Abstract
Obesity adversely affects outcome in pediatric acute lymphocytic leukemia and acute myeloid leukemia (AML). We asked if obesity, measured by body mass index (BMI), affected outcome in 329 adult AML patients treated with high-dose cytarabine and idarubicin-containing regimens administered according to actual body weight. Age≥60, unfavorable karyotype, secondary AML, and positive smoking status had adverse impact on overall survival in a multivariate analysis, while BMI did not. We conclude that high BMI should not be a barrier to administer high-dose cytarabine-containing regimens for AML induction.
Keywords: Acute myeloid leukemia, Obesity, Body mass index, Outcome
Introduction
The prevalence of obesity has increased substantially over the past few decades and has more than doubled in adults in the USA [1]. Furthermore, obesity is a known risk factor for adult hematological malignancies [2]. Chemotherapy dosages for obese patients are often empirically reduced on the basis of ideal body weight because of concerns about excessive toxicity; however, dose reductions may compromise treatment outcomes [3, 4]. There is a paucity of information on the influence of body mass index (BMI) on the toxicity of intensive chemotherapy in adult AML and on progression-free survival (PFS) and overall survival (OS) following intensive chemotherapy [5, 6]. We compared toxicity data and survival outcomes across three BMI groups [normal (NL), overweight (OW), and obese (OB)], in 329 adults with AML, treated with similar AML induction chemotherapy administered by actual weights, to find out if obesity is a prognostic factor in AML.
Patients and methods
We queried the leukemia database for patients treated with high-dose cytarabine and idarubicin-containing regimens at the Roswell Park Cancer Institute (RPCI) between June 1990 and December 2008. Three hundred twenty-nine AML (except acute promyelocytic leukemia) patients eligible for high-dose cytarabine and idarubicin-containing regimens were found. The following patient data were scored: age, gender, weight, height, karyotype, smoking history, disease presentation (de novo vs. secondary), time of relapse, and allogeneic transplant. Patients’ heights and weights were recorded at the time of induction chemotherapy. BMI was calculated using the World Health Organization (WHO) classification definition [7] and was categorized as follows: NL as BMI<25 kg/m2, OW as BMI between 25.0 and 29.9 kg/m2, and OB as BMI≥30.0 kg/m2. In this population, only one patient (18.48 kg/m2) was classified as underweight according to the WHO definition (BMI<18.5) and was therefore included in NL BMI category. The RPCI Scientific Review Committee and the Institutional Review Board approved the study.
Treatment
Induction chemotherapy consisted of high-dose cytarabine [3 g/m2 (1.5 g/m2 for age ≥50) every 12 h×12 doses] and idarubicin (12 mg/m2×3 doses) based on actual body weight. Sixty-one of 329 patients received priming with arsenic trioxide (0.15 to 0.65 mg/kg) prior to high-dose cytarabine and idarubicin on a phase I clinical trial. Consolidation therapy varied over time. All patients had adequate renal, hepatic, and cardiac function prior to treatment initiation. Of note, 34 patients underwent allogeneic hematopoietic stem cell transplantation during first remission.
Statistical analyses
Response criteria were standard [8]. PFS was defined as time from diagnosis until time of relapse. OS was defined as time from diagnosis until time of last follow-up or death.
Univariate analyses
Descriptive statistics and chi-square tests were used to explore associations between body mass index (BMI) and other characteristics of interest. The log rank (Mantel–Cox) test within the Kaplan–Meier (KM) analysis was used to assess statistically significant differences in mean PFS and OS (in months) according to various participant characteristics. All analyses were stratified by participant BMI at diagnosis (NL, OW, and OB).
Pulmonary and cardiac toxicities, infection, and multi-organ failure toxicities were obtained from medical records and were categorized according to the common terminology criteria for adverse events version 3. Furthermore, patients were categorized as having no toxicity or at least mild toxicity in any of the preceding areas. Differences in toxicity levels were assessed using chi-square analysis.
