0.1 or 0.3 μM) were administered individually first and then in combination (Cap+AITC); 20 min elapsed between two challenges for recovery. The order of AITC and Cap applications was randomized between different neurons (e.g., Fig. 2, left).
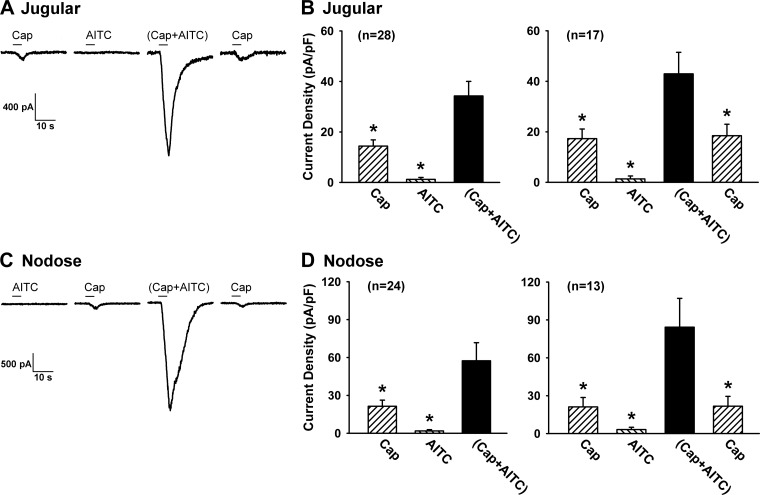
Fig. 2.
Synergistic effect generated by a simultaneous application of Cap and AITC in isolated vagal pulmonary sensory neurons. A and C: experimental records illustrating the inward currents evoked by Cap (0.3 μM), AITC (30 μM), Cap+AITC, a combination of Cap and AITC at the same concentrations, and Cap (repeated after washout) in a jugular (31 pF) and a nodose (19.5 pF) neuron, respectively. B and D, left: group data of current densities evoked by Cap (0.1 or 0.3 μM), AITC (30 μM), and Cap+AITC; the number in parenthesis is the total number of neurons studied. B and D, right: to test whether the effect was reversible, the response to Cap was measured again after washout in a majority of these neurons (number shown in parentheses). The duration of each chemical application was 4 s, as depicted by the horizontal bar. An interval of 20 min elapsed between 2 challenges for recovery. Data are means ± SE. *P < 0.05, significantly different from the response to Cap+AITC.
Series 3.
To investigate whether the interaction between TRPA1 and TRPV1 activations also existed in response to another selective agonist of TRPA1, the identical protocol as Series 2 was repeated when AITC was replaced by cinnamaldehyde (CA; 150–300 μM).
Series 4.
To determine whether the responses of these neurons were uniquely generated by the interaction between TRPA1 and TRPV1 activations, either AITC or Cap was replaced by one of these known chemical activators of vagal pulmonary sensory neurons: phenylbiguanide (PBG; 10 μM), an agonist of 5-HT3 receptor, and ATP (0.3 or 1 μM), an agonist of P2X2 and P2X3 receptors (18).
Series 5.
To investigate the role of extracellular Ca2+ in the interaction between TRPA1 and TRPV1 channels, the responses to Cap, AITC, and Cap+AITC were recorded and compared when each neuron was perfused by regular ECS (containing 1.8 mM Ca2+), Ca2+-free ECS, and regular ECS again (washout for recovery).
Series 6.
To determine whether and how the interaction was influenced by the sequence of TRPA1 and TRPV1 activations, AITC alone was applied to neurons immediately preceding the Cap application; for comparison, Cap alone was applied immediately preceding the AITC application in other neurons.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemical (St. Louis, MO). A stock solution of Cap (1 mM) was prepared in 1% ethanol, 1% Tween 80, and 98% saline. Stock solutions of ATP and PBG were prepared in saline at the concentrations of 10 mM and 2 mM, respectively. Stock solutions of AITC and CA were prepared in DMSO at the concentrations of 0.1 M. Solutions of these chemicals at desired concentrations were then prepared daily by dilution with ECS, except in certain parts of Series 1 and 5 with the Ca2+-free ECS.
Statistical Analysis
Data were analyzed with a one-way repeated-measures ANOVA, followed by a post hoc Fisher's test, unless mentioned otherwise. A P value <0.05 was considered significant. All data are means ± SE.
RESULTS
In this study, a total of 168 pulmonary sensory neurons were isolated from nodose and jugular ganglia of 35 rats (164 ± 10 g) and identified by the fluorescence intensity of DiI. The whole-cell capacitances of these neurons were 18.1 ± 0.7 pF (n = 168); the majority of them (150 out of 168) were small in size (capacitance ≤30 pF); 55% (n = 93) of these cells were nodose neurons, and 45% (n = 75) were jugular neurons. To normalize the responses between cells of different sizes, the current density (current/capacitance; pA/pF) was calculated for comparison.
Study Series 1
Concentration-response relationships of both TRPA1 and TRPV1 agonists in isolated pulmonary sensory neurons.
