Abstract
Reactive stromal changes that occur in different human cancers might play a role in local tumor spreading and progression. Studies done on various human cancers have shown activated stromal cell phenotypes, modified extracellular matrix (ECM) composition, and increased microvessel density. Furthermore, they exhibit biological markers consistent with stroma at the site of wound repair. In prostate cancer, stroma is composed of fibroblasts, myofibroblasts, endothelial cells and immune cells. Predominant cells in the tumorous stroma are, however, fibroblasts/myofibroblasts. They are responsible for the synthesis, deposition and remodeling of the ECM. Epithelial tumorous cells, in interaction with stromal cells and with the help of various molecules of ECM, create a microenvironment suitable for cancer cell proliferation, movement, and differentiation. In this review, we discussed the role of different stromal components in prostate cancer as well as their potential prognostic and therapeutic significance.
KEYWORDS: Prostate cancer, stroma, myofibroblast, extracellular matrix components
INTRODUCTION
Prostatic cancer is the most common malignancy in men and the second cause of cancer death in developed countries.
The prostatic gland is composed of two distinctive compartments: epithelial and stromal. They mutually interact via androgen receptors and this interplay is important for prostate development and differentiation [1-3]. Analogously, prostate cancer is composed of both malignant epithelial cells and supportive stroma whose transformation is important for the growth and development of the tumor. Cancerous stroma is composed of fibroblasts, myofibroblasts, endothelial cells and immune cells. Predominant cellular types are, however, fibroblasts or myofibroblasts, which play an important role in synthesis, deposition and remodeling of the extracellular matrix. Tumorous epithelial cells, in interaction with stromal cells, and with the help of various molecules of extracellular matrix (ECM) create a microenvironment suitable for cancer cell proliferation, movement, and differentiation [4-13].
Reactive stromal changes occurring in different human cancers might play a role in local tumor spread and progression. Studies on different human cancer specimens have demonstrated activated stromal cell phenotypes, modified ECM composition, and increased microvessel density. Furthermore, they exhibit biological markers consistent with stroma at the site of wound repair [1, 5, 7, 12,13].
Tumor cell populations have several important features: capacity for self-renewal, ability to survive under different stress conditions and potential to produce metastases, the latter resulting in increased cancer aggressiveness and widespread dissemination. It appears that not only all the cells of tumor stroma, but also the other stromal components can potentially affect tumorigenesis. They play a key role in enhancement of tumor progression by stimulating angiogenesis and promoting cancer cell survival, proliferation, and invasion. In epithelial-stromal transformation, a panel of highly motile, independent cells capable of invasion and metastasis is involved [5-15].
Presently, it is evident that both malignant transformation and tumor progression are not exclusively regulated by disruption of oncogenes and tumor suppressor genes in neoplastic cells. Other factors, such as the modified interaction between stromal and epithelial compartments that influence androgen receptors, and studies on molecular pathways, signal molecules, and molecules of ECM involved in prostate carcinogenesis are crucial to the better understanding of cancer development and progression [1-14].
Different models were used to study cell to cell and ECM interactions in prostate cancer: cell line cultures, animal models, and prostate cancer tissue specimens obtained at intraoperative consultations and tissue processed and embedded in paraffin blocks. Preclinical animal models on rats, mice and dogs were established in an attempt to mimic the initial steps of prostate carcinogenesis as well as carcinomatous progression and metastatic potential [14-17].
ANDROGEN RECEPTORS
Androgen plays a significant role in the development of the prostate gland, whereas stromal cells are crucial to maintaining its proper function. In order to become differentiated, prostatic epithelial cells require the presence of androgen receptors both in stromal and epithelial parts of prostate [2-4,8].
It is well known that this interaction performed via androgen receptors (AR) is also important in prostate carcinogenesis. Epithelial AR deprivation therapy is used in prostate cancer, but it cannot completely suppress the growth of the tumor. Stromal AR, thus, appear to have a more important role than AR in epithelial tumorous cells. Activated stromal AR affect stromal myofibroblasts, through which prostate carcinoma progression is promoted, and seem to be significant even in androgen-resistant tumors [18-21]. These stromal receptors may be possible targets for future anticancer therapies and are the subject of many studies done on in vitro cell lines, tissue recombination experiments, and androgen receptor knockout animal models.
FIBROBLASTS AND MYOFIBROBLASTS
Myofibroblasts are dynamic stromal cells found at the site of pathologic tissue remodeling. Carcinoma cells have the ability to transform fibroblasts into reactive myofibroblasts, which synthesize different ECM components: collagen, fibronectin, tenascin, versican, galectin, laminin and others. Myofibroblasts can also express proteases and secrete growth factors that support angiogenesis. They are crucial cells that create a tumor-promoting reactive stroma setting, and can stimulate cancer cell growth and migration [22-24].
Prostate cancer-reactive stroma is composed of a myofibroblasts and fibroblasts mixture, with a significant decrease in fully differentiated smooth muscle, whereas normal prostate stroma consists predominantly of smooth muscle [25-27]. Proteins of the ECM play role in cell adhesion and cell signaling, and remodeling of ECM influences cancer spread and invasion. The “new” microenvironment, created in this way, is continuously changing to support the formation of glandular structures and tumor structures, as shown in some animal models [21, 27-29].