Multivariable analyses
Cox proportional hazard modeling was used to assess prognostic factors of OS and was limited to the 267 patients for whom complete data for all variables in the model were available. Tests for interactions between variables of interest and groups were performed using Cox proportional hazard modeling for both PFS and OS outcomes. Data were censored at the time of allogeneic transplantation. Models of PFS and OS were adjusted for: age (<60 years vs. ≥60 years), gender (female, male), AML presentation (de novo vs. secondary), WBC count at diagnosis (<100×109/L vs. ≥100×109/L), smoking history (never, previous smoker, current smoker), treatment decade (1990–1999 vs. 2000–2008), and karyotype [9] (intermediate, unfavorable, favorable, and unknown). OS was analyzed among 267 patients, including 208 events and 59 censors; PFS was analyzed among 181 patients and included 130 events and 51 censors. Survival curves stratified by BMI at diagnosis were obtained from Cox proportional hazard models after simultaneous adjustment for the covariates listed previously by plotting at the mean of each covariate. All significance testing was based upon a p value of <0.05. Analyses were completed using SPSS version 14.0.
Results
There were 188 (57%) males and 141 (43%) females (median age 57 years, range 18–85). The median follow-up was 12.9 months (range<1–195). A total of 34 patients (10.3%) underwent allogeneic transplantation. Patients’ BMIs ranged from 18.48 to 60.61 kg/m2. Overall, approximately 16% of patients reached 5 years OS. BMI was similar among patients who did or did not proceed to transplantation. Other characteristics are shown in Table 1. There were significantly less smokers among the NL BMI patients (P=0.023) and more males among the OW BMI patients (P=0.006). No other differences were noted.
Table 1.
Selected characteristics of the total study cohort and stratified by participant BMI status (normal vs. overweight vs. obese)
| Total
|
Normal n=106 BMI<25.0 |
Overweight n=119 BMI 25.0–29.9 |
Obese n=104 BMI ≥30.0 |
p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | Number | Percent | ||
| BMI | NA | ||||||||
| Normal | 106 | 32.2% | |||||||
| Overweight | 119 | 36.2% | NA | NA | NA | ||||
| Obese | 104 | 31.6% | |||||||
| Smoking (n=281) | 0.023 | ||||||||
| Never | 101 | 35.9% | 22 | 25.3% | 40 | 40.0% | 39 | 41.5% | |
| Previous | 118 | 42.0% | 37 | 42.5% | 39 | 39.0% | 42 | 44.7% | |
| Current | 62 | 22.1% | 28 | 32.2% | 21 | 21.0% | 13 | 13.8% | |
| Gender | 0.006 | ||||||||
| Female | 141 | 42.9% | 46 | 43.4% | 39 | 32.8% | 56 | 53.8% | |
| Male | 188 | 57.1% | 60 | 56.6% | 80 | 67.2% | 48 | 46.2% | |
| Age | 0.323 | ||||||||
| <60 years | 178 | 54.1% | 57 | 53.8% | 59 | 49.6% | 62 | 59.6% | |
| ≥60 years | 151 | 45.9% | 49 | 46.2% | 60 | 50.4% | 42 | 40.4% | |
| AML presentation | 0.119 | ||||||||
| De novo | 229 | 69.6% | 68 | 64.2% | 81 | 68.1% | 80 | 76.9% | |