The concentration-current relationships of Cap (n = 15) and AITC (n = 20) were obtained in isolated pulmonary sensory neurons, as shown in Fig. 1. Within these concentration ranges, the responses to Cap and AITC were reproducible in the same neurons. The response to AITC was not measured beyond the concentration of 100 μM because the application of 300 μM AITC consistently resulted in desensitization of pulmonary sensory neurons to subsequent application of AITC in our preliminary study.
Only low concentrations of Cap (0.1 or 0.3 μM) and AITC (30 μM) were used in the later study series; the higher concentration of Cap (0.3 μM) was applied only in the neurons that did not respond to the lower concentration (0.1 μM).
In some of these neurons, the concentration-current relationships of both Cap and AITC were obtained again after they were perfused with Ca2+-free ECS for 10 min; although the response to a given concentration of Cap was reduced in the Ca2+-free condition, the response to Cap increased progressively in a concentration-dependent manner (n = 6; Fig. 1C). A similar change was also seen in the concentration-current relationship of AITC in the Ca2+-free condition (n = 11; Fig. 1F).
Study Series 2 and 3
Positive interaction of simultaneous TRPA1 and TRPV1 activations in vagal pulmonary sensory neurons.
Application of Cap (0.1 or 0.3 μM; 4 s) immediately evoked a small current in both pulmonary jugular and nodose neurons (14.4 ± 2.5 pA/pF in jugular neurons, n = 28; 21.4 ± 4.9 pA/pF in nodose neurons, n = 24; Fig. 2); the latency of the response was 1.3 ± 0.04 s (e.g., Fig. 2A). Application of AITC (30 μM; 4 s) either did not evoke any response in 87% of the cells (e.g., Fig. 2) or induced a small current in the remaining 13% (e.g., Fig. 6B), with a relatively longer latency (2.9 ± 0.4 s) compared with that of Cap; the average responses were 1.2 ± 0.8 pA/pF in jugular neurons (n = 28) and 1.8 ± 1.0 pA/pF in nodose neurons (n = 24). However, when Cap and AITC of the same concentrations were applied in combination for 4 s, the response was markedly amplified, and the current density was elevated to 34.3 ± 5.8 pA/pF in jugular neurons (P < 0.01; n = 28) and 57.4 ± 14.4 pA/pF in nodose neurons (P < 0.01; n = 24), with a response latency (1.4 ± 0.04 s) comparable to that of Cap alone (Fig. 2). In addition, the duration of current evoked by the combined challenge of AITC and Cap (15.1 ± 1.2 s) was significantly longer than that of Cap (5.9 ± 0.5 s; P < 0.01) or AITC (6.3 ± 1.1 s; P < 0.01) when they were applied individually in the same neurons. The responses to Cap alone completely returned to control when the test was repeated 20 min later (Fig. 2, B and D, right), and the potentiated responses to the combined challenge were also reproducible. Because the potentiated responses were similar between nodose and jugular neurons (Fig. 2, B and D), the data from these two groups were pooled together for the later study series.
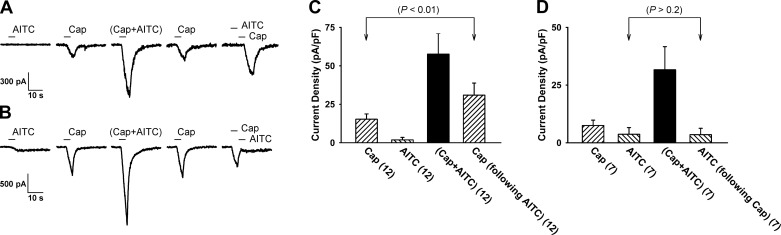
Fig. 6.
Responses evoked by separate but immediately successive application of TRPA1 or TRPV1 agonists. A: experimental records illustrating the synergistic effect generated by a combined application of AITC (30 μM) and Cap (0.3 μM), and Cap+AITC in a jugular neuron (24.8 pF); 20 min after confirming that the response to Cap had returned to control, the current evoked by the same concentration of Cap immediately following an application of AITC alone was clearly potentiated in the same neuron. B: experimental record illustrating that an application of Cap alone immediately preceding AITC did not potentiate the AITC-evoked current. C (n = 12) and D (n = 7): group data (means ± SE) collected under the experimental conditions described in A and B, respectively; the P values were determined by the paired t-test comparing the response to the corresponding challenge of Cap or AITC at control.
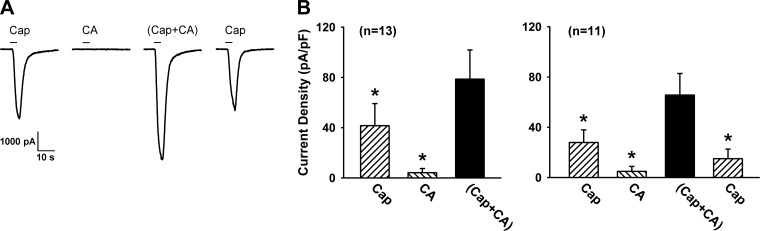
A similar pattern of this synergistic effect of simultaneous TRPA1 and TRPV1 activations was also found when AITC was replaced by CA (150–300 μM), another selective agonist of TRPA1 (Fig. 3). The current density evoked by a combination of Cap and CA (78.6 ± 23.2 pA/pF) was significantly greater than the mathematical sum of the responses to Cap (41.6 ± 17.5 pA/pF) and CA (4.1 ± 3.4 pA/pF) when they were applied individually in the same neurons (P < 0.05; n = 13) (Fig. 3).
