Abstract
IMRT and helical tomotherapy for head and neck cancer (HNC) treatment are associated with higher doses to certain non-target tissues than traditional static beam techniques. We hypothesized that this may lead to higher acute mucosal and hematologic toxicities. This analysis was limited to 178 patients receiving ≥ 60 Gy with concurrent weekly cisplatin. Radiation delivery used 3D-CRT in 41 patients (23%), conventional IMRT in 56 patients (31%), and helical tomotherapy in 81 patients (46%). Acute mucositis rates, weekly hematologic parameters, and ability to deliver planned chemotherapy cycles were examined for each patient during their course of chemoradiotherapy. Analysis showed patients were well balanced with regard to sex, age, and stage. Treatment time, as assessed by delivered monitor units, varied significantly between the 3D-CRT (median=502), IMRT (median=1087), and tomotherapy (median=6757) cohorts. Acute mucositis grades did not significantly differ between the three subsets. Through six weeks of chemoradiotherapy, the median decline in hemoglobin was 15.6%, the median decline in platelets was 30.6%, and the median decline in leukocytes was 51.5%, but these drops were not significantly different between treatment cohorts. Chemotherapy was discontinued or held secondary to hematologic toxicity in 12% of 3D-CRT patients, 5% of IMRT patients and 15% of tomotherapy patients (p=0.14). In conclusion, HNC patients undergoing high dose radiation with concurrent weekly cisplatin chemotherapy, the longer beam-on times and larger volumes of low-to-moderate radiation doses to non-target tissues associated with modern IMRT delivery techniques do not appear to result in increased acute hematologic or mucosal toxicities.
Keywords: Mucositis, neutropenia, IMRT, helical tomotherapy, head and neck cancer
Introduction
Radiotherapy plays a central role in the management of head and neck squamous cell carcinoma (HNSCC). In recent years concomitant chemoradiotherapy has become the standard of care for patients with locoregionally-advanced disease who are fit for aggressive treatment and in whom non-operative management is preferred. Based on studies from 1965-1993, the MACH-NC meta-analysis demonstrated an absolute survival benefit of 4% at 5 years with the addition of chemotherapy for patients undergoing radiotherapy (1). The recently updated meta-analysis by this group demonstrated that concurrent chemoradiotherapy can improve overall survival by 6.5%, and showed that these benefits extend to the postoperative setting for high-risk patients (2). However, these benefits come at the expense of an approximate doubling of grade 3 or greater acute toxicities (3, 4); worsening of mucositis and hematologic toxicities account for the vast majority of these more prevalent toxicities.
In parallel with the common incorporation of concomitant chemotherapy during HNSCC radiotherapy, significant progress in radiotherapy delivery has been realized. Intensity-modulated radiotherapy (IMRT), through inverse planning algorithms that optimize treatment field design and deliver variable beam intensity, has the ability to limit dose to normal structures during HNSCC radiotherapy (5). These efforts have primarily focused on minimizing late radiation-induced xerostomia by sparing parotid glands. However, low-to-moderate doses to selected non-target tissues are commonly increased in IMRT plans for HNSCC, and can increase acute toxicities when compared to 3D-CRT, including fatigue (6), mucositis (7), and alopecia (8). Additional work has demonstrated that the addition of concurrent chemotherapy may influence the relative merit of IMRT and helical tomotherapy radiation plans in comparison to 3D-CRT plans (9), by lowering the tolerance threshold of normal tissues to low-to-moderate radiation dose. These studies demonstrate that certain toxicities beyond xerostomia may be greater in patients undergoing IMRT versus traditional radiotherapy, particularly in the setting of concurrent chemotherapy. The impact of low-dose radiation on acute toxicities in HNSCC chemoradiotherapy has not yet been well characterized.
At the University of Wisconsin, LINAC-based IMRT was instituted in 2001 for head and neck cancer radiation delivery. Subsequently, helical tomotherapy was developed at our institution (10) and became the predominant method of IMRT delivery in 2005. These advanced delivery methods require longer beam-on times, and are associated with larger volumes of tissue exposed to low-dose radiation. We hypothesized that the prolonged radiation exposure and more prominent distribution of low-dose radiation could potentially have an adverse impact on hematocytes as they circulate through the head and neck vasculature in patients receiving IMRT, and/or increase mucositis as compared to 3D-CRT in patients undergoing chemoradiotherapy.
