Summary
Factor (F)VIIIa, a heterotrimer comprised of A1, A2, and A3C1C2 subunits, is labile due to the tendency of the A2 subunit to dissociate from the A1/A3C1C2 dimer. As dissociation of the A2 subunit inactivates FVIIIa activity, retention of A2 defines FVIIIa stability and thus, FXase activity. Earlier results showed that replacing residues D519, E665, and E1984 at the A2 domain interface with Ala or Val reduced rates of FVIIIa decay, increasing FXa and thrombin generation (Wakabayashi et al., Blood 112: 2761, 2008). We now show the enhanced FVIIIa stability of these variants results from increases in inter-A2 subunit affinity. Using a FVIIIa reconstitution assay to monitor inter-subunit affinity by activity regeneration, the apparent Kd value for the interaction of wild type (WT) A2 subunit with WT A1/A3C1C2 dimer (43 ± 2 nM) was significantly higher than values observed for the A2 point mutants D519A/V, E665A/V, and E1984A/V which ranged from ∼5 to ∼19 nM. Val was determined to be the optimal hydrophobic residue at position 665 (apparent Kd = 5.1 ± 0.7 nM) as substitutions with Ile or Leu at this position increased the apparent Kd value by ∼3- and ∼7-fold, respectively. Furthermore, the double mutant (D519V/E665V) showed an ∼47-fold lower apparent Kd value (0.9 ± 0.6 nM) than WT. Thus these hydrophobic mutations at the A2 subunit interfaces result in high binding affinities for the A2 subunit and correlate well with previously observed reductions in rates in FVIIIa decay.
Keywords: Factor VIII, Factor VIIIa, inter-subunit binding affinity, protein stability
Introduction
Factor (F)VIII is a plasma protein that is absent or defective in patients with hemophilia A. FVIII is translated as a single chain polypeptide that undergoes post-translational modification and cleavage to produce the heterodimer that circulates in plasma. The heterodimer is made up of a heavy chain (HC) consisting of the A1(a1)A2(a2)B domains and a light chain (LC) consisting of the (a3)A3C1C2 domains. HC and LC are associated in a relatively stable, non-covalent and copper-dependent interaction at the A1-A3C1C2 interface. FVIII is activated by limited proteolysis catalyzed by thrombin and FXa that produces the active FVIIIa heterotrimer made up of the A1, A2, and A3C1C2 subunits. The FVIIIa heterotrimer serves as a cofactor to FIXa in the propagation stage of clotting. FVIIIa and FIXa assemble into the FXase complex on a negatively charged phospholipid surface, and FVIIIa increases the catalytic efficiency of FIXa in the conversion of FX to FXa by several orders of magnitude (See Ref. (1) for review).
FVIIIa is inactivated as the A2 subunit spontaneously dissociates from the A1/A3C1C2 dimer at physiologic concentrations. Weak electrostatic interactions between A2 and both A1 and A3C1C2 subunits contribute to the instability of the FVIIIa heterotrimer. Dissociation of the A2 subunit leads to dampening of FXase activity (2,3). The mechanism of A2 dissociation is not well defined. Several point mutations to FVIII at the A2-A1 and A2-A3C1C2 interfaces have been shown to promote A2 dissociation in FVIIIa (4-6). These FVIII variants are characterized by a one-stage/two-stage assay discrepancy (7,8) in which the latter assay, which examines FVIIIa stability, reveals significant reductions in activity compared to WT FVIIIa due to increased rates of dissociation of the A2 subunit.
Using the ceruloplasmin-based homology model (9), we previously identified acidic residues localized in hydrophobic pockets at the A2-A1 and A2-A3C1C2 interfaces with spatial separations from nearby potential hydrogen-bond partners exceeding 2.8 Å, suggesting that they did not contribute to hydrogen bond interactions at the A2 interface (10). Thus, these residues that include D519, an A2 residue at the A2-A1 interface, E665, an A2 residue at the A2-A3C1C2 interface, and E1984, an A3 residue at the A2-A3C1C2 interface were proposed to destabilize the inter-A2 subunit interaction. Mutating these residues individually to either Ala of Val in an attempt to increase the buried hydrophobic surface area and decrease the buried hydrophilic surface area at the A2 interface showed that the D519A/V, E665A/V and E1984A/V variants had greater FVIII stability to thermal and chemical denaturation, and reduced rates of FVIIIa decay (10,11). This latter parameter reflected prolonged FXase activity and consequent greater amounts of thrombin in a thrombin generation assay. Furthermore, a variant in which both D519 and E665 were mutated to Val (D519V/E665V) showed the most dramatic increases in FVIII and FVIIIa stability and thrombin generation as compared with variants representing all possible double mutation combinations at these three sites (11). In this current study, we show by a functional, FVIIIa reconstitution assay that the high FVIIIa stability observed in these variants results from a greater affinity for the A2 subunit.