| Secondary | 100 | 30.4% | 38 | 35.8% | 38 | 31.9% | 24 | 23.1% | |
| WBC count | 0.811 | ||||||||
| <100×109/L | 296 | 90.0% | 97 | 91.5% | 106 | 89.1% | 93 | 89.4% | |
| ≥100×109/L | 33 | 10.0% | 9 | 8.5% | 13 | 10.9% | 11 | 10.6% | |
| Karyotype (n=315) | 0.585 | ||||||||
| Favorable | 25 | 7.9% | 5 | 4.9% | 10 | 8.8% | 10 | 10.0% | |
| Intermediate | 144 | 45.7% | 47 | 46.1% | 56 | 49.6% | 41 | 41.0% | |
| Unfavorable | 114 | 36.2% | 41 | 40.2% | 37 | 32.7% | 36 | 36.0% | |
| Unknown | 32 | 10.2% | 9 | 8.8% | 10 | 8.8% | 13 | 13.0% | |
| Mortality | 0.277 | ||||||||
| Alive | 63 | 19.1% | 15 | 14.2% | 25 | 21.0% | 23 | 22.1% | |
| Dead | 266 | 80.9% | 91 | 85.8% | 94 | 79.0% | 81 | 77.9% | |
| Decade of treatment | 0.066 | ||||||||
| 1990–1999 | 213 | 64.7% | 74 | 69.8% | 81 | 68.1% | 58 | 55.8% | |
| 2000–2008 | 116 | 35.3% | 32 | 30.2% | 38 | 31.9% | 46 | 44.2% | |
| 5-year survival | 0.346 | ||||||||
| No | 277 | 84.2% | 93 | 87.7% | 96 | 80.7% | 88 | 84.6% | |
| Yes | 52 | 15.8% | 13 | 12.3% | 23 | 19.3% | 16 | 15.4% | |
P value based on chi-square analysis, comparing BMI categories. Boldface P values represent chi-square analyses that are statistically significant at p<0.05 level
AML acute myeloid leukemia, WBC white blood cell
As shown in Table 2, there were significantly less (P=0.014) pulmonary complications for OW patients compared to NL or OB patients with regards to grade one pulmonary toxicities. However, no other statistically significant differences were discovered in the other (cardiac and infectious) toxicities among the different BMI groups. Pairwise comparisons found statistically significant differences in reporting pulmonary complications for both NW patients and OB patients compared to OW patients (NL vs. OW: p=0.023; OW vs. OB: p=0.010, not shown). However, there were no statistically significant differences in pulmonary toxicity between NL and OB patients (p=0.782), and no differences were found for either cardiac complications or infection between BMI subgroups. Overall, day 30 mortality was 5.7% for NL, 6.7% for OW, and 7.7%% for OB patients and was not statistically different (p= 0.840). Similarly, no statistically significant differences in 60-day mortality were observed by BMI category (p=0.277).
Table 2.
Frequency of reporting pulmonary toxicity, cardiac toxicity, or infectious complications (none vs. any) within 30 days of starting treatment by BMI status (normal: BMI<25.0; overweight: BMI 25.0–29.9; obese: BMI≥30.0)
| Toxicity
|
Overall
|
Normal
|
Overweight
|
Obese
|
p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | Number | Percent | Number | Percent | ||
| Pulmonary | |||||||||
| None | 172 | 52.4% | 50 | 47.6% | 75 | 63.0% | 47 | 45.2% | 0.014 |
| Anya | 156 | 47.6% | 55 | 52.4% | 44 | 37.0% | 57 | 54.8% | |
| Cardiac | |||||||||
| None | 176 | 53.7% | 54 | 51.4% | 69 | 58.0% | 53 | 51.0% | 0.494 |
| Any | 152 | 46.3% | 51 | 48.6% | 50 | 42.0% | 51 | 49.0% | |