The origin of carcinoma fibroblasts and myofibroblasts, including the origin of stromal fibroblasts and bone marrow-derived mesenchymal stem cells, remains questionable, so as the epithelial-endothelial-mesenchymal transition process [9,12,14]. Mechanical force has been studied as one of the factors in conversion of fibroblasts into myofibroblasts. It was investigated with different cell types employing microfluid platforms. The expansion of tumor cells induces mechanical changes in multiple fibroblastic populations in the tumor microenvironment, exerting force on surrounding tissue and inducing local compressive stress [30].
Fibroblast and myofibroblastic stromal changes in prostate carcinoma could be easily quantified by a simple histochemical method (Mallory or Masson trichrome staining) (Figure 1) as well as by an immunohistochemical procedure using antibodies to vimentin, α- smooth muscle actin and desmin [5,25]. Loss of the smooth-muscle cells, quantified immunohistochemically by intensity of the stromal changes, and the appearance of the stromal fibro- and myofibroblasts was associated with a shorter disease-free period and the worse outcome [31,32].
Figure 1.
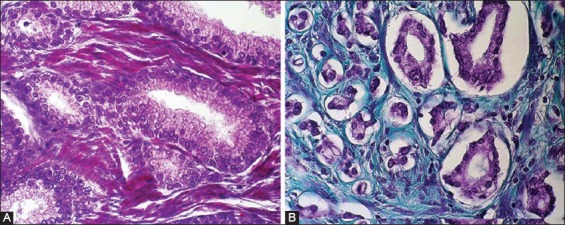
Mallory or Masson trichrome staining in A) benign prostate hyperplasia (x400) and B) prostate cancer (x400).
A relatively recently described phenomenon, that might be related to the fibro- or myofibroblasts and their products, is the appearance of the so-called periacinar halos, retraction clefting or cleft-like spaces within a neoplastic prostatic tissue. The neoplastic cells of prostatic cancer often appear pulled away from the surrounding stroma, leaving halos around the acini [33-36]. Some authors have suggested that this retraction artifact might serve as an additional criterion in the diagnosis of carcinoma, especially when prominent and identified in the half or more of the gland [33-37]. The most pronounced periacinar retraction was noticed in the association with Gleason pattern 3 adenocarcinoma. Some authors have suggested that this phenomenon is probably only an artifact [38]. Conversely, other authors attributed the periacinar retraction to the stromal changes present in prostatic adenocarcinoma and did not consider these clefts to be a simple artifact. Similarly to the retraction artifact, it has been shown that the stromal reaction in prostatic carcinoma is more pronounced in Gleason pattern 3 [25,39]. Furthermore, periacinar retraction artifact was also proposed as an additional and helpful diagnostic criterion in breast and urothelial carcinoma [40,41]. In addition, recent results revealed that the retraction artefact in prostatic carcinoma correlates with different clinicopathological features of the tumor as well as with the biochemical recurrence-free survival, pointing out that the presence and the extent of the retraction artifact could predict worse outcome in patients with prostatic adenocarcinoma [42]. The similar prognostic significance of retraction artifact has been reported in breast carcinoma [43,44].
COLLAGENS
Collagens are fibrillar proteins that form a three-dimensional frame of ECM and are important in the cell signaling processes and metabolism. They are a fundamental part of stromal changes that affect tumor progression, cell survival, apoptosis and cell invasion [9]. Collagens are produced by fibroblasts and myofibroblasts. In prostate cancer, the network of collagen fibers is loose, and its organization is disturbed. Metabolic changes in carcinomatous stroma are increased compared to metabolic changes present in normal prostate stroma. Type I collagen is thorn and results in formation of biologically active collagen I peptides, which then facilitate proliferation and angiogenesis. Also, collagen type I can induce a reduction of E-cadherin-mediated cell–cell adhesion and the loss of E-cadherin, which is important for invasion capability. Type I collagen slitting is also required for angiogenesis at tumor sites. Decreased density of collagen and reticular fibers were found in human prostate tumor stroma [45-47]. Therefore, the assessment of changes in fibrillar components that affect the stromal environment in prostate cancer may help in the evaluation of tumor aggressiveness [29].
ELASTIC FIBERS
Elastic fibers are important for tissue flexibility. Similar to collagens, after elastin degradation, elastin peptides induce stromal cells (fibroblasts, macrophages, lymphocytes, smooth muscle cells and endothelial cells) via the elastin–laminin receptor. There are limited data on the role of elastin and its receptors in tumor invasion, but they are disorganized in the stroma of prostate cancer.
Elastin and its peptides are factors involved in tumor invasion, because these molecules are known to stimulate receptor signaling and chemotaxis. This could explain the morphometric changes reported in certain tumor cell lines invading elastic lamina [29, 46].