Fig. 3.
Synergistic effect generated by a simultaneous application of cinnamaldehyde (CA) and Cap in vagal pulmonary sensory neurons. A: experimental records illustrating the inward currents evoked by Cap (0.1 μM), CA (150 μM), Cap+CA, a combination of Cap and CA at the same concentrations, and Cap (repeated after washout) in a jugular neuron (33.8 pF). B, left: group data (n = 13) of current densities evoked by Cap (0.1 or 0.3 μM), CA (150–300 μM), and Cap+CA. B, right: to test whether the effect was reversible, group data were obtained when the response to Cap was measured again after washout in 11 of the 13 cells. Data are means ± SE. *P < 0.05, significantly different from the response to Cap+CA. For more detailed explanations, see legend to Fig. 2.
Study Series 4
A lack of positive interaction when either AITC or Cap was replaced by a non-TRPA1 and non-TRPV1 chemical activator of vagal pulmonary sensory neurons.
To determine whether both TRPA1 and TRPV1 activations are essential in generating the synergistic effect, the study was repeated when either AITC or Cap was replaced by one of the non-TRPA1 and non-TRPV1 chemical activators of these neurons, ATP (0.3 or 1.0 μM) and PBG (10 μM) (e.g., see Fig. 4, A and B). These studies showed that neither the peak amplitude nor the duration of the current evoked by a combined application of ATP with either AITC or Cap was greater than the mathematical sum of the responses when they were applied individually in the same neurons (Fig. 4, C and D). Similarly, there was no positive interaction when a combined application of PBG with AITC or Cap was applied (Fig. 4, E and F).
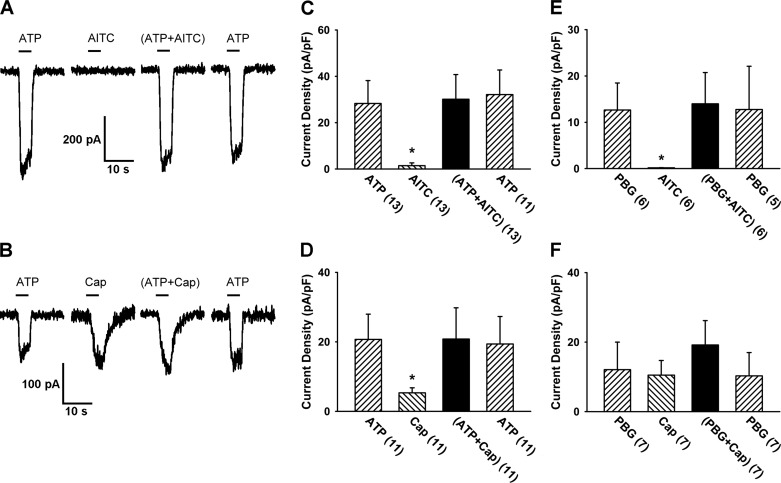
Fig. 4.
A lack of positive interaction when either Cap or AITC was replaced by a non-transient receptor potential ankyrin 1 (non-TRPA1) and non-TRP vanilloid 1 (non-TRPV1) channel activator of vagal chemical activator of vagal pulmonary sensory neurons. A: experimental records illustrating inward currents evoked by ATP (1 μM), AITC (30 μM), ATP+AITC, and ATP (repeated after washout) in a nodose neuron (23 pF). B: inward currents evoked by ATP (1 μM), Cap (0.3 μM), ATP+Cap, and ATP (repeated after washout) in a nodose neuron (15.1 pF). Both ATP and phenylbiguanide (PBG; 10 μM) are non-TRPA1 and non-TRPV1 chemical activators of vagal pulmonary sensory neurons. C–F: group data of the current densities evoked when either Cap or AITC was replaced by ATP (0.3 or 1.0 μM) or PBG (10 μM); the number in parenthesis is the number of neurons studied under that condition. Data are means ± SE. *P < 0.05, significantly different from the response to the combined application.
To determine whether the lack of potentiating effect was due to those maximal currents that were already reached by these chemical activators, a higher concentration of ATP or PBG was applied individually; the current density evoked by ATP at 3 μM was significantly higher than that by ATP at 1 μM (225%, P < 0.05; n = 9), and the current density evoked by PBG at 30 μM was significantly higher than that by PBG at 10 μM (252%, P < 0.05; n = 6).
Study Series 5
The positive interaction was completely absent when Ca2+ was removed from the ECS.
In our preliminary study using the ratiometric-image technique (Axon Imaging Workbench; Axon Instruments), we have demonstrated that the Ca2+-free ECS (containing 5 mM of EGTA) completely blocked the Ca2+ influx (measured by the fura-2 340/380 ratio) evoked by Cap (0.3 μM) in pulmonary sensory neurons (Lin RL and Lee LY, unpublished observations).
