Abstract
Objective
To determine whether d-dimer would be increased in children with traumatic brain injury (TBI), specifically mild abusive head trauma (AHT).
Study design
D-dimer was measured using multiplex bead technology in 195 children <4 years old (n=93 controls without TBI, n=102 cases with TBI) using previously collected serum. D-dimer was then measured prospectively in a clinical setting in 44 children (n=24 controls, n=20 cases). Receiver operator curves (ROC) were generated for prospective data.
Results
In both the retrospective and prospective cohorts, median (25th–75th percentile) d-dimer was significantly higher in cases vs. controls. An ROC demonstrated an area under the curve (AUC) of 0.91 (95% CI: 0.83 – 0.99) in the prospective cohort. At a cut-off of 0.59μg/L, the sensitivity and specificity for identification of a case was 90% and 75%, respectively.
Conclusions
Our data suggest that serum d-dimer may be able to be used to identify which young children at risk for AHT might benefit from a head computer tomography or other additional evaluation. Additional data are needed in order to better identify the clinical scenarios which may result in false positive or false negative d-dimer concentrations.
Keywords: abusive head trauma, biomarker
Abusive head trauma (AHT) is the leading cause of death from traumatic brain injury (TBI) in infants.1 Proper diagnosis of AHT is difficult even for experienced, discerning clinicians because often no history of trauma is provided, children present with non-specific symptoms, such as vomiting or fussiness, and the physical examination is normal. 2, 3 As a result, misdiagnosis is common and can result in catastrophic medical and social consequences. The importance of timely diagnosis of AHT cannot be overemphasized: if unrecognized, child abuse can be an escalating form of trauma that often ends in disability or even death.4, 5
The frequency with which AHT is misdiagnosed and the resulting morbidity and mortality are compounded by the lack of a well-established screening test to help physicians identify children who might benefit from evaluation with cranial computed tomography (CT). The role of this screening test would, therefore, be to identify the subset of infants and toddlers for whom the treating physician should ‘think brain’ and order a head CT.
Over the past 10 years, we have reported on the possible use of both brain and non-brain specific serum biomarkers as screening tools for infants at increased risk of missed AHT.6–9 During the same period, considerable efforts have been made to develop a biomarker panel for adults with TBI that could be used as diagnostic and/or prognostic adjuncts.10, 11 Development of useful biomarkers or panels of biomarkers for clinical practice is, however, a long and arduous process.12
There are numerous serum biomarkers which are already part of clinical practice and are United States Food and Drug Administration (FDA)-approved for other uses. D-dimer is a fibrin degradation product which gets its name because it contains two cross-linked D fragments of the fibrin protein. It was introduced into clinical practice in the 1990s, primarily as a test to rule out deep vein thrombosis and pulmonary embolus13,14 and to diagnose disseminated intravascular coagulation.15 As early as 1982, it was recognized that pediatric TBI could result in perturbations of the coagulation system, although this study did not specifically look at d-dimer concentrations.16 Several studies have demonstrated that increased serum d-dimer concentrations are associated with poor outcome after TBI in adults.17,18 There is only a single pediatric study evaluating serum d-dimer after pediatric TBI. In that study, Swanson et al19 measured serum d-dimer concentrations in 57 children with a history of closed head injury who underwent a head CT to evaluate for intracranial hemorrhage. Forty of the 57 children had a Glasgow Coma Scale (GCS) score of 15; 20 of the 57 had an abnormal head CT. Increased serum d-dimer concentrations were strongly associated with an abnormal head CT (p < .0001) and this association was stronger than the association with the GCS score. The negative predictive value (NPV) of a serum d-dimer concentration <0.50μg/L was 94% for intracranial hemorrhage. As a result, the authors proposed that children with a serum d-dimer concentration <0.50μg/L could safely be discharged without a head CT. Interestingly, despite the encouraging results and a supportive accompanying editorial comment, there have been no follow-up studies.
The objective of the current study was, therefore, to determine whether a serum d-dimer concentration could help discriminate between children with and without brain injury, specifically mild AHT. In order to accomplish this objective, we used both retrospective and prospective cohorts. We hypothesized that children with brain injury would have increased serum d-dimer concentrations compared with controls.
