Abstract
Natural gas hydrates are icy crystalline materials that contain hydrocarbons, which are the primary energy source for this civilization. The abundance of naturally occurring gas hydrates leads to a growing interest in exploitation. Despite their potential as energy resources and in industrial applications, there is insufficient understanding of hydrate kinetics, which hinders the utilization of these invaluable resources. Perturbation of liquid water structure by solutes has been proposed to be a key process in hydrate inhibition, but this hypothesis remains unproven. Here, we report the direct observation of the perturbation of the liquid water structure induced by amino acids using polarized Raman spectroscopy, and its influence on gas hydrate nucleation and growth kinetics. Amino acids with hydrophilic and/or electrically charged side chains disrupted the water structure and thus provided effective hydrate inhibition. The strong correlation between the extent of perturbation by amino acids and their inhibition performance constitutes convincing evidence for the perturbation inhibition mechanism. The present findings bring the practical applications of gas hydrates significantly closer, and provide a new perspective on the freezing and melting phenomena of naturally occurring gas hydrates.
Gas hydrates are ice-like solids in which gases are enclosed in a hydrogen-bonded water crystal lattice1, and have attracted worldwide attention as potential clean energy resources because of their natural abundance2. The amount of hydrocarbons stored in naturally occurring gas hydrates approaches that of all other fossil fuel reserves in the world combined3. Gas hydrates form under low temperature and high pressure conditions, so are usually found under marine sediments and permafrost. The Arctic region contains substantial natural gas and oil reserves4, as well as mineral resources and fisheries5, so the increased availability of sea routes has resulted in increased interest in Arctic exploration6.
Gas hydrate formation and inhibition phenomena are also important to gas and oil industry. Natural gas transportation lines often provide thermodynamic conditions that are favorable for gas hydrate formation7. In addition, a massive increase in the amount of CO2 emission from fuel combustions leads to a growing interest in CO2 transportation through pipelines. Unfortunately, this process sometimes causes pipeline blockages, which leads to unavoidable delays in operation, the need for repairs, and severe financial losses. A variety of hydrate prevention methods1 such as thermal heating, depressurization, and mechanical elimination have been suggested; one simple method is the injection of hydrate inhibitors1. The injection of thermodynamic hydrate inhibitors such as alcohols and glycols is regarded as a robust method, since these compounds permanently inhibit hydrate formation by directly changing the temperature and pressure conditions of formation. However, this method requires the injection of huge amounts of these compounds, so the use of kinetic hydrate inhibitors (KHIs) has been suggested as a promising alternative because they can delay hydrate nucleation and growth at much lower concentrations8. One significant issue for the utilization of KHIs is the prediction of when gas hydrates will nucleate, and then how quickly they will grow. Despite strenuous efforts, gas hydrate inhibition phenomena are still poorly understood because of their inherent complexity.
It was originally thought that KHIs adsorb directly onto the hydrate surface. The morphologies of hydrate crystals were found experimentally to be changed by the presence of KHIs such as polymers9,10, antifreeze proteins11, and quaternary ammonium zwitterions12. In addition, Monte Carlo13 and molecular dynamics14,15 simulations have been performed to investigate the formation of hydrogen bonds between oxygen species in KHIs and hydrogen on hydrate surfaces. However, other studies have disputed this mechanism16,17,18,19 because it was found that some KHIs do not make direct contact with hydrate surfaces. Several more recent studies have proposed that perturbation of the structure of liquid water by KHIs is another mechanism that can be used to interpret gas hydrate inhibition phenomena12,15. In general, such perturbation is a universal phenomenon in which a solute dissolved in water induces changes in the structure of water20,21,22,23. Simulation results have showed that the hydrophilic moieties of KHIs disrupt the structure of water and render it incompatible with the hydrate structure, and that this disruption is responsible for hydrate inhibition12. However, there have been almost no experimental assessments of this hypothesis. Our recent study proposed that the KHI abilities of some hydrophobic amino acids with respect to CO2 hydrate inhibition are attributed to the perturbation of the structure of the surrounding water24, but no experimental evidence for this proposal has yet been reported.
In this study, we directly observed the perturbation of the structure of liquid water by amino acids, and investigated the influence of this perturbation on CO2 hydrate nucleation and growth kinetics. The findings of this study provide strong experimental evidence in support of the hypothesis that perturbation plays a critical role in the inhibition of gas hydrate formation.