After the positive interaction of a combined application of Cap and AITC was confirmed (e.g., see Fig. 5A), the same experimental protocol was repeated in the same neurons after they had been perfused with the Ca2+-free ECS for 10 min (e.g., see Fig. 5B). It was evident that the positive interaction was completely abolished when Ca2+ was removed from the ECS. The current density evoked by a combination of Cap and AITC (both prepared in Ca2+-free ECS) was not different from the mathematical sum of the responses to Cap and AITC when they were perfused individually (P > 0.05; n = 16) (Fig. 5, B and D). The remaining inward current evoked by Cap during the Ca2+-free ECS perfusion was probably mediated by other cations (e.g., Na+) (7, 36).
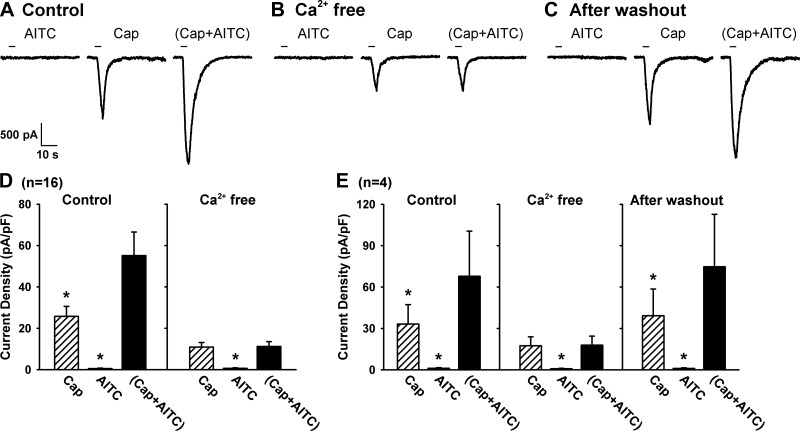
Fig. 5.
Role of extracellular Ca2+ in the positive interaction of TRPA1 and TRPV1 channels. A: experimental records illustrating the positive interaction of TRPA1 and TRPV1 channels during control in a jugular neuron (27.4 pF). B: positive interaction was completely abolished after the same neuron was perfused by Ca2+-free ECS for 10 min. C: positive interaction returned after the same neuron was perfused by regular ECS again for 20 min. D: group data of the responses (n = 16) when neurons were perfused with regular ECS (control) and Ca2+-free ECS, respectively. E: in 4 of these 16 neurons, group data were also obtained after the Ca2+-free ECS was washed out. Data are means ± SE. *P < 0.05, significantly different from the response to Cap+AITC.
In 4 of these 16 neurons, we further investigated whether the positive interaction between TRPA1 and TRPV1 channels would return after they were perfused again by the ECS containing the regular Ca2+ concentration (1.8 mM) for 20 min. As illustrated in Fig. 5, C and E, the potentiating effect of a combined application of AITC and Cap was completely recovered after the Ca2+ concentration in ECS was returned to its normal level. These data clearly indicated an important role of extracellular Ca2+ in generating the synergistic effect of simultaneous activations of TRPA1 and TRPV1 channels.
Study Series 6
Responses to separate but immediately successive applications of TRPA1 or TRPV1 agonist.
The response to Cap alone was also potentiated when AITC alone was applied immediately preceding the Cap application in these neurons. The current density evoked by Cap alone was 15.3 ± 3.4 pA/pF, but the response to the same Cap challenge immediately following the application of AITC alone was significantly augmented (31.0 ± 7.8 pA/pF; P < 0.01, n = 12; paired t-test) in the same neurons (Fig. 6, A and C). However, reversing the order did not potentiate the response to AITC; the current density evoked by AITC alone was 3.7 ± 2.9 pA/pF, and the response to the same AITC challenge was not altered (3.5 ± 2.8 pA/pF; P > 0.2, n = 7; paired t-test) immediately following the application of Cap (Fig. 6, B and D). Neurons studied in this series were also included in Series 2.
An overall analysis of the pattern and the variability of positive interaction.
Data analysis in the studies described above revealed that the magnitude and pattern of the potentiating effect generated by simultaneous TRPA1 and TRPV1 activations varied among these neurons. When the data of all the neurons studied with the same protocol (Series 2, 5, and 6; n = 68) were pooled together, four different patterns of the potentiated responses can be identified based on the following characteristic features of the responses, and the distribution of these neurons in each category was as follows.
MAGNITUDE OF PEAK CURRENT POTENTIATION.
In ∼10% (7 out of 68) of the neurons studied, the peak current evoked by a combined Cap+AITC application was more than fivefold greater than the mathematic sum of the responses to separate applications of AITC and Cap (e.g., Fig. 7A, a); in a majority (69%) of the neurons, it was 1.2- to 5-fold greater (e.g., Fig. 7A, b) and not different or only slightly greater (<1.2-fold) in 21% (e.g., Fig. 7A, c).