Methods
D-dimer was measured first in a retrospective cohort using multiplex bead technology. The use of the retrospective cohort allowed for d-dimer to be screened as a potential marker in a large number of patients using a very small volume of serum for each patient. A prospective cohort was then used to assess the feasibility of d-dimer measurement in clinical practice. Given the young age of children at risk for AHT, the ability to collect an adequate amount of blood for d-dimer measurement is an important consideration. In addition, translation of normal versus pathologic concentrations of d-dimer from multiplex bead technology to a clinical laboratory can only be done using samples measured in a clinical laboratory. Previously processed and/or frozen samples cannot be used to measure d-dimer in a clinical laboratory and therefore prospectively collected specimens were needed.
Retrospective Cohort
Subjects in the retrospective cohort were selected from a serum bank which has been established at the Safar Center for Resuscitation Research. This serum bank contains hundreds of serum samples from cases and controls which were collected prospectively as part of several IRB-approved studies.
Controls were selected from the serum bank if they were less than 4 years old and did not have brain injury. The majority of these subjects did not have a head CT because there was no clinical indication for one. The head CT was normal in those children who did have one. Cases were selected from the serum bank if they were less than 4 years of age and had an abnormal head CT secondary to trauma (eg, children with atraumatic abnormalities such as hydrocephalus or brain tumor were not included). All cases in the retrospective cohort were evaluated by the Children’s Hospital of Pittsburgh of UPMC Child Protection Team for concerns of abuse; some were determined to be the result of abuse and others were not. Determining whether an injury is the result of abuse based on the conclusion of a Child Protection Team is frequently used in studies of AHT.4, 20, 21 In each case of AHT, the time of injury was defined as the time when the child’s symptoms started or the time when a caretaker sought medical care for the child, whichever came first; this is an approach we have used previously.8, 9
Prospective cohort
Subjects were eligible for enrollment if they were less than 4 years of age and were enrolled in a prospective, IRB-approved parent study of children at increased risk for AHT. Consent was obtained for controls and for subjects in which there was no concern for abuse. An IRB approved waiver of informed consent was used in cases of suspected AHT. Subjects were classified as controls if the head CT was either normal or not done and were classified as cases if the head CT was abnormal. Unlike in the retrospective cohort, in which all cases had TBI, subjects in the prospective cohort were included as cases if they had an isolated skull fracture, or if there was any intracranial abnormality, whether it was traumatic or not. This distinction is important because, in clinical practice, children with brain abnormalities, such as hydrocephalus or a brain tumor, may present with the same non-specific symptoms as children with AHT. Prior to using a biomarker such as d-dimer in clinical practice, it is imperative to evaluate what type of abnormalities can and cannot be identified by d-dimer. AHT, as well as the time of injury, was defined in this cohort in the same way as in the retrospective cohort.
Measures
For all subjects in both cohorts, demographic data including age, sex, and mechanism of injury (abuse or not abuse) were collected. Race was classified as white or not-white. As part of clinical care, a GCS score was assigned for all trauma patients. CT scans and/or brain MRI were evaluated by a pediatric neuroradiologist as part of routine clinical care. Subjects were designated as a case or control based on whether the neuroimaging was abnormal or normal, respectively. For the prospective cohort, data were also collected about the presence of any extra-cranial injuries as well as the reason for presentation for medical care.
Biomarker measurement
Retrospective cohort: Blood was collected as soon as possible after arrival to the hospital. Blood samples were spun at 5,000 RPM for 10 minutes. Serum was then removed and stored at −70°C until analysis. D-dimer concentrations were measured using a d-dimer single-plex assay (Affymetrix, Inc., Santa Clara, CA) according to the manufacturer’s instructions. Because of the recognized kit-to-kit variability, 5% of samples were overlapped between kits and the values among kits were normalized using a scaling procedure which utilizes both concentration standards (CS) and quality control (QC) samples duplicated across plates.
Prospective cohort: Blood was collected in a 3.2% sodium citrate tube and processed in the CLIA (Clinical Laboratory Improvement Amendments) -certified hospital laboratory at Children’s Hospital of Pittsburgh of UPMC according to standard procedure. Normal d-dimer concentrations are defined as less than 0.5μg/L Fibrinogen Equivalent Units (FEU).
Statistical Analyses
SPSS 21.0 was used for all analyses. Descriptive statistics were used to characterize the sample. Mann-Whitney tests were used to evaluate the difference between groups. Spearman correlations were used to assess the relationship between d-dimer concentrations and clinical variables, including time after injury and GCS score. For all analyses, p < 0.05 was considered significant. Receiver operator curves (ROC) were generated for the prospective data. Area under the curve (AUC) was calculated. Coordinate points of each curve were evaluated for various levels of sensitivity and specificity.