Results and discussion
Model amino acid system
The five amino acids alanine, aspartic acid, asparagine, phenylalanine, and histidine were tested as solutes (Table 1) as they have distinct side chains in their molecular structures. If we think of alanine as the basis of this system, aspartic acid, asparagine, phenylalanine, and histidine have additional carboxylic acid (−COOH), amide (−CONH2), phenyl (−C6H5), and imidazole (−C3H3N2) groups, respectively. The hydrophobicity, acidity, and solubility of an amino acid in water strongly depend on its side chain properties. Thus, we can vary the physicochemical properties of the test compound by simply selecting a suitable molecule.
Table 1. The physicochemical properties of the tested amino acids.
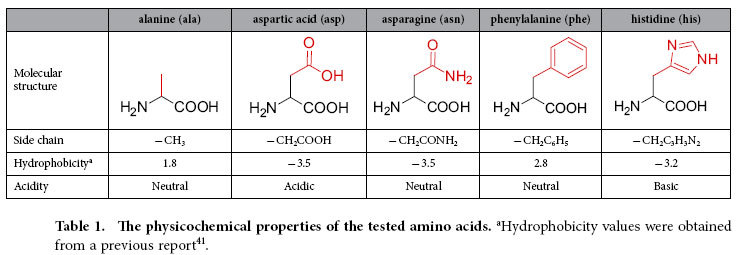
An important consideration in the investigation of the interactions of an amino acid side chain with water molecules is the charge distribution within the amino acid molecule. Although the carboxylic acid and amine groups that are commonly attached to the central carbon atoms of amino acids can form carboxylate and ammonium ions respectively, the electric charge distributions of such side chains are entirely different from those of the respective ions (see Supplementary Fig. S1). In particular, the hydrophilic nature of the side chains and their ability to form ionized or resonance structures induces polarization of the amino acids (Supplementary information). The molecular polarization of an amino acid is expected to increase the strength of its interactions with water molecules, and hence the extent of the perturbation of the structure of liquid water.
Perturbation of liquid water structure
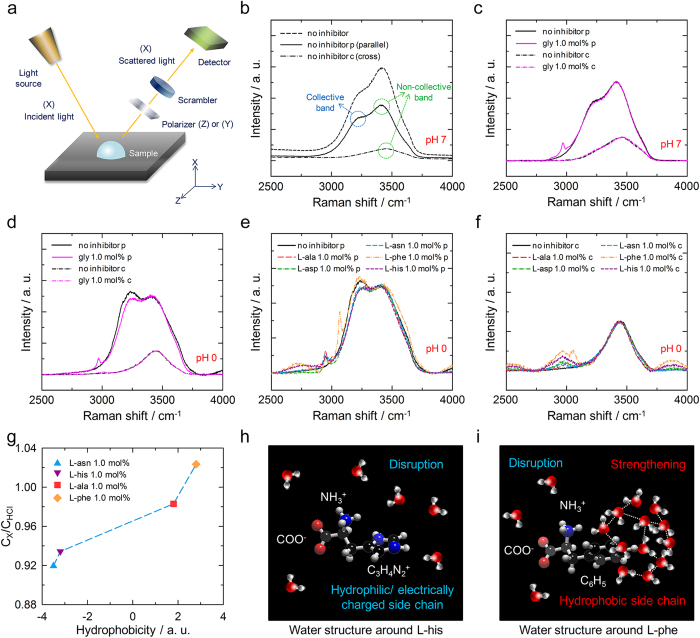
To examine the perturbations of liquid water structure by amino acids, polarized Raman spectra of aqueous solutions of the amino acids were obtained. In these experiments, scattered light from the aqueous amino acid solution passed through a polarizer plate and a scrambler (Fig. 1a); this experimental set-up is very similar to that used in previous studies25,26,27. The polarization geometries were X(ZZ)X and X(ZY)X for the parallel and cross positions respectively. In the Raman spectrum of pure liquid water (no inhibitor), two broad bands centered near 3250 and 3400 cm−1 were observed (Fig. 1b), which were attributed to symmetric and asymmetric OH stretching respectively28,29. When a polarizer was applied to extract the polarized Raman spectra for the parallel and cross positions, only the lower frequency band was highly polarized. This lower frequency band has previously been assigned to the collective in-phase stretching motion of strongly hydrogen-bonded water30,31, and is thus due to the intrinsic dynamics of liquid water molecules32,33. The ratio of the intensity of this band to that of the non-collective band can be used to quantify the fraction of such water34. As this ratio increases, the water molecules become more strongly connected to each other, whereas a decrease in this ratio indicates the disruption of the hydrogen bond network25,26.