Materials and methods
Nomenclature
The FVIII protein sequence is numbered from the mature N-terminus using reference sequence NP000123.1. According to Human Genome Variation Society (HGVS), FVIII variants designated in the text at residues D519, E665, and E1984 correspond to p.D538, p.E684, and p.E2003 respectively (12).
Materials
Recombinant FVIII (Kogenate) was a generous gift from Dr. Lisa Regan of Bayer (Berkeley, CA). Phospholipid vesicles containing 20% phosphatidylcholine (PC), 50% phosphatidylethanolamine (PE), and 30% phosphatidylserine (PS) were prepared as previously described(13). The reagents α-thrombin (Haematologic Technologies, Inc., Essex Junction, VT, USA), FIXa-β and FX (Enzyme Research Laboratories, South Bend, IN, USA), and the chromogenic FXa substrate Pefachrome Xa (Pefa-5523, CH3OCO-D-CHA-Gly-Arg-pNA AcOH; Centerchem, Norwalk, CT) were purchased from the indicated vendors.
Construction, Expression, and Purification of WT A1 and A3C1C2, and Variant A3C1C2 Subunits
WT A1 and A3C1C2 subunits were purified from Kogenate FVIII as previously described (14). FVIII mutants with Ala and Val mutations at E1984 were individually constructed as B-domainless FVIII (15). Recombinant WT and variant FVIII forms were stably expressed in baby hamster kidney (BHK) cells and purified as described previously (16). After transfection, there were no significant differences in the amounts of FVIII secreted among the variants. E1984A FVIII and E1984V FVIII were cleaved with thrombin (60 nM), and each thrombin-activated FVIII was incubated overnight with EDTA (50 mM) to separate the A1 and A3C1C2 subunits (17). The A1 subunit was removed by a heparin column (18), and A2 was removed by an affinity column using immobilized R8B12 monoclonal antibody (2). A3C1C2 subunit was further purified and concentrated on a MonoS column (GE Healthcare) equilibrated with 10 mM MES, pH 6.0, 0.1 M NaCl, 0.01% Tween20, and 0.02% NaN3 (buffer A). The adsorbed A3C1C2 subunit was eluted from the MonoS column using an NaCl gradient (0 – 0.8 M) in 20 mM HEPES, pH 7.2, 0.01% Tween20, 0.02% NaN3. Alternatively, E1984A A3C1C2 was purified from FVIII by removing A2 with the R8B12 affinity column after thrombin (60 nM) digest, and removing contaminants and A1 using a MonoS column as described above. Purity and concentration were determined by band density quantification using gels stained from SDS-PAGE or Western blots. Protein concentrations were verified by Bradford assay using bovine serum albumin (BSA) as a standard (19).
Construction, Expression and Purification of WT and Variant A2 Subunits
Amino acid substitutions in the isolated A2 domain were introduced in the pFastBac-wtA2 plasmid, in which A2 contains a TEV cleavage site and His6 tag (20), using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). D519 and E665 were each replaced with Ala and Val as single mutations, and a double mutant was constructed in which both D519 and E665 were replaced with Val. In addition, E665 in A2 was also replaced with Leu, Ile, and Phe. Baculovirus expressed-A2 (bA2) constructs were prepared and purified from High-Five cells (Invitrogen) using the Bac-to-Bac Baculovirus Expression system (Invitrogen) as previously described (20). Briefly, WT bA2 and each bA2 variant were purified from the supernatant of baculovirus infected High-Five cells. The supernatant was concentrated on a heparin column, and bA2 was isolated by the His6 tag on a Talon™ IMAC column. AcTEV protease was used remove the His6 tag by cleavage at the TEV site, and the protease was removed and protein concentrated by column chromatography. The purity of bA2 and concentration were determined by band density quantification of stained gels from SDS-PAGE or Western blots. Protein concentrations were verified by Bradford assay using BSA as a standard (19).
Purity and Concentration by SDS-PAGE Gel Staining and Western Blotting Band Density Quantification
FVIII subunits were electrophoresed on a 10% polyacrylamide gel at constant voltage. Gels were stained with Gelcode Blue (Thermo Electron Corporation, Waltham, MA) or transferred to a polyvinyl fluoride membrane to be probed with anti-A2 (R8B12) and anti-A3 (2D2) monoclonal antibodies. The chemifluorescence substrate (ECF substrate; GE Healthcare, Piscataway, NJ, USA) reaction with a secondary antibody (anti-mouse IgG linked to alkaline phosphatase) was detected by a fluorescence signal using a phosphoimager (Storm 860; GE Healthcare). The band densities from dilutions of each purified subunit and standards were quantified and were used to determine subunit concentration. Protein concentrations were verified by Bradford assay using BSA as a standard (19).
