Abstract
Objective
Describe the parent-reported prevalence of ADHD treatments among a national sample of children with special health care needs (CSHCN), and assess the alignment of ADHD treatment to current American Academy of Pediatrics (AAP) guidelines.
Methods
Parent-reported data from the 2009–10 National Survey of Children with Special Health Care Needs allowed for weighted national and state-based prevalence estimates of medication, behavioral therapy, and dietary supplement use for ADHD treatment among CSHCN, aged 4–17 years with current ADHD. National estimates were compared across demographic groups, ADHD severity, and comorbidities. Medication treatment by drug class was described.
Results
Of CSHCN with current ADHD, 74.0% had past week medication treatment and 44.0% had past year behavioral therapy. In the past year, 10.2% used dietary supplements for ADHD. Overall, 87.3% had received past week medication treatment or past year behavioral therapy (both=30.7%, neither=12.7%). Among preschool-aged CSHCN with ADHD, 25.4% received medication treatment alone, 31.9% received behavioral therapy alone, 21.2% received both treatments, and 21.4% received neither treatment. Central nervous system stimulants were the most common medication class (84.8%) among CSHCN with ADHD, followed by the selective norepinephrine reuptake inhibitor atomoxetine (8.4%).
Conclusions
These estimates benchmark clinical practice for the period directly preceding the AAP’s 2011 ADHD guidelines. Most children with ADHD received medication treatment or behavioral therapy; just under one-third received both. Multi-modal treatment was most common among CSHCN with severe ADHD and those with comorbidities. About half of preschoolers received behavioral therapy, the recommended first-line treatment for this age group.
Keywords: Medication treatment, behavioral therapy, Public Health, epidemiology
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental disorder (1) commonly managed by pediatricians.(2) Professional guidelines describe best practices for diagnosis (1) and treatment.(3, 4) The 2011 American Academy of Pediatrics’ (AAP) guidelines were expanded to include all children aged 4–18, with special considerations for preschoolers and adolescents. AAP now recommends behavioral therapy first for preschool-aged children, with short-acting methylphenidate prescribed if therapy does not sufficiently improve symptoms. For older children, an FDA-approved ADHD medication with or without behavioral therapy is recommended.(5) In 2011, 6.4 million children aged 4–17 years (11%) had a parent report of an ADHD diagnosis by a health care provider.(6) Prevalence estimates of parent-reported ADHD increased 33% from 1997 to 2008.(7) As ADHD diagnoses have increased, so has the prevalence of children taking medication for ADHD (medicated ADHD).(2, 8, 9) While there is earlier evidence of increased stimulant usage among preschoolers with ADHD (10), less is known on recent treatment patterns for very young children. Many children with ADHD not treated with medication receive behavioral or alternative therapies; however, little is known about the prevalence of these therapies. Epidemiological research suggests that families of black and Hispanic children with ADHD are less likely to choose pharmacological treatments and regard behavioral treatments more positively(11, 12); however, population-based data on the relative frequency of non-pharmacological ADHD treatment across racial and ethnic groups are needed to fully characterize ADHD treatment or identify potential treatment gaps.(6)
The goals of this study were to: 1) describe the prevalence of medication, behavioral therapy, and dietary supplement use for ADHD among a national sample of children with special health care needs with ADHD; 2) identify differences in prevalence by relevant demographic factors; and 3) assess the alignment of treatment patterns to AAP’s age-group-specific guidelines.(3)
Methods
The 2009–2010 National Survey of Children with Special Health Care Needs (NS-CSHCN) is a nationally representative, population-based telephone survey conducted by the Centers for Disease Control and Prevention with funding and direction from the Health Resources Services Administration; data were collected from July 2009 to March 2011.(13) A knowledgeable parent or guardian in the household responded to a 5-part screener to determine whether any children aged 2 to 17 years in the household met the criteria of having a special health care need. Children were considered to be CSHCN if they met one or more of the following criteria because of a medical, behavioral or other health condition that had lasted or was expected to last 12 or more months: needs or uses medicine other than vitamins prescribed by a doctor; needs or uses more medical care, mental health or educational services than is usual for most children of the same age; is limited or prevented in any way in his or her ability to do the things most children of the same age can do; needs or gets special therapy, such as physical, occupational or speech therapy; or has any kind of emotional, developmental or behavioral problem for which he or she needs treatment or counseling.(14) Nationally, 372,698 children were screened for a special health care need; of these, detailed interviews were completed regarding 40,242 CSHCN (1 randomly selected per eligible household). The overall response rate was 25.5%, which incorporates a 44% response rate among those contacted by landline and 15.2% among those contacted by cellphone. The interview completion rate among eligible families was 84% of landline households and 77% of cell phone families (81% overall).
The survey included questions on family demographics, health care services, and presence of selected health conditions, including ADHD. Parents were asked whether their child had ever been diagnosed with ADHD by a doctor or other health care provider, and if so, whether the child currently had ADHD. For those with current ADHD, follow-up questions assessed level of parent-reported severity (mild, moderate, or severe) and ADHD medication use during the past week and past year. Children completing the survey in the last five of six quarters were also asked a series of more specific ADHD treatment questions. If medication was used in the past week, the parent was asked to list medication(s) taken; because many respondents were at home during the telephone survey, they were asked to read the medication names directly from the medication bottles. Medications were grouped into six categories: central nervous system stimulants (CNS Stimulants), selective norepinephrine reuptake inhibitors (SNRI), selective serotonin reuptake inhibitors (SSRI), atypical antipsychotics, centrally acting alpha-agonist hypotensive agents, and centrally acting alpha-adrenergic receptor agonists (Appendix; online). Parents were also asked whether their child had received “behavioral treatment for ADD or ADHD, including classroom management, peer interventions, social skills training, or cognitive-behavioral therapy” in the past year and whether their child had taken dietary supplements to treat ADHD in the past week or past year.