LAMININS
Laminins are heterotrimeric molecules made up by one α, one β and one γ chain. Thus far, five α-chains, three β-chains and three γ-chains have been described. These chains combine into at least 14 different types of laminin. The distribution of these laminin isoforms varies between tissues, but in most basal membranes (BMs) more than one type of laminin is present. Laminins are associated with cell differentiation, preservation of cell shape and movement, maintenance of tissue phenotypes, promotion of tissue survival and are present in the basal lamina. Their functions in tumor invasion are the subject of extensive research [48-50]. Some studies reported significant decrease in the expression of laminin in carcinoma comparing to the adjacent prostate tissue (Figure 2) [39]. It has also been shown that membrane type 1 matrix metalloproteases are modifying the laminin-rich basal membrane, playing thus a role in transformation of prostate intraepithelial neoplasm into invasive cancer through their capacity to degrade laminin [39, 51,52].
Figure 2.
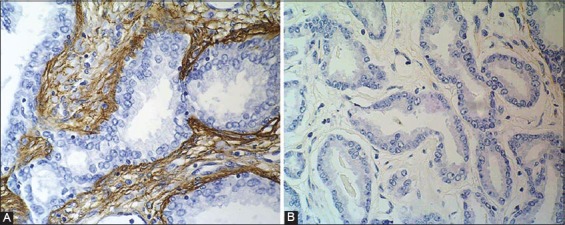
Immunohistochemical laminin staining in A) benign prostate hyperplasia, showing positive cells in stroma (x400) and B) prostate cancer, showing negative cells in stroma (x400).
TENASCIN-C
Tenascin-C is a large (180–300 kDa), hexameric multidomain glycoprotein and located mainly in the ECM. It is involved in tissue interactions during embryogenesis, wound healing, inflammation, and oncogenesis. Tenascin-C is considered to be an anti-adhesive molecule in the stroma, modulating adhesion between cells via fibronectin, an important factor in the cell to ECM adhesion. Tenascin expression in prostatic tissue is considered to decrease or disappear after the maturation of the gland is finished. Some studies reported that tenascin expression is increased in prostate cancer stroma and tenascin-C significantly increased in stroma around neoplastic glands. These data suggest a potential role of tenascin-C in the regulation of tumor cell proliferation, invasion, and metastasis [52-57].
In our experience, the expression of tenascin-C was significantly increased in carcinomatous tissue compared to the adjacent peritumourous tissue and BPH. Tenascin-C was predominantly expressed in stroma around neoplastic glands but was also expressed in the wall of medium-sized blood vessels, which served as an internal positive control. However, in only a few cases we noted a weak positive tenascin reaction in the cytoplasm of neoplastic epithelial cells (Figure 3) [39].
Figure 3.
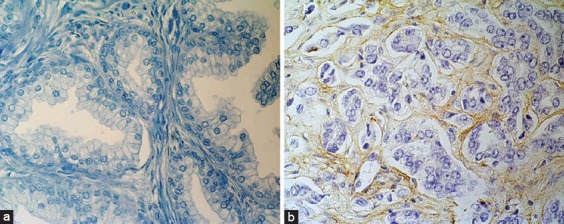
Immunohistochemical tenascin-C staining in A) benign prostate hyperplasia, showing negative cells in stroma (x400) and B) prostate cancer, showing positive cells in stroma (x400).
GALECTIN-3
Galectin-3 interacts with the intracellular glycoproteins, cell surface molecules, and the extracellular matrix proteins. According to the present data, it is down-regulated in prostate cancer. Van den Brule et al. suggested that galectin-3 might play an anti-tumor role when present in the nucleus, whereas it could favor tumor progression when expressed in the cytoplasm of the tumorous epithelial cells [58]. Other authors did not confirm these results, but it was suggested that the expression of galectin-3 in cytoplasm of the epithelial cells correlates positively with tumor progression [59]. It is shown that galectin-3 can inhibit anticancer drug-induced apoptosis through regulation of Bad protein and suppression of the mitochondrial apoptosis pathway [60]. It could be one of the target proteins for cancer treatment. The presence of galectin-3 in the stroma, however, indicated an unfavorable prognosis [58-62].
In our experience, the expression of galectin-3 was significantly decreased in carcinoma compared to adjacent peritumourous tissue and BPH (Figure 4).
Figure 4.
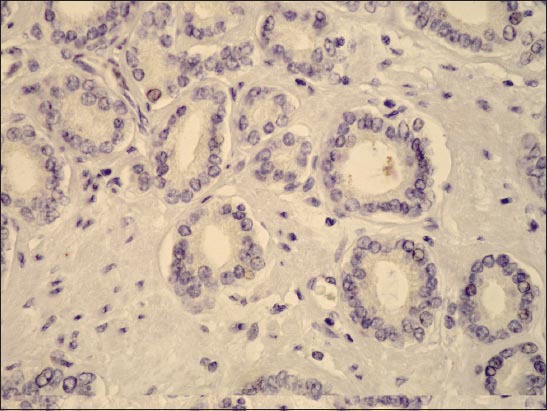
Immunohistochemical galectin-3 staining in prostate cancer, showing negative cells in stroma (x400).