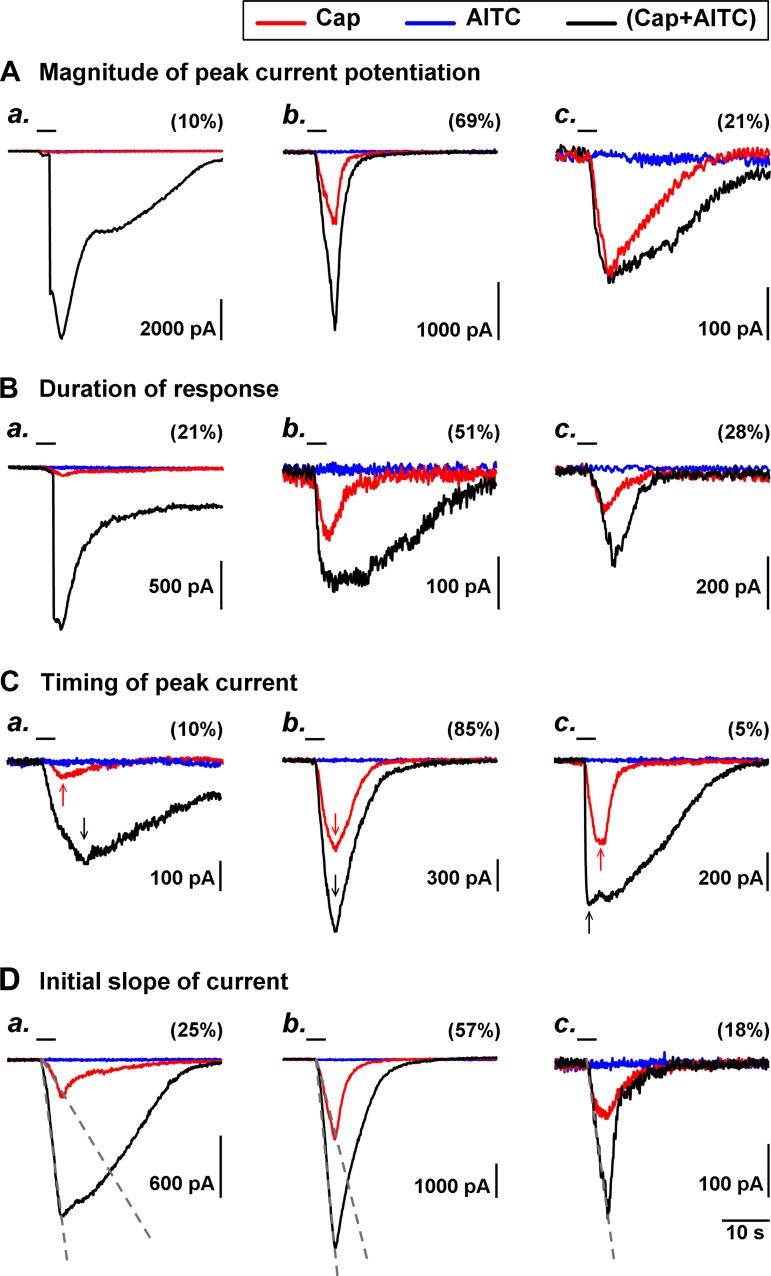
Fig. 7.
Different patterns and variability of the synergistic effect of simultaneous activations of TRPA1 and TRPV1 channels. Data of all the neurons in Series 2, 5, and 6 were pooled (n = 68), and 4 different patterns of potentiated responses were identified and illustrated in A–D; the distribution of these neurons in each subcategory was expressed as the percentage in parenthesis. Typical examples in each subcategory are illustrated in a, b, and c, in which 3 traces (experimental records) from the same neuron were superimposed for comparison. Blue, AITC alone; red, Cap alone; black, Cap+AITC. Arrows in C were added to mark the time that the evoked current reached its peak. The gray dashed lines in D were added to show the initial slope of current trajectory. For more detailed explanations, see results and Fig. legend 2.
DURATION OF RESPONSE.
The current evoked by Cap+AITC was markedly prolonged in most of the neurons. In 21% of all the neurons studied, the duration of the current evoked by Cap+AITC lasted for >20 s longer than that of either Cap or AITC alone (e.g., Fig. 7B, a), 3–20 s longer in 51% (e.g., Fig. 7B, b), and only 0–3 s longer in the remaining 28% (e.g., Fig. 7B, c).
TIMING OF PEAK CURRENT.
In the vast majority (85%) of the neurons, the current evoked by Cap+AITC reached the peak at about the same time as that evoked by Cap alone (e.g., Fig. 7C, b); the current evoked by the combined application took longer time to reach the peak in 10% (e.g., Fig. 7C, a) and reached the peak sooner in only 5% (e.g., Fig. 7C, c).
INITIAL SLOPE OF THE CURRENT.