Weighted analyses using SAS-callable SUDAAN (Version 11.0.0, RTI International, NC) were conducted to produce estimates of current ADHD prevalence among CSHCN aged 4–17 years as well as prevalence of ADHD medication use overall and by medication class, behavioral therapy, and dietary supplement use to treat ADHD among CSHCN at the national and state level. National estimates of combination therapy (defined as past week medication treatment and past year behavioral therapy) were also described. National treatment estimates were compared using chi-square tests, stratified by child sex, child age, child race/ethnicity, US region of residence, health insurance status, presence of a medical home (a model of primary care that is patient-centered, comprehensive, team-based, coordinated, accessible, and focused on quality and safety),(15) parent-perception of ADHD severity, and presence of current co-occurring mental or developmental conditions (referred to as co-occurring conditions). Co-occurring conditions included in this analysis were depression, anxiety, behavioral or conduct problems (such as oppositional defiant disorder or conduct disorder), autism spectrum disorders, developmental delay, and intellectual disability. Multiple imputation was used by NCHS to create values for respondents with missing data on household income (7.9% missing), parental education (0.9%), race/ethnicity (0.7%), and household language (0.5%); imputed values were incorporated into the analyses for these variables.(13)
Results
Of all completed NS-CSHCN interviews (40,242), 9,459 CSHCN aged 4–17 years were reported to have current ADHD, had valid responses to the ADHD treatment questions, and had complete data on sex. The demographic profile of the sample and this population is shown in Table 1; online. Among all CSHCN, the weighted estimate of parent-reported current ADHD was 32.3% (CI: 31.5–33.2).
Table 1.
Characteristics of children with special health care needs aged 4–17 who currently have attention-deficit/hyperactivity disorder (ADHD) by parent report, National Survey of Children with Special Health Care Needs: US, 2009–10
| Unweighted n | Weighted % | 95% CI | |
|---|---|---|---|
| Boys | 6,769 | 69.8 | (68.1, 71.5) |
| Girls | 2,690 | 30.2 | (28.5, 31.9) |
| Age 4–5 | 280 | 3.4 | (2.8, 4.1) |
| Age 6–11 | 4235 | 45.1 | (43.3, 46.9) |
| Age 12–17 | 4944 | 51.5 | (49.7, 53.3) |
| Non-Hispanic White, single race1 | 6,845 | 64.9 | (63.0, 66.7) |
| Non-Hispanic Black, single race1 | 896 | 15.1 | (13.7, 16.6) |
| Hispanic, any race(s)1 | 871 | 12.9 | (11.5, 14.5) |
| Non-Hispanic other single race or multiple races1 | 847 | 7.1 | (6.2, 8.2) |
| Highest education of a resident parent | |||
| Less Than High School1 | 575 | 12.3 | (10.8, 13.8) |
| High School Graduate1 | 1,680 | 22.4 | (20.9, 24.0) |
| More Than High School1 | 7,204 | 65.3 | (63.5, 67.1) |
| % Federal Poverty Level | |||
| <100% 1 | 1,896 | 25.0 | (23.4, 26.6) |
| 100%–200%1 | 1,955 | 22.6 | (21.1, 24.2) |
| >200%1 | 5,608 | 52.5 | (50.6, 54.3) |
| English as Primary Household Language1 | 9,292 | 96.9 | (95.9, 97.6) |
| Other Language As Primary1 | 167 | 3.1 | (2.4, 4.1) |
| Region of U.S. | |||
| Northeast | 1,614 | 17.0 | (15.8, 18.3) |
| Midwest | 2,191 | 23.0 | (21.8, 24.4) |
| South | 3,572 | 43.4 | (41.8, 45.1) |
| West | 2,082 | 16.6 | (15.2, 18.1) |
| Type of Health Insurance | |||
| Non-Public Insurance | 5,263 | 48.7 | (46.9, 50.5) |
| Public Insurance | 3,937 | 48.6 | (46.8, 50.4) |
| No Insurance | 247 | 2.7 | (2.2, 3.3) |
| Medical Home | 3,836 | 38.5 | (36.8, 40.3) |
| No Medical Home | 5,344 | 61.5 | (59.7, 63.2) |
| Parent-Reported ADHD Severity | |||
| Mild | 3,420 | 33.7 | (32.1, 35.4) |
| Moderate | 4,279 | 45.2 | (43.4, 47.0) |
| Severe | 1,719 | 21.1 | (19.6, 22.7) |
| Any Co-occurring Conditions2 | 5,381 | 59.4 | (57.7, 61.2) |
| No Co-occurring Condition | 4,013 | 40.6 | (38.8, 42.3) |
Multiple imputation used to estimate value for respondents with missing data on household income (7.9% missing), parental education (0.9%), race/ethnicity (0.7%), and household language (0.5%).
Co-occurring conditions included parent-reported depression, anxiety, behavioral or conduct problems (such as oppositional defiant disorder or conduct disorder), autism spectrum disorder, developmental delay and intellectual disability.
Medication Treatment
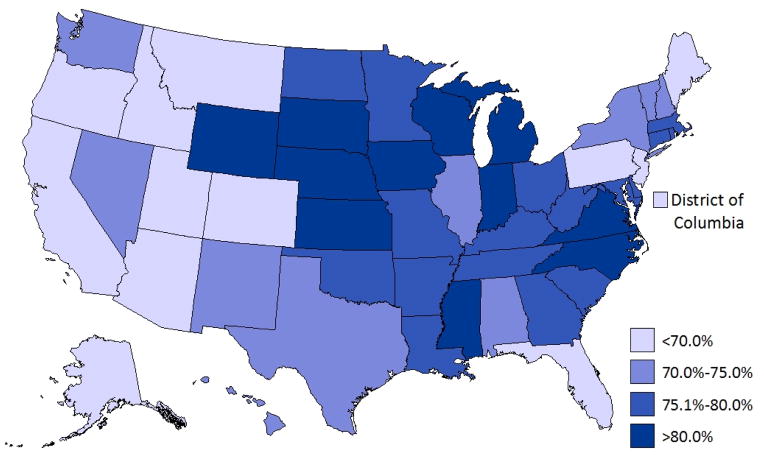
Of the CSHCN reported by their parents to have current ADHD, 82.6% (CI: 81.1—84.1) had taken medication for ADHD in the past year and 74.0% (CI: 72.3—75.7) had taken medication for ADHD in the past week. Young CSHCN 4–5 years old with current ADHD were least likely to have taken medication in the past week. A larger percentage of boys had taken medication in the past week compared to girls, although this difference did not reach statistical significance (p=0.06; Table 2). CSHCN who were uninsured, without a medical home, lived in the West, with mild ADHD, or with a co-occurring condition were less likely to have taken medication in the past week (Table 2; Figure 1). Compared to non-Hispanic white CSHCN, CSHCN of other racial and ethnic groups were less likely to have taken medication for ADHD in the past week. State-level prevalence of current medication treatment among CSHCN with ADHD ranged from 56.6% (California) to 87.5% (Michigan; Figure 1).
