Abstract
The wound-healing potential of Phaleria macrocarpa was evaluated by monitoring the levels of inflammatory mediators, collagen, and antioxidant enzymes. Experimentally, two-centimeter-wide full-thickness-deep skin excision wounds were created on the posterior neck area of the rats. The wounds were topically treated with gum acacia as a vehicle in the control group, intrasite gel in the reference group, and 100 and 200 mg/mL P. macrocarpa fruit extract in the treatment group. Granulation tissues were excised on the 15th day and were further processed for histological and biochemical analyzes. Wound healing was evaluated by measuring the contractions and protein contents of the wounds. Cellular redistribution and collagen deposition were assessed morphologically using Masson’s trichrome stain. Superoxide dismutase (SOD) and catalase (CAT) activities, along with malondialdehyde (MDA) level were determined in skin tissue homogenates of the dermal wounds. Serum levels of transforming growth factor beta 1 (TGF-β1) and tumor necrosis factor alpha (TNF-α) were evaluated in all the animals. A significant decrease in wound area was caused by a significant increase in TGF-β1 level in the treated groups. Decrease in TNF-α level and increase in the collagen formation were also observed in the treated groups. Topical treatment with P. macrocarpa fruit extract increased the SOD and CAT activities in the healing wounds, thereby significantly decreasing MDA level. The topical treatment with P. macrocarpa fruit extract showed significant healing effect on excision wounds and demonstrated an important role in the inflammation process by increasing antioxidant enzyme activities, thereby accelerating the wound healing process and reducing tissue injury.
KEYWORDS: Antioxidant enzyme, inflammatory mediator, Phaleria macrocarpa, wound healing
INTRODUCTION
A wound is a sharp injury that damages the dermal layer of the skin. There are two types of the wounds: closed wound and open wound [1]. Wound healing from either accidental injury or surgical intervention involves the activity of a complex network of blood cells, tissues cytokines, and growth factors. Increased cellular activity is apparent in wound healing, thereby causing an increased metabolic demand for nutrients. The deficiencies in some nutrition factors such as vitamin A or C could impede the cellular differentiation, immune function and collagen formation leading to a delay in wound healing process [2]. Therefore, it is not surprising that the researchers continuously attempt to find drugs from natural sources in order to cure wounds [3]. Since more than 50% of proteins in scar tissues consist of collagen, collagen production is essential in the process of healing. Fibroblasts regulate the synthesis of collagen and other proteins throughout the repairing process [4]. Numerous cytokines, such as transforming growth factor beta (TGF-β), platelet-derived growth factors (PDGF) and epidermal growth factor (EGF), are responsible for fibroblast stimulation for collagen synthesis. Inflammation is a normal phase of the wound-healing process, being crucial in removing contaminating microorganisms. Both bacteria and endotoxins can lead to an extended increase of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1), prolonging thus the inflammatory phase. If this phase continues, the wound may reach its chronic stage and may fail to heal. The prolonged inflammation can lead to increased levels of matrix metalloproteinases, a family of proteases that can reduce the extracellular matrix [5]. A series of studies showed that nitric oxide and reactive oxygen species (ROS) are pivotal regulators of wound healing [6]. ROS are beneficial for protection from microbial invasion; intracellular signal transduction requires a low level of ROS. Furthermore, hydrogen peroxide at low levels is essential for efficient wound angiogenesis. However, excessive ROS is poisonous because of its high reactivity. Hyperoxia and hypoxia both increase ROS production, but increasing ROS level eliminates the beneficial effect of ROS and can result in additional tissue damage [7].
Phaleria macrocarpa is a traditional medicinal plant from New Guinea, Papua Island, and Indonesia. P. macrocarpa grows throughout the year in tropical areas and reaches a height of 1 m to 6 m. It is a tree with stems, leaves, roots, flowers, and fruits. Its fruit has an ellipse shape and is about 3 cm in diameter. The fruit is green before ripening and red when fully ripe [8]. P. macrocarpa has been used to treat cancer, diabetes, ulcers and hypercholesterolemia [9-12]. Previous studies reported the isolation of many active compounds from various parts of P. macrocarpa, including phalerin, mangiferin, benzophenone glucopyranosides, mahkoside, kaempferol-3-O-β-D-glucoside, dodecanoic acid, and palmitic acid [13]. We aimed to investigate the healing efficacy of P. macrocarpa fruit extract on excision wounds of rats and to elucidate possible mechanisms underlying such healing effect.