Materials and Methods
Patients
An institutional review board (IRB)-approved prospective database was maintained detailing records of all head and neck cancer patients at the University of Wisconsin from 1992 onward. From this database, 281 patients with stage III-IVb HNSCC who received concurrent chemotherapy were identified. Patients with SCC tumors of the nasopharynx, oropharynx, oral cavity, larynx, hypopharynx, and unknown primary were included, whether treated with definitive chemoradiotherapy or with postoperative chemoradiotherapy. For improved uniformity, only patients receiving radiation doses ≥ 60 Gy with concurrent weekly cisplatin at 30 mg/m2 (a common off protocol institutional standard (11) were included for study. Between 2001 and 2010, 178 patients met these criteria and were analyzed.
Treatment details
All patients underwent full pretreatment evaluation including either direct or fiberoptic visualization of the oral cavity, oropharynx, nasopharynx, hypopharynx, and larynx, dental evaluation, radiographic imaging of the head and neck, primarily consisting of computed tomography (CT) with magnetic resonance imaging (MRI) for selected patients, radiographic imaging of the chest (X-ray or CT), and complete blood counts.
Conventional 3D-CRT
Patients underwent a planning CT with intravenous contrast in our department, with customized Aquaplast masks (Aquaplast, Wyckoff, NJ) for immobilization. Planning CTs were obtained with 2.5 mm slices, and treatment planning was then performed on the Pinnacle3 treatment-planning system (Philips Radiation Oncology Systems, Fitchburg, WI). The 3D-CRT treatments comprehensively covered the primary tumor bed and draining cervical lymphatics and supraclavicular regions, with opposed lateral beams matched to a half-beam blocked AP supraclavicular field with appropriate spinal cord shielding. Six MV photons were utilized with a shrinking-field technique, with 6-9 MeV electrons to boost level V nodal stations as appropriate after off-cord photon field reductions.
LINAC-based IMRT and helical tomotherapy IMRT
Treatment planning for patients receiving IMRT used the same positioning and immobilization procedures as outlined above. For tomotherapy plans, all volumes were contoured in Pinnacle and then transferred into the TomoTherapy Hi-Art treatment planning system (TomoTherapy, Madison, WI). Patients receiving LINAC-based 7-field step-and-shoot IMRT were fitted with a custom maxillofacial bite tray to which an infrared light reflecting fiducial array was attached to facilitate high precision localization and delivery of treatments using dily optical guidance as described in (12). Patients treated with helical tomotherapy IMRT underwent daily megavoltage CT treatment scans for position verifcations and adjustment as needed before each radiation treatment delivery. The high-risk clinical target volume (CTV1) typically encompassed the GTV plus a margin, and included the high risk draining regional lymphatics. In patients with unilateral neck metastasis, the contralateral N0 neck was designated as a low-risk CTV2. An additional lower-risk CTV (CTV3) was created when appropriate for the contralateral low neck and supraclavicular lymph nodes for patients treated with helical tomotherapy; the low neck and supraclavicular lymph nodes were commonly treated with an AP field for patients receiving LINAC-based IMRT. A CTV to planning target volume (PTV) margin of 3 mm was utilized to accommodate organ motion and setup error. Differential doses were assigned, with gross disease receiving 70 Gy to the tumor PTV, high-risk nodal PTV1 receiving 60 Gy, lower-risk PTV2 receiving 54 Gy, and lowest-risk PTV3 (when utilized) receiving 50 Gy. Treatments were delivered with 6 MV photons.
Chemotherapy
Patients received intravenous cisplatin at 30 mg/m2 weekly throughout the course of radiation. Hematologic surveys (hemoglobin, hematocrit, platelet count, and white blood cell count without differential) and serum creatinine levels were monitored weekly throughout treatment, with cisplatin held if the white blood cell count was ≤ 1500/μL or platelets fell ≤ 100,000, or for any grade 3 non-hematologic toxicity occurring during the preceding week. Bone-marrow stimulating drugs (i.e. epoetin, darbepoetin, filgastrim, pegfilgastrim) were not used during weekly cisplatin administration.
