Abstract
Parainfluenza infection is a cause of significant morbidity and mortality in allogeneic hematopoietic stem cell transplant (HSCT) patients. DAS181 is a novel antiviral agent with activity against influenza and parainfluenza. We report the first 2 cases, to our knowledge, of successful DAS181 use in ventilated HSCT patients with severe parainfluenza lung disease.
Keywords: parainfluenza, DAS181, HSCT, mechanical ventilation
DAS181, a sialidase fusion protein, cleaves sialic acid-containing receptors used by influenza and parainfluenza viruses and prevents infection of respiratory cells. Parainfluenza virus (PIV) infection in hematopoietic stem cell transplant (HSCT) patients is a major cause of morbidity and mortality (1). DAS181 has been evaluated in phase 2 trials for influenza and is currently undergoing phase 2 investigation for parainfluenza in non-immunocompromised individuals (2, 3). The powder formulation of DAS181 is inhaled, and has been used successfully in ambulatory patients with allogeneic HSCT. However, this formulation does not allow for drug administration to intubated patients (4, 5). Herein, we report the first 2 cases to our knowledge of patients treated with DAS181 via a nebulized version of the drug, expanding its use to intubated HSCT patients with severe lower respiratory tract disease.
Case 1
A 64-year-old woman underwent a non-myeloablative allogeneic HSCT 6 years before this presentation. Her transplant was complicated by chronic graft-versus-host disease (GVHD) of her skin, conjunctivae, and oral mucosa that required ongoing immunosuppression with prednisone and sirolimus. She had been progressively ill with respiratory symptoms for 2 weeks before presentation, despite oral levofloxacin treatment for a presumed bronchitis exacerbation. She presented to an outside hospital with worsening dyspnea and diminishing exercise capacity, and developed atrial fibrillation with a rapid ventricular response.
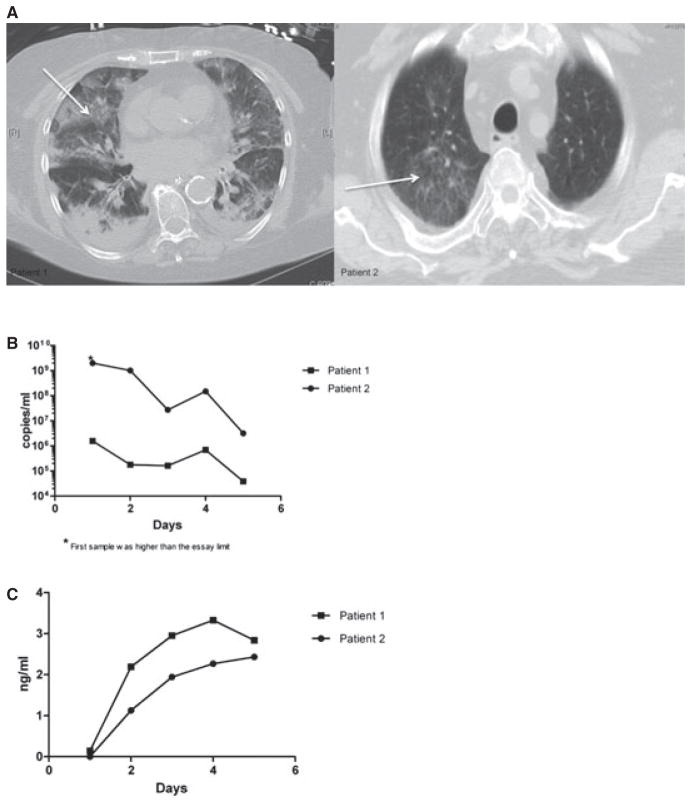
Subsequently, she was transferred to Brigham and Women’s Hospital and required mechanical ventilation. Her chest computed tomography (CT) on admission showed diffuse bilateral ground-glass opacities (Fig. 1A, left), and she was treated empirically for possible bacterial pneumonia. PIV-1 was detected by qualitative polymerase chain reaction (PCR) in nasopharyngeal swab and in bronchoalveolar lavage (BAL) specimens on hospital day (HD) 1 and HD 2 respectively.
Fig. 1.
(A) Computed tomography chest images upon admission from Case 1 (left) and Case 2 (right) demonstrated ground-glass opacities (arrows) in lung parenchyma. (B) Decrease in parainfluenza virus load by quantitative polymerase chain reaction in tracheal samples obtained daily during the 5-day treatment course on both patients. (C) Plasma concentration of DAS181 during the course of treatment remained low.
Because of the lack of improvement, despite empirical antibacterial treatment, on HD 6, Emergency Investigational New Drug (E-IND) permission for DAS181 treatment was obtained from the US Food and Drug Administration (FDA). With approval from the hospital’s institutional review board (IRB), consent was acquired from the patient’s healthcare proxy.