Results
Retrospective cohort
A total of 195 subjects (n=93 controls, n=102 cases) were enrolled retrospectively. These subjects received medical care at CHP between April 2002 and September 2012. There was no difference in the proportion of males (56% vs. 57%) or the proportion of white children (83% vs. 75%) between cases and controls. Cases were slightly, although statistically significantly, older than controls (Median [25th–75th percentile] age in months 5.4 [2.6/16.1] vs. 4.4 [2.1/8.1], p = 0.01).
Of the 102 cases, 66 (64.5%) had AHT with a GCS score of 13–15, 18 (17.5%) had AHT with a GCS score of less than 13, 12 (12%) had non-AHT with a GCS score of 13–15 and the remaining 6 (6%) had non-AHT with a GCS score less than 13. Therefore, 82% of the cases were children with AHT and 76% of the cases had a GCS score of 13–15. Of the cases, 37 (36%) had an isolated skull fracture or a skull fracture with an underlying subdural hemorrhage, but no intraparenchymal injury, 55 (54%) had a subdural hemorrhage not associated with a skull fracture, and the remaining 10 (9.8%) had other abnormalities, such as chronic subdural hemorrhages.
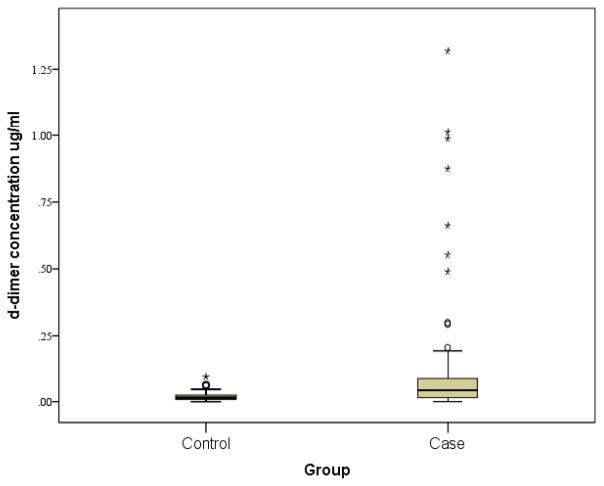
Median (25th–75th percentile) d-dimer was higher in cases vs. controls 0.43 (0.15/0.89) vs. 0.15 (0.83/0.25)μg/L. The corresponding Mann-Whitney test was significant (p < 0.001) (Figure 1). There was no correlation between d-dimer concentrations and age, between d-dimer and GCS score, or between d-dimer and injury mechanism (AHT vs. non-AHT).
Figure 1.
Serum d-dimer concentrations in the retrospective cohort
In cases, the median (range) time after injury (in hours) when blood was collected was 11.4 (0.6–184) hours.
Prospective cohort
A total of 44 subjects (n=24 controls, n=20 cases) were enrolled prospectively (Table I). These subjects received medical care at CHP between April 2013 and February 2014. There was no difference in the proportion of males, the proportion of white children or the mean age between cases and controls. Of the 24 controls, all presented with non-specific symptoms such as vomiting, fussiness, or a seizure. Of the 20 cases, 4 presented with a history of trauma, 4 presented with soft tissue swelling of the scalp without a history of trauma and 16 presented with non-specific symptoms and no history of trauma. Brain injuries among the 20 cases included acute subdural hemorrhages and/or intraparenchymal injury consistent with AHT (n=9), isolated skull fracture (n=3), a skull fracture with a underlying subdural or epidural hemorrhage without parenchymal injury (n=4), isolated chronic subdural hemorrhage (n=2), findings consistent with Sturge-Weber syndrome (n=1) and a skull fracture with an associated carotid artery tear and middle cerebral artery stroke (n=1). Therefore of 20 cases, 14 (9 subjects with AHT, 4 subjects with a skull fracture and underlying hemorrhage and one subject with a stroke) had an acute intracranial injury. Mean (SD) GCS score was 12.4 (4.4) with a median of 15. Thirty-five percent (7/20) of cases had extra-cranial fractures; 4% (1/24) of the controls had extra-cranial fractures.
Table 1.