Figure 1. Perturbation of liquid water structure by amino acids.
(a) Experimental set-up for performing polarized Raman spectroscopy. (b) Raman and polarized Raman spectra of pure liquid water. The OH stretching band near 3250 cm−1 was due to collective motion of hydrogen-bonded water. Polarized Raman spectra of an aqueous glycine solution (c) under neutral conditions and (d) under acidic conditions. Polarized Raman spectra of the aqueous amino acid solutions under acidic conditions (e) for the parallel position and (f) for the cross position. The amino acids affected the collective motion of water. (g) Correlation between the extents of perturbation due to the amino acids and their hydrophobicities. Decreases and increases in CX/CHCl indicate disruptions and strengthenings respectively of the water structure. Hypothetical representations of the liquid water structure (h) around L-histidine and (i) around L-phenylalanine. The hydrophilic and electrically charged side chain of L-histidine disrupted the water structure, whereas the hydrophobic side chain of L-phenylalanine strengthened it.
On the addition of 1.0 mol% glycine to water, the two broad OH stretching bands in both the parallel and cross spectra were only slightly affected (Fig. 1c), consistent with a previous report27, and an additional band due to the CH2 symmetric stretching of glycine near 2970 cm−1 was present35. This result has been interpreted as follows: the interaction of the amino acid side chains with water is shielded by large overlapping hydration shells around the ammonium and carboxylate ions, and the accessibility of water to the side chains is considerably reduced by the self-association of amino acids via electrostatic interactions at neutral pH27. To investigate the influence of the amino acid side chains on the structure of water, an aqueous HCl solution was used to induce the deionization of the carboxylate ions. An acidic environment is also close to the actual conditions for CO2 hydrate formation due to the presence of carbonic acids in water36. An increase in CO2 pressure accelerates the formation of carbonic acids, thus significantly decreasing the pH. According to the previous report, the pH values of the CO2 hydrate system can be decreased to around 3.18 with 35 bar of CO2 at 283.15 K36. Under acidic conditions, the addition of 1.0 mol% glycine reduced the intensity of the collective band, indicating the disruption of the water structure (Fig. 1d). Since glycine has no side chain, it seems likely that the carboxylate and ammonium ions produced this disruption of the water hydrogen bond network owing to their hydrophilic nature and electric charges.
These experiments were repeated with the solutions containing various amino acids. The effects of the presence of the amino acids on the parallel spectra varied with the properties of their side chains (Fig. 1e); the OH stretching bands in the cross spectra were not affected (Fig. 1f). L-alanine, L-aspartic acid, L-asparagine, and L-histidine were found to definitely reduce the intensity of the collective band, indicating the disruption of the water structure. In contrast, L-phenylalanine, the most hydrophobic of the tested amino acids, was found to increase the band intensity by strengthening the hydrogen bond network. To quantify the extent of each perturbation, the C value of each parallel spectrum was calculated from the ratio of the intensities of the collective band and the non-collective band. A C value larger than 1 indicates the strengthening of the water structure, whereas a value less than 1 indicates disruption. The relative C values of the aqueous HCl solutions containing amino acids with respect to that of HCl solution were plotted against the hydrophobicities of the amino acids (Fig. 1g). The extent of perturbation was strongly correlated with the hydrophobicity, consistent with a previous report27. This trend clearly indicates that hydrophilic and/or electrically charged side chains disrupt the water structure, as depicted (Fig. 1h). While L-phenylalanine also contains carboxylate and ammonium ions, the strengthening of the structure of water around the hydrophobic side chains would be greater than the disruption around the ionic moieties (Fig. 1i).
These experimental results demonstrate that the structure of liquid water is perturbed by the presence of the amino acids, and that the extent of perturbation varies with the side chain properties. Therefore, the amino acids tested were found to be suitable for the investigation of the influence of perturbation on hydrate inhibition.