MATERIALS AND METHODS
Plant extraction
Seedless P. macrocarpa fruits were harvested as voucher specimens (SK1929/11) at the Selangor housing area in the herbarium of University Putra in Malaysia. The P. macrocarpa fruits were dried and crushed. The resulting powder (100 g) was placed in a conical flask and soaked in 900 mL 95% ethanol for three days at room temperature (30 ± 2 °C). The suspension was shaken intermittently for the powder to dissolve completely in ethanol, thereby creating a dark brown mixture. After three days, the mixture was filtered using a filter paper (Whatman, 185 mm), and the filtrate was distilled under reduced pressure in a rotary evaporator. The extract was kept at -20 °C prior to use [14].
Animals
Healthy adult Sprague-Dawley (SD) rats weighing 250 - 300 g were obtained from the animal house unit of the Faculty of Medicine in the University of Malaya, Malaysia. The rats were maintained in wire bottom cages at 22 ± 3 °C and 50% to 60 % humidity under a 12 h–12 h light–dark cycle for at least one week prior to the experiment. The cages were maintained according to standard housing conditions, and access to standard diet and water was provided ad libitum during the experiment. The experimental protocol was approved by the animal ethics committee under Ethic No. PM 1 November 2011 MAA. All of the animal care criteria prepared by the National Academy of Sciences and outlined in the Guide for the Care and Use of Laboratory Animals were applied throughout the experiment [15].
Experimental design
The adult female SD rats were randomly divided into four groups, with five rats in each group. Each rat was housed separately. The animals were anesthetized. The skin was shaved using an electrical shaver and disinfected with 70% alcohol. A uniform wound area with 2cm diameter was excised from the napes of the rats using a round seal, as described by Morton et al. [16]. Incision of the muscle layer was avoided, and skin tension was kept constant during the procedure. All rats were treated twice a day, as follows: the vehicle control group was treated with 0.2 mL gum acacia; the reference drug group was treated with 0.2 mL intrasite gel; one of the treated groups was topically administered 0.2 mL of the 100 mg/mL plant extract; and the other treated group was topically administered 0.2 mL of the 200 mg/mL plant extract. Contraction of the wound area was measured at days 0, 5, 10, and 15 after incision. At day 15, a high dose of anesthesia was administered to all the experimental animals, and the skin from the healed wound area was excised in order to obtain a homogenous tissue for histopathological examination. Blood samples were also collected to measure other parameters.
Histological evaluation of wound healing
A piece of skin from the healed wound area was fixed in 10% formalin and then dehydrated with increasing alcohol concentrations in an automated tissue processing machine. The processed tissue was embedded in paraffin for tissue sectioning. A microtome was used to cut the tissue into 5 µm sections for staining with hematoxylin–eosin solutions and Masson’s Trichrome stain according to standard protocols [17].
Measurement of TNF-α and TGF-β1 levels by enzyme-linked immunosorbent assay (ELISA)
The blood samples of the rats were collected in tubes. The blood samples were allowed to clot for 30 min at 25°C and were then centrifuged at 2000 × g for 15 min at 4 °C. The serum was collected and preserved at -80°C prior to use. TNF-α and TGF-β1 levels were measured using ELISA kits [Thermo Scientific (Cat.# ER3TNFA) and Abnova (Cat.# KA0279 version: 04), respectively] according to the detailed instructions of the manufacturers. The assay had a sensitivity limit of ≤ 15 pg/mL and ≤ 7.8 pg/mL for TNF-α and TGF-β1, respectively.