Reconstitution of A1/A3C1C2 from Purified Subunits
A1/A3C1C2 was reconstituted using 240 nM or 480 nM A1 subunit and 1 μM A3C1C2 subunit in 20 mM HEPES pH 7.2, 100 mM NaCl, 5 mM CaCl2, 0.01% Tween20, and 100 μg/mL of BSA overnight at 4 °C. The reconstituted A1/A3C1C2 dimer was then incubated at 37°C for 2 hours before use in FVIIIa reconstitution. Alternatively, 20 nM A3C1C2 subunit was reconstituted with 500 nM of A1 subunit and was incubated overnight at 4°C. As the Kd for the interaction between A1 and A3C1C2 is approximately 60 nM (21), A1/A3C1C2 was reconstituted using a molar excess of one subunit over the other to ensure full reconstitution. The concentration of the limiting component was used as the dimer concentration.
Chromogenic FXa Generation Assays
The rate of conversion of FX to FXa was monitored in a purified system at 23°C. Reconstituted FVIIIa (2 nM) was added to a reaction mixture of 40 nM FIXa and 20 μM PSPCPE in 20 mM HEPES pH 7.2, 100 mM NaCl, 5 mM CaCl2, 0.01% Tween20, and 100 μg/mL BSA. After 2 minutes, 300 nM FX was added to the mixture, and the reaction was stopped with 100 mM EDTA after an additional 2 minutes. FXa generated was determined using the FXa chromogenic substrate, Pefachrome Xa (0.46 mM final concentration). Reactions were read for 3 minutes at 385 nm using a Vmax microtiter plate reader (Molecular Devices).
A2 Subunit Affinity for A1/A3C1C2 by Reconstitution Assay
WT and variant bA2 (20 nM) were reconstituted with indicated concentrations of the A1/A3C1C2 dimer. Alternatively, WT FVIIIa and FVIIIa mutants with a substitution in the A3C1C2 subunit were first reconstituted with A1 subunit as described above and then the reconstituted A1/A3C1C2 dimer (20 nM) was reacted with varying concentrations of A2 subunit. Reconstitution reactions were incubated at room temperature for 2 hours. The rate of FXa generated per nM of A1/A3C1C2 or nM of A2 was determined using a FXa generation assay.
Data Analysis
Kd and activity parameters for FXa generation were calculated from initial rate data by fitting the data using non-linear least-squares regression analysis to a single-site ligand binding model using the equations,
where AT is the total concentration of A2, A is the concentration of free A2, AB is the concentration of FVIIIa which is proportional to FXa generation rate, BT is the total concentration of A1/A3C1C2 and K is a conversion factor from the concentration of reconstituted FVIIIa to FVIIIa activity measured by FXa generation. Since Kd values are obtained from curve fits of functional data, we refer to these as apparent Kd values.
Results
bA2 subunits
We have previously observed that mutations, individually or in combination, to convert acidic residues buried in hydrophobic pockets at the interface of the A2 domain to Ala or Val result in marked reductions in the rate of FVIIIa decay. As such, we would predict proteins with these mutations to show enhanced affinity for the A2 subunit, and this change in affinity should correlate with the magnitude reduction in FVIIIa decay. To test this hypothesis, we assessed the inter-A2 subunit affinity following a titration of FVIIIa reconstitution using a FVIIIa subunit harboring the mutation recombined with the complementary WT subunits.
For mutations at D519 and E665, both of which localize to the A2 domain and interface with the A1 and A3 domains respectively, variants were prepared using a baculovirus expression system as described in Methods and materials. We have previously shown that WT A2 expressed in this system yields equivalent results to A2 subunit purified from FVIIIa expressed in mammalian (BHK) cell culture (20). The bA2 variants D519A, D519V, E665A, E665V and the double mutant, D519V/E665V, were purified as described above and subjected to SDS-PAGE to assess their electrophoretic mobility and purity. Results shown in Figure 1 indicate that each of the variants migrates to a position that is essentially indistinguishable from the A2 subunit (∼43 kDa) purified from FVIIIa and shows the presence of little if any contaminating proteins.
Figure 1. SDS-PAGE of purified WT bA2 and bA2 variants.
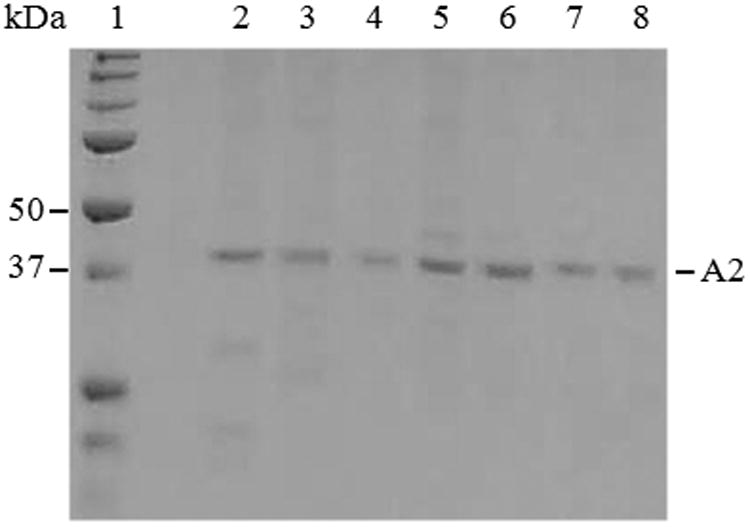
Approximately 0.5 μg of FVIIIa-derived A2 subunit and the WT and variant bA2 forms were applied to a 10% polyacrylamide gel with a molecular weight marker (lane 1), and bands were visualized using GelCode blue. Samples are A2 derived from FVIIIa (lane 2), WT bA2 (lane 3), and representative bA2 variants: D519A (lane 4), D519V (lane 5), E665A (lane 6), E665V (lane 7), and D519VE665V (lane 8).