Table 2.
Prevalence of treatment for attention-deficit/hyperactivity disorder (ADHD) among children with special health care needs aged 4–17 years who have parent-reported current ADHD by demographic subgroups, National Survey of Children with Special Health Care Needs: US, 2009–10
| Took ADHD medication in past week | Used behavioral therapy in past year | Used dietary supplements in past year | ||||
|---|---|---|---|---|---|---|
| Characteristic | % (95% CI) | PR1 | % (95% CI) | PR1 | % (95% CI) | PR1 |
| Overall | 74.0 (72.3—75.7) | 44.0 (42.2—45.8) | 10.2 (9.2—11.4) | |||
| Boys | 75.1 (73.1—77.1) | 1.05 (1.00—1.11) | 44.4 (42.3—46.5) | 1.03 (0.94—1.13) | 10.2 (9.0—11.6) | 1.00 (0.78—1.28) |
| Girls | 71.5 (68.3—74.5) | Ref. | 43.2 (39.9—46.5) | Ref. | 10.2 (8.3—12.6) | Ref. |
| Age 4–5 | 46.6 (36.9—56.7) | Ref. | 53.2 (42.9—63.1) | Ref. | 15.3 (9.8—23.2) | Ref. |
| Age 6–11 | 77.2 (74.5—79.6) | 1.66 (1.33—2.06) | 47.5 (44.7—50.2) | 0.89 (0.73—1.09) | 11.4 (9.8—13.3) | 0.75 (0.47—1.18) |
| Age 12–17 | 73.1 (70.8—75.3) | 1.57 (1.26—1.95) | 40.4 (38.0—42.8) | 0.76 (0.62—0.93) | 8.8 (7.5—10.4) | 0.57 (0.36—0.91) |
| Non-Hispanic White2 | 78.2 (76.5—79.8) | Ref. | 40.9 (39.0—42.9) | Ref. | 9.6 (8.5—10.8) | Ref. |
| Non-Hispanic Black2 | 66.8 (61.5—71.7) | 0.85 (0.79—0.92) | 49.4 (44.1—54.7) | 1.21 (1.07—1.36) | 10.7 (7.7—14.8) | 1.12 (0.79—1.59) |
| Hispanic2 | 69.5 (63.3—75.0) | 0.89 (0.81—0.97) | 50.8 (44.5—57.0) | 1.24 (1.09—1.42) | 13.1 (9.4—18.0) | 1.37 (0.97—1.94) |
| Other2 | 59.8 (51.4—67.6) | 0.76 (0.67—0.88) | 48.6 (41.3—55.9) | 1.19 (1.01—1.39) | 9.7 (6.4—14.4) | 1.01 (0.66—1.55) |
| Parental education | ||||||
| Less Than High School2 | 74.6 (68.6—79.8) | 1.01 (0.93—1.10) | 41.0 (34.5—47.8) | 0.91 (0.77—1.08) | 9.6 (6.1—14.7) | 0.83 (0.53—1.31) |
| High School Graduate2 | 74.6 (70.9—77.9) | 1.01 (0.96—1.07) | 43.0 (39.1—46.9) | 0.96 (0.86—1.06) | 6.6 (4.9—8.8) | 0.57 (0.42—0.78) |
| More Than High School2 | 73.8 (71.7—75.7) | Ref. | 44.9 (42.9—47.0) | Ref. | 11.6 (10.3—13.0) | Ref. |
| <100% Poverty Level2 | 74.3 (71.0—77.3) | 1.01 (0.96—1.06) | 47.6 (43.9—51.4) | 1.13 (1.03—1.25) | 8.0 (6.1—10.4) | 0.69 (0.52—0.93) |
| 100%-200% Poverty Level2 | 74.7 (71.0—78.0) | 1.01 (0.96—1.07) | 44.6 (40.6—48.6) | 1.06 (0.95—1.18) | 9.6 (7.5—12.3) | 0.83 (0.63—1.10) |
| >200% Poverty Level2 | 73.6 (71.2—76.0) | Ref. | 42.1 (39.7—44.5) | Ref. | 11.5 (10.1—13.2) | Ref. |
| English as Primary Language2 | 74.5 (72.7—76.1) | Ref. | 43.5 (41.7—45.3) | Ref. | 10.2 (9.1—11.4) | Ref. |
| Other Language As Primary2 | 61.5 (48.8—72.9) | 0.83 (0.68—1.01) | 59.4 (47.1—70.6) | 1.36 (1.11—1.68) | 11.2 (6.6—18.4) | 1.10 (0.65—1.87) |
| Northeast | 70.2 (66.2—73.9) | 1.12 (1.01—1.24) | 53.2 (48.9—57.4) | 1.08 (0.95—1.24) | 10.2 (8.0—12.8) | 0.73 (0.51—1.03) |
| Midwest | 81.0 (78.1—83.4) | 1.29 (1.17—1.42) | 42.9 (39.7—46.2) | 0.87 (0.77—1.00) | 7.1 (5.7—8.9) | 0.51 (0.36—0.72) |
| South | 76.2 (73.8—78.5) | 1.21 (1.11—1.33) | 39.1 (36.5—41.7) | 0.80 (0.70—0.90) | 10.5 (8.9—12.3) | 0.75 (0.55—1.02) |
| West | 62.7 (57.1—68.0) | Ref. | 49.1 (43.9—54.3) | Ref. | 14.0 (10.7—18.0) | Ref. |
| Non-Public Insurance | 74.4 (72.1—76.5) | Ref. | 38.2 (35.9—40.5) | Ref. | 11.2 (9.8—12.7) | Ref. |
| Public Insurance | 75.1 (72.4—77.6) | 1.01 (0.96—1.06) | 49.9 (47.1—52.7) | 1.31 (1.20—1.42) | 8.9 (7.3—10.7) | 0.79 (0.63—1.00) |
| No Insurance | 50.0 (40.1—59.9) | 0.67 (0.55—0.82) | 44.0 (34.4—54.0) | 1.15 (0.91—1.45) | 17.9 (10.6—28.6) | 1.60 (0.95—2.69) |
| Medical Home | 81.3 (79.1—83.3) | Ref. | 34.3 (31.8—36.9) | Ref. | 7.1 (5.8—8.6) | Ref. |
| No Medical Home | 70.3 (67.9—72.6) | 0.86 (0.83—0.90) | 49.9 (47.5—52.3) | 1.45 (1.33—1.59) | 12.0 (10.5—13.7) | 1.70 (1.34—2.16) |
| Mild ADHD | 64.8 (61.8—67.7) | Ref. | 31.7 (29.0—34.6) | Ref. | 9.3 (7.5—11.5) | Ref. |