Heterogeneous nucleation kinetics
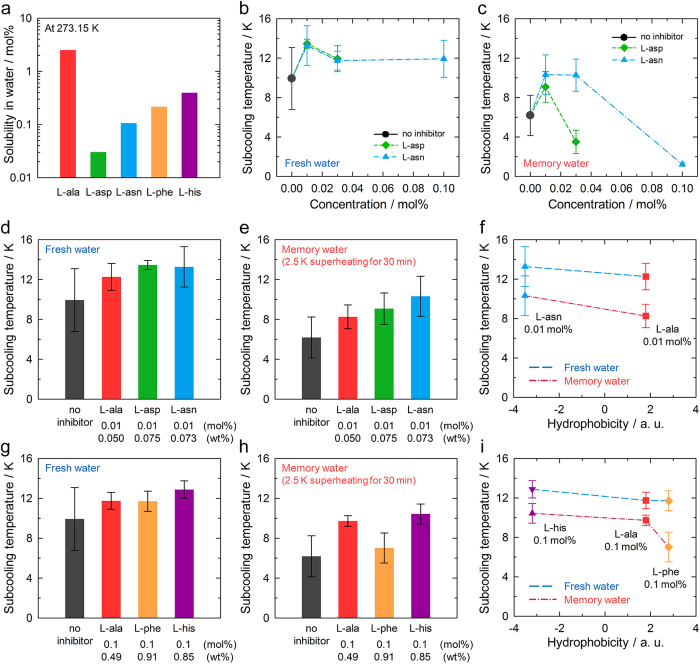
To examine the effects of the amino acids on hydrate nucleation, the subcooling temperature, which is the difference between the onset temperature and the phase equilibrium temperature at the onset pressure, was obtained for each amino acid (Supplementary information). Hydrate nucleation is accompanied by a sharp increase in temperature during cooling, as described in our previous report24. When investigating heterogeneous nucleation kinetics, an important consideration is the solubility of the KHI in water under hydrate forming conditions. The solubility values of the tested amino acids in water at 273.15 K were plotted (Fig. 2a); this temperature was chosen as it is the temperature with the lowest solubility values. On increasing the concentrations of L-aspartic acid and L-asparagine, the nucleation kinetics did not change significantly (Fig. 2b). The measurements were repeated by using memory water with a previous hydrate history1, since this method gives more reliable results37. Memory water was prepared by dissociating the hydrates, and thus having a thermal hysteresis for hydrate formation. Therefore, hydrate nucleation occurs much easier in the memory water. In the memory water system, however, as the concentrations of L-aspartic acid and L-asparagine exceeded their solubilities, the subcooling temperature values suddenly dropped (Fig. 2c), indicating the nucleation kinetics was accelerated. This failure of hydrate inhibition may be attributed to the presence of undissolved amino acids that precipitated during hydrate dissociation, which could provide additional sites for heterogeneous hydrate nucleation. The inhibition performances of the amino acids were therefore measured at concentrations much lower than their solubility values.
Figure 2. Heterogeneous nucleation kinetics of the CO2 hydrates.
(a) The solubilities of the amino acids in water at 273.15 K42,43. CO2 hydrate nucleation kinetics (b) in fresh water and (c) in memory water. Memory water was obtained from the dissociation of CO2 hydrate at a temperature 2.5 K above the phase equilibrium temperature for 30 min. The injection of KHIs at concentrations closed their solubilities led to failures in hydrate inhibition. CO2 hydrate nucleation kinetics in the presence of 0.01 mol% amino acids (d) in fresh water and (e) in memory water. (f) The correlation of subcooling temperatures at the onset of CO2 hydrate nucleation with the hydrophobicities. CO2 hydrate nucleation kinetics in the presence of 0.1 mol% amino acids (g) in fresh water and (h) in memory water. (i) The correlation of subcooling temperatures with the amino acid hydrophobicities. The values for the average and standard deviation are shown. L-phenylalanine had negligible influence on nucleation kinetics, especially in memory water, whereas all the other tested amino acids were found to be effective in inhibiting CO2 hydrate nucleation. The data for the system with no inhibitor or 0.1 mol% L-alanine were obtained from our previous report24.