Outcomes and analyses
Patient and treatment factors were analyzed, and outcomes including mucositis grade, chemotherapy discontinuation, and narcotic usage were identified from the database. Total monitor units for each radiation plan were recorded. Hematologic parameters, including WBC, hemoglobin, and platelets, were analyzed from the medical record prior to chemotherapy administration (baseline) and weekly throughout the chemoradiotherapy course. In cases of chemotherapy discontinuation, medical record review was performed to assess whether discontinuation was for hematologic toxicity or non-hematologic reasons. For continuous variables, univariate analysis of variance (ANOVA) testing was performed to assess differences between treatment groups. Where significant differences were detected, post hoc comparisons between subsets were performed via the Bonferroni method. Chi-squared tests were utilized to compare categorical variables.
Results
Baseline patient characteristics are summarized in Table I. The three treatment cohorts were well balanced for age, sex, and stage. T stage was T0 in 12 patients (6.7%), T1 in 29 patients (16.3%), T2 in 58 patients (32.6%), T3 in 45 patients (25.3%), and T4 in 34 patients (19.1%); these distributions did not differ significantly between treatment groups. AJCC TNM stage was IVa-b in 157 of 178 patients (88.2%) and did not differ between the radiation delivery treatment groups. Tumors were predominantly of the oropharynx (n=108; 60.7%), with the remainder of tumors from the larynx (n=26), hypopharynx (n=13), unknown primary (n=12), nasopharynx (n=10), and oral cavity (n=10); these distributions did not differ significantly between radiation delivery treatment groups. Patients treated with helical tomotherapy had slightly lower baseline hemoglobin (p=0.055) and white blood cell counts (p=0.015) than patients treated with 3D-CRT or LINAC-IMRT (Table I).
Table I.
Patient and baseline hematologic parameters in patients undergoing chemoradiotherapy, stratified by radiation delivery modality.
| Variable | 3D-CRT | LINAC-IMRT | Tomotherapy | P |
|---|---|---|---|---|
| Number of patients | 41 | 56 | 81 | -- |
| Median Age (Range, years) | 54 (39-78) | 53 (36-77) | 57 (39-77) | 0.30 |
| Sex (% male) | 75.6% | 83.9% | 75.3% | 0.44 |
| Baseline hemoglobin (mean) | 14.0 | 14.2 | 13.7 | 0.055 |
| Baseline white blood cell count (mean) | 7.7 | 8.6 | 7.1 | 0.015 |
| Baseline platelets (mean) | 255,000 | 255,500 | 261,100 | 0.912 |
3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
Significant differences in radiation treatment parameters were noted across the three treatment groups (Table II). Total monitor units delivered increased markedly between the 3D-CRT, IMRT, and helical tomotherapy subsets. Postoperative patients managed with chemoradiation comprised a larger segment of the helical tomotherapy cohort as compared to either the 3D-CRT or LINAC-IMRT groups. As such, mean radiation dose was slightly lower in the helical tomotherapy subset as compared to the other two groups. Fraction number varied significantly in our subsets, due to the use of simultaneous integrated boosts in the IMRT-treated patients, and total number of days in the radiation course similarly showed a significant difference across subsets.
Table II.
Radiation parameters in patients undergoing chemoradiotherapy stratified by radiation delivery modality.
| Variable | 3D-CRT | LINAC-IMRT | Tomotherapy | P |
|---|---|---|---|---|
| Monitor units (median; range) | 502 (253-572) | 1087 (500-1578) | 6703 (4783-11221) | <0.001 |
| Postoperative treatment | 14.6% | 5.4% | 34.6% | <0.001 |
| Mean radiation dose (Gy) | 69.8 | 69.2 | 68.4 | 0.031 |
| Fraction number (mean) | 39.2 | 33.0 | 32.4 | <0.001 |
| Total days (mean) | 45.7 | 46.2 | 42.9 | 0.007 |
3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
Hematologic dynamics during chemoradiotherapy are depicted in Figures 1-3, presented as a percentage of baseline to account for any pre-therapy differences. The median decline in hemoglobin was 15.6% through six weeks of chemoradiotherapy, with no significant differences between radiation delivery subsets (Figure 1). Across all patients, the median decline in leukocytes was 51.5%, with no significant differences noted between treatment subsets (Figure 2). The median decline in platelet count was 30.6% in patients undergoing chemoradiotherapy, with no significant differences between radiation delivery modalities (Figure 3).
Figure 1.