CONNEXINS
Connexins are transmembrane proteins that form intercellular channels important for cell communication and adhesion. It is also a tumor suppressor gene (protein) [63-65]. Dysfunction of connexins plays a role in prostate carcinogenesis and its expression is often reduced during tumor progression and metastasis. In study of Benko et al. decreased connexin 43 (Cx43) expression was related to the prostate cancer progression. Lower Cx43 expression was associated with shorter follow-up time, indicating a shorter disease-free survival and higher preoperative PSA values [66].
SYNDECAN-2
Syndecan-2 (SDC2) is a heparan-sulfate glycosaminoglycan, which participates in cell adhesion and migration, and is known to play a role in cancer progression and neoangiogenesis [67,68]. SDC2 overexpression in prostate cancer was significantly associated with the established features indicative of worse prognosis, such as a higher preoperative PSA value, a higher Gleason score, positive surgical margins and the extraprostatic extension. Expression of SDC2 was also associated with the biochemical disease progression [69].
METALLOPROTEINASES
An elevated levels of metalloproteinases were registered in the stroma of various cancers. Matrix metalloproteinases (MMP) are produced by both tumor epithelial and stromal cells. MMPs are able to degrade a variety of ECM molecules, and regulate signaling pathways that control cell growth, survival, invasion, inflammation, and angiogenesis. In normal tissue MMP activity is carefully controlled, but in cancer their control mechanisms are altered. In a recently published study, it was found that expression of MMP-2, MMP-3, and MMP-9 was increased, favoring tumor progression [51,52].
ANGIOGENESIS
Angiogenesis is important for tissue growth as well as for tumor generation, progression, and its malignant behavior. Neovascularization in prostate carcinoma develops not only through cell-to-cell interaction, but also via multiple autocrine, paracrine and mechanical factors, and is similar to the one seen in the wound repair process [8-12]. As previously mentioned, prostate cancer stroma is composed of ‘reactive’ fibroblasts and myofibroblasts, responsible for extracellular matrix remodeling and increase in local vascular density. Stromal cells are capable of regulating angiogenesis by various protein and cytokine molecules. Protein ps20 is known to enhance endothelial cell motility and its synthesis is stimulated by TGF-β [70]. Another humoral factor, vascular endothelial growth factor (VEGF), is also reported to act as an endothelial cell mitogen and can be synthesized by both epithelial cells and myofibroblasts. Some studies confirmed that VEGF expression in prostate cancer correlates to a PSA level and Gleason score [71, 72].
GROWTH FACTORS, PEPTIDES AND RECEPTORS
Prostate cancer stromal cells express epidermal growth factor (EGF) as well as transforming growth factor-α (TGF-α). These factors are also synthesized by malignant epithelial cells and signal through epidermal growth factor receptors (EGFR). Autocrine expression of EGF and TGF-α affects the autonomous growth of human prostate cancer. Also, it seems that EGF plays an important role in stimulation of invasiveness of prostate cancer by promoting chemomigration of tumorous cells. The EGFR family-related oncogenes HER-2/neu, HER-3, and HER-4 are also differentially expressed in the stroma of prostate cancer. HER-4 receptor protein is strongly expressed in normal epithelial cells, but not in cancer.
Transforming growth factor-β (TGF-β) increases cancer growth and metastasis because of the altered expression of TGF-β receptors. This signaling pathway is downregulated in prostate cancer. The expression of TGF-β RI and RII proteins is reduced in both the primary cancer and lymph node metastases [73,74].
Human cancer cells acquire autocrine expression of fibroblasts growth factor-2 (FGF-2), which encourage cancer cell proliferation and elevates the titer of FGF-2 in patients’ serum. FGF-2 regulates changes in ECM by modulating expression of proteases and promoting the synthesis of collagen, fibronectin, and proteoglycans.
Prostatic stromal cells secrete insulin-like growth factor-I and II (IGF-I, II), which stimulate the growth of epithelial tumor cells via the EGFR signal transduction cascade. However, its importance in prostate carcinogenesis is still unclear.
It appears that nerve growth factor (NGF) is produced by both stromal and epithelial tumor cells, but prostate cancer cells that produce autocrine NGF are able to escape paracrine dependence of stromal cell-derived NGF. Upregulation of autocrine neurotrophin expression may be associated with invasion along the perineural space and metastasis [75].
Vascular endothelial growth factor (VEGF) promotes angiogenesis in prostate carcinoma.
Platelet-derived growth factors (PDGF) contribute to cell proliferation, survival, transformation, and chemotaxis. Prostate cancer expresses both PDGF-A and the PDGF-A receptor, which are presumed to play a role in malignant transformation in prostate cancer. PDGF-B has not been detected in this type of carcinoma [74-77].
CYTOKINES AND RECEPTORS
Hepatocyte growth factor (HGF) is expressed in the stroma of the human prostate. It stimulates proliferation and motility of cancer cells, interacting through c-met protooncogene product located in the epithelial cells.
Other cytokines, such as interleukins -1 (IL-1), -2 (IL-2), or interferon-alpha, -beta, and -gamma are also expressed during prostatic carcinogenesis. Studies have reported contradictory results about IL-6 signaling and IL-6 receptors in cancer cells in vitro and in tissue. IL-10 upregulates expression of tissue inhibitor of metalloproteinase-1, -2 and -9 which is consistent with its overall inhibitory effect on cancer cells [78].