Binding affinity between bA2 subunit and WT A1/A3C1C2 dimer determined by reconstitution assays
Factor VIIIa reconstitution assays used a fixed concentration of the WT or variant bA2 forms combined with variable concentrations of the A1/A3C1C2 dimer that had been previously reconstituted from the WT isolated A1 and A3C1C2 subunits. The reason for using reconstituted A1/A3C1C2 rather than A1/A3C1C2 isolated as the dimer from WT FVIIIa was to eliminate any possible contamination of A2 subunit in the A1/A3C1C2 preparation. Indeed FXa generation assays using high concentrations of the reconstituted A1/A3C1C2 alone showed essentially no activity in the absence of added A2 subunit (data not shown).
FVIIIa reconstitution reactions were assessed by FXa generation assays as described in Methods and materials. Results from assays where bA2 (20 nM) was titrated with varying concentrations of A1/A3C1C2 dimer are shown in Figure 2. WT bA2 and each bA2 variant yielded a saturable level of FXa generated in the presence of excess dimer. We observed that the variants demonstrated maximal levels of FXa generation at lower dimer concentrations; with the D519V/E665V bA2 double mutant achieving maximal FXa generation at the lowest level of added dimer. These activity curves were fitted to a single-site ligand binding model to estimate the inter-A2 subunit affinity for each variant and results are presented in Table 1.
Figure 2. A2 binding in FVIIIa variants with mutations in A2 subunit by reconstitution assay.
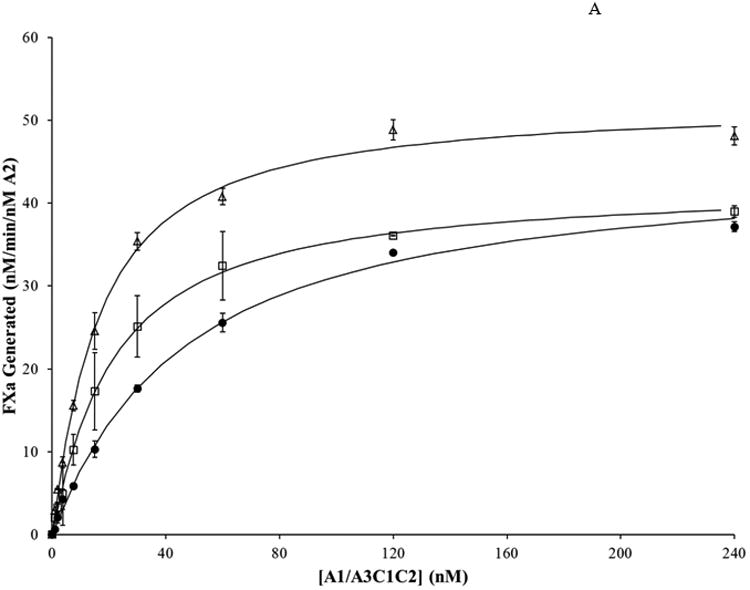
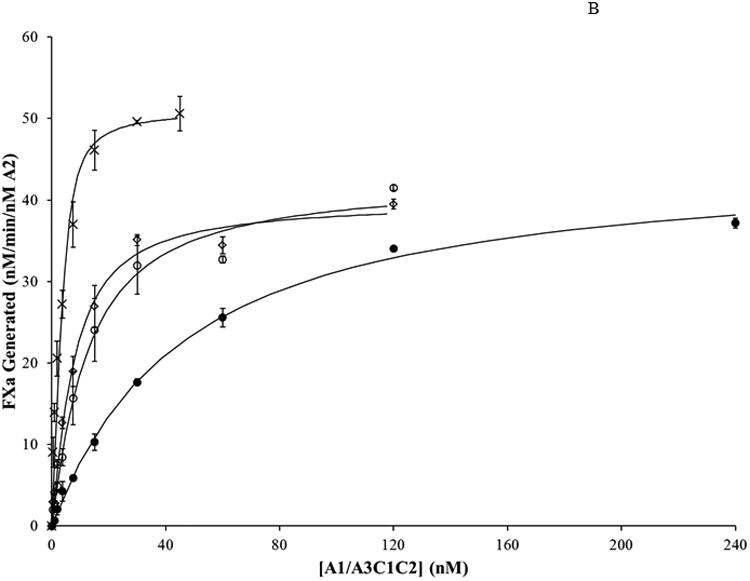
WT FVIIIa and FVIIIa mutants with a substitution in the A2 subunit were reconstituted at varying A1/A3C1C2 concentrations. WT and bA2 variants (20 nM) were reconstituted with the indicated concentrations of A1/A3C1C2 prepared in the presence of excess (1 μM) A3C1C2. Reconstitution reactions were incubated at room temperature for 2 hours. Rates of FXa generated per nM of A2 were determined and the data fitted to a single site binding equation as described in Methods and materials. Results were averaged from 2 (D519A, E665A) or 3 (WT, D519V, E665V, D519VE665V) separate determinations. A. WT, closed circles; D519A, open squares; E665A, open triangles; B. WT, closed circles; D519V, open circles; E665V, open diamonds; D519V/E665V, crosses.