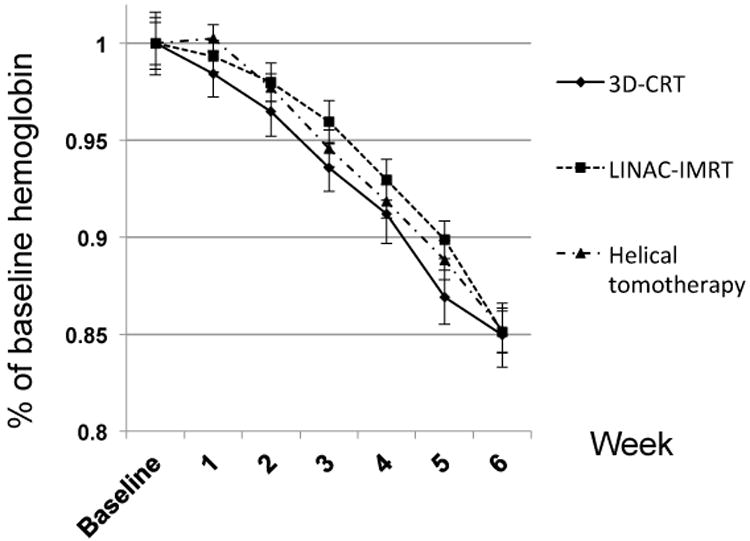
Dynamics of hemoglobin during chemoradiotherapy for head and neck cancer, stratified by radiation delivery modality. 3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
Figure 3.
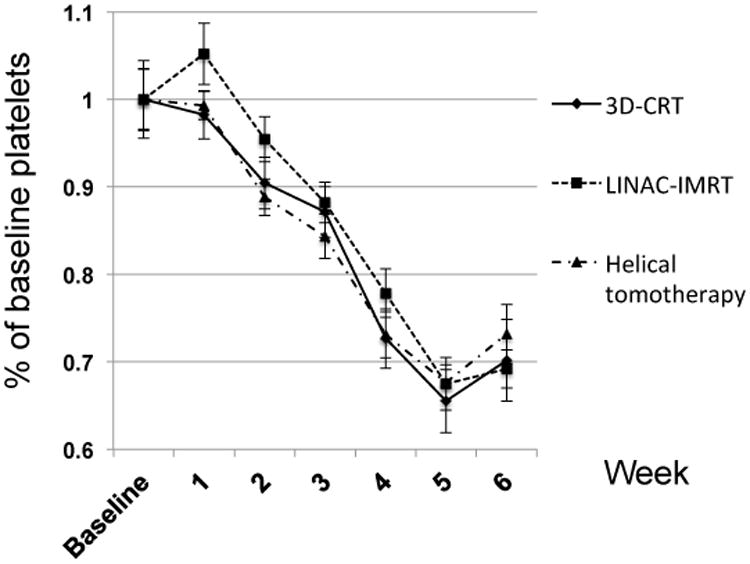
Dynamics of platelet counts during chemoradiotherapy for head and neck cancer, stratified by radiation delivery modality. 3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
Figure 2.
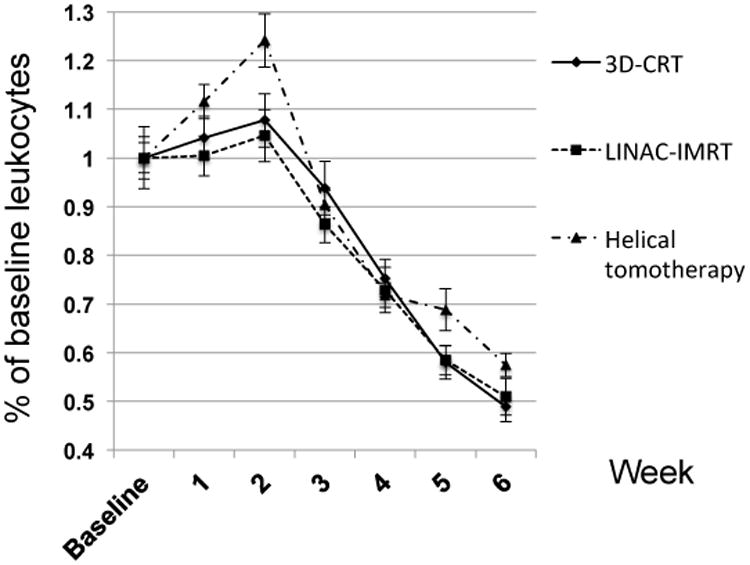
Dynamics of leukocyte counts during chemoradiotherapy for head and neck cancer, stratified by radiation delivery modality. 3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
The average number of cycles of chemotherapy delivered across all 178 patients was 5.6, with no differences in the number of cycles delivered between treatment groups (Table III). Chemotherapy was discontinued early in 29.8% of all patients, with no differences detected between treatment arms. Hematologic toxicities caused chemotherapy to be held or discontinued in 11.2% of patients, with no differences between treatment subsets noted.