Demographics of the prospective cohort
| Controls (n=24) | Cases (n=20) | p-value | |
|---|---|---|---|
| Age [mean (SD)] (in months) | 6.4 (8.24) | 11.6 (8.3) | NS |
| Race (% white) | 75% (18/24) | 85% (17/20) | NS |
| Sex (% male) | 58% (14/24) | 50% (10/20) | NS |
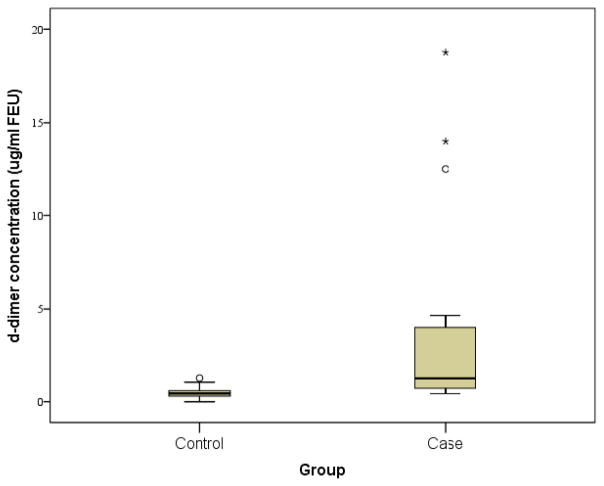
Median (25th–75th percentile)_d-dimer concentrations were higher in cases vs. controls (1.25 [0.70/4.25] vs. 0.44 [0.29/0.60] μg/L) FEU. The corresponding Mann-Whitney was significant at p < 0.000. Blood was collected at a mean (SD) of 25.2 (20.2) hours after injury in cases. In univariate analyses, there was a strong relationship between d-dimer concentrations and age (r = 0.41, p = 0.006), between GCS score and d-dimer concentration (r = −0.62, p < 0.000) and between GCS score and age (r = −0.38, p = 0.012). Because of the small sample size and skew of the data, it was not possible to evaluate further the relationships between GCS score, age, and d-dimer concentrations.
There was no relationship between GCS score and the time after injury when samples were collected. There was no difference between the d-dimer concentration and the presence or absence of extra-cranial fractures.
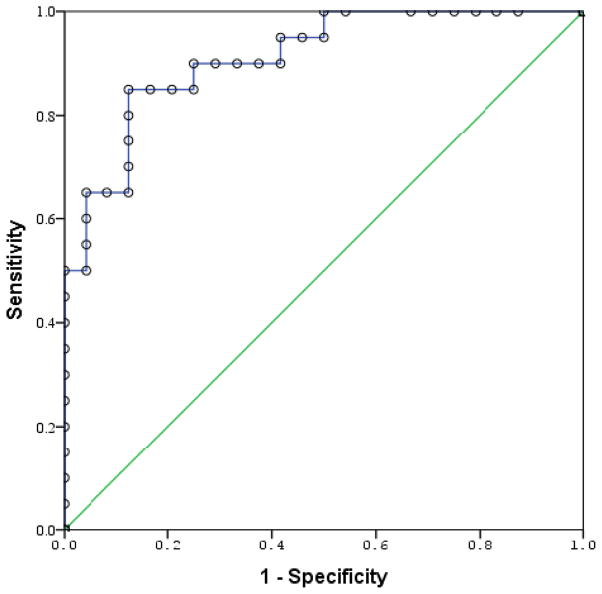
An ROC demonstrated an area under the curve (AUC) of 0.91 (95% CI: 0.83 – 0.99). The probability of correctly classifying a randomly chosen subject as a case or control based on the serum d-dimer concentration score was, therefore, greater than 90%. At a cut-off of 0.59μg/L, the sensitivity and specificity for identification of a case was 90% and 75%, respectively.
Discussion
This exploratory study suggests that d-dimer may be able to be used as a marker of brain injury in infants and young children who are at high risk for AHT. The AUC of >0.90 in the prospective sample demonstrates that d-dimer is able to discriminate cases from controls with close to perfect accuracy and may be more accurate than many of the non-FDA approved biomarkers that we have previously evaluated in this population.8, 9, 22, 23 Because d-dimer is FDA-approved, it can already be measured as part of clinical practice both in both pediatric and adult hospitals. Although AHT is often treated at pediatric hospitals, the majority of children with AHT are initially evaluated in adult hospitals; this is also where they are most likely to be misdiagnosed.24 Furthermore, use of a CLIA-approved microtainer allows for d-dimer measurement using less than 2 mL of blood, an important consideration in infants and young children.