The slope of the initial current trajectory was determined (as shown in Fig. 7D), and a ratio between that evoked by Cap+AITC and that by Cap alone was calculated in each neuron. For example, in the neuron shown in Fig. 7D, b, the slope of the initial current evoked by Cap+AITC and Cap alone was 1,134 pA/s and 451 pA/s, respectively; the ratio was 2.5 (1,134/451). In all the neurons studied, this ratio was >5.0 in 25% of the neurons (e.g., Fig. 7D, a), between 1.2 and 5.0 in 57% (e.g., Fig. 7D, b), and <1.2 in the remaining 18% (e.g., Fig. 7D, c).
DISCUSSION
This study demonstrated a distinct synergistic effect of simultaneous TRPA1 and TRPV1 activations in isolated rat vagal pulmonary sensory neurons. The current density evoked by a combination of low concentrations of AITC and Cap was more than twofold greater than the mathematical sum of the responses to AITC and Cap when they were administered separately at the same concentrations in the same neurons. Thus these results have provided convincing evidence that a positive interaction between these two TRP channels occurs at the pulmonary sensory neurons. The mechanisms underlying this effect are not fully understood, but extracellular Ca2+ plays a critical role because the positive interaction between TRPA1 and TRPV1 was completely abrogated when Ca2+ was removed from the ECS. The absence of a positive interaction in the Ca2+-free ECS (Fig. 5) was not due to a loss of the charge carrier for the current because the concentration-current relationships of Cap and AITC were still clearly present under the Ca2+-free condition (Fig. 1).
It was previously reported that extracellular Ca2+ can gate and sensitize TRPV1 expressed in HEK293 and oocytes via an electrostatic interaction with two glutamates (E600 and E648) located near the channel pore (1, 8). TRPA1 has also been shown to be directly gated by Ca2+ that activates TRPA1 by binding the EF-hand domain in its NH2 terminus (10, 40). More importantly, Ca2+ is the primary ion species conducted through both TRPA1 and TRPV1 upon activation, which triggers an abrupt influx of Ca2+ and a rapid rise of intracellular Ca2+ concentration (7, 12, 36). Wang and coworkers (38) have suggested that the entry of extracellular Ca2+ via TRPA1 into the cell further potentiates the TRPA1 channel activity because its effect was abolished when the Ca2+ permeability was blocked by a selective mutation of Asp918 near the pore region of TRPA1. This proposition is further supported by the observation that TRPA1 channels are activated when the solution containing Ca2+ was applied to the cytosolic face in the excised patch preparation or when Ca2+ was dialyzed into cells (8, 10, 40). Therefore, it seems plausible that the Ca2+ influx through the opening of TRPV1 may in turn potentiate the sensitivity of TRPA1 located in the close vicinity, resulting in a positive interaction between these two channels (31).
The role of intracellular Ca2+ as a mediator regulating the interaction between the TRPA1 and TRPV1 channels has also been proposed in the nociceptor neurons during acute inflammatory hyperalgesia (3, 4, 23). For example, autacoids such as bradykinin can bind to G protein-coupled receptors located on the nociceptor neuronal membrane and activates phospholipase C (PLC), which in turn cleaves the phospholipid phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate, leading to the release of Ca2+ from intracellular stores. PLC can also activate protein kinase C, increasing the sensitivity of TRPV1. Both the Ca2+ influx through TRPV1 and the internal Ca2+ release can then activate TRPA1 (4). Spahn and coworkers (34) recently reported that TRPV1 can be sensitized after sustained stimulation of TRPA1 (for 2 min) by cinnamaldehyde or mustard oil in dorsal root ganglion nociceptor neurons. Their study suggested that the TRPA1 activation triggers the signaling cascade involving activation of Ca2+-sensitive adenylyl cyclase, increase in cAMP, activation of protein kinase A (PKA), and phosphorylation of TRPV1 (34). The cAMP/PKA signaling cascade has also been shown to be involved in the inflammatory mediator (e.g., PGE2)-induced TRPV1 hypersensitivity (14, 19). These intracellular second-messenger signaling cascades are an attractive hypothesis for explaining the TRPA1-TRPV1 interaction. However, the rapidity of the onset of this positive interaction observed in our study is distinctly different from the observations reported in those previous studies and seems to argue against a major role of these signaling pathways. In 82% of all the neurons studied, the combined application of AITC and Cap evoked an abrupt and marked increase in the slope of the initial current trajectory, compared with that evoked by Cap alone (Fig. 7D, a and b). This increase in the slope of the evoked current occurred abruptly and immediately after the cell was exposed to the combined application of AITC and Cap, indicating that an increasing number of TRPV1 and/or TPRA1 channels were opened from the outset of the response.