| Moderate ADHD | 77.1 (74.7—79.3) | 1.19 (1.13—1.26) | 45.6 (43.0—48.3) | 1.44 (1.29—1.60) | 9.8 (8.3—11.4) | 1.05 (0.80—1.36) |
| Severe ADHD | 82.7 (78.2—86.5) | 1.28 (1.19—1.37) | 60.5 (56.0—64.8) | 1.91 (1.70—2.14) | 12.7 (10.2—15.5) | 1.36 (1.01—1.83) |
| Co-occurring condition3 | 71.2 (68.8—73.4) | 0.91 (0.87—0.95) | 55.9 (53.5—58.3) | 2.10 (1.89—2.34) | 12.4 (10.9—14.0) | 1.74 (1.36—2.23) |
| No Co-occurring condition | 78.2 (75.6—80.5) | Ref. | 26.6 (24.1—29.3) | Ref. | 7.1 (5.7—8.8) | Ref. |
PR = Prevalence ratio. PR in bold indicates statistically significant difference than reference group at the α=0.05 level.
Multiple imputation used to estimate value for respondents with missing data on household income (7.9% missing), parental education (0.9%), race/ethnicity (0.7%), and household language (0.5%).
Co-occurring conditions included parent-reported depression, anxiety, behavioral or conduct problems (such as oppositional defiant disorder or conduct disorder), autism spectrum disorder, developmental delay and intellectual disability (parent-reported).
Figure 1.
Parent-reported ADHD medication use in the past week among children (4–17 years) with special health care needs and ADHD: US, 2009–10
Past Year Behavioral Therapy
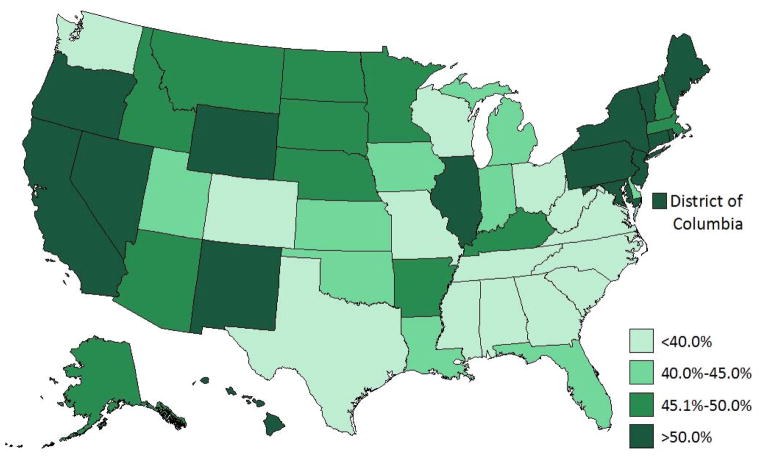
Among CSHCN with current ADHD, 44.0% (CI: 42.2—45.8) had received behavioral therapy in the past year. CSHCN with ADHD who were 12 years of age or older were less likely than the youngest children (4–5 years of age) to have received behavioral therapy in the previous year. CSHCN with current ADHD living below the federal poverty level were more likely to receive behavioral therapy than those above 200% of the federal poverty level. Other groups more likely to have received behavioral therapy included those that had public insurance (relative to non-public insurance), did not have a medical home, and had moderate or severe ADHD (compared to mild ADHD) (Table 2; Figure 2). Behavioral therapy in the past year was less common among non-Hispanic white CSHCN compared to other racial/ethnic groups and was less common in the South than in the West. CSHCN with ADHD and a co-occurring condition were twice as likely as those without a co-occurring condition to have had behavioral therapy in the past year. State-level prevalence of behavioral therapy in the past year among CSHCN with ADHD ranged from 32.5% (Tennessee) to 60.6% (Hawaii; Figure 2). There was an inverse correlation between the state-based estimates of past year behavioral therapy and past week medication for ADHD (r= −0.399, p <0.01).
Figure 2.
Parent-reported behavioral therapy for ADHD in the past year among children (4–17 years) with special health care needs and ADHD: US, 2009–10
Dietary Supplement Use
Of CSHCN with current ADHD, 10.2% (CI: 9.2—11.4) took dietary supplements for ADHD in the past year; 6.4% (CI: 5.6—7.4) in the past week. CSHCN with ADHD who lived in the Midwest (compared to the West) or were 12 years of age or older (compared to those 4–5 years) were less likely to have taken dietary supplements within the past year. Those who did not have a medical home and those with a co-occurring condition were more likely to have received dietary supplements within the past year (Table 2). Dietary supplement use was less common among those whose parents were high school graduates (compared to those who had more than a HS education), with household incomes below 100% of the federal poverty level (compared to those with a FPL over 200%), and had public insurance (relative to non-public insurance). State-level estimates of dietary supplement usage in the past year among CSHCN with ADHD ranged from 3.7% (Missouri) to 18.1% (Montana; Figure 3; online).
Figure 3.