| Infection | |||||||||
| None | 119 | 36.3% | 41 | 39.0% | 43 | 36.1% | 35 | 33.7% | 0.719 |
| Any | 209 | 63.7% | 64 | 61.0% | 76 | 63.9% | 69 | 66.3% | |
p value based on chi-square analysis comparing toxicities (none vs. any) of patients with differing BMI categories
Patient who suffered mild, moderate, severe, life-threatening, or death within 30 days of starting treatment were categorized as having “any” toxicity
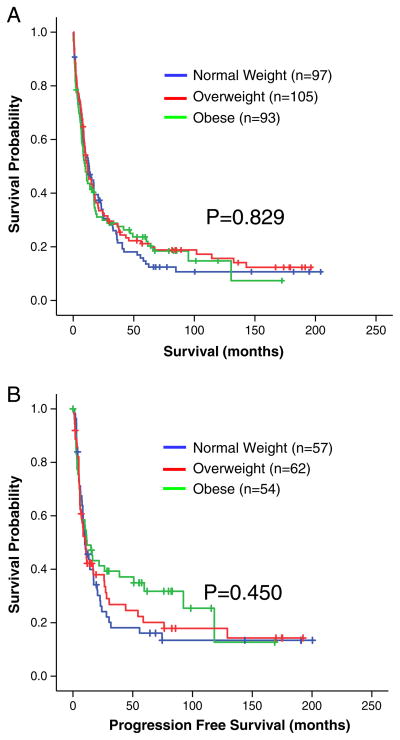
There was no statistically significant difference in OS for NL (36.5 months), OW (49.5 months), or OB patients (41.0 months) (P=0.471) based on univariate KM analysis (Table 3). Patients who received arsenic trioxide did not show difference in PFS or OS in Cox proportional model when compared to patients who did not receive arsenic trioxide. However, statistically significant differences in OS were observed for smoking status, age, karyotype, AML presentation, and treatment decade based on univariate analysis. Similarly, no significant differences in PFS were observed between BMI strata (Table 3); PFS for NL, OW, and OB were 37.6 vs. 51.9 vs. 55.4 months, respectively, (P=0.247) based on KM analysis. Additional analysis was performed excluding 34 patients who underwent allogeneic transplantation (n=295). No statistically significant differences in OS and PFS were noted between BMI subgroups (Fig. 1a and 1b) When patients were separated by decade of diagnosis, 213 patients (64.7%) were treated between 1990 and 2000, and 116 patients (35.3%) were treated after 2000. The mean OS in the 1990s was 35.3 months (95% CI: 27.4 to 43.2), compared to 41.9 months (95% CI: 34.0 to 49.9) in the 2000s (P<0.01).
Table 3.
Mean overall survival and progression-free survival for various characteristics based on Kaplan–Meier analysis
| Overall survival (n=329)
|
Progression-free survival (n=207)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Number of individuals | Number of events | Mean (95% CI) | p value | Number of individuals | Number of events | Mean (95% CI) | p value | |
| BMI | ||||||||
| Normal | 106 | 91 | 36.5 (24.6–48.4) | 0.47 | 66 | 54 | 37.6 (21.2–54.0) | 0.25 |
| Overweight | 119 | 94 | 49.5 (36.5–62.6) | 76 | 54 | 51.9 (34.0–69.7) | ||
| Obese | 104 | 81 | 41.0 (28.8–53.2) | 65 | 44 | 55.4 (37.6–73.3) | ||
| Smoking status | ||||||||
| Never | 101 | 71 | 61.2 (45.6–76.8) | <0.01 | 66 | 42 | 65.3 (45.1–85.5) | 0.02 |
| Former | 118 | 106 | 29.2 (20.6–37.7) | 73 | 62 | 33.8 (20.9–46.1) | ||
| Current | 62 | 45 | 53.1 (32.9–73.3) | 45 | 29 | 58.0 (31.8–84.3) | ||
| Age | ||||||||
| <60 years | 178 | 119 | 69.0 (55.7–82.3) | <0.01 | 126 | 75 | 75.0 (57.9–92.1) | <0.01 |
| ≥60 years | 151 | 147 | 20.8 (15.1–26.5) | 81 | 77 | 22.9 (14.5–31.2) | ||
| Sex | ||||||||
| Female | 141 | 107 | 53.0 (39.9–66.2) | 0.10 | 92 | 62 | 63.6 (43.5–79.7) | 0.10 |
| Male | 188 | 159 | 37.9 (29.0–46.9) | 115 | 90 | 41.8 (28.9–54.7) | ||
| Cytogenetics | ||||||||