The functional interaction between TRPA1 and TRPV1 may also be linked to specific features of the channel structure. TRP channels from members of different subfamilies can form heteromultimeric complexes through which physical interactions between these TRP channels are found (16, 30). Recent studies have reported that functional heteromeric channels can be formed from TRPA1 and TRPV1 on the cytoplasmic membrane, and these channel complexes exhibit pharmacological and electrophysiological properties that are different from these individual channels (2, 32, 33, 35). The agonist-induced conformational changes could lead to alteration of gating properties of these channels and modulate their activities (33). Thus activation of TRPV1 can modulate the intrinsic properties of the TRPA1 channel, probably through direct interaction of the channels within the heteromeric TRPA1-TRPV1 complex. Indeed, their study indicated that single-channel properties of TRPA1 are modulated by TRPV1 activity, independent of the change of intracellular Ca2+ (35). Although it seems probable that a TRPA1-TRPV1 interaction occurring within the heteromeric TRPA1-TRPV1 complexes can contribute to the synergistic effect observed in our study, the relative importance of these heteromeric channels should be evaluated based on our finding of the critical dependence of extracellular Ca2+ in the positive interaction in pulmonary sensory neurons (Fig. 5). Furthermore, the extent of the expression of these channel complexes in pulmonary nodose and jugular neurons also requires further investigation. Nonetheless, a possible involvement of this heteromeric TRPA1-TRPV1 complex certainly merits further investigation considering the recent finding that the number of neurons expressing Tmem100, a two-transmembrane protein and a potentiating modulator of this channel complex, is increased in dorsal root ganglia under inflammatory condition (39).
In this study, we purposely chose relatively low (near threshold) concentrations of AITC and Cap (Fig. 1) to avoid desensitization and to evaluate the responses of these neurons to TRPA1 and TRPV1 activations near physiological and pathophysiological conditions. As such, when AITC was applied alone, it failed to evoke any measurable current in 87% of the pulmonary sensory neurons and evoked only a very small current in the remaining 13%. However, when the same concentration of AITC was applied simultaneously with Cap, a pronounced amplification of the response was evoked in 79% of all the neurons studied (Fig. 7A, a and b), which is a distinct contrast to the minimal stimulatory effect generated by this low concentration of AITC alone. The selectivity of this positive interaction between TRPA1 and TRPV1 was clearly illustrated by the absence of any synergistic effect when either AITC or Cap was replaced by ATP or PBG, a non-TRPA1 and non-TRPV1 chemical activator of these neurons (Fig. 4).
We cannot determine in this study the relative contributions of TRPA1 and TRPV1 to the amplified current response evoked by the combined application. In 85% of the neurons, the current evoked by the combined Cap+AITC application reached the peak precisely at the same time as that by Cap alone (Fig. 7C, b), which appears to indicate that the primary current was conducted through TRPV1 channels during the combined application. In our study of replacing the combination of AITC and Cap with separate but successive applications of these two chemical agonists, the Cap-evoked current was significantly augmented immediately following the application of AITC alone, whereas the AITC-evoked response was not altered immediately following the Cap application (Fig. 6). These contrasting results seem to suggest the possibility that the activation of TRPA1 by AITC exerted a sensitizing effect on TRPV1, and this effect sustained for a few seconds even after the extracellular AITC was washed away. One possible explanation is that the stimulatory effect of the simultaneous application of AITC and Cap was amplified by an activation of TRPA1. Once it is activated, its intracellular signaling activity perpetuated even after the extracellular AITC was removed.
This synergistic effect generated by AITC and Cap was not limited to these two specific chemical activators of TRPA1 and TRPV1; for example, the potentiating effect was also clearly shown when AITC was replaced by cinnamaldehyde, another known chemical activator of TRPA1 (Fig. 3). Furthermore, the positive interaction could also be demonstrated when Cap was replaced by high temperature (>39.8°C), a natural biological activator of TRPV1 (27); in a small number of neurons studied, when the temperature of ECS was elevated from room temperature (25.6°C) to 40.2°C and maintained for 60 s, the current response to the same concentration of AITC (30 μM for 4 s) was also markedly elevated (unpublished observations).
The positive interaction between TRPA1 and TRPV1 channels demonstrated in this study may serve a regulatory function in modulating the sensitivity of vagal pulmonary C-fiber neurons, which is important in maintaining various airway functions under both physiological and pathophysiological conditions for the following reasons. These two TRP channels are colocalized abundantly in a majority of vagal pulmonary C-fiber afferent nerves (25) that are primarily responsible for eliciting pulmonary protective reflexes against certain inhaled irritants (e.g., acid aerosol, cigarette smoke, sulfur dioxide, etc.) (21). A recent study has demonstrated a pronounced potentiation of the pulmonary chemoreflex responses generated by stimulation of these afferents by a combined challenge of AITC and Cap in anesthetized rats (22). More importantly, both TRPA1 and TRPV1 can be activated by a number of chemical mediators (e.g., TRPA1 by reactive oxygen species and bradykinin, TRPV1 by proton and anandamide), and these mediators are known to be released endogenously and locally during airway inflammatory reactions (3, 5, 15, 24, 37). Hence, there is a distinct possibility that these TRP channels can be activated simultaneously during airway inflammation. Increasing evidence reported in recent studies suggests that TRPA1 and TRPV1 expressed in these C-fibers play a pivotal role in the manifestation of various symptoms of airway hypersensitivity, a common pathophysiological feature in patients with airway inflammatory diseases. Indeed, stimulation of bronchopulmonary C-fiber afferents is known to elicit cough, dyspneic sensation, airway hypersecretion, and reflex bronchoconstriction via the centrally mediated cholinergic reflexes and the local axon reflex via the tachykininergic mechanisms (21). Taken together, a synergistic effect of simultaneous activations of TRPA1 and TRPV1 expressed on vagal bronchopulmonary C-fiber afferents may trigger exaggerated sensory and reflex responses during airway inflammation and results in airway hypersensitivity.