Although Swanson et al19 suggested a cut-off of 0.50μg/L to maximize the NPV, the positive predictive value (PPV) may be more clinically relevant for our clinical dilemma because of the difference in the clinical challenge being addressed in the two studies. In the Swanson study, all subjects underwent head CT as part of standard practice and the goal of the d-dimer was to determine which subjects might not need to undergo head CT. In contrast, in our target population, the role of d-dimer would be to identify the subset of infants and toddlers who should undergo head CT in order to evaluate for unsuspected brain injury and who might otherwise be discharged from medical care without imaging (e.g. a ‘think brain’ scenario vs. a ‘rule out brain’ scenario). This is consistent with the fact that of the 16 controls with a d-dimer concentration <0.5μg/L, only 7(44%) underwent neuroimaging as part of clinical care. Similarly, when d-dimer is used clinically to determine which patients do not need to undergo evaluation for pulmonary embolus or deep vein thrombosis,13, 14 the NPV is the relevant statistic, and the PPV is used when d-dimer is used to identify disseminated intravascular coagulation.15
The PPV of d-dimer at a cut-off of 1.0μg/L was 87% in our small cohort. Interestingly, the two false positives were in children with conditions known to cause increase d-dimer concentrations: aortic stenosis 25 and sickle cell disease.26–28 The NPV at this cut-off was 88%; two false negative subjects included one with an isolated skull fracture without intracranial injury and one with chronic subdural hemorrhages without evidence of acute injury. Although both of these injuries can be seen in children with child abuse, it would be surprising if a serum biomarker of brain injury were increased in these injuries; an isolated skull fracture is not associated with brain injury and even a serum biomarker with a long serum half-life would not be present in the serum for weeks after an injury. It is important to recognize that d-dimer cannot be used to make a diagnosis of abuse, but only to alert the treating physician to the possibility of brain injury.
In the current study, we measured d-dimer at a single time point. Because most of our subjects had a GCS score of 15, only one blood sample was collected as part of clinical care. Additional study is needed to determine whether serial measurement of d-dimer could be useful in the cohort of children with initial borderline d-dimer concentrations which will not provide a clear clinical direction. The feasibility of serial blood sampling in the emergency department might be problematic even if research data were to suggest an advantage to serial d-dimer measurement. There are no studies to our knowledge which have looked at serial d-dimer concentrations after adult or pediatric TBI, although there are limited data related to serial d-dimer in critically ill adult trauma patients.29
Finally, we were surprised that in our prospective sample, there was a strong relationship between d-dimer concentrations and age, between GCS score and d-dimer concentration and between GCS score and age. This relationship was not seen in the larger, retrospective cohort. The lack of a relationship is unlikely due to a type II error given the large sample size. Additional prospective data are needed to further evaluate these differences.
In summary, our data suggest that a single serum d-dimer may be able to identify infants and toddlers who would benefit from neuroimaging to evaluate for brain injury. Although we recognize that a combination of biomarkers will likely to be more accurate than a single biomarker,9, 30 d-dimer may have the ability to provide an interim solution until more accurate markers are available for clinical use. In addition, d-dimer should be strongly considered for inclusion in any biomarker panel which is being developed, either within the panel, or as a separate assay, given its clinical availability. Additional data are needed in order to better identify those clinical scenarios in which false positive and false negative d-dimer concentrations are likely and to assess whether a combination of d-dimer with certain clinical variables might provide higher accuracy than d-dimer alone.
Figure 2.
Serum d–dimer concentrations in the prospective cohort
Figure 3.
Received operator characteristic curve demonstrating ability to discriminate cases from controls. AUC = 0.91 (95% CI: 0.83 – 0.99)
Acknowledgments
Funded by the National Institutes of Health (R01HD055986 [to R.B.]).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rachel P. Berger, Email: rachel.berger@chp.edu, Associate Professor of Pediatrics, Safar Center for Resuscitation Research, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, Pittsburgh, PA 15224, University of Pittsburgh, Phone: 412-692-8664, Fax: 412-692-8399.
Janet Fromkin, Email: janet.fromkin@chp.edu, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, Pittsburgh, PA 15224, University of Pittsburgh, Phone: 412-692-8664, Fax: 412-692-8399.
Pam Rubin, Email: Pamela.rubin@chp.edu, Children’s Hospital of Pittsburgh of UPMC, 4401 Penn Avenue, Pittsburgh, PA 15224, University of Pittsburgh, Phone: 412-692-8664, Fax: 412-692-8399.