Homogenate tissue sample preparations to determine superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) levels
To assess the levels of SOD (Cayman Item No. 706002), CAT (Cayman Item No. 707002), and MDA (Cayman Item No. 10009055) in the homogenate skin tissues, the skin tissues were weighed, minced, and homogenized on ice in 5 mL to 10 mL cold phosphate-buffered saline using a Teflon homogenizer. The homogenized mixtures were centrifuged at 10.000 ×g for 15 min at 4 °C. The supernatants were collected in sterile tubes and maintained at -80 °C prior to use. The assays for SOD, CAT, and MDA were performed according to the detailed instructions of the manufacturers. Protein concentration was determined using the Bradford method, with bovine serum albumin as a standard [18].
Statistical analysis
Values were expressed as mean ± standard deviation. Statistical examination of data was performed using one-way ANOVA, and a Post Hoc LSD test was conducted to compare the treated groups with the control groups using a p-value ≤ 0.05 as a level of significance.
RESULTS
Wound contraction
Results from the macroscopic evaluation of wound healing rate on day 15 after incision revealed that the wounds dressed with P. macrocarpa showed considerable signs of dermal healing and significantly healed faster compared with the vehicle-treated control group (gum acacia in normal saline) as presented on Figure 1.
Figure 1.
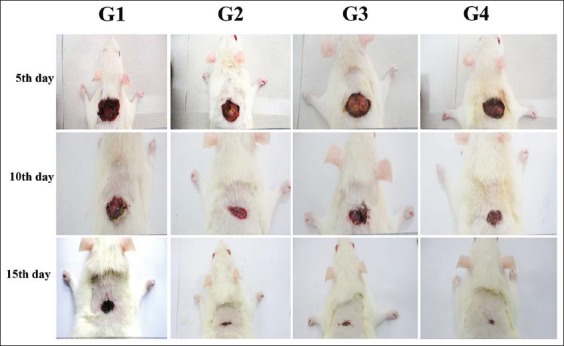
Macroscopic appearance of excision wound healing area treatment (G1) 0.2 mL of vehicle control (gum acacia in normal saline); (G2) 0.2 mL Intrasite; (G3) 0.2 mL of 100 mg/mL of P. macrocarpa; (G4) 0.2 mL of 200 mg/mL of P. macrocarpa.
A measurement of wound healing progression showed that the plant extract induced wound healing (Figure 2). An excision wound margin was traced after wound creation using a transparent paper, and the wound area was measured using a graphing paper. Wound contraction was measured at 5-day-intervals until day 15. In the excision wound model, the wounds of the animal groups treated with 100 and 200 mg/mL P. macrocarpa fruit extract contracted by 25.6 ± 10.8 and 19.2 ± 8.7 mm2, respectively, at significantly higher rates at day 15 (p ≤ 0.05) compared with the vehicle-treated control group (55 ± 10.3 mm2).
Figure 2.
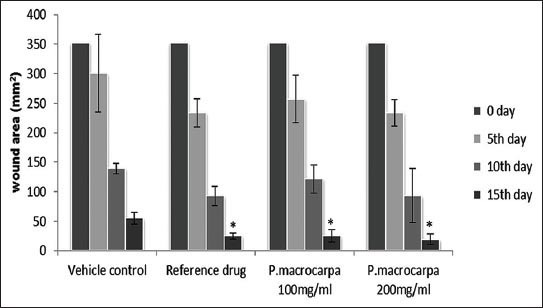
Effects of P. macrocarpa on the progression of wound healing (wound area mm2) in rats (Data expressed as mean ± SD, 5 rats/group. (* Significant (p ≤ 0.05) compared with vehicle control).
Histopathological evaluation of healed wound area
The histology of the wound tissues on the 15th day after wounding was evaluated. Wound enclosure was found to be smaller in the P. macrocarpa fruit extract-treated groups (Figure 3) and the granulation tissues contained comparatively less inflammatory cells and more collagen, fibroblast, and blood proliferating capillaries than the vehicle-treated control group, as revealed by Masson’s Trichrome staining (Figure 4).
Figure 3.