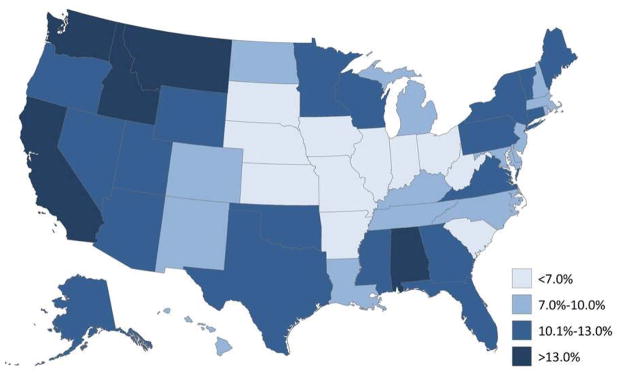
Parent-reported dietary supplement use for ADHD in the past year among children (4–17 years) with special health care needs and ADHD: US, 2009–10
Combination Therapy
Overall, 30.7% (CI: 29.1—32.4) of CSHCN with current ADHD were receiving both medication and behavioral therapy for ADHD (Table 3). The percentage of CSHCN receiving either or both medication treatment or behavioral therapy to treat ADHD was 87.3% (CI: 85.9—88.6); an additional 1.2% (CI: 0.9—1.6) were taking dietary supplements alone for ADHD treatment, such that 88.6% (CI: 87.2—89.8) of CSHCN with ADHD were receiving one or more of the three therapies. Of the potential combinations of medication and behavioral therapy, medication treatment alone was the most common current treatment modality overall and for most demographic subgroups (Table 3). Nearly half (43.3%) of CSHCN with ADHD in the following demographic groups were receiving medication treatment without behavioral therapy: non-Hispanic white CSHCN, CSHCN living in the Midwest or South, CSHCN with non-public health insurance, and CSHCN with mild ADHD; over half of CSHCN with a medical home or without a co-occurring condition were receiving medication without behavioral therapy.
Table 3.
Mutually exclusive combinations of past year behavioral therapy and past week medication treatment for attention-deficit/hyperactivity disorder (ADHD) among children with special health care needs aged 4–17 years who have parent-reported current ADHD by demographic subgroups, National Survey of Children with Special Health Care Needs: US, 2009–10
| Behavioral Therapy and Medication Treatment | Medication | Treatment Only | Behavioral | Therapy Only | Neither Behavioral Therapy Nor Medication Treatment | ||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | p-value1 |
| Overall | 30.7 | (29.1—32.4) | 43.3 | (41.6—45.1) | 13.3 | (12.1—14.6) | 12.7 | (11.4—14.1) | |
| Boys | 31.8 | (29.9—33.8) | 43.3 | (41.2—45.5) | 12.6 | (11.2—14.1) | 12.3 | (10.7—14.0) | |
| Girls | 28.2 | (25.3—31.3) | 43.3 | (40.1—46.6) | 15.0 | (12.6—17.7) | 13.5 | (11.4—16.0) | 0.14 |
| Age 4–5 | 21.2 | (15.1—29.1) | 25.4 | (17.4—35.5) | 31.9 | (23.5—41.8) | 21.4 | (13.5—32.4) | |
| Age 6–11 | 34.7 | (32.2—37.3) | 42.5 | (39.8—45.2) | 12.8 | (11.0—14.7) | 10.1 | (8.1—12.5) | <0.001 |
| Age 12–17 | 27.9 | (25.7—30.1) | 45.3 | (42.8—47.7) | 12.5 | (10.9—14.4) | 14.3 | (12.7—16.1) | |
| Non-Hispanic White2 | 29.9 | (28.1—31.8) | 48.3 | (46.3—50.3) | 11.0 | (9.8—12.3) | 10.8 | (9.6—12.1) | |
| Non-Hispanic Black2 | 32.3 | (27.7—37.2) | 34.5 | (29.6—39.7) | 17.1 | (13.5—21.5) | 16.2 | (12.4—20.9) | <0.001 |
| Hispanic2 | 33.8 | (28.1—40.0) | 35.7 | (30.0—41.9) | 17.0 | (12.5—22.7) | 13.5 | (10.0—18.0) | |
| Other2 | 29.0 | (23.6—35.1) | 30.7 | (25.4—36.7) | 19.6 | (14.8—25.5) | 20.7 | (13.1—31.1) | |
| Parental education | |||||||||
| Less Than High School2 | 32.3 | (26.2—39.0) | 42.3 | (35.7—49.1) | 8.7 | (5.9—12.6) | 16.7 | (12.4—22.3) | |
| High School Graduate2 | 29.9 | (26.5—33.5) | 44.7 | (40.8—48.6) | 13.1 | (10.5—16.2) | 12.3 | (10.0—15.1) | 0.10 |
| More Than High School2 | 30.7 | (28.9—32.6) | 43.1 | (41.1—45.1) | 14.2 | (12.7—15.9) | 12.0 | (10.5—13.7) | |
| <100% Poverty Level2 | 34.7 | (31.2—38.4) | 39.6 | (36.0—43.3) | 12.9 | (10.8—15.4) | 12.8 | (10.6—15.5) | |
| 100%-200% Poverty Level2 | 32.5 | (28.9—36.3) | 42.2 | (38.3—46.2) | 12.1 | (9.7—14.9) | 13.2 | (10.7—16.3) | 0.03 |
| >200% Poverty Level2 | 28.0 | (26.0—30.2) | 45.6 | (43.2—48.0) | 14.0 | (12.2—16.0) | 12.4 | (10.5—14.5) | |
| English as Primary Language2 | 30.4 | (28.8—32.0) | 44.1 | (42.3—45.9) | 13.1 | (11.9—14.5) | 12.4 | (11.1—13.9) | <0.001 |
| Other Language As Primary2 | 40.8 | (27.8—55.1) | 20.8 | (13.6—30.5) | 18.6 | (10.6—30.8) | 19.8 | (12.5—30.1) | |
| Northeast | 36.3 | (32.2—40.6) | 33.9 | (29.9—38.1) | 16.9 | (14.1—20.2) | 12.9 | (10.4—16.0) | |
| Midwest | 32.7 | (29.7—35.9) | 48.2 | (44.9—51.5) | 10.2 | (8.3—12.4) | 8.9 | (7.2—11.1) | <0.001 |
| South | 27.8 | (25.5—30.2) | 48.4 | (45.7—51.2) | 11.3 | (9.7—13.2) | 12.5 | (10.8—14.4) | |
| West | 29.9 | (25.5—34.8) | 32.8 | (28.7—37.3) | 19.2 | (15.2—24.0) | 18.1 | (13.6—23.7) | |
| Non-Public Insurance | 26.0 | (24.0—28.0) | 48.4 | (46.1—50.8) | 12.2 | (10.6—14.0) | 13.4 | (11.8—15.2) | |
| Public Insurance | 36.1 | (33.5—38.7) | 39.0 | (36.4—41.8) | 13.9 | (12.1—15.9) | 11.0 | (9.1—13.4) | <0.001 |
| No Insurance | 22.5 | (15.8—31.0) | 27.5 | (19.5—37.3) | 21.5 | (14.1—31.4) | 28.6 | (20.2—38.7) | |
| Medical Home | 25.5 | (23.3—27.9) | 55.8 | (53.1—58.4) | 8.8 | (7.4—10.4) | 9.9 | (8.4—11.7) | <0.001 |
| No Medical Home | 34.2 | (31.9—36.5) | 36.2 | (33.9—38.5) | 15.8 | (14.0—17.7) | 13.9 | (12.1—16.0) | |