In the measurements of the heterogeneous nucleation kinetics, the subcooling temperatures of the solutions containing amino acids were about 2–4 K higher than those of the solution without inhibitor (Fig. 2d,e), indicating that L-alanine, L-aspartic acid, and L-asparagine delay hydrate nucleation. Interestingly, L-aspartic acid and L-asparagine were found to be more effective in hydrate inhibition than L-alanine, and their inhibition performances were correlated with their hydrophobicities (Fig. 2f). This correlation is attributed to the disruption of the water structure by their hydrophilic side chains, as indicated by the Raman spectra. As expected, L-histidine was also found to be more effective in hydrate inhibition than L-alanine, owing to its hydrophilic side chain (Fig. 2g,h). However, L-phenylalanine had almost no influence on the kinetics of hydrate nucleation, especially in memory water. The structure of water around the hydrophobic L-phenylalanine side chain was strengthened, even though the structure was disrupted around the carboxylate and ammonium ions. The competition between the disruption and strengthening of the water structure can affect the nucleation kinetics. The inhibition performances of L-alanine, L-phenylalanine, and L-histidine were strongly correlated with their hydrophobicities (Fig. 2i). It is obvious that the nucleation inhibition performances of the amino acids increased with decreasing hydrophobicity, with a similar trend to that found in our previous study24. Surprisingly, this trend is also very similar to the trend in the extent of perturbation obtained from the polarized Raman spectra (Fig. 1g). The strong correlation of the nucleation inhibition performances of the amino acids with the extents of their perturbations of water structure suggests that water structure is an important factor in hydrate nucleation. Amino acids with hydrophilic and/or electrically charged side chains are likely to be effective in delaying hydrate nucleation because of their disruptions of water structure, whereas those with hydrophobic side chains have negligible effects on kinetics due to the competition between the strengthening of the water structure around side chains and the disruption of the water structure around ionic moieties.
Growth kinetics
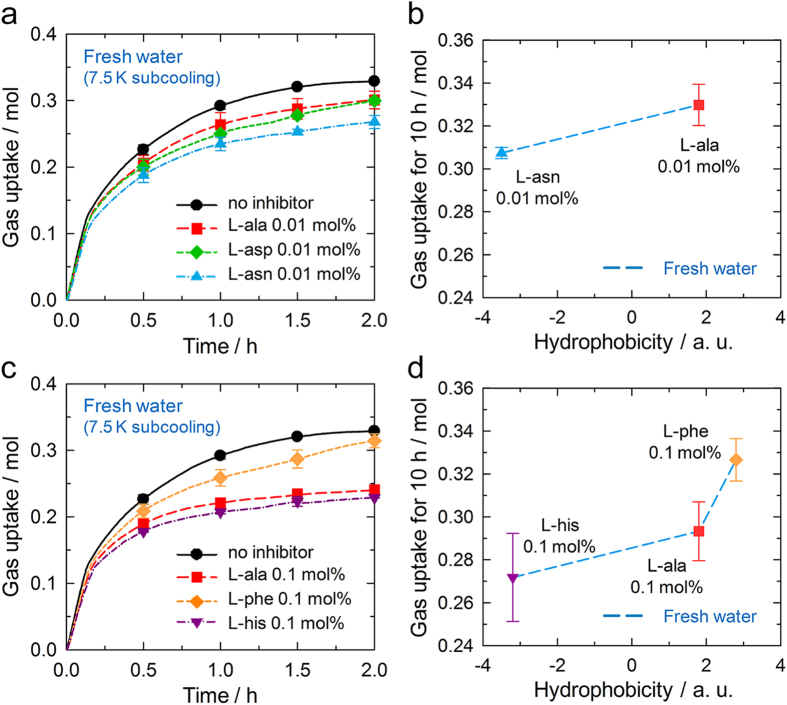
Further investigations of the kinetics of hydrate growth were performed. In the solution without inhibitor, rapid growth was initiated immediately after the onset of hydrate nucleation, and the growth rate gradually decreased over time (Fig. 3a). On the addition of 0.01 mol% amino acids, however, an inflection point was observed after the rapid growth over the first 7 min, and then the growth rate fell below that of the solution without inhibitor. L-aspartic acid and L-asparagine exhibited better growth inhibition performances than L-alanine, which can be attributed to their hydrophilic side chains. Their growth inhibition performances were also correlated with their hydrophobicities (Fig. 3b). The addition of 0.1 mol% amino acids produced similar results (Fig. 3c). These experimental results show that these amino acids delayed hydrate growth as well as hydrate nucleation. Although L-phenylalanine also reduced the rate of hydrate growth, it was less effective than L-alanine owing to its hydrophobic side chain. The ability to retard hydrate growth was strongly correlated with the hydrophobicity (Fig. 3d), consistent with the results of our previous studies24,38. All of the tested amino acids were found to delay hydrate growth.
Figure 3. Growth kinetics of the CO2 hydrates.
(a) CO2 hydrate growth kinetics in the presence of 0.01 mol% amino acids in fresh water. The gas uptake measurements were initiated at the onset of CO2 hydrate nucleation. (b) The correlation between the amount of gas uptake over 10 h and amino acid hydrophobicity. (c) CO2 hydrate growth kinetics in the presence of 0.1 mol% amino acids in fresh water. (d) The correlation of gas uptake values with the amino acid hydrophobicities. The lines are the average values of the gas uptake, which were obtained every 10 s. The symbols and their error bars indicate the average and standard deviation, respectively. All the tested amino acids were found to inhibit CO2 hydrate growth. The data for the solutions with no inhibitor or 0.1 mol% L-alanine were obtained from our previous report24.