Table III.
Chemotherapy parameters, hematologic, and mucosal toxicity rates in patients undergoing chemoradiotherapy stratified by radiation delivery modality.
| Variable | 3D-CRT | LINAC-IMRT | Tomotherapy | P |
|---|---|---|---|---|
| Cycles of chemotherapy | 5.4 | 5.6 | 5.7 | 0.39 |
| Chemotherapy discontinued | 22.0% | 30.4% | 33.3% | 0.27 |
| Chemotherapy held due to hematologic Toxicity | 12.2% | 5.4% | 14.8% | 0.14 |
| Mucositis grade (mean) | 2.7 | 2.5 | 2.6 | 0.20 |
| Narcotic Usage | 94.7% | 94.5% | 97.1% | 0.74 |
3D-CRT=3-dimensional conformal radiotherapy; LINAC-IMRT=linear-accelerator based intensity-modulated radiotherapy.
Additionally, no differences in mean mucositis grades or narcotic usage were detected between arms (Table III). To analyze these differences in a more homogeneous subgroup of patients, mucositis rates and narcotic usage were further assessed within oropharynx patients (n=108). Among oropharynx patients treated with 3D-CRT, grade 2 mucositis occurred in 12.5% and grade 3 in 83.3%; among LINAC-IMRT patients grade 2 mucositis occurred in 40% and grade 3 in 57.5%; and among helical tomotherapy-treated patients grade 2 mucositis occurred in 30% and grade 3 in 70%. These rates were not significantly different by χ2 analysis (p=0.149). Furthermore, narcotic usage rates in oropharynx patients were not different between 3D-CRT patients (100%), LINAC-IMRT patients (95%) or helical tomotherapy (100%) (p=0.228).
Discussion
Helical tomotherapy and LINAC-based IMRT deliver significantly higher monitor units than 3D-CRT in patients undergoing chemoradiotherapy for HNSCC, and are known to be associated with higher volumes of low-dose radiation to normal tissues beyond conventional treatment portals than 3D-CRT. IMRT utilization rates in HNSCC continue to rise, from 14% in 2002 to 53% in 2005 (13), and higher in 2012; however, IMRT itself has not been demonstrated to improve survival outcomes in HNSCC compared to 3D-CRT in SEER-medicare database reviews. Therefore, consideration of normal tissue toxicities, including and beyond salivary glands, in IMRT HNSCC radiotherapy warrants careful consideration. We demonstrate that acute hematologic and mucosal toxicities are similar between HNSCC patients undergoing high-dose chemoradiotherapy across patients receiving radiotherapy from 3D-CRT, LINAC-IMRT, and helical tomotherapy techniques. More advanced radiotherapy techniques did not worsen mucositis rates, reduce number of chemotherapy cycles delivered, or exacerbate acute hematologic toxicities, but also were not associated with improved outcomes in these toxicity measures as compared to 3D-CRT.
During the period of study, differences in chemoradiotherapy utilization and delivery were detectable in this analysis. Based on two randomized studies demonstrating benefits to concurrent chemoradiotherapy as compared to radiotherapy in the postoperative setting (3, 4), the subset of patients treated with helical tomotherapy (2005 onward) in this analysis included more postoperative patients that the 3D-CRT or LINAC-IMRT subsets. Likewise, the utilization of simultaneous integrated boosts with more advanced radiation delivery results in a significant reduction in the number of fractions, and shortened overall radiation course length, as compared to patients receiving 3D-CRT. Despite these differences detected in this analysis, the cumulative doses of chemotherapy delivered to patients undergoing chemoradiotherapy were remarkably similar when assessed by radiation delivery modality, suggesting that advanced radiation delivery techniques do not necessarily promote HNSCC therapy intensification by allowing improved delivery of chemotherapy.