| Mild ADHD | 16.6 | (14.6—18.7) | 48.2 | (45.3—51.2) | 15.1 | (12.9—17.7) | 20.1 | (17.7—22.7) | |
| Moderate ADHD | 32.6 | (30.2—35.2) | 44.5 | (41.8—47.1) | 13.0 | (11.2—14.9) | 10.0 | (8.4—11.7) | <0.001 |
| Severe ADHD | 49.6 | (45.3—53.9) | 33.1 | (29.3—37.2) | 10.9 | (8.5—13.8) | 6.4 | (3.6—11.2) | |
| Co-occurring condition3 | 38.9 | (36.7—41.2) | 32.3 | (30.1—34.6) | 17.0 | (15.3—18.9) | 11.8 | (10.1—13.8) | <0.001 |
| No Co-occurring condition | 18.7 | (16.6—21.0) | 59.5 | (56.7—62.2) | 7.9 | (6.3—9.9) | 13.9 | (12.1—16.0) | |
Chi-square p-value testing for difference in distribution among the four treatment categories (past year behavioral therapy and past week medication; past week medication alone; past year behavioral therapy alone; neither past week medication nor past year behavioral therapy) between demographic groups.
Multiple imputation used to estimate value for respondents with missing data on household income (7.9% missing), parental education (0.9%), race/ethnicity (0.7%), and household language (0.5%).
Co-occurring conditions included parent-reported depression, anxiety, behavioral or conduct problems (such as oppositional defiant disorder or conduct disorder), autism spectrum disorder, developmental delay and intellectual disability.
Descriptively, the CSHCN subgroups that had the highest rates of medication and behavioral therapy were those in the Northeast, with public insurance, with severe ADHD, and with a co-occurring condition (Table 3). The CSHCN subgroups with the highest rates of neither ADHD medication treatment nor behavioral therapy were children who were aged 4–5, of other races, spoke a primary language other than English, lived in the West, did not have health insurance, and had mild ADHD. There was not a statistically significant difference in treatment modalities between boys and girls.
Of CSHCN who were receiving both medication treatment and behavioral therapy, 14.2% (95% CI: 12.1%—16.5%) were also taking dietary supplements. Among CSHCN receiving medication but not behavioral therapy, 5.1% (95% CI: 4.1%—6.4%) also took dietary supplements, while 18.4% (95% CI: 14.4%—23.3%) of CSHCN receiving behavioral therapy but not medication took dietary supplements. Overall, 9.3% (95% CI: 6.9%—12.5%) of CSHCN who received neither medication nor behavioral therapy were taking dietary supplements.
Among CSHCN aged 4–5, 25.4% (95% CI: 17.4—35.5) were receiving medication treatment alone, 31.9% (95% CI: 23.5—41.8) were receiving behavioral therapy alone, and 21.2% (95% CI: 15.1—29.1) were receiving both treatments (Table 3). The remaining 21.4% (95% CI: 13.5—32.4) of CSHCN aged 4–5 received neither behavioral therapy nor medication.
Medication Treatment by Medication Class
Of CSHCN with ADHD who took medication for ADHD in the past week, 84.8% (CI: 83.3—86.3) took a central nervous system stimulant (CNSS) medication; a little more than half of these being methylphenidate formulations (55.8%; CI: 53.6—58.0). Of all CSHCN taking medication for ADHD, 47.3% (CI: 45.3—49.4) took methylphenidate formulations. The second most commonly reported medication class was selective norepinephrine reuptake inhibitors (SNRI), for the use of the SNRI atomoxetine, which was reported for 8.4% (CI: 7.4—9.6) of CSHCN who were taking medication for ADHD. A smaller proportion of children were taking medication from each of four other drug classes reported for the treatment of ADHD (atypical antipsychotics: 5.7%, CI: 4.7—6.8; SSRIs: 3.1%, CI: 2.4—4.0; centrally-acting alpha-agonist hypotensive agents (Clonidine): 4.1%, CI: 3.3—5.0; and centrally acting alpha-adrenergic receptor agonists (Guanfacine): 4.3%, CI: 3.6—5.2).
Discussion
This study establishes the relative national prevalence of reported ADHD treatments, specifically medication treatment and behavioral therapy, as well as the use of dietary supplements for the treatment of ADHD among US CSHCN in 2009–10. In this national sample of CSHCN, approximately 87% of children with parent-reported current ADHD were receiving either medication treatment or behavioral therapy, with an additional 1.2% receiving dietary supplements alone as an alternative treatment. Medication treatment alone was the most common treatment for ADHD, with nearly half of CSHCN with current ADHD taking medication alone for treatment. The most common drug class of ADHD medications was CNSS, with nearly 85% taking a medication in this drug class. The inverse relationship observed between state-level medication and behavioral treatment estimates informs previous discussions about the geographic variability in ADHD medication treatment (6, 16–18) and suggests that states that have lower state-wide estimates of behavioral therapy tend to have higher estimates of medication treatment.