John Snyder, Email: snyderjd@upmc.edu, UPMC Hillman Cancer Center, 5115 Centre Avenue, Pittsburgh, PA 15232, Phone: 412-623-7748, Fax: 412-623-1415.
Rudolph Richichi, Statistical Analysis and Measurement Consultants, Inc., P.O. Box 224, Lanexa, VA 23089.
Patrick Kochanek, Email: kochanekpm@ccm.upmc.edu, Safar Center for Resuscitation Research, 3434 Fifth Avenue, Pittsburgh PA 15260, Phone: 412-383-1900.
References
- 1.Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 2008;34:S157–S62. doi: 10.1016/j.amepre.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Ettaro L, Berger RP, Songer T. Abusive head trauma in young children: characteristics and medical charges in a hospitalized population. Child Abuse Negl. 2004;28:1099–111. doi: 10.1016/j.chiabu.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Sieswerda-Hoogendoorn T, Boos S, Spivack B, Bilo RA, van Rijn RR. Educational paper: Abusive Head Trauma part I. Clinical aspects. European journal of pediatrics. 2012;171:415–23. doi: 10.1007/s00431-011-1598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay TC. Analysis of missed cases of abusive head trauma. JAMA : the journal of the American Medical Association. 1999;281:621–6. doi: 10.1001/jama.281.7.621. [DOI] [PubMed] [Google Scholar]
- 5.Sheets LK, Leach ME, Koszewski IJ, Lessmeier AM, Nugent M, Simpson P. Sentinel injuries in infants evaluated for child physical abuse. Pediatrics. 2013;131:701–7. doi: 10.1542/peds.2012-2780. [DOI] [PubMed] [Google Scholar]
- 6.Berger R, Hymel K, Gao W. The use of biomarkers after inflicted traumatic brain injury: Insight into etiology, pathophysiology and biochemistry. Clinical Pediatric Emergency Medicine. 2006;7:186–93. [Google Scholar]
- 7.Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Developmental neuroscience. 2006;28:327–35. doi: 10.1159/000094158. [DOI] [PubMed] [Google Scholar]
- 8.Berger RP, Dulani T, Adelson PD, Leventhal JM, Richichi R, Kochanek PM. Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: a possible screening tool. Pediatrics. 2006;117:325–32. doi: 10.1542/peds.2005-0711. [DOI] [PubMed] [Google Scholar]
- 9.Berger RP, Ta’asan S, Rand A, Lokshin A, Kochanek P. Multiplex assessment of serum biomarker concentrations in well-appearing children with inflicted traumatic brain injury. Pediatr Res. 2009;65:97–102. doi: 10.1203/PDR.0b013e31818c7e27. [DOI] [PubMed] [Google Scholar]
- 10.Castellanos M, Serena J. Applicability of biomarkers in ischemic stroke. Cerebrovasc Dis. 2007;24(Suppl 1):7–15. doi: 10.1159/000107374. [DOI] [PubMed] [Google Scholar]
- 11.Niyonkuru C, Wagner AK, Ozawa H, Amin K, Goyal A, Fabio A. Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: a practical example. J Neurotrauma. 2013;30:938–45. doi: 10.1089/neu.2012.2578. [DOI] [PubMed] [Google Scholar]
- 12.Berger RP, Houle JF, Hayes RL, Wang KK, Mondello S, Bell MJ. Translating biomarkers research to clinical care: applications and issues for rehabilomics. PM R. 2011;3:S31–8. doi: 10.1016/j.pmrj.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Barnes DM, Wakefield TW, Rectenwald JE. Novel Biomarkers Associated with Deep Venous Thrombosis: A Comprehensive Review. Biomarker insights. 2008;3:93–100. [PMC free article] [PubMed] [Google Scholar]
- 14.Corwin MT, Donohoo JH, Partridge R, Egglin TK, Mayo-Smith WW. Do emergency physicians use serum D-dimer effectively to determine the need for CT when evaluating patients for pulmonary embolism? Review of 5,344 consecutive patients. AJR American journal of roentgenology. 2009;192:1319–23. doi: 10.2214/AJR.08.1346. [DOI] [PubMed] [Google Scholar]
- 15.Boisclair MD, Lane DA, Wilde JT, Ireland H, Preston FE, Ofosu FA. A comparative evaluation of assays for markers of activated coagulation and/or fibrinolysis: thrombin-antithrombin complex, D-dimer and fibrinogen/fibrin fragment E antigen. Br J Haematol. 1990;74:471–9. doi: 10.1111/j.1365-2141.1990.tb06337.x. [DOI] [PubMed] [Google Scholar]