Recent randomized clinical trial data demonstrates significant benefit in terms of salivary function preservation with the use of IMRT in HNSCC (6), providing strong rationale for the use of IMRT when parotid avoidance is feasible. However, fatigue was significantly more prevalent in the IMRT group than in the 3D-CRT group (74% vs 41%; p=0.0015), which the authors postulate might be due to greater radiation doses to non-tumor tissues such as the posterior fossa in IMRT-treated patients. Additionally, functional grade 3 mucositis/stomatitis was 60% in the IMRT arm, versus 44% in the 3D-CRT arm (no p-value reported). Likewise, patients receiving IMRT versus non-IMRT techniques on RTOG 0234 had higher rates of oral mucositis (45% vs. 26%; p=0.006), potentially related to increased volumes of tissue exposed to low-dose radiation in the presence of sensitizing chemotherapy (7). Dosimetric studies have demonstrated dose to oral mucosa can be higher using IMRT versus traditional field design without specific oral mucosa dose objectives (14); such increases in low-to-moderate doses can take on additional significance in the presence of concurrent chemotherapy. Chemotherapy has been demonstrated to increase the risk of mucosal Grade 3 toxicity approximately 4 times over radiation alone, and is equivalent to an additional 6.2 Gy to the oral mucosa (15). We did not observe differences in rates of mucositis between 3D-CRT patients and IMRT-treated patients. It has been our institutional policy to put a dose limitation of mean oral cavity dose to ≤ 30 Gy, which may contribute to the lack of increased oral mucositis in patients treated with IMRT in this study, as the mean dose to the oral cavity has been shown to be an important risk factor for oral mucositis development (16). While some groups, as above (7), have showed higher mucositis rates with IMRT as compared to non-IMRT techniques, and we have shown no difference between IMRT techniques and 3D-CRT, it warrants mentioning that other studies have demonstrated lower mucositis rates with IMRT in oropharyngeal patients. Al-Mamgani et al (17) demonstrated in a matched-pair analysis reduced rates of grade 3 acute toxicity with IMRT as compared to 3D-CRT, as well as lower rates of grade ≥2 late dysphagia and xerostomia with IMRT utilization. Therefore, continued investigations into the impact of low- to moderate- non-target radiation doses that accompany IMRT delivery in HNSCC remain important.
Radiation is a well-known cause of myelosuppression, and hematologic toxicity can be dose-limiting with concurrent cisplatin chemoradiotherapy. Lymphocytes are exquisitely radiosensitive, and radiation-induced lymphopenia can be long-lasting (18). Lymphopenia occurs rapidly as lymphocytes die in interphase following radiation exposure. Additionally, circulating blood contains up to 10% of blood progenitor cells (19), which may be sensitive to radiation when passing through the head and neck vasculature during radiation delivery. When small fields of bone marrow are radiated, unexposed bone marrow can compensate for inactivated marrow. However, concurrent chemotherapy can limit compensatory hematopoiesis (20). Despite these factors, the dramatically longer beam-on time associated particularly with helical tomotherapy, as compared to 3D-CRT, was not associated with increased acute hematologic toxicities in this analysis. The lack of complete blood counts with differentials limited our ability to assess whether differences in lymphopenia were present between these cohorts; nonetheless, clinically significant changes were undetectable.
Limitations to this study include the heterogeneous patient population in terms of tumor subsite. While no significant differences between treatment groups were detected, mucositis rates appreciated by clinicians can differ depending on the primary tumor subsite, and this may contribute to the lack of mucositis differences noted between treatment cohorts; additionally, DVH analyses were not performed in these patients. As mentioned above, complete blood counts with differentials were lacking in most patients. Differentials may have allowed specific analysis of lymphocytes, and may have enhanced the sensitivity of this analysis for differences in hematologic parameters between treatment cohorts, though clinically significant differences were not detected. Furthermore, additional acute toxicities such as fatigue, alopecia, and skin toxicity were not able to be assessed in this analysis, but these endpoints warrant consideration when assessing toxicities in IMRT-treated patients as compared to those treated with static-beam fields.
In conclusion, the longer beam-on times and larger volumes of low-to-moderate radiation doses to non-target tissues associated with modern IMRT delivery techniques do not appear to result in increased acute hematologic or mucosal toxicities in HNSCC patients undergoing high dose radiation with concurrent weekly cisplatin chemotherapy. However, prospective studies utilizing advanced radiation techniques in the setting of concurrent chemotherapy should assess possible toxicities from low-to-moderate radiation doses to tissues not classically affected by 3D conformal radiotherapy.