CONCLUSIONS
Many mechanisms are involved in the biology of prostate cancer growth and progression. Collaboration between epithelial and stromal compartments, that both interact under the influence of androgen and other hormonal factors, is among the most important, resulting in a formation of microenvironment suitable for cancer growth and progression. Consequently, the interaction of different extracellular matrix proteins, glycoproteins, metalloproteinases, growth factors and their receptors is altered. A new suitable environment is created for neovascularization and survival of resistant cell clones capable of self-renewal, invasion and metastasis. In addition, these stromal changes could serve as valuable additional tools in diagnosis and prognosis of prostate cancer. Further investigation is needed for the novel therapeutic possibilities that could influence stromal cells in the tumor.
REFERENCES
- [1].Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol. 1997;79(S2):18–26. doi: 10.1111/j.1464-410x.1997.tb16917.x. DOI: 10.1111/j.1464-410X.1997.tb16917.x. [DOI] [PubMed] [Google Scholar]
- [2].Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol. 2004;92(4):221–236. doi: 10.1016/j.jsbmb.2004.10.017. DOI: 10.1016/j.jsbmb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- [3].Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol. 2008;288(1-2):30–37. doi: 10.1016/j.mce.2008.02.024. DOI: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- [4].Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17(4):411–419. doi: 10.1023/a:1006129420005. DOI: 10.1023/A:1006129420005. [DOI] [PubMed] [Google Scholar]
- [5].Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- [6].Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. DOI: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- [7].Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. DOI: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- [8].Cano P, Godoy A, Escamilla R, Dhir R, Onate SA. Stromal-epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res. 2007;67(2):511–519. doi: 10.1158/0008-5472.CAN-06-1478. DOI: 10.1158/0008-5472.CAN-06-1478. [DOI] [PubMed] [Google Scholar]
- [9].Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166(6):2472–2483. DOI: 10.1016/S0022-5347(05)65620-0. [PubMed] [Google Scholar]
- [10].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. DOI: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [11].Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70(9-10):473–485. doi: 10.1046/j.1432-0436.2002.700902.x. DOI: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- [12].Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107(1):1–10. doi: 10.1002/ijc.11335. DOI: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- [13].Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Bio. 2005;15(2):132–137. doi: 10.1016/j.semcancer.2004.08.002. DOI: 10.1016/j.semcancer.2004.08.002. [DOI] [PubMed] [Google Scholar]
- [14].Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A, et al. Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122(5):1849–1868. doi: 10.1172/JCI59218. DOI: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM, et al. The current state of preclinical prostate cancer animal models. Prostate. 2008;68(6):629–639. doi: 10.1002/pros.20726. DOI: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hensley PJ, Kyprianou N. Modeling prostate cancer in mice: limitations and opportunities. J Androl. 2012;33(2):133–44. doi: 10.2164/jandrol.111.013987. DOI: 10.2164/jandrol.111.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zou M, Jiao J, Zou Q, Xu Y, Cheng M, Xu J, et al. Multiple metastases in a novel LNCaP model of human prostate cancer. Oncol Rep. 2013;30(2):615–622. doi: 10.3892/or.2013.2305. DOI: 10.3892/or.2013.2305. [DOI] [PubMed] [Google Scholar]
- [18].Cunha GR, Tuohimaa P, Visakorpi T. Steroids and prostate cancer. J Steroid Biochem Mol Biol. 2004;92(4):219–220. doi: 10.1016/j.jsbmb.2004.10.001. DOI: 10.1016/j.jsbmb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [19].Richard C, Kim G, Koikawa Y, Salm SN, Tsujimura A, Wilson EL, et al. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate. 2002;50(2):83–91. doi: 10.1002/pros.10035. DOI: 10.1002/pros.10035. [DOI] [PubMed] [Google Scholar]
- [20].Wen S, Chang HC, Tian J, Shang Z, Niu Y, Chang C. Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer. Am J Pathol. 2015;185:293–301. doi: 10.1016/j.ajpath.2014.10.012. DOI: 10.1016/j.ajpath.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Adisetiyo H, Liang M, Liao CP, Jeong JH, Cohen MB, Roy-Burman P, et al. Dependence of castration-resistant prostate cancer (CRPC) stem cells on CRPC-associated fibroblasts. J Cell Physiol. 2014;229(9):1170–1176. doi: 10.1002/jcp.24546. DOI: 10.1002/jcp.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell–stroma interface. Annu Rev Cell Dev Biol. 2009;25:567–595. doi: 10.1146/annurev.cellbio.24.110707.175315. DOI: 10.1146/annurev.cellbio.24.110707.175315. [DOI] [PubMed] [Google Scholar]