| Intermediate | 144 | 112 | 51.4 (39.4–63.7) | <0.01 | 103 | 77 | 51.8 (36.4–67.2) | <0.01 |
| Unfavorable | 114 | 101 | 27.8 (17.3–38.3) | 62 | 51 | 32.7 (16.7–48.8) | ||
| Favorable | 25 | 10 | 124.2 (90.9–157.5) | 22 | 7 | 117.8 (76.2–159.5) | ||
| Unknown | 32 | 29 | 25.6 (14.2–37.0) | 17 | 14 | 25.3 (13.5–37.0) | ||
| WBC count | ||||||||
| <100×109/L | 296 | 240 | 44.7 (36.6–52.8) | 0.24 | 186 | 138 | 50.0 (38.7–61.4) | 0.61 |
| ≥100×109/L | 33 | 26 | 37.8 (17.3–58.2) | 21 | 14 | 52.7 (23.3–82.1) | ||
| AML presentation | ||||||||
| De novo | 229 | 147 | 54.3 (44.1–64.5) | <0.01 | 147 | 99 | 57.2 (43.6–70.9) | <0.01 |
| Secondary | 100 | 75 | 21.8 (13.1–30.5) | 37 | 34 | 25.5 (9.9–41.1) | ||
| Treatment | ||||||||
| 1990–1999 | 213 | 193 | 35.3 (27.4–43.2) | <0.01 | 129 | 111 | 38.8 (27.6–50.0) | <0.01 |
| 2000–2008 | 116 | 73 | 41.9 (34.0–49.9) | 78 | 41 | 46.7 (36.4–57.1) | ||
Mean survival and 95% CI were calculated from Kaplan–Meier analyses of overall survival time in months; p values based on log rank (Mantel–Cox) test of equality between different factor levels of each covariate. Boldface entries represent p values significant at P<0.05
CI confidence interval, WBC white blood cell count at diagnosis, AML acute myeloid leukemia
Fig. 1.
a Curves based on Kaplan-Meier analyses assessing differences in overall survival by BMI status at diagnosis, excluding respondents who underwent allogeneic transplantation. Normal weight: n=97 (n=14 censored); Overweight: n=105 (n=18 censored); Obese: n=93 (n=19 censored). The + sign reflects censored patients. b Curves based on Kaplan-Meier analyses assessing differences in progression free survival by BMI status at diagnosis, excluding respondents who underwent allogeneic transplantation. Normal weight: n=57 (n=11 censored); Overweight: n=62 (n=15 censored); Obese: n=54 (n=17 censored). The + sign reflects censored patients
In multivariate analysis, age≥60 (p=0.004), unfavorable karyotypes (p<0.001), WBC count≥100×109/L (p=0.018), secondary presentation (p=0.047), and smoking history (former: p=0.001; current: p=0.048) had adverse impact on OS. There were no statistically significant differences in OS for either OW patients (HR=0.88, 95% CI: 0.62–1.23, p=0.446) or OB patients (HR=1.04, 95% CI: 0.73–1.46, p=0.845) compared to NL patients. Similarly, no statistically significant differences in PFS were observed for either OW patients (HR=0.89, 95% CI: 0.58–1.36, p=0.582) or OB patients (HR=0.78, 95% CI: 0.50–1.23, p=0.284) in a multivariate analysis. However, a positive smoking history (former: p=0.004) and unfavorable karyotype (p=0.002) remained detrimental to PFS. Diagnosis after 2000 was associated with longer PFS (HR=0.60, 95% CI: 0.38–0.94, p=0.026) but did not affect OS (HR=0.78, 95% CI: 0.55–1.12, p=0.179). In the multivariate analysis, no interactions between BMI and various patient characteristics were observed on either PFS or OS (Table 4).
Table 4.