In summary, this study demonstrated a distinct positive interaction occurring abruptly between TRPA1 and TRPV1 when they were activated simultaneously by their respective agonists at near-threshold concentrations in vagal pulmonary sensory neurons. Despite that AITC at this low concentration evoked only minimal or undetectable response in these neurons, it consistently amplified the Cap-evoked current in the same neurons. This synergistic effect was dependent on the extracellular Ca2+, and the rapid onset of the action further suggests that the interaction probably occurred locally at the sites of these channels. Our results propose that the TRPA1-TRPV1 interaction may play a part in regulating the sensitivity of pulmonary sensory neurons during airway inflammatory reaction, but its functional role and importance remain to be determined under various physiological and pathophysiological conditions.
GRANTS
This study was supported in part by National Institutes of Health (NIH) grants HL-96914 and UL1TR0000117. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.-C.H. and L.-Y.L. conception and design of research; C.-C.H. and L.-Y.L. performed experiments; C.-C.H. and L.-Y.L. analyzed data; C.-C.H. and L.-Y.L. interpreted results of experiments; C.-C.H. and L.-Y.L. prepared figures; C.-C.H. and L.-Y.L. drafted manuscript; C.-C.H. and L.-Y.L. edited and revised manuscript; C.-C.H. and L.-Y.L. approved final version of manuscript.
ACKNOWLEDGMENTS
Authors thank Drs. Brian Delisle and Qihai Gu for critical reading of the draft of this manuscript and valuable suggestions, Ruei-Lung Lin for performing the preliminary experiments to verify the blocking effect of EGTA on Cap-evoked intracellular Ca2+ transient, and Reynold Tapia for technical assistance.
The preliminary report of this study has been presented as an abstract in the 8th International Cough Symposium (London, United Kingdom) in June 2014.
REFERENCES
- 1.Ahern GP, Brooks IM, Miyares RL, Wang XB. Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci 25: 5109–5116, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akopian AN. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr Pharm Biotechnol 12: 89–94, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124: 1269–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 75: 181–200, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 106: 9099–9104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience 154: 1467–1476, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. [DOI] [PubMed] [Google Scholar]
- 10.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 282: 13180–13189, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 166: 510–521, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol 2: 563–608, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Geppetti P, Materazzi S, Nicoletti P. The transient receptor potential vanilloid 1: role in airway inflammation and disease. Eur J Pharmacol 533: 207–214, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol 89: 1985–1993, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Gu Q, Lee LY. Characterization of acid signaling in rat vagal pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 291: L58–L65, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 118: 917–928, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Jammes Y, Fornaris E, Mei N, Barrat E. Afferent and efferent components of the bronchial vagal branches in cats. J Auton Nerv Syst 5: 165–176, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol 295: L858–L865, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwong K, Lee LY. PGE(2) sensitizes cultured pulmonary vagal sensory neurons to chemical and electrical stimuli. J Appl Physiol 93: 1419–1428, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 9: 243–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee LY, Yu J. Sensory nerves in lung and airways. Compr Physiol 4: 287–324, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Lin YJ, Lin RL, Ruan T, Khosravi M, Lee LY. A synergistic effect of simultaneous TRPA1 and TRPV1 activations on vagal pulmonary C-fiber afferents. J Appl Physiol 118: 273–281, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell 124: 1123–1125, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 4: e7596, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassenstein C, Kwong K, Taylor-Clark T, Kollarik M, Macglashan DM, Braun A, Undem BJ. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J Physiol 586: 1595–1604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One 7: e42454, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni D, Lee LY. Effect of increasing temperature on TRPV1-mediated responses in isolated rat pulmonary sensory neurons. Am J Physiol Lung Cell Mol Physiol 294: L563–L571, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflügers Arch 464: 425–458, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Park JY, Hwang EM, Yarishkin O, Seo JH, Kim E, Yoo J, Yi GS, Kim DG, Park N, Ha CM, La JH, Kang D, Han J, Oh U, Hong SG. TRPM4b channel suppresses store-operated Ca2+ entry by a novel protein-protein interaction with the TRPC3 channel. Biochem Biophys Res Commun 368: 677–683, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Patil MJ, Jeske NA, Akopian AN. Transient receptor potential V1 regulates activation and modulation of transient receptor potential A1 by Ca2+. Neuroscience 171: 1109–1119, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Mol Pharmacol 80: 117–123, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci 29: 1568–1578, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spahn V, Stein C, Zollner C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol Pharmacol 85: 335–344, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Staruschenko A, Jeske NA, Akopian AN. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J Biol Chem 285: 15167–15177, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 586: 3447–3459, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283: 32691–32703, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron 85: 833–846, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 10: 277–279, 2007. [DOI] [PubMed] [Google Scholar]