An intriguing point is that the trend in the growth inhibition performances of the amino acids was very similar to that found for the heterogeneous nucleation kinetics. It is especially noteworthy in that there have been several previous reports of discrepancies between the nucleation and growth inhibition performances of KHIs39,40. These discrepancies have been attributed to the fact that hydrate growth occurs on hydrate surfaces, whereas heterogeneous hydrate nucleation mostly initiates from solid nucleating impurities1. Therefore, it is likely that the effects of amino acids are independent of surface properties. These findings imply that perturbation is a better explanation for hydrate inhibition by amino acids than adsorption onto the hydrate surface, because perturbation arises in the presence of KHIs due to the interruption of hydrogen bonds between water molecules and not the presence of any surface. In addition, the growth inhibition performances of the amino acids were found to be closely related to the extent of perturbation. This correlation strongly supports the perturbation inhibition mechanism.
Conclusion
In summary, we have directly observed perturbation of the structure of liquid water by amino acids, and investigated the influence of this perturbation on the kinetics of gas hydrate nucleation and growth. Amino acids with hydrophilic and/or electrically charged side chains were found to disrupt the structure of liquid water, whereas those with hydrophobic side chains strengthened this structure. The KHI efficacies of the amino acids were strongly correlated with their hydrophobicities, and thus with the extents of perturbation. These findings indicate that perturbation phenomena play a vital role in hydrate inhibition, and also imply that any molecule dissolved in water will affect the structure of water and hydrate kinetics because of its intrinsic hydrophobic or hydrophilic nature. The fundamental insights into gas hydrate kinetics and the mechanism of hydrate inhibition gained in this study are beneficial for understanding the freezing and melting phenomena of gas hydrates. In addition, the present findings will assist a variety of industrial applications including gas storage, separation, and transportation.
Methods
Polarized Raman spectroscopy
The experimental set-up for the polarized Raman spectroscopy measurements is presented (Fig. 1a). LabRam Aramis (Horiba Jobin Yvon) Raman spectroscopy was performed under ambient conditions. The incident light was provided by a 514 nm Ar-ion laser with 2 mW power. A scrambler was used to remove instrumental errors. A droplet of each aqueous amino acid solution (0.1 mL) was loaded onto a polymer holder covered with aluminum foil, and the scan was performed from 2500 cm−1 to 4000 cm−1 for 60 s with a band resolution of 2 cm−1. The baselines were adjusted, and then all spectra were normalized.
In previous studies25,26,27, the extent of perturbation has been calculated with an area-based method. The intensity of the collective band is extracted from the intensity of the polarized Raman spectra for the parallel and cross positions. Then, the C value is calculated through integration of the intensity of the spectra, and then normalized. However, this method can produce erroneous results. An adjustment is involved in the extraction of the collective band because the difference spectrum obtained from the polarized Raman spectra for the parallel and cross positions is very sensitive to the degree of depolarization. In addition, the resulting spectrum of the collective band is assumed to be symmetric, and the entire spectrum is defined by the results for the low-frequency region.
In this study, we quantified the extent of perturbation by using an intensity-based method. The key idea of this method is very similar to that of the previous method in that the C value is obtained from the difference between the intensities of the collective and non-collective bands. This method simply uses the maximum band intensity, and thus does not distort the raw experimental data. The C value is defined by the following equation (1):
 |
where IL is the maximum intensity of the lower frequency band (~3250 cm−1) in the parallel component, and IH is that of the higher frequency band (~3400 cm−1). The lower frequency and higher frequency bands correspond to the collective and non-collective bands respectively. Then, the relative C value of an aqueous amino acid solution is defined as follows:
 |
where CX is the C value of the aqueous amino acid solution, CW is that of pure water, and CHCl is that of the aqueous HCl solution.
Additional Information
How to cite this article: Sa, J.-H. et al. Gas hydrate inhibition by perturbation of liquid water structure. Sci. Rep. 5, 11526; doi: 10.1038/srep11526 (2015).