Planned duration of treatment with DAS181 was 5 days. A solution of DAS181 was made and delivered by an in-line Aeroneb® nebulizer (Aerogen, Galway, Ireland) at a dose of 3.2 mg during the first 3 days. Because of improvement in her respiratory status, she was extubated on the third day, and the final 2 doses of DAS181, at 4.5 mg each, were given via an Aeroneb® nebulizer and a face mask (Table 1). Treatment with DAS181 substantially decreased PIV load as measured by quantitative PCR (Fig. 1B).
Table 1.
Ventilation parameters improved with DAS181 treatment
| Case no.
|
Day
|
RR
|
PEEP
|
FiO2 (%)
|
SaO2 (%)
|
PIV Ag
|
PIV qPCR
|
|---|---|---|---|---|---|---|---|
| 1 | 1 | 20 | 5 | 40 | 94 | + | |
| 4 | 22 | Extubated | 100 | 94 | |||
| 5 | 20 | 100 | 92 | − | |||
| 2 | 1 | 22 | 12 | 50 | 93 | + | |
| 4 | 24 | 10 | 40 | 97 | |||
| 5 | 20 | 8 | 40 | 97 | − | ||
| 9 | 17 | Extubated | 40 | 96 | − |
RR, respiratory rate in breaths per minute; PEEP, positive end expiratory pressure; FiO2, fraction of inspired oxygen; SaO2, oxygen saturation measured by pulse oximetry; PIV Ag, parainfluenza virus antigen detection test; PIV qPCR parainfluenza qualitative polymerase chain reaction assay.
A surveillance nasopharyngeal swab obtained after treatment completion was negative for PIV by qualitative PCR. Plasma drug levels were obtained daily while she was receiving DAS181 and ranged from 2.19 to 3.43 ng/mL (Fig. 1C). The only treatment-related and expected effect noted was an asymptomatic increase in her serum alkaline phosphatase (ALP), which peaked at 240 mg/dL on day 6 following DAS181 initiation.
Three days after completing DAS181, her respiratory status worsened; pulmonary complications of GVHD, secondary nosocomial pneumonia, pulmonary emboli and PIV recurrence were considered. However, she declined re-intubation and any further diagnostic testing; her treatment goals were transitioned to comfort care. She passed away on HD 20.
Case 2
A 69-year-old man with myelodysplastic syndrome underwent HSCT 8 months before this presentation. Transplant conditioning was done with busulfan, fludarabine, and anti-thymocyte globulin. His course was complicated by GVHD, for which he was on prednisone 10 mg daily. A bone marrow biopsy 4 months after HSCT demonstrated recurrence of myelodysplasia, and he was taken off immunosuppression, while prophylaxis with acyclovir, fluconazole, and atovaquone continued.
He presented to Beth Israel Deaconess Medical Center with fever, hemoptysis, and respiratory distress, and was found to be hemodynamically unstable. Chest CT demonstrated ground-glass opacities in the right upper lobe (Fig. 1A, right), and he required mechanical ventilation. He was started on empirical antimicrobial therapy, which included vancomycin, imipenem-cilastatin, and voriconazole. On HD 4, PIV-3 antigen was detected from BAL fluid via direct immunofluorescence staining using monoclonal antibodies specific for PIV-3. Cultures from multiple blood, urine, and BAL samples did not demonstrate other pathogens.
Because of the lack of clinical improvement with supportive treatment and confirmation of ongoing PIV-3 infection on repeat BAL sampling, an E-IND for DAS181 was approved by the FDA and IRB, and consent was granted by the patient’s healthcare proxy. On HD 5, DAS181 was administered via nebulizer, while the patient continued to be on ventilation for a 5-day course. A DAS181 solution was made and instilled via an Aeroneb® in-line nebulizer. Initial dose was 3.2 mg daily for the first 3 days, and was increased to 4.5 mg daily during the last 2 days of treatment. His ventilation parameters, including respiratory rate, positive end-expiratory pressure, and oxygenation, progressively improved and he was successfully extubated 3 days after completing this treatment (Table 1).
During the treatment course, tracheal aspirates were obtained daily and PIV-3 load was quantified by PCR and decreased substantially while on treatment with DAS181 (Fig. 1B). On the last day of treatment, viral antigen was no longer detectable in tracheal aspirates by immunofluorescence staining, and viral culture remained negative. Drug levels in plasma were closely monitored and remained within a low level, 1.13–2.43 ng/mL (Fig. 1C). No adverse effects were observed, other than slight ALP elevation, peaking at 169 mg/dL, with no associated elevation of aminotransferases or bilirubin. The patient was discharged home after a prolonged hospital stay.