Table 1. Apparent Kd of A2 in WT FVIIIa and FVIIIa variants.
| FVIIIa subunit mutation(s) | Kd (app) | Kd (app) Fold difference to WT | Bmax |
|---|---|---|---|
| nM | nM/min/nM A2 | ||
| WT (A2) | 43.3 ± 2.29 | 1.00 | 45.2 ± 0.68 |
| D519A | 18.9 ± 0.83 | 0.44 | 42.3 ± 0.50 |
| D519V | 10.0 ± 1.58 | 0.23 | 42.7 ± 1.70 |
| E665A | 13.8 ± 1.05 | 0.32 | 52.3 ± 0.97 |
| E665V | 5.14 ± 0.72 | 0.12 | 40.0 ± 1.13 |
| E665L | 44.0 ± 2.14 | 0.98 | 31.4 ± 0.52 |
| E665I | 17.5 ± 3.43 | 0.40 | 50.7 ± 1.21 |
| D519V/E665V | 0.93 ± 0.55 | 0.02 | 51.2 ± 3.19 |
| nM/min/nM A1/A3C1C2 | |||
| WT (A3C1C2) | 56.3 ± 2.59 | 1.00 | 47.4 ± 0.62 |
| E1984A | 15.3 ± 1.12 | 0.29 | 62.2 ± 0.95 |
| E1984V | 14.2 ± 2.13 | 0.25 | 75.2 ± 2.27 |
Apparent (app) Kd for WT FVIIIa and FVIIIa variants were determined as described in “Methods and materials.” Bmax values represent rates of FXa generation at saturating FVIIIa subunit. Results were averaged from 2-4 separate determinations and are presented with standard deviation values.
Affinity (Kd) values presented represent a functional, apparent Kd value inasmuch as this assay does not directly measure the interaction between A2 subunit and A1/A3C1C2 dimer, but rather measures the extent of FVIIIa reconstitution following assembly of the FXase complex and catalysis of FXa. We observed an apparent Kd value of ∼43 nM for the WT bA2 interaction which is similar to a value determined in a prior report using a similar assay (∼30 nM (20)). A very early study determined the affinity of the A1/A3C1C2 for the A2 subunit to be ∼260 nM (14). We attribute this discrepancy with the more recent studies to the lower quality of the reagents used. The inter-subunit Kd of 260 nM for A2 was determined using an A2 subunit that was purified using a somewhat cumbersome and lengthy procedure from the FVIIIa trimer (14). We believe this method was somewhat detrimental to the activity of the A2 protein. We currently use a much improved purification scheme for the A2 subunit from FVIIIa (20), as well as the A2 obtained from the baculovirus expression system, the latter of which provides A2 subunit free from any contaminating A1 and A3C1C2 subunits. Each of the single bA2 variants showed reduced Kd values relative to WT with D519A, D519V, and E665A values reduced by ∼2.3-, ∼4.3-, and ∼3.1-fold, respectively. Furthermore, the Val replacement for E665 showed a greater reduction in apparent Kd than the Ala replacement at this site (∼8.4 and 3.1-fold reductions, respectively). However, the D519V/E665V double mutant showed the highest inter-A2 subunit affinity with an apparent Kd that was reduced ∼47-fold relative to the WT bA2. This latter result indicated that replacing acidic residues with hydrophobic ones at A2 domain interface with A1 and A3 domains yields a greater inter-A2 subunit affinity than either substitution individually. Maximal levels of FXa generated for these point mutation variants did not appreciably differ from that of WT, whereas the value for the double mutation was modestly increased.
Binding affinity for additional E665 variants
Results shown above indicate that the E665V bA2 variant possessed the highest inter-subunit affinity of the point mutants tested, and this affinity was ∼2-fold greater than that of the E665A variant. Additionally, a previous study (10) showed that E665V had the slowest FVIIIa decay rate of the point mutants at 4 nM FVIIIa, and this decay rate was ∼3-fold slower than that of E665A. These observations suggest a contribution of residue size in addition to hydrophobic character for the enhanced affinity at E665 relative to WT. While a Val mutation at D519 resulted in a similar ∼2-fold increase in A2 affinity over the Ala mutation, D519V and D519A FVIIIa decay rates were relatively indistinguishable (70% and 60% of WT, respectively) (10). To further explore contribution of residue size at E665, the bA2 variants were constructed to replace the Glu residue with Leu, Ile or Phe, thereby increasing the specific volume of the residue side chain compared with Val. While the expression levels for E665I and E665L bA2 proteins were similar to the WT and other variants, the E665F variant did not express, suggesting that a Phe residue at this site may not be tolerable for A2 expression.