ADHD treatment results in important and measurable improvements in the core symptoms of ADHD, with published effect sizes ranging from 0.6 for behavioral strategies and 0.7 for non-stimulant medications to 1.0 for stimulant medications.(3) In this study, just under one-third of CSHCN with ADHD were receiving both medication and behavioral therapy for ADHD. Children with severe ADHD and those with comorbidities were among the most likely to receive combination treatment. However, 56.0% of CSHCN had not received behavioral therapy in the past year. This is an important finding, given previous research suggesting the benefits of multi-modal (medication plus behavioral therapy) treatment for childhood ADHD on core and peripheral features of the disorder and AAP’s recommendation of combination therapy for children 6–11 years of age.(3, 19–21) Future research is warranted that investigates barriers to receipt of behavioral therapies for ADHD, including a lack of access due to eligibility requirements, lack of availability in the geographic area, or cost barriers (including insurance coverage).
This study revealed that non-Hispanic white CSHCN were more likely to receive medication treatment for ADHD, whereas CSHCN of all other races and ethnicities were more likely to receive behavioral therapy for ADHD, while rates of combination therapy (medication and behavioral therapy) were similar across racial groups. These findings are consistent with research suggesting more favorable perceptions of behavioral therapy and less favorable views of medications among black and Hispanic parents as compared to white parents.(22) Approximately one in five CSHCN of “other” races and children who were reported to speak a primary language other than English were neither receiving medication nor behavioral therapy for ADHD, representing a potential service gap.
For preschool-aged (4–5 years) CSHCN with an ADHD diagnosis the prevalence rates of each of the four treatment groups (medication alone, medication plus behavioral therapy, behavioral therapy alone, and neither behavioral nor medication treatment) were similar. Although recommended as a first-line treatment for ADHD,(3) half (53.2%) of preschool-aged CSHCN with ADHD received behavioral therapy in the past year. These data provide an important benchmark for clinical practice, as these data were collected in the years just before the release of the 2011 AAP diagnostic and treatment guidelines for ADHD.(5) AAP guidelines currently recommend that behavioral therapy should be used as first-line treatment for ADHD among preschoolers, with methylphenidate prescribed when persistent, significant functional impairment persists. A recent comparative effectiveness study identified four behavioral interventions for preschool-aged children with ADHD,(23) but these and other high quality interventions may not be available to children in medically underserved settings in which there is limited access. Ongoing surveillance and future research that studies ADHD practice patterns for preschoolers can contribute to understanding the barriers to the provision of behavioral services, which may currently limit clinical practice from being more closely aligned with best practices.
Strengths of this study include analysis of a large, nationally representative sample of CSHCN; collection of ADHD medication treatment by asking parents to read the name of the medication directly from available prescription bottles, thereby reducing recall bias; and concurrent collection of medication treatment, behavioral therapy, and dietary supplement use for ADHD treatment. However, the results should be considered within the context of certain limitations. The analyses were based on parent-reported data that were not clinically validated. As previously reported, the vast majority of children with ADHD meet CSHCN criteria (14), however approximately 13% of children with ADHD do not meet the criteria (24) and are therefore not represented by these data; included children with ADHD who met CSHCN criteria may be more impaired and could have different treatment patterns than children with ADHD who do not meet CSHCN criteria. The behavioral therapy question was somewhat broad, asking about “behavioral treatment for ADHD, such as classroom management, peer interventions, social skills training, or cognitive-behavioral therapy”; the data did not lend itself to differentiating treatment intensity or treatment quality and low intensity school-based interventions were therefore counted with intensive multi-month, structured behavioral therapy programs. Further, some children were likely receiving other treatments for ADHD that were not included in the survey (e.g., neurofeedback); therefore, the total prevalence of any treatment for ADHD is likely underestimated. Finally, the response rates of the NS-CSHCN (landline: 43.7%, cell-phone: 15.2%, combined: 25.5%) were low and could have led to biased results, despite the fact that weighting accounted for non-response.
This study represents the first nationally-representative US study to estimate behavioral treatment and dietary supplement use for the treatment of ADHD, alongside estimates of medication for ADHD treatment. The relative percentages reveal that ADHD medication was the most common single ADHD treatment in 2009–10, followed by combination therapy (medication and behavioral therapy), and then behavioral therapy alone. Future research can help improve understanding of the barriers to the provision of behavioral therapy for childhood ADHD, particularly among the preschool population.
Supplementary Material
Acknowledgments
Funding Source:
None other than salaries at the authors’ home institutions.
Abbreviations
- ADHD
attention-deficit/hyperactivity disorder
- CSHCN
children with special health care needs
Appendix. Categories of medications taken in past week to treat attention-deficit/hyperactivity disorder (ADHD) by parent report, National Survey of Children with Special Health Care Needs: 2009–10
| Drug Class* | Medications Included^ |
|---|---|
| Central nervous system stimulants (CNS Stimulants) | Adderall, Adderall XR, Amphetamine Concerta Daytrana Patch Dexedrine, Dexedrine Spansule, Dextrostat, Dextro-Amphetamine Demethylphenidate Focalin, Focalin XR Metadate, Metadate CD Methylin Methylphenidate Ritalin, Ritalin LA, Ritalin SR Vyvanse, Lisdexamfetmine |
| Selective norepinephrine reuptake inhibitors (SNRI) | Strattera, Atomoxetine |
| Selective serotonin reuptake inhibitors (SSRI) | Celexa, Citalopram Fluoxetine Prozac Sertraline Zoloft |
| Atypical antipsychotics | Abilify Risperdal, Risperidone, Risperdol |
| Centrally acting alpha-agonist hypotensive agents | Clonidine |
| Centrally acting alpha 2A adrenergic receptor agonists | Guanfacine, Intuniv, Tenex |
Drug class was determined from http://www.nlm.nih.gov/medlineplus/medlineplus.html
Seroquel, Trazadone, and Wellbutrin were reported by a small number of families, but were excluded from analyses due to low sample size.