- [23].Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract. 1996;192(7):712–717. doi: 10.1016/S0344-0338(96)80093-8. DOI: 10.1016/S0344-0338(96)80093-8. [DOI] [PubMed] [Google Scholar]
- [24].Noel A, Foidart JM. The role of stroma in breast carcinoma growth in vivo. J Mammary Gland Biol Neoplasia. 1998;3(2):215–225. doi: 10.1023/a:1018703208453. DOI: 10.1023/A:1018703208453. [DOI] [PubMed] [Google Scholar]
- [25].Tomas D, Kruslin B. The potential value of (myo)fibroblastic stromal reaction in the diagnosis of prostatic adenocarcinoma. Prostate. 2004;61(4):324–331. doi: 10.1002/pros.20109. DOI: 10.1002/pros.20109. [DOI] [PubMed] [Google Scholar]
- [26].Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Ver. 2012;31(1-2):195–208. doi: 10.1007/s10555-011-9340-x. DOI: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- [27].Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, et al. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer- associated fibroblasts in human prostate cancer. Biomaterials. 2013;34(20):4777–4785. doi: 10.1016/j.biomaterials.2013.03.005. DOI: 10.1016/j.biomaterials.2013.03.005. [DOI] [PubMed] [Google Scholar]
- [28].Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-induced EMT during cancer progression. Cell Tissue Res. 2012;347(1):85–101. doi: 10.1007/s00441-011-1199-1. DOI: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gonçalves BF, Campos SG, Costa CF, Scarano WR, Góes RM, Taboga SR. Key participants of the tumor microenvironment of the prostate: an approach of the structural dynamic of cellular elements and extracellular matrix components during epithelial-stromal transition. Acta Histochem. 2015;117(1):4–13. doi: 10.1016/j.acthis.2014.10.009. DOI: 10.1016/j.acthis.2014.10.009. [DOI] [PubMed] [Google Scholar]
- [30].Ao M, Brewer BM, Yang L, Franco Coronel OE, Hayward SW, Webb DJ, et al. Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci Rep. 2015;5:8334. doi: 10.1038/srep08334. DOI: 10.1038/srep08334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tomas D, Spajić B, Milošević M, Demirović A, Marušić Z, Krušlin B. Intensity of stromal changes predicts biochemical recurrence-free survival in prostatic carcinoma. Scand J Urol Nephrol. 2010;44(5):284–290. doi: 10.3109/00365599.2010.485578. DOI: 10.3109/00365599.2010.485578. [DOI] [PubMed] [Google Scholar]
- [32].Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9(13):4792–4801. [PubMed] [Google Scholar]
- [33].Krušlin B, Tomas D, Rogatsch H, Novosel I, Čupić H, Belicza M, et al. Periacinar retraction clefting in the prostatic needle core biopsies: an important diagnostic criterion or a simple artifact? Virchows Arch. 2003;443(4):524–527. doi: 10.1007/s00428-003-0862-7. DOI: 10.1007/s00428-003-0862-7. [DOI] [PubMed] [Google Scholar]
- [34].Krušlin B, Tomas D, Rogatsch H, Reljić A, Vučić M, Baličević D, et al. Correlation of periacinar retraction clefting in needle core biopsies and corresponding prostatectomy specimens. Int J Surg Pathol. 2005;13(1):67–72. doi: 10.1177/106689690501300109. DOI: 10.1177/106689690501300109. [DOI] [PubMed] [Google Scholar]
- [35].Ulamec M, Tomas D, Ensinger C, Čupić H, Belicza M, Mikuz G, et al. Periacinar retraction clefting in proliferative prostatic atrophy and prostatic adenocarcinoma. J Clin Pathol. 2007;60(10):1098–1101. doi: 10.1136/jcp.2006.044784. DOI: 10.1136/jcp.2006.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fávaro WJ, Hetzl AC, Reis LO, Ferreira U, Billis A, Cagnon VH. Periacinar retraction clefting in nonneoplastic and neoplastic prostatic glands: artifact or molecular involvement. Pathol Oncol Res. 2012;18(2):285–292. doi: 10.1007/s12253-011-9440-5. DOI: 10.1007/s12253-011-9440-5. [DOI] [PubMed] [Google Scholar]
- [37].Varma M, Lee MW, Tamboli P, Zarbo RJ, Jimenez RE, Salles PG, et al. Morphologic criteria for the diagnosis of prostatic adenocarcinoma in needle biopsy specimens. A study of 250 consecutive cases in a routine surgical pathology practice. Arch Pathol Lab Med. 2002;126(5):554–561. doi: 10.5858/2002-126-0554-MCFTDO. [DOI] [PubMed] [Google Scholar]
- [38].Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla AL. 3th ed. Washington, DC: AFIP; 1998. Tumors of the prostate gland, seminal vesicles, male urethra, and penis. [Google Scholar]