Multivariate analysis for OS (n=234) and progression-free survival (n=148) by selected characteristics based on Cox proportional hazard modeling excluding 34 patients receiving an allogeneic transplant
| Characteristic | Overall survival
|
Progression-free survival
|
||||
|---|---|---|---|---|---|---|
| Number | HR (95% CI) | p value | Number | HR (95% CI) | p value | |
| BMI | ||||||
| Normal | 75 | 1.00 | 46 | 1.00 | ||
| Overweight | 80 | 0.94 (0.65–1.35) | 0.733 | 55 | 0.95 (0.60–1.52) | 0.830 |
| Obese | 79 | 1.16 (0.80–1.67) | 0.442 | 47 | 0.85 (0.51–1.42) | 0.532 |
| Smoking status | ||||||
| Never | 83 | 1.00 | 50 | 1.00 | ||
| Former | 101 | 1.56 (1.10–2.20) | 0.012 | 62 | 1.72 (1.07–2.75) | 0.024 |
| Current | 50 | 1.31 (0.84–2.05) | 0.234 | 36 | 1.32 (0.72–2.43) | 0.378 |
| Gender | ||||||
| Female | 99 | 1.00 | 63 | 1.00 | ||
| Male | 135 | 1.05 (0.77–1.45) | 0.757 | 85 | 1.19 (0.77–1.84) | 0.429 |
| Age | ||||||
| <60 years | 126 | 1.00 | 85 | 1.00 | ||
| ≥60 years | 108 | 1.52 (1.05–2.21) | 0.028 | 63 | 1.25 (0.77–2.02) | 0.371 |
| Presentation | ||||||
| De novo | 169 | 1.00 | 118 | 1.00 | ||
| Secondary | 65 | 1.36 (0.97–1.89) | 0.073 | 30 | 1.12 (0.69–1.81) | 0.662 |
| WBC count | ||||||
| <100×109/L | 210 | 1.00 | 134 | 1.00 | ||
| ≥100×109/L | 24 | 2.20 (1.37–3.55) | 0.001 | 14 | 2.22 (1.15–4.30) | 0.018 |
| Karyotype | ||||||
| Intermediate | 105 | 1.00 | 74 | 1.00 | ||
| Unfavorable | 86 | 2.01 (1.45–2.79) | <0.001 | 42 | 1.73 (1.10–2.73) | 0.017 |
| Favorable | 23 | 0.41 (0.20–0.83) | 0.014 | 20 | 0.32 (0.13–0.75) | 0.009 |
| Unknown | 20 | 1.09 (0.64–1.87) | 0.744 | 12 | 1.13 (0.55–2.32) | 0.740 |
| Decade of treatment | ||||||
| 1990–1999 | 144 | 1.00 | 93 | 1.00 | 0.015 | |
| 2000–2008 | 90 | 0.73 (0.50–1.07) | 0.110 | 55 | 0.53 (0.32–0.89) | |
Thirty four allogeneic transplant patients were excluded from the model. Gender, age, AML presentation, WBC count, karyotype, and smoking status were entered into the model concurrently to assess hazard ratio. P value based on Cox proportional hazard modeling after adjusting for all other covariates. Boldface entries represent statistically significant hazard ratios compared to referent group (p<0.05)
AML acute myeloid leukemia, CI confidence interval, WBC white blood cell
Discussion
Obesity has been associated with an increased risk of developing adult AML, but no survival data have been systematically studied [1, 10–12]. Establishing an association between obesity and outcomes could have significant implications for AML management [13]. We observed no association between BMI and outcome in adults with AML. However, we did see a trend toward improved survival for OW young (18–39 year old) populations (P=0.07). This may represent the limitations of BMI as a predictive tool since many AML patients will likely be in the OW group [14].
Specifically, BMI makes no distinction between muscles versus fat mass, and therefore, muscular individuals can be categorized in a higher BMI status. Other methods to assess obesity status are waist circumference [16–18], neck circumference [19, 20], and hip-to-waist ratio [21]. All have been used to predict outcome. For example, a 20% increase in the risk of colon cancer recurrence or mortality was observed for every 10 cm increase in waist circumference [18]. This may represent improved classification complimentary to the BMI that is in current use. Furthermore, sensitivity analyses using an age- and gender-weighted BMI classification tool based on NHANES-1 classifications were set forth by Must et al [15] but were not widely adopted.