In experiments similar to those described above, the E665L and E665I bA2 subunits were titrated with variable levels of A1/A3C1C2 dimer. Subsequently, rates of FXa generated in each of the reactions were determined using a FXa generation assay. The FXa generation curves were fitted to a single-site binding equation (Figure 3) to determine the apparent Kd values for the inter-A2 subunit affinity (Table 1). Replacing E665 with Leu did not significantly alter the affinity of A2 for A1/A3C1C2, whereas replacing E665 with an Ile yielded a modest (2-fold) increase in affinity. These results suggest that of the hydrophobic residues tested, Val at position 665 is optimal for inter-subunit affinity. Interestingly, the extent of maximal FXa generated for the E665L variant was ∼30% lower than that of WT. This effect may result from the extended structure of the Leu side chain negatively impacting orientation of A2 subunit within the FXase complex.
Figure 3. A2 binding in FVIIIa variants with E665L and E665I mutations.
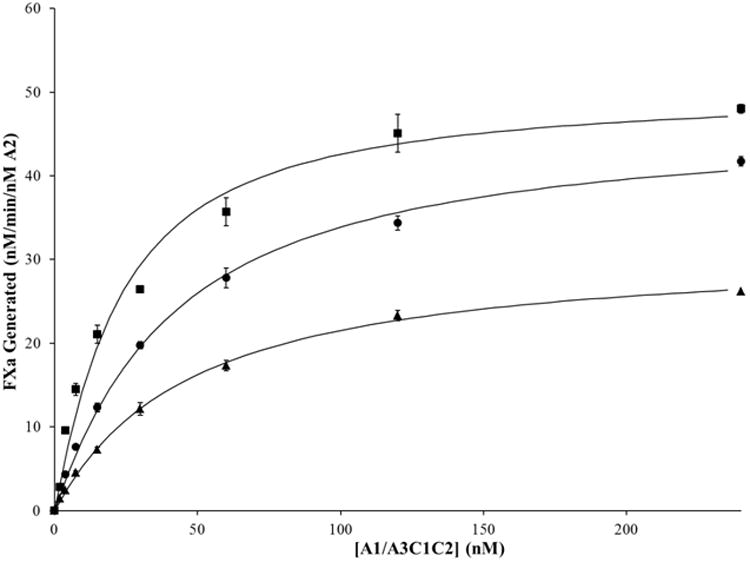
WT FVIIIa and FVIIIa mutants with a substitution in the A2 subunit were reconstituted from WT and bA2 (20 nM) and the indicated concentrations of A1/A3C1C2, and FXa generation assays performed as described in the legend to Figure 2. Results were averaged from 4 separate determinations. WT, closed circles; E665L, closed triangles; E665I, closed squares.
Binding affinity between WT bA2 subunit and variant A1/A3C1C2 dimer determined by reconstitution assays
For high stability variants containing a mutation in the A3C1C2 subunit, the WT A3C1C2 and A3 domain variant E1984A and E1984V subunits were purified as described in Methods and materials. Each of the A3C1C2 subunits were reconstituted with excess WT A1 as opposed to excess A3C1C2 due to limiting concentrations of variant A3C1C2. The dimer was reconstituted as described in Methods and materials. The purpose of this reconstitution rather than purification of A1/A3C1C2 dimers from the FVIIIa forms was to eliminate the presence of A2 subunit that may contaminate the dimer preparations. The reconstituted dimers (20 nM) were titrated with 0-240 nM WT bA2. Progress curves from FXa generation assays were fitted to the single-site binding equation (Figure 4) to estimate the apparent Kd values for A2 in WT FVIIIa and FVIIIa variants (Table 1). The slight differences in Kd values determined in the two WT experimental sets can be attributed to standard deviation and/or slight discrepancies in the concentration determination of the limiting component. We note however, that the results obtained with the complete complement of WT subunits yielded Kd values that were significantly greater than values for the point mutants with the exception of the E665L variant. Similar to the variants with substitutions in the A2 subunit, each of the substitutions made in A3C1C2 resulted in a similar decrease in Kd app for the A2 subunit (3.7- and 4-fold for E1984A and E1984V, respectively) as compared to WT FVIIIa. Unlike the disparity in values seen with the E665 Ala and Val variants, these results suggested that specific volume differences between Ala and Val did not impact the inter-A2 subunit interaction. Furthermore, the modest increases in maximal FXa generation observed with these variants were consistent with previously observed increases in thrombin generation parameter values for the variants (11).
Figure 4. A2 binding in FVIIIa variants with mutations in A3C1C2 subunit by reconstitution assay.