- 16.Miner ME, Kaufman HH, Garham SH, Haar FH, Gildenberg PL. Disseminated intravascular coagulation fibrinolytic syndrome following head injury in children: frequency and prognostic implications. J Pediatr. 1982;10:687–91. doi: 10.1016/s0022-3476(82)80565-9. [DOI] [PubMed] [Google Scholar]
- 17.Chhabra G, Sharma S, Subramanian A, Agrawal D, Sinha S, Mukhopadhyay AK. Coagulopathy as prognostic marker in acute traumatic brain injury. Journal of emergencies, trauma, and shock. 2013;6:180–5. doi: 10.4103/0974-2700.115332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Defazio MV, Rammo RA, Robles JR, Bramlett HM, Dietrich WD, Bullock MR. The Potential Utility of Blood-Derived Biochemical Markers as Indicators of Early Clinical Trends Following Severe Traumatic Brain Injury. World neurosurgery. 2014;81:151–8. doi: 10.1016/j.wneu.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swanson CA, Burns JC, Peterson BM. Low plasma D-dimer concentration predicts the absence of traumatic brain injury in children. J Trauma. 2010;68:1072–7. doi: 10.1097/TA.0b013e3181d7a6f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hymel KP, Abshire TC, Luckey DW, Jenny C. Coagulopathy in pediatric abusive head trauma. Pediatrics. 1997;99:371–5. doi: 10.1542/peds.99.3.371. [DOI] [PubMed] [Google Scholar]
- 21.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, Sinal SH. A population-based study of inflicted traumatic brain injury in young children. JAMA : the journal of the American Medical Association. 2003;290:621–6. doi: 10.1001/jama.290.5.621. [DOI] [PubMed] [Google Scholar]
- 22.Berger RP, Hayes RL, Richichi R, Beers SR, Wang KK. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J Neurotrauma. 2012;29:162–7. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger RP, Adelson PD, Pierce MC, Dulani T, Cassidy LD, Kochanek PM. Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J of Neurosurgery. 2005;103:61–8. doi: 10.3171/ped.2005.103.1.0061. [DOI] [PubMed] [Google Scholar]
- 24.Letson M, Cooper J, Deans K, Scribano P, Makoroff K, Feldman K, et al. Prior opportunities to diagnose abuse in children with abusive head trauma. Pediatric Academic Societies Annual Meeting; Washington, DC. 2013. [Google Scholar]
- 25.Natorska J, Marek G, Hlawaty M, Sadowski J, Tracz W, Undas A. Fibrin presence within aortic valves in patients with aortic stenosis: association with in vivo thrombin generation and fibrin clot properties. Thrombosis and haemostasis. 2011;105:254–60. doi: 10.1160/TH10-09-0612. [DOI] [PubMed] [Google Scholar]
- 26.Francis RB., Jr Elevated fibrin D-dimer fragment in sickle cell anemia: evidence for activation of coagulation during the steady state as well as in painful crisis. Haemostasis. 1989;19:105–11. doi: 10.1159/000215901. [DOI] [PubMed] [Google Scholar]
- 27.Devine DV, Kinney TR, Thomas PF, Rosse WF, Greenberg CS. Fragment D-dimer levels: an objective marker of vaso-occlusive crisis and other complications of sickle cell disease. Blood. 1986;68:317–9. [PubMed] [Google Scholar]
- 28.Shah N, Thornburg C, Telen MJ, Ortel TL. Characterization of the hypercoagulable state in patients with sickle cell disease. Thrombosis research. 2012;130:e241–5. doi: 10.1016/j.thromres.2012.08.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen JP, Rowe DW, Enderson BL. Contrasting post-traumatic serial changes for D-dimer and PAI-1 in critically injured patients. Thrombosis research. 1999;94:175–85. doi: 10.1016/s0049-3848(98)00211-4. [DOI] [PubMed] [Google Scholar]
- 30.Lo TY, Jones PA, Minns RA. Pediatric brain trauma outcome prediction using paired serum levels of inflammatory mediators and brain-specific proteins. J Neurotrauma. 2009;26:1479–87. doi: 10.1089/neu.2008.0753. [DOI] [PubMed] [Google Scholar]