Supplementary Material
Acknowledgments
The present study was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST) (Grant No. 2014-003266). We also acknowledge the Research Institute of Industrial Science & Technology for financial support. Synchrotron powder X-ray diffraction experiments at Pohang Accelerator Laboratory were supported in part by MEST and the Pohang University of Science & Technology. Raman measurements were supported by the Raman research center.
Footnotes
Author Contributions J.H.S. and K.H.L. designed the experiments; J.H.S. recorded the polarized Raman spectra; J.H.S. and G.H.K. carried out heterogeneous nucleation kinetics and growth kinetics experiments; J.H.S., G.H.K. and D.A. performed synchrotron powder X-ray diffraction experiments; J.H.S., K.H. and K.H.L. interpreted the experimental results and wrote the manuscript. All authors discussed the results and commented on the manuscript.
References
- Sloan E. D. & Koh C. A. Clathrate hydrates of natural gases (CRC Press, Boca Raton, FL, 2008). [Google Scholar]
- Boswell R. & Collett T. S. Current perspectives on gas hydrate resources. Energy Environ. Sci. 4, 1206–1215 (2011). [Google Scholar]
- Klauda J. B. & Sandler S. I. Global distribution of methane hydrate in ocean sediment. Energy Fuels 19, 459–470 (2005). [Google Scholar]
- Gautier D. L. et al. Assessment of undiscovered oil and gas in the Arctic. Science 324, 1175–1179 (2009). [DOI] [PubMed] [Google Scholar]
- Kullerud L. Sustainability: A green Arctic. Nature 478, 179–180 (2011). [DOI] [PubMed] [Google Scholar]
- Stephenson S. R., Smith L. C. & Agnew J. A. Divergent long-term trajectories of human access to the Arctic. Nature Clim. Change 1, 156–160 (2011). [Google Scholar]
- Hammerschmidt E. G. Formation of gas hydrates in natural gas transmission lines. Ind. Eng. Chem. 26, 851–855 (1934). [Google Scholar]
- Kelland M. A. History of the development of low dosage hydrate inhibitors. Energy Fuels 20, 825–847 (2006). [Google Scholar]
- Makogon T. Y., Larsen R., Knight C. A. & Sloan E. D. Melt growth of tetrahydrofuran clathrate hydrate and its inhibition: method and first results. J. Cryst. Growth 179, 258–262 (1997). [Google Scholar]
- Larsen R., Knight C. A. & Sloan E. D. Clathrate hydrate growth and inhibition. Fluid Phase Equilib. 150-151, 353–360 (1998). [Google Scholar]
- Zeng H., Wilson L. D., Walker V. K. & Ripmeester J. A. Effect of antifreeze proteins on the nucleation, growth, and the memory effect during tetrahydrofuran clathrate hydrate formation. J. Am. Chem. Soc. 128, 2844–2850 (2006). [DOI] [PubMed] [Google Scholar]
- Storr M. T., Taylor P. C., Monfort J.-P. & Rodger P. M. Kinetic inhibitor of hydrate crystallization. J. Am. Chem. Soc. 126, 1569–1576 (2004). [DOI] [PubMed] [Google Scholar]
- Carver T. J., Drew M. G. B. & Rodger P. M., Inhibition of crystal growth in methane hydrate. J. Chem. Soc. Faraday Trans. 91, 3449–3460 (1995). [Google Scholar]
- Freer E. M. & Sloan E. D. An engineering approach to kinetic inhibitor design using molecular dynamics simulations. Ann. N. Y. Acad. Sci. 912, 651–657 (2000). [Google Scholar]
- Anderson B. J., Tester J. W., Borghi G. P. & Trout B. L. Properties of inhibitors of methane hydrate formation via molecular dynamics simulations. J. Am. Chem. Soc. 127, 17852–17862 (2005). [DOI] [PubMed] [Google Scholar]
- Moon C., Taylor P. C. & Rodger P. M. Clathrate nucleation and inhibition from a molecular perspective. Can. J. Phys. 81, 451–457 (2003). [Google Scholar]
- Kvamme B., Kuznetsova T. & Aasoldsen K. Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J. Mol. Graph. Model. 23, 524–536 (2005). [DOI] [PubMed] [Google Scholar]
- Hawtin R. W. & Rodger P. M. Polydispersity in oligomeric low dosage gas hydrate inhibitors. J. Mater. Chem. 16, 1934–1942 (2006). [Google Scholar]
- Moon C., Hawtin R. W. & Rodger P. M. Nucleation and control of clathrate hydrates: insights from simulation. Faraday Discuss. 136, 367–382 (2007). [DOI] [PubMed] [Google Scholar]