- [39].Tomas D, Ulamec M, Hudolin T, Bulimbašić S, Belicza M, Krušlin B. Myofibroblastic stromal reaction and expression of tenascin-C and laminin in prostate adenocarcinoma. Prostate Cancer Prostatic Dis. 2006;9(4):414–419. doi: 10.1038/sj.pcan.4500874. DOI: 10.1038/sj.pcan.4500874. [DOI] [PubMed] [Google Scholar]
- [40].Irie J, Manucha V, Ioffe OB, Silverberg SG. Artefact as the pathologist's friend: peritumoral retraction in in situ and infiltrating duct carcinoma of the breast. Int J Surg Pathol. 2007;15(1):53–59. doi: 10.1177/1066896906295690. DOI: 10.1177/1066896906295690. [DOI] [PubMed] [Google Scholar]
- [41].McKenney JK, Gomez JA, Desai S, Lee MW, Amin MB. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am J Surg Pathol. 2001;25(3):356–362. doi: 10.1097/00000478-200103000-00010. DOI: 10.1097/00000478-200103000-00010. [DOI] [PubMed] [Google Scholar]
- [42].Tomas D, Spajić B, Milošević M, Demirović A, Marušić Z, Krušlin B. Extensive retraction artefact predicts biochemical recurrence-free survival in prostatic carcinoma. Histopathology. 2011;58(3):447–454. doi: 10.1111/j.1365-2559.2011.03769.x. DOI: 10.1111/j.1365-2559.2011.03769.x. [DOI] [PubMed] [Google Scholar]
- [43].Acs G, Khakpour N, Kiluk J, Lee MC, Laronga C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. Am J Surg Pathol. 2015;39(3):325–337. doi: 10.1097/PAS.0000000000000339. DOI: 10.1097/PAS.0000000000000339. [DOI] [PubMed] [Google Scholar]
- [44].Acs G, Paragh G, Chuang ST, Laronga C, Zhang PJ. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol. 2009;33(2):202–210. doi: 10.1097/PAS.0b013e318185e171. DOI: 10.1097/PAS.0b013e318185e171. [DOI] [PubMed] [Google Scholar]
- [45].Taylor RA, Risbridger GP. Prostatic tumor stroma a key player in cancer progression. Curr Cancer Drug Targets. 2008;8(6):490–497. doi: 10.2174/156800908785699351. DOI: 10.2174/156800908785699351. [DOI] [PubMed] [Google Scholar]
- [46].Pupa SM, Menard S, Forti S, Tagliabue E. New insight into the role of extracellular matrix during tumor onset and progression. J Cell Physiol. 2002;192(3):259–267. doi: 10.1002/jcp.10142. DOI: 10.1002/jcp.10142. [DOI] [PubMed] [Google Scholar]
- [47].Taboga SR, Vidal BC. Collagen fibers in human prostatic lesions: histochemistry and anisotropies. J Submicrosc Cytol Pathol. 2003;35(1):11–16. [PubMed] [Google Scholar]
- [48].LeBleu VS, MacDonald B, Kalluri R. Structure and function of base- ment membranes. Exp Biol Med. 2007;232(9):1121–1129. doi: 10.3181/0703-MR-72. DOI: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- [49].Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Bio. 2002;12(3):197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- [50].Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218(2):213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. DOI: 10.1002/(SICI)1097-0177(200006)218:2<213:AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [51].Sounni NE, Noel A. Membrane type-matrix metalloproteinase and tumor progression. Biochimie. 2005;87(3-4):329–342. doi: 10.1016/j.biochi.2004.07.012. DOI: 10.1016/j.biochi.2004.07.012. [DOI] [PubMed] [Google Scholar]
- [52].Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, Nagle RB, et al. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia. 2005;7(4):380–389. doi: 10.1593/neo.04619. DOI: 10.1593/neo.04619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chiquet-Ehrismann R. Tenascin and other adhesion-modulating proteins in cancer. Semin Cancer Biol. 1993;4(5):301–310. [PubMed] [Google Scholar]
- [54].Erickson HP. Tenascin-C, tenascin-R, and tenascin-X: a family of talented proteins in search of their functions. Curr Opin Cell Biol. 1993;5(5):869–876. doi: 10.1016/0955-0674(93)90037-q. DOI: 10.1016/0955-0674(93)90037-Q. [DOI] [PubMed] [Google Scholar]
- [55].Shiraishi T, Kato H, Komada S, Imai H, Hirokawa Y, Kusano I, et al. Tenascin expression and postnatal development of the human prostate. Int J Dev Biol. 1994;38(2):391–395. [PubMed] [Google Scholar]
- [56].Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol. 2005;37(5):1066–1083. doi: 10.1016/j.biocel.2004.12.002. DOI: 10.1016/j.biocel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [57].Vollmer G. Biologic and oncologic implications of tenascin-C/hexabrachion proteins. Crit Rev Oncol/Hematol. 1997;25(3):187–210. doi: 10.1016/s1040-8428(97)00004-8. DOI: 10.1016/S1040-8428(97)00004-8. [DOI] [PubMed] [Google Scholar]
- [58].Van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89(4):361–367. doi: 10.1002/1097-0215(20000720)89:4<361::aid-ijc8>3.0.co;2-u. DOI: 10.1002/1097-0215(20000720)89:4<361:AID-IJC8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [59].Takenaka Y, Fukumori T, Raz A. Galectin-3 and metastasis. Glycoconj J. 2004;19(7-9):543–549. doi: 10.1023/B:GLYC.0000014084.01324.15. DOI: 10.1023/B:GLYC.0000014084.01324.15. [DOI] [PubMed] [Google Scholar]