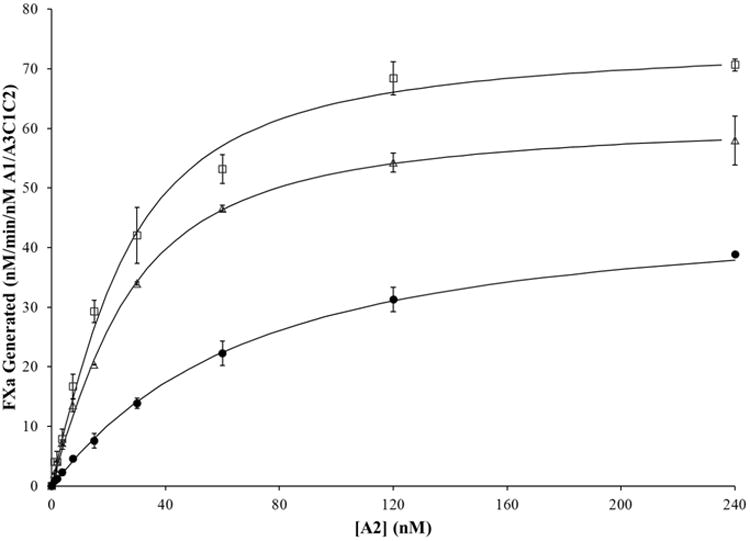
WT FVIIIa and FVIIIa mutants with a substitution in the A3C1C2 subunit were reconstituted at varying A2 concentrations. 20 nM of WT, E1984A, and E1984V A1/A3C1C2 were reconstituted with the indicated concentrations of WT bA2 and excess (500 nM) A1. Reconstitution reactions were incubated at room temperature for 2 hours. Rates of FXa generated per nM A1/A3C1C2 were determined and the data fitted to a single site binding equation as described in Methods and materials. Results were averaged from 2 separate determinations. WT, closed circles; E1984A, open triangles; E1984V, open squares.
Discussion
FVIIIa activity is labile due to the tendency for A2 subunit to dissociate under physiological conditions. We have previously shown that replacing D519, E665, and E1984, alone (10) and in combination (11), with either alanine or valine resulted in decreased rates of FVIIIa decay, as well as increased FVIII thermostability and increased thrombin generation in plasma. In the present study, we show that the enhanced stability observed in these FVIII variants results from an increased inter-A2 subunit affinity, as determined by functional assay using FVIIIa reconstitution from isolated subunits. D519, E665, and E1984 are conserved in human, canine, porcine, mouse, rabbit and bat FVIII, and D519 is also conserved in rat FVIII. Considering that these residues are detrimental to FVIII and FVIIIa stability, conservation of D519, E665, and E1984 suggests that their role in A2 dissociation has an important function, presumably for the dissociation of FVIIIa leading to down-regulation of FXase.
The extent of affinity enhancement observed correlates well with previous results monitoring the rates of FVIIIa activity decay (10). For example, at 4 nM FVIIIa the D519A and E665A variant activity decayed at a rate that was ∼60% the rate of WT FVIIIa, while the E665V decayed at a rate ∼20% that of WT and this decay rate was similar to that of either the Ala or Val mutation at E1984 (10). Examination of the functional affinity data (Table 1) suggest somewhat of a gradient of the apparent Kd values for the point mutants with E665V < E1984V ≈ E665A≈ D519V ≈ E1984A < D519A <WT. Furthermore, the D519V/E665V FVIIIa (1.5 nM) possessed an activity decay rate that was ∼14% the value observed for WT FVIIIa (11). This marked reduction in FVIIIa decay parallels the enhanced affinity for the double mutation.
While the above variants show reduced rates of FVIIIa decay as a result of increased affinity for the bA2 subunit, other variants have been described where covalent interaction of the A2 subunit to eliminate its dissociation have demonstrated increased FVIIIa activity using both in vitro and in vivo assays. Pipe and Kaufman produced a high stability FVIIIa by eliminating the thrombin cleavage site at Arg740 and covalently linking the A2 and A3 domains using a 54 amino acid linker consisting of B-domain residues. This variant, termed IR8, maintained 38% initial clotting activity for over 4 hours after thrombin activation (22). Physiological studies showed that IR8 ectopically expressed in platelets was more effective than WT FVIII in cuticular bleeding assays and FeCl3 carotid artery models in hemophilia A mice (23). Gale et al. (24) showed that covalently linking the A2 and A3 domains by a disulfide bond also increases FVIIIa stability. In this study, a disulfide bond was generated between A2 residue Y664C and A3 residue T1826C, and this variant retained 90% clotting activity three hours after activation. As WT FVIIIa only retained 5% activity after 40 minutes, the disulfide bond variant showed sustained FVIIIa stability (24). A whole blood rotational thromboelastogram (ROTEG) assay showed that this increase in FVIIIa stability resulted in faster rates of clot formation and better clot quality (25).