Our results are in contrast to those observed in pediatric AML. Specifically, a retrospective pediatric study showed poor outcome in obese and underweight children treated on Children’s Cancer Group-2961 (CCG-2961) [5]. Both underweight and overweight children with AML had increased treatment toxicities and decreased OS. However, this study utilized a pediatric scale, overweight (>95th percentile) or underweight (<1.0 percentile), and not the BMI WHO classification, which may explain the discrepancy between our report and CCG-2961. Furthermore, obesity is related to incidence, complications, and prognosis in acute promyelocytic leukemia [10, 22–24], regardless of age. The reason for these discrepancies is not clear.
However, our results are similar to a large retrospective study in the transplant setting of AML patients [25]. In that study encompassing 4,215 patients, BMI was not an adverse prognostic indicator for outcome, regardless of donor source.
Cardiac and pulmonary complications in obese populations have been extensively studied, and obesity’s negative impact was clearly documented in patients with non-malignant diseases [26, 27]. Infectious complications can be increased due to decreased mobility, and glucose intolerance due to obesity. In our study, there was a statistical difference in pulmonary complications between OW vs. OB and OW vs. NL populations. However, this difference did not appear to be a consistent finding throughout different grades of pulmonary toxicities in the different BMI categories. Of note, we detected a trend toward OS advantage in the young (18–39 years old) OW population, which may have been due to less pulmonary toxicity in this group.
We observed that patients diagnosed and treated in the 1990s had a significantly inferior OS compared to >2000s. When stratified by BMI status, OS was significantly longer in the NL and OB groups treated after 2000 but, interestingly, not in the OW group. We suspect that the survival benefits most likely represent improvements in supportive care and possibly allogeneic stem cell transplantation methods. This progressive chronologic survival improvement benefit was seen previously in a large AML population study in Sweden where all ages, except patients older than 80 years, had survival benefits with progressive chronologic time points [28].
A major caveat of this study is the sample size, compared to studies conducted in stem cell transplantation and pediatric AML. However, AML is a rare disease, and this sample size was sufficient to detect differences in other well-accepted AML prognostic factors such as age, WBC count, karyotype, etc., suggesting that if indeed BMI had a role, we should have been able to detect it. Another caveat of this study is its retrospective design and the need to collect data from physician notes within each patient’s medical chart.
Interestingly, patients who were former smokers had worse survival outcomes compared to current smokers. A possible explanation for this phenomenon is that patients who quit smoking did so because of significant deterioration of their health prior to AML diagnosis such as pulmonary complications. One of the limitations of the smoking data is the lack of temporality in assessing time since smoking cessation as the current data only assessed smoking status at diagnosis. Therefore, it is possible that the “former smokers” continued smoking until just prior to diagnosis or current smokers quit just after diagnosis with AML. Although this limitation of our data is likely to result in misclassification, smoking status was not the main exposure of interest studied in this manuscript.
In summary, obesity has been shown to adversely affect outcome in pediatric AML [5] and acute promyelocytic leukemia [39] but not in adult AML patients, based on our results. Therefore, we conclude that BMI is not a poor prognostic factor in AML and high BMI should not be a barrier for intensive induction chemotherapy.
Acknowledgments
This work was supported partially by grants from the National Cancer Institute Grant CA16056 (HJL, ASL, AJH, LAF, SNJS, AWB, MB, MRB, ESW, MW), the Szefel Foundation, Roswell Park Cancer Institute (ESW), the Nancy C. Cully Endowment for Leukemia Research (MW), and the Heidi Leukemia Research Fund, Buffalo, NY (MW).
Contributor Information
Hun Ju Lee, Leukemia Section, Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA.
Andrea S. Licht, Department of Health Behavior, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
Andrew J. Hyland, Department of Health Behavior, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
Laurie A. Ford, Leukemia Section, Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
Sheila N. J. Sait, Clinical Cytogenetics Laboratory, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
AnneMarie W. Block, Clinical Cytogenetics Laboratory, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
Maurice Barcos, Department of Pathology, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA.