Acknowledgments
None
Disclosure of funding: KRK is supported in part by NIH Grant 1UL1RR25011, and has served as a consultant to SK Pharma, unrelated to this work
WAT has received grant aid and an honorarium from Philips Radiation Oncology Systems, and a grant from Accuary, is a Scientific Advisory board member for ViewRay Inc., and receives royalties from patents through the Wisconsin Alumni Research Fund (WARF), all unrelated to this work.
Footnotes
Conflicts of Interest: TJK, SRR, KPC, HMG, and PMH have no disclosures
References
- 1.Pignon JP, Bourhis J, Domenge C, Designe LI. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 2.Pignon JP, Ie Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 5.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 6.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. doi:S1470-2045(10)70290-4 [pii] 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khuntia D, Harris J, Bentzen SM, et al. Increased oral mucositis after IMRT versus non-IMRT when combined with cetuximab and cisplatin or docetaxel for head and neck cancer: Preliminary results of RTOG 0234. Int J Radiat Oncol Biol Phys. 2008;72(Suppl):S33. [Google Scholar]
- 8.Rosenthal DI, Chambers MS, Fuller CD, et al. Beam path toxicities to non-target structures during intensity-modulated radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;72:747–755. doi: 10.1016/j.ijrobp.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogelius IS, Westerly DC, Cannon GM, et al. Intensity-modulated radiotherapy might increase pneumonitis risk relative to three-dimensional conformal radiotherapy in patients receiving combined chemotherapy and radiotherapy: a modeling study of dose dumping. Int J Radiat Oncol Biol Phys. 2011;80:893–899. doi: 10.1016/j.ijrobp.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackie TR. History of tomotherapy. Phys Med Biol. 2006;51:R427–453. doi: 10.1088/0031-9155/51/13/R24. [DOI] [PubMed] [Google Scholar]
- 11.Traynor AM, Richards GM, Hartig GK, et al. Comprehensive IMRT plus weekly cisplatin for advanced head and neck cancer: the University of Wisconsin experience. Head & neck. 2010;32:599–606. doi: 10.1002/hed.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tome WA, Meeks SL, McNutt TR, et al. Optically guided intensity modulated radiotherapy. Radiother Oncol. 2001;61:33–44. doi: 10.1016/s0167-8140(01)00414-5. [DOI] [PubMed] [Google Scholar]
- 13.Yu JB, Soulos PR, Sharma R, et al. Patterns of Care and Outcomes Associated with Intensity-Modulated Radiation Therapy versus Conventional Radiation Therapy for Older Patients with Head-and-Neck Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 14.Sanguineti G, Endres EJ, Gunn BG, Parker B. Is there a “mucosa-sparing” benefit of IMRT for head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2006;66:931–938. doi: 10.1016/j.ijrobp.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 15.Sanguineti G, Sormani MP, Marur S, et al. Effect of radiotherapy and chemotherapy on the risk of mucositis during intensity-modulated radiation therapy for oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;83:235–242. doi: 10.1016/j.ijrobp.2011.06.2000. [DOI] [PubMed] [Google Scholar]
- 16.Werbrouck J, De Ruyck K, Duprez F, et al. Acute normal tissue reactions in head-and-neck cancer patients treated with IMRT: influence of dose and association with genetic polymorphisms in DNA DSB repair genes. Int J Radiat Oncol Biol Phys. 2009;73:1187–1195. doi: 10.1016/j.ijrobp.2008.08.073. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mamgani A, Van Rooij P, Tans L, et al. Toxicity and Outcome of Intensity-Modulated Radiotherapy versus 3-Dimensional Conformal Radiotherapy for Oropharyngeal Cancer: A Matched-Pair Analysis. Technol Cancer Res Treat. 2012 Oct 19; doi: 10.7785/tcrt.2012.500305. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Carr BI, Metes DM. Peripheral blood lymphocyte depletion after hepatic arterial 90Yttrium microsphere therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1179–1184. doi: 10.1016/j.ijrobp.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 20.Rose BS, Liang Y, Lau SK, et al. Correlation Between Radiation Dose to (18)F-FDG-PET Defined Active Bone Marrow Subregions and Acute Hematologic Toxicity in Cervical Cancer Patients Treated With Chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1185–1191. doi: 10.1016/j.ijrobp.2011.09.048. [DOI] [PubMed] [Google Scholar]