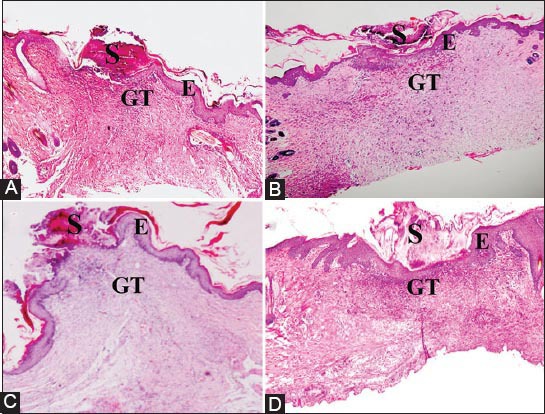
Hematoxylin and eosin-stained sections of the wound at the 15th day after wounding in rats. (A) 0.2mL of vehicle, gum acacia in normal saline; (B) 0.2mL of Intrasite gel; (C) 0.2mL of P. macrocarpa (100mg/mL); (D) 0.2mL of P. macrocarpa (200mg/mL); S = Scab, E = Epidermis, GT = granulation tissue (magnification 20x).
Figure 4.
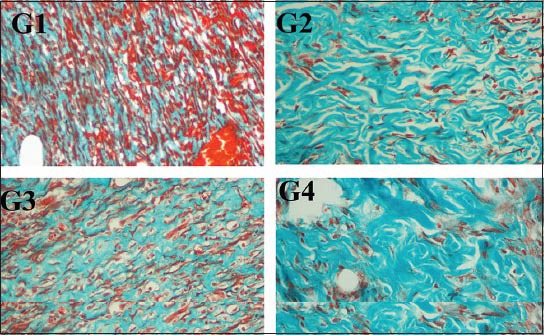
Masson’s Trichrome staining of granulation tissue in the wound area of all animal groups at the 15th day after wounding in rats (G1) Skin dressed with vehicle shows more inflammatory cells; (G2) Skin dressed with Intrasite gel shows less inflammatory cells and more collagen produce compared to vehicle group; (G3) Skin dressed with 0.2 mL of P. macrocarpa 100 mg/mL; (G4) Skin dressed with 0.2 mL of P. macrocarpa 200 mg/mL show significantly decreased inflammatory cells and increase in the collagen production compared to vehicle (magnification 20x).
Assessment of TGF-β1 and TNF-α in the wounded rat undergoing treatment
TGF-β1 and TNF-α are important inflammatory cytokines for the wound-healing process. Therefore, in this study TGF-β1 and TNF-α levels in the treated groups were determined to evaluate the effect of P. macrocarpa fruit extract on inflammatory mediators. The groups treated with 100 and 200 mg/mL P. macrocarpa fruit extracts showed significantly increased TGF-β1 levels (42.06 ± 2.03 and 58.09 ± 1.74 pg/mL, respectively; p ≤ 0.05) compared with the control group treated with the vehicle (23.13 ± 1.24 pg/mL; Figure 5). By contrast, TNF-α levels significantly decreased in the groups treated with 100 and 200 mg/mL P. macrocarpa fruit extracts (112.3 ± 23.4 and 95.2 ± 9.4 pg/mL, respectively; p ≤ 0.05) compared with the control group treated with the vehicle (185.5 ± 28.1 pg/mL; Figure 6).
Figure 5.
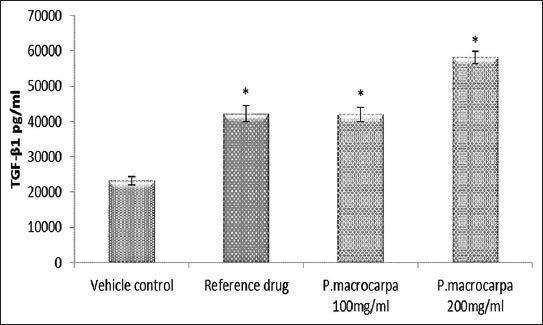
Effects of P. macrocarpa on the TGF-β1 serum level in the treated wounded rats. Data expressed as mean ± SD, 5 rats/group (* Significant p ≤ 0.05 compared with vehicle control).
Figure 6.