- Luu D. V. & Cambon L. Perturbation of liquid-water structure by ionic substances. J. Mol. Struct. 237, 411–419 (1990). [Google Scholar]
- Head-Gordon T. & Stillinger F. H. An orientational perturbation theory for pure liquid water. J. Chem. Phys. 98, 3313–3327 (1993). [Google Scholar]
- Finney J. L., Soper A. K. & Turner J. Z. Water perturbation close to non-polar groups in aqueous solutions. Pure Appl. Chem. 65, 2521–2526 (1993). [Google Scholar]
- Mancinelli R. et al. Perturbation of water structure due to monovalent ions in solution. Phys. Chem. Chem. Phys. 9, 2959–2967 (2007). [DOI] [PubMed] [Google Scholar]
- Sa J.-H. et al. Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation. Sci. Rep. 3, 2428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Maeda Y. & Kitano H. Raman spectroscopic study on water in polymer gels. J. Phys. Chem. 97, 3619–3622 (1993). [Google Scholar]
- Maeda Y. et al. Raman spectroscopic study of water in aqueous polymer solutions. J. Phys. Chem. 97, 13903–13906 (1993). [Google Scholar]
- Ide M., Maeda Y. & Kitano H. Effect of hydrophobicity of amino acids on the structure of water. J. Phys. Chem. B 101, 7022–7026 (1997). [Google Scholar]
- Carey D. M. & Korenowski G. M. Measurement of the Raman spectrum of liquid water. J. Chem. Phys. 108, 2669–2675 (1998). [Google Scholar]
- Auer B. M. & Skinner J. L. IR and Raman spectra of liquid water: Theory and interpretation. J. Chem. Phys. 128, 224511 (2008). [DOI] [PubMed] [Google Scholar]
- Whalley E. A detailed assignment of the O–H stretching bands of ice I. Can. J. Chem. 55, 3429–3441 (1977). [Google Scholar]
- Green J. L., Lacey A. R. & Sceats M. G. Spectroscopic evidence for spatial correlations of hydrogen bonds in liquid water. J. Phys. Chem. 90, 3958–3964 (1986). [Google Scholar]
- Yashkichev V. I. Model of the collective motion of water molecules in water. J. Struct. Chem. 12, 291–292 (1971). [Google Scholar]
- Ohmine I. Liquid water dynamics: Collective motions, fluctuation, and relaxation. J. Phys. Chem. 99, 6767–6776 (1995). [Google Scholar]
- Raichlin Y., Millo A. & Katzir A. Investigations of the structure of water using mid-IR fiberoptic evanescent wave spectroscopy. Phys. Rev. Lett. 93, 185703 (2004). [DOI] [PubMed] [Google Scholar]
- Furić K., Mohaček V., Bonifačić M. & Štefanić I. Raman spectroscopic study of H2O and D2O water solutions of glycine. J. Mol. Struct. 267, 39–44 (1992). [Google Scholar]
- Sa J.-H. et al. Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ. Sci. Technol. 45, 5885–5891 (2011). [DOI] [PubMed] [Google Scholar]
- Duchateau C. et al. Laboratory evaluation of kinetic hydrate inhibitors: a procedure for enhancing the repeatability of test results. Energy Fuels 23, 962–966 (2009). [Google Scholar]
- Sa J.-H. et al. Abnormal incorporation of amino acids into the gas hydrate crystal lattice. Phys. Chem. Chem. Phys. 16, 26730–26734 (2014). [DOI] [PubMed] [Google Scholar]
- Ohno H. et al. Interaction of antifreeze proteins with hydrocarbon hydrates. Chem. Eur. J. 16, 10409–10417 (2010). [DOI] [PubMed] [Google Scholar]
- Townson I., Walker V. K., Ripmeester J. A. & Englezos P. Bacterial inhibition of methane clathrate hydrates formed in a stirred autoclave. Energy Fuels 26, 7170–7175 (2012). [Google Scholar]
- Kyte J. & Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982). [DOI] [PubMed] [Google Scholar]
- Dunn M. S., Ross F. J. & Read L. S. The solubility of the amino acids in water. J. Biol. Chem. 103, 579–595 (1933). [Google Scholar]
- Dalton J. B. & Schmidt C. L. A. The solubilities of certain amino acids and related compounds in water, the densities of their solutions at twenty-five degrees, and the calculated heats of solution and partial molal volumes. II. J. Biol. Chem. 109, 241–248 (1935). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.