Discussion
PIV infections in HSCT patients cause substantial morbidity and mortality, and have no effective treatments (1, 6). Ribavirin and intravenous immunoglobulins have been used with no proven clinical benefit, and in vitro data have not been encouraging regarding the use of available neuraminidase inhibitors in PIV infections (1, 7).
DAS181 is a novel inhaled agent, currently undergoing evaluation for influenza and parainfluenza infections (2, 3). DAS181 is a fusion molecule, consisting of a sialidase catalytic domain, obtained from Actinomyces viscosus, bound to a surface anchoring sequence, which is critical to prolonging the drug’s attachment to respiratory epithelial cells (8, 9). Cleavage of sialic acid interferes with viral entry, preventing infection of epithelial cells (8, 9). DAS181 is the first antiviral compound that functions by hindering a host-pathogen interaction via the blockade of host-cell sialic acid receptors, located on the surface of respiratory cells (3, 4).
We report the first 2 cases, to our knowledge, of DAS181 use to treat HSCT patients with severe PIV lower respiratory tract disease requiring mechanical ventilation. Drug delivery was accomplished via a nebulized version of the drug, unlike published reports with ambulatory patients who received a powder formulation (4, 5, 10). Administration took place via the endotracheal route or with a face mask once daily. Both patients tolerated drug delivery well without any bronchospasm. Although a 5-day course was given to both patients, optimal treatment duration is yet to be determined.
In Case 1, PIV recurrence was considered in the differential of her clinical deterioration, and recurrence has been reported in an allogeneic HSCT patient following DAS181 treatment. Thus, it is possible that longer treatment duration may reduce risk of relapse in these critically ill patients (4). Both patients metabolized the drug well, and plasma concentrations remained at a low level, indicating drug delivery to the lung with low-level systemic absorption.
Asymptomatic elevations in ALP, with baseline normalization post treatment, are known to occur with DAS181 administration. These elevations have been attributed to reduced ALP clearance, resulting from increased protein desialylation from systemic exposure to the drug (2).
A significant decrease of PIV in tracheal aspirates and nasopharyngeal swabs was observed in both patients, correlating with clinical improvement. This use of PCR as a virologic endpoint has been validated by recent influenza studies as a reliable, sensitive, dynamic, and quantifiable test in the clinical setting of viral respiratory tract infections (11). However, in Case 2, while the PCR quantified 106 copies/mL of PIV in the tracheal aspirate on day 5 of treatment, a viral culture from the same sample was negative. This disparity may suggest the presence of nonviable virus. Therefore, PCR, immunohistochemistry staining, and viral cultures may be used in combination to determine virologic endpoints (2, 11). As with our patients, previously published investigational-use cases of DAS181 are reporting promising outcomes (4, 5, 10). Our findings support further evaluation of DAS181 in ventilated immunosuppressed patients with PIV infection.
References
- 1.Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119(12):2738–2745. doi: 10.1182/blood-2011-08-371112. quiz 2969. [DOI] [PubMed] [Google Scholar]
- 2.Moss RB, Hansen C, Sanders RL, Hawley S, Li T, Steigbigel RT. A phase II study of DAS181, a novel host directed antiviral for the treatment of influenza infection. J Infect Dis. 2012;206(12):1844–1851. doi: 10.1093/infdis/jis622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Allergy and Infectious Diseases. DAS181 in Patients with Parainfluenza. Bethesda, MD: ClinicalTrials.gov; 2013. Available at: http://clinicaltrials.gov/ct2/show/NCT01441024. [Google Scholar]
- 4.Chen YB, Driscoll JP, McAfee SL, et al. Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clin Infect Dis. 2011;53(7):e77–e80. doi: 10.1093/cid/cir501. [DOI] [PubMed] [Google Scholar]
- 5.Guzman-Suarez BB, Buckley MW, Gilmore ET, et al. Clinical potential of DAS181 for treatment of parainfluenza-3 infections in transplant recipients. Transpl Infect Dis. 2012;14(4):427–433. doi: 10.1111/j.1399-3062.2012.00718.x. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–578. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 7.Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115(7):1688–1698. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicholls JM, Moss RB, Haslam SM. The use of sialidase therapy for respiratory viral infections. Antiviral Res. 2013;98(3):401–409. doi: 10.1016/j.antiviral.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscona A, Porotto M, Palmer S, et al. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J Infect Dis. 2010;202(2):234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drozd DR, Limaye AP, Moss RB, et al. DAS181 treatment of severe parainfluenza type 3 pneumonia in a lung transplant recipient. Transpl Infect Dis. 2013;15(1):E28–E32. doi: 10.1111/tid.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ison MG, de Jong MD, Gilligan KJ, et al. End points for testing influenza antiviral treatments for patients at high risk of severe and life-threatening disease. J Infect Dis. 2010;201(11):1654–1662. doi: 10.1086/652498. [DOI] [PubMed] [Google Scholar]