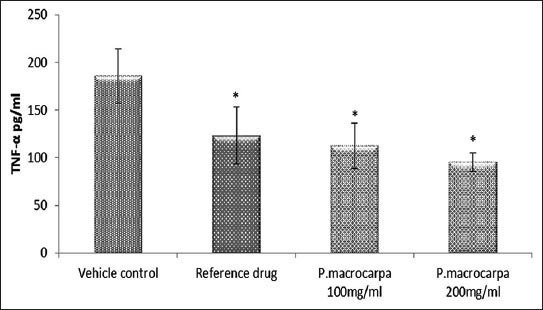
Effects of P. macrocarpa on the TNF-α serum level in the treated wounded rats. Data expressed as mean ± SD, 5rats/group (* Significant p ≤ 0.05 compared with vehicle control).
SOD, CAT, and MDA levels in the healed wound area
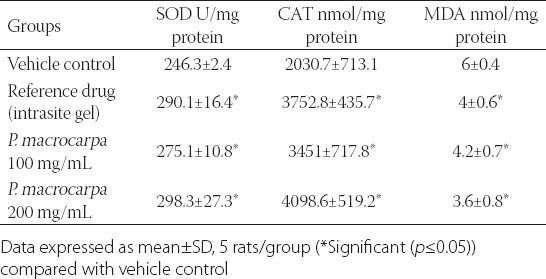
The levels of antioxidant enzymes, SOD and CAT, and the natural product of lipid peroxidation, MDA, were estimated in this study to evaluate wound-healing progress and determine the effect of the P. macrocarpa fruit extract (Table 1). The results revealed that SOD and CAT levels significantly increased (p ≤ 0.05) in P. macrocarpa fruit extract-treated groups compared with the vehicle-treated control group. By contrast, MDA levels significantly decreased (p ≤ 0.05) in P. macrocarpa fruit extract-treated groups compared with the vehicle-treated control group.
Table 1.
Effects of P. macrocarpa on the SOD, CAT activity and MDA level in the healed wound area at day 15 post-treatment in the rats
DISCUSSION
One of the main findings of this study considers topical application of P. macrocarpa fruit extracts on skin excision wounds in rats. The Study showed that topical application resulted in an improved wound contraction rate and considerable healing time reduction compared with that of the control group. These results may be attributed to the enhanced wound healing progression and the display of noticeable wound margin hydration resulting from tissue regeneration. Histological evaluation of the wound areas of treated groups confirmed the increase in cellular infiltration and angiogenesis and the increase in fibroblasts and collagen depositions. The mechanisms of topical P. macrocarpa action on the wound area, which were due to the chemotactic influence of the plant extract, may have attracted inflammatory cells. The mitogenic activity of the plant extract possibly increased cellular proliferation and significantly contributed to wound healing. P. macrocarpa-treated groups demonstrated significantly smaller wound areas at day 15 after wounding compared with the vehicle-treated control group.
TGF-β1 plays an important role as an inflammatory mediator in the initiation of wound healing by activating and stimulating macrophages to secrete cytokines that further act as fibroblast growth factors (PDGF, TNF-α and interleukin-1). In the proliferative phase, macrophages secrete TGF-β1, T lymphocytes, and platelets. TGF-β1 is considered to be a major control signal that regulates fibroblast functions. TGF-β1 affects extracellular matrix precipitation by the following activities: enhancing the gene transcriptions for proteins essential in the production of matrix, mainly collagen, fibronectin, and proteoglycans; inhibiting the production of proteases, the enzymes that cause breakdown of extracellular matrix; and stimulating the metalloprotease inhibitor [4].