- [60].Fukumori T, Oka N, Takenaka N, Nangia-Makker P, Elsamman E, Kasai T, et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 2006;66(6):3114–3119. doi: 10.1158/0008-5472.CAN-05-3750. DOI: 10.1158/0008-5472.CAN-05-3750. [DOI] [PubMed] [Google Scholar]
- [61].Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, et al. Decreased galectin-3 expression in prostate cancer. Prostate. 2000;44(2):118–123. doi: 10.1002/1097-0045(20000701)44:2<118::aid-pros4>3.0.co;2-u. DOI: 10.1002/1097-0045(20000701)44:2<118:AID-PROS4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- [62].Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174(4):1515–1523. doi: 10.2353/ajpath.2009.080816. DOI: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang M, Berthoud VM, Beyer EC. Connexin43 increases the sensitivity of prostate cancer cells to TNFa-induced apoptosis. J Cell Sci. 2007;120(2):320–329. doi: 10.1242/jcs.03343. DOI: 10.1242/jcs.03343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Govindarajan R, Zhao S, Song XH, Guo RJ, Wheelock M, Johnson KR, et al. Impaired trafficking of connexins in androgenindependent human prostate cancer cell lines and its migration by a-catenin. J Biol Chem. 2002;277(51):50087–50097. doi: 10.1074/jbc.M202652200. DOI: 10.1074/jbc.M202652200. [DOI] [PubMed] [Google Scholar]
- [65].Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D’hondt C, Bultynck G, et al. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta. 2011;1815(1):13–25. doi: 10.1016/j.bbcan.2010.08.004. DOI: 10.1016/j.bbcan.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [66].Benko G, Spajić B, Demirović A, Stimac G, Kru Sbreve Lin B, Tomas D. Prognostic value of connexin43 expression in patients with clinically localized prostate cancer. Prostate Cancer Prostatic Dis. 2011;14(1):90–95. doi: 10.1038/pcan.2010.51. DOI: 10.1038/pcan.2010.51. [DOI] [PubMed] [Google Scholar]
- [67].Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res. 2005;96(5):488–500. doi: 10.1161/01.RES.0000159708.71142.c8. DOI: 10.1161/01.RES.0000159708.71142.c8. [DOI] [PubMed] [Google Scholar]
- [68].Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, et al. Syndecan- 2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun. 2009;384(2):231–235. doi: 10.1016/j.bbrc.2009.04.093. DOI: 10.1016/j.bbrc.2009.04.093. [DOI] [PubMed] [Google Scholar]
- [69].Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis. 2010;13(1):78–82. doi: 10.1038/pcan.2009.43. DOI: 10.1038/pcan.2009. [DOI] [PubMed] [Google Scholar]
- [70].Ruiz M, Pettaway M, Stoeltzing O, Ellis L, Bar-Eli M. Activator protein 2 alpha inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Res. 2004;64(2):631–638. doi: 10.1158/0008-5472.can-03-2751. DOI: 10.1158/0008-5472.CAN-03-2751. [DOI] [PubMed] [Google Scholar]
- [71].Chung LWK, Hsieh CL, Law A, Sung SY, Gardner TA, Egawa M, et al. New targets for therapy in prostate cancer: modulation of stromal–epithelial interactions. Urology. 2003;(5):44–54. doi: 10.1016/s0090-4295(03)00796-9. DOI: 10.1016/S0090-4295(03)00796-9. [DOI] [PubMed] [Google Scholar]
- [72].Cooper CR, McLean L, Mucci NR, Poneza P, Pienta KJ. Prostate cancer cell adhesion to quiescent endothelial cells is not mediated by beta-1 integrin subunit. Anticancer Res. 2000;20(6B):4159–4162. [PubMed] [Google Scholar]
- [73].Wong YC, Tam NNC. Differentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation. 2002;70(9-10):633–645. doi: 10.1046/j.1432-0436.2002.700916.x. DOI: 10.1046/j.1432-0436.2002.700916.x. [DOI] [PubMed] [Google Scholar]
- [74].Hsieh CL, Gardner TA, Miao L, Balian G, Chung LWK. Cotargeting tumor and stroma in a novel chimeric tumor model involving the growth of both human prostate cancer and bone stromal cells. Cancer Gene Ther. 2004;11(2):148–155. doi: 10.1038/sj.cgt.7700665. DOI: 10.1038/sj.cgt.7700665. [DOI] [PubMed] [Google Scholar]
- [75].Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, et al. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res. 2003;63(16):4786–4791. [PubMed] [Google Scholar]
- [76].Desmouliere A, Geinoz, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. DOI: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].West AF, O’Donnell M, Charlton RG, Neal DE, Leung HY. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer. 2001;85(4):576–583. doi: 10.1054/bjoc.2001.1971. DOI: 10.1054/bjoc.2001.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate. 2000;42(2):150–160. doi: 10.1002/(sici)1097-0045(20000201)42:2<150::aid-pros10>3.0.co;2-h. DOI: 10.1002/(SICI)1097-0045(20000201)42:2<150:AID-PROS10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]