Maria R. Baer, Leukemia Section, Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA. University of Maryland Greenebaum Cancer Center, Baltimore, MD, USA
Eunice S. Wang, Leukemia Section, Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA
Meir Wetzler, Email: meir.wetzler@roswellpark.org, Leukemia Section, Department of Medicine, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, USA.
References
- 1.Calle EE, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15 (10):1083–1101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter RJ, et al. Dosing chemotherapy in obese patients: actual versus assigned body surface area (BSA) Cancer Treat Rev. 2009;35(1):69–78. doi: 10.1016/j.ctrv.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Sparreboom A, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25(30):4707–4713. doi: 10.1200/JCO.2007.11.2938. [DOI] [PubMed] [Google Scholar]
- 5.Lange BJ, et al. Mortality in overweight and underweight children with acute myeloid leukemia. JAMA. 2005;293(2):203–211. doi: 10.1001/jama.293.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Lange BJ, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood. 2008;111 (3):1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 10.Larsson SC, Wolk A. Overweight and obesity and incidence of leukemia: a meta-analysis of cohort studies. Int J Cancer. 2008;122(6):1418–1421. doi: 10.1002/ijc.23176. [DOI] [PubMed] [Google Scholar]
- 11.Ross JA, et al. Body mass index and risk of leukemia in older women. Cancer Epidemiol Biomarkers Prev. 2004;13(11 Pt 1):1810–1813. [PubMed] [Google Scholar]
- 12.Tedesco J, et al. High prevalence of obesity in acute promyelocytic leukemia (APL): implications for differentiating agents in APL and metabolic syndrome. Ther Adv Hematol. 2011;2(3):141–145. doi: 10.1177/2040620711408490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang A, et al. Obesity and weight control practices in 2000 among veterans using VA facilities. Obes Res. 2005;13(8):1405–1411. doi: 10.1038/oby.2005.170. [DOI] [PubMed] [Google Scholar]
- 14.Obesity trends among US adults. CDC; 2009. [Google Scholar]
- 15.Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) and triceps skinfold thickness. Am J Clin Nutr. 1991;53(4):839–846. doi: 10.1093/ajcn/53.4.839. [DOI] [PubMed] [Google Scholar]
- 16.Appel SJ, Harrell JS, Davenport ML. Central obesity, the metabolic syndrome, and plasminogen activator inhibitor-1 in young adults. J Am Acad Nurse Pract. 2005;17(12):535–541. doi: 10.1111/j.1745-7599.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 17.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55 (2):285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haydon AM, et al. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busetto L, et al. Obstructive sleep apnea syndrome in morbid obesity: effects of intragastric balloon. Chest. 2005;128(2):618–623. doi: 10.1378/chest.128.2.618. [DOI] [PubMed] [Google Scholar]
- 20.Hatipoglu N, et al. Neck circumference: an additional tool of screening overweight and obesity in childhood. Eur J Pediatr. 2010;169(6):733–739. doi: 10.1007/s00431-009-1104-z. [DOI] [PubMed] [Google Scholar]
- 21.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56(5):303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 22.Estey E, et al. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661–1664. doi: 10.1038/sj.leu.2400783. [DOI] [PubMed] [Google Scholar]
- 23.Jeddi R, et al. High body mass index is an independent predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leuk Res. 2010;34(4):545–547. doi: 10.1016/j.leukres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Jeddi R, et al. Predictive factors of all-trans-retinoic acid related complications during induction therapy for acute promyelocytic leukemia. Hematology. 2008;13(3):142–146. doi: 10.1179/102453308X316112. [DOI] [PubMed] [Google Scholar]
- 25.Navarro WH, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transpl. 2010;16(10):1442–1450. doi: 10.1016/j.bbmt.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque AK, et al. Pulmonary and cardiovascular complications of obesity: an autopsy study of 76 obese subjects. Arch Pathol Lab Med. 2008;132(9):1397–1404. doi: 10.5858/2008-132-1397-PACCOO. [DOI] [PubMed] [Google Scholar]
- 27.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87(4):407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 28.Derolf AR, et al. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113 (16):3666–3672. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]