In this study, significantly increased TGF-β1 levels and significantly decreased TNF-α levels were observed in the P. macrocarpa fruit extract-treated groups compared with the vehicle-treated control group at day 15 after wounding. These results clearly indicate that the effect of the plant extract in accelerating wound healing is possibly due to the reduction of the inflammatory phase and its rapid transition to the proliferative phase, during which enhanced wound contraction was observed. To provide supplementary evidence for this suggestion, previous studies reported that during the second and third week of healing, fibroblasts started to adopt myofibroblasts phenotypic properties via large parcels of actin containing microfilaments, which are organized along the cytoplasmic cover of the plasma membrane, thereby establishing cell-to-cell and cell–matrix linkages [19]. Many studies on the use of medicinal plants in treating wounds have reported the significant role of TGF-β1 in accelerating wound healing [20]. Eleutherine indica extract accelerates wound healing by stimulating the release of TGF-β, which is bound to the fibroblast receptors and initiates TGF-β-Smad-mediated collagen production. Smad, which belongs to a family of proteins that interacts with TGF-β type 1 receptors, transduced receptor signals to specific target gene in the nucleus and directly interacts with activated TGF-β receptors [21]. Similar results were produced in Polygonumcu spidatum-treated wounds. The results from immunohistochemical analyzes showed that TGF-β1 levels significantly increased in the groups treated with the plant extract at day 1, 3, and 7 after wounding [22].
ROS are deleterious to the wound healing process because of their harmful effects on cells and tissues. Free radical scavenging enzymes are cytoprotective enzymes that play essential roles in the reduction, deactivation and removal of ROS, as well as in the regulation of the wound healing. SOD converts superoxide to hydrogen peroxide, which is then transformed into the water by CAT in lysosomes [23]. In the present study, homogenate tissues from wounds treated with P. macrocarpa showed a significantly decreased MDA and inflammatory TNF-α levels, thereby indicating that SOD and CAT activities significantly increased in response to oxidative stress. These findings agreed with those of previous studies [24-25]. The reduced SOD activity in the wound homogenates obtained from the vehicle-treated control group was attributed to the increased production of reactive oxygen radicals, which could decrease the antioxidant activity of the enzyme [26].
The presence of the antioxidant and anti-inflammatory properties may be among the factors that contributed to the wound-healing potential of the P. macrocarpa extracts. The topical application of P. macrocarpa fruit extract restored the antioxidant enzyme activities, which prevented the deleterious effects of free radicals. Previous studies have reported the antioxidant and anti-inflammatory abilities of various plants, as well as their ability to induce antioxidant enzyme activities for the prevention or treatment of various diseases, such as cancer, diabetes, atherosclerosis, hyperglycemia, and hypercholesterolemia [27].
CONCLUSION
Topical treatment with P. macrocarpa fruit extract improved the activities of wound healing by reducing cellular damage and accelerating the wound healing process.
ACKNOWLEDGEMENTS
Authors thank University of Malaya, Malaysia for supporting this work by the research finance grant (PV046/2012A).
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. DOI: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- [2].Mackay D, Miller AL. Nutritional Support for Wound Healing. Alter Med Rev. 2003;8(4):359–377. [PubMed] [Google Scholar]
- [3].Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, et al. Impaired wound contraction in stromelysin-1–deficient mice. Ann Surg. 1999;230(2):260–265. doi: 10.1097/00000658-199908000-00017. DOI: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283–289. doi: 10.2741/1184. DOI: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- [5].Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25(1):19–25. doi: 10.1016/j.clindermatol.2006.12.005. DOI: 10.1016/j.clindermatol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [6].Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric oxide. 2002;7(1):1–10. doi: 10.1016/s1089-8603(02)00002-2. DOI: 10.1016/S1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- [7].Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. DOI: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hendra R, Ahmad S, Sukari A, Shukor MY, Oskoueian E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) boerl fruit. Int J Mol Sci. 2011;12(6):3422–3431. doi: 10.3390/ijms12063422. DOI: 10.3390/ijms12063422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Soo C, Chong, Mohamad A, Dollah, Pei PC, Abdullah M. Phaleria macrocarpa (Scheff.) Boerl fruit aqueous extract enhances LDL receptor and PCSK9 expression in vivo and in vitro. J Ethnopharmacol. 2011;137:817–827. doi: 10.1016/j.jep.2011.06.041. DOI: 10.1016/j.jep.2011.06.041. [DOI] [PubMed] [Google Scholar]