Footnotes
CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Health Resources and Services Administration.
Conflicts of Interest:
None of the authors have any conflicts of interest to disclose.
Contributors’ Statements
Susanna N. Visser: Dr. Visser led the conceptualized and design of the study, interpreted the data for the work, drafted the initial manuscript, and led all manuscript revisions.
Melissa L. Danielson, Joseph Holbrook: Ms. Danielson and Dr. Holbrook participated in the design of the work, carried out the analyses, and critically reviewed and revised the manuscript.
Rebecca H. Bitsko and Laura Schieve: Drs. Bitsko and Schieve made substantial contributions to the early conceptualization of this work, participated in the interpretation of data for the work, and critically reviewed the manuscript for important intellectual content.
Reem Gandhour and Stephen J. Blumberg: Drs. Ghandour and Blumberg both participated in the acquisition and interpretation of the data for the work and critically reviewed and revised the manuscript for important intellectual content.
Mark Wolraich and Steven Cuffe: Drs. Wolraich and Cuffe participated in the design of the work, with an emphasis on grouping the medication treatment data for analysis and ensuring appropriate design to allow for the evaluation of the alignment of study outcomes to the AAP diagnostic guidelines, interpreted the data, and critically reviewed the manuscript for important intellectual content.
All coauthors provided final approval of the submitted version of this manuscript and agree to be accountable for all aspects of the work.
Financial Disclosure:
Dr. Cuffe is receiving support for research on navigated transcranial magnetic stimulation from Nextstim Oy. The other authors have no financial disclosures.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing; 2013. [Google Scholar]
- 2.McDonald DC, Jalbert SK. Geographic Variation and Disparity in Stimulant Treatment of Adults and Children in the United States in 2008. Psychiatric Services. 2013 Nov 1;64(11):1079–86. doi: 10.1176/appi.ps.004442012. Epub 2013/08/06. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–22. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics’ Subcommittee on Attention-Deficit/Hyperactivity Disorder Steering Committee on Quality Improvement and Management. Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, et al. ADHD: Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2011. 2011 Nov 1;128:1007–22. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, et al. Trends in the Parent-Report of Health Care Provider-Diagnosed and Medicated Attention-Deficit/Hyperactivity Disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. 2014;53:34–46.e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–42. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children — United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59:1439–43. [PubMed] [Google Scholar]
- 9.Zuvekas SH, Vitiello B. Stimulant medication use in children: A 12-year perspective. Am J Psychiatry. 2012;169:160–6. doi: 10.1176/appi.ajp.2011.11030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. Jama. 2000 Feb 23;283:1025–30. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]
- 11.Stevens J, Harman JS, Kelleher KJ. Ethnic and regional differences in primary care visits for Attention-Deficit Hyperactivity Disorder. Journal of Developmental & Behavioral Pediatrics. 2004;25:318–25. doi: 10.1097/00004703-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Pham AV, Carlson JS, Kosciulek JF. Ethnic Differences in Parental Beliefs of Attention-Deficit/Hyperactivity Disorder and Treatment. Journal of Attention Disorders 2010. 2010 May 1;13:584–91. doi: 10.1177/1087054709332391. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention National Center for Health Statistics State and Local Area Integrated Telephone Survey. 2009–2010 National Survey of Children with Special Health Care Needs Frequently Asked Questions. 2011 [updated December 2011]. Available from: http://www.cdc.gov/nchs/slaits/cshcn.htm.
- 14.Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002 Jan-Feb;2:38–48. doi: 10.1367/1539-4409(2002)002<0038:icwshc>2.0.co;2. Epub 2002/03/13. eng. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Family Physicians (AAFP), American Academy of Pediatrics (AAP), American College of Physicians (ACP), American Osteopathic Association (AOA) Joint Principles of the Patient Centered Medical Home. 2007. Feb, [Google Scholar]
- 16.Visser SN, Blumberg SJ, Danielson ML, Bitsko RH, Kogan MD. State-based and demographic variation in parent-reported ADHD medication rates, 2007–2008. Preventing Chronic Disease. 2013;10:20073. doi: 10.5888/pcd9.120073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulton BD, Scheffler RM, Hinshaw SP, Levine P, Stone S, Brown TT, et al. National variation of ADHD diagnostic prevalence and medication use: Health care providers and education policies. Psychiatric Services. 2009;60:1075–83. doi: 10.1176/ps.2009.60.8.1075. [DOI] [PubMed] [Google Scholar]
- 18.Arns M, van der Heijden KB, Arnold LE, Kenemans JL. Geographic Variation in the Prevalence of Attention-Deficit/Hyperactivity Disorder: The Sunny Perspective. Biological psychiatry. 2013 Oct 15;74:585–90. doi: 10.1016/j.biopsych.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Multimodal Treatment Study of Children with ADHD Cooperative Group. A 14-month randomized clinical trial of treatment strategies for Attention Deficit Hyperactivity Disorder. Archives of General Psychiatry. 1999 Dec;56:1073–86. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 20.Pelham WE, Gnagy EM. Psychosocial and combined treatments for ADHD. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:225–36. [Google Scholar]
- 21.Majewicz-Hefley A, Carlson JS. A Meta-Analysis of Combined Treatments for Children Diagnosed With ADHD. Journal of Attention Disorders 2007. 2007 Feb 1;10:239–50. doi: 10.1177/1087054706289934. [DOI] [PubMed] [Google Scholar]
- 22.Pham AV, Carlson JS, Kosciulek JF. Ethnic differences in parental beliefs of attention-deficit/hyperactivity disorder and treatment. J Atten Disord. 2010 May;13:584–91. doi: 10.1177/1087054709332391. Epub 2009/05/06. eng. [DOI] [PubMed] [Google Scholar]
- 23.Charach A, Dashti B, Carson P, Booker L, Lim CG, Lillie E, et al. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. [PubMed] [Google Scholar]
- 24.Visser SN, Danielson ML, Ghandour RM. Frequency of medication treatment, behavioral therapy, and dietary supplements among a national sample of children with special health care needs with ADHD. American Public Health Association Annual Meeting; October 29, 2012; San Francisco, CA. 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.