- [10].Riwanto I, Budijitno S, Dharmana E, Handojo D, Prasetyo SA, Eko A, et al. Effect of Phaleria macrocarpa supplementation on apoptosis and tumor growth of C3H mice with breast cancer under treatment with adriamycin-cyclophosphamide. Int Surg. 2011;96(2):164–170. doi: 10.9738/1404.1. DOI: 10.9738/1404.1. [DOI] [PubMed] [Google Scholar]
- [11].Ali R B., Atangwho IJ, Kaur N, Abraika OS, Ahmad M, Mahmud R, et al. Bioassay-Guided Antidiabetic Study of Phaleria macrocarpa Fruit Extract. Molecules. 2012;17(5):4986–5002. doi: 10.3390/molecules17054986. DOI: 10.3390/molecules17054986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Abood WN, Abdulla MA, Ismail S. Involvement of Inflammatory Mediators in the Gastroprotective Action of Phaleria macrocarpa against Ethanol-Induced Gastric Ulcer. World Applied Sciences Journal. 2014;30:344–350. [Google Scholar]
- [13].Tandrasasmita OM, Lee JS, Baek SH, Tjandrawinata RR. Induction of cellular apoptosis in human breastcancer by DLBS1425, a Phaleria macrocarpa compound extract, via downregulation of PI3-kinase/AKT pathway. Cancer Biol Ther. 2010;8(10):814–823. doi: 10.4161/cbt.10.8.13085. DOI: 10.4161/cbt.10.8.13085. [DOI] [PubMed] [Google Scholar]
- [14].Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J. 2007;1(13):1–4. doi: 10.1186/1752-153X-1-13. DOI: 10.1186/1752-153X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. The 1996 guide for the care and use of laboratory animals. ILAR J. 1997;38(1):41–48. doi: 10.1093/ilar.38.1.41. DOI: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- [16].Morton JJ, Malone MH. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196(1):117–126. [PubMed] [Google Scholar]
- [17].Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidantive stress in stomach disorders. J Clin Biochem Nutr. 2012;50(1):35–39. doi: 10.3164/jcbn.11-115SR. DOI: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1):248–254. doi: 10.1016/0003-2697(76)90527-3. DOI: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [19].Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister J-J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Bio Cell. 2004;15(9):4310–4320. doi: 10.1091/mbc.E04-05-0386. DOI: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang T, Yin L, Yang J, Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur J Pharm. 2007;567(3):177–185. doi: 10.1016/j.ejphar.2007.02.033. DOI: 10.1016/j.ejphar.2007.02.033. [DOI] [PubMed] [Google Scholar]
- [21].Upadhyay A, Chattopadhyay P, Goyary D, Mazumder PM, Veer V. Eleutherine indica L. accelerates in vivo cutaneous wound healing by stimulating Smad-mediated collagen production. J Ethnopharmacol. 2013;146(2):490–494. doi: 10.1016/j.jep.2013.01.012. DOI: 10.1016/j.jep.2013.01.012. [DOI] [PubMed] [Google Scholar]
- [22].Wu X-b, Luo X-q, Gu S-y, Xu J-h. The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol. 2012;141(3):934–937. doi: 10.1016/j.jep.2012.03.040. DOI: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- [23].Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4(1):5. doi: 10.1186/1475-2840-4-5. DOI: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gouthamchandra K, Mahmood R, Manjunatha H. Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol. 2010;30(1):11–18. doi: 10.1016/j.etap.2010.03.005. DOI: 10.1016/j.etap.2010.03.005. [DOI] [PubMed] [Google Scholar]
- [25].Nevin K, Rajamohan T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol Physiol. 2010;23(6):290–297. doi: 10.1159/000313516. DOI: 10.1159/000313516. [DOI] [PubMed] [Google Scholar]
- [26].El-Razek FA, El-Metwally E, Shehab G, Hassan A, Gomaa A. Effects of cactus pear (Opuntia ficus indica) juice on oxidative stress in diabetic cataract rats. Saudi J Health Sci. 2012;1(1):23. DOI: 10.4103/2278-0521.94980. [Google Scholar]
- [27].Ali R B, Atangwho IJ, Kuar N, Mohamed EAH, Mohamed AJ, Asmawi MZ, et al. Hypoglycemic and anti-hyperglycemic study of Phaleria macrocarpa fruits pericarp. J Med Plant Res. 2012;6(10):1982–1990. [Google Scholar]