In an earlier study, we showed that the non-covalent D519V/E665V FVIII variant, which shows the highest inter-A2 subunit affinity of the variants tested in this report, increased thrombin generation parameters such as thrombin peak value and endogenous thrombin potential ∼2.2-fold and 1.7-fold relative to WT, respectively (11). Recently, this variant was evaluated in several injury models in the hemophilia A mouse (26). This study reported that the variant showed a >4-fold increase in maximal platelet-fibrin deposition after laser injury to the cremaster muscle arteriole and ∼2-fold increase in protection from bleeding in a tail clip and tail vein transection assays as compared with WT FVIII. Thus the capacity to increase the retention of A2 subunit in FVIIIa by limiting its dissociation appears to have clear benefits in enhancing thrombin generation in vitro and in vivo, suggesting a potential role for these reagents as a therapeutic for hemophilia A.
Our reconstitution assay allows determination of apparent Kd values for inter-A2 subunit interactions in FVIIIa. Of our variants, D519V/E665V had the lowest apparent Kd compared to WT FVIII and, as indicated above, has proven to be effective as a therapeutic treatment for several vascular injury models in the hemophilia A mouse (26). Overall, this line of investigation indicates that apparent Kd measurements involving A2 subunit would be an effective tool for predicting the translational potential of FVIIIa variants with altered A2 interactions.
What is known about this topic?
Replacing the acidic residues D519, E665, and E1984 in hydrophobic patches at the A2-A1/A3C1C2 interface in FVIII with either Ala or Val results in increased FVIII and FVIIIa stability.
What does this paper add?
The increased FVIII/FVIIIa stability observed in D519A/V, E665A/V, E1984A/V, and D519V/E665V is due to an increased apparent affinity for the A2 subunit.
A gradient of A2 affinity was observed based on specific point mutations and combination of mutations ranging from ∼2-fold to ∼47-fold lower Kd values (higher affinity) compared to WT FVIIIa.
Acknowledgments
We thank Dr. Lisa Regan of Bayer Corp. for the FVIII concentrate (Kogenate) and the 2D2 monoclonal antibody. A preliminary account of this work was presented at the XXIV Congress of the International Society on Thrombosis and Haemostasis in Amsterdam, the Netherlands on July 3, 2013.
This work was supported by NIH Grant HL38199 (to P. J. F.) and NIH predoctoral trainee-ship GM068411 from the Institutional Ruth L. Kirschstein National Research Award (to M. M.).
Abbreviations
- FVIIIa
factor VIIIa
- FXase
factor Xase
- WT
wild type
- FIXa
factor IXa
- FVIII
factor VIII
- HC
heavy chain
- LC
light chain
- FXa
factor Xa
- FIXa
factor IXa
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- FX
factor X
- BHK
baby hamster kidney
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- bA2
baculovirus expressed A2
- IMAC
immobilized metal affinity chromatography
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid]
- BSA
bovine serum albumin
- ROTEG
whole blood rotational thromboelastogram
Footnotes
Conflicts of interest: None declared.
References
- 1.Fay PJ. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 2004;18:1–15. doi: 10.1016/s0268-960x(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 2.Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266:8957–8962. [PubMed] [Google Scholar]
- 3.Lollar P, Parker CG. pH-dependent denaturation of thrombin-activated porcine factor VIII. J Biol Chem. 1990;265:1688–1692. [PubMed] [Google Scholar]
- 4.Pipe SW, Eickhorst AN, McKinley SH, Saenko EL, Kaufman RJ. Mild hemophilia A caused by increased rate of factor VIII A2 subunit dissociation: evidence for nonproteolytic inactivation of factor VIIIa in vivo. Blood. 1999;93:176–183. [PubMed] [Google Scholar]
- 5.Pipe SW, Saenko EL, Eickhorst AN, Kemball-Cook G, Kaufman RJ. Hemophilia A mutations associated with 1-stage/2-stage activity discrepancy disrupt protein-protein interactions within the triplicated A domains of thrombin-activated factor VIIIa. Blood. 2001;97:685–691. doi: 10.1182/blood.v97.3.685. [DOI] [PubMed] [Google Scholar]
- 6.Hakeos WH, Miao H, Sirachainan N, Kemball-Cook G, Saenko EL, Kaufman RJ, et al. Hemophilia A mutations within the factor VIII A2-A3 subunit interface destabilize factor VIIIa and cause one-stage/two-stage activity discrepancy. Thromb Haemost. 2002;88:781–787. [PubMed] [Google Scholar]
- 7.Duncan EM, Duncan BM, Tunbridge LJ, Lloyd JV. Familial discrepancy between the one-stage and two-stage factor VIII methods in a subgroup of patients with haemophilia A. Br J Haematol. 1994;87:846–848. doi: 10.1111/j.1365-2141.1994.tb06749.x. [DOI] [PubMed] [Google Scholar]
- 8.Rudzki Z, Duncan EM, Casey GJ, Neumann M, Favaloro EJ, Lloyd JV. Mutations in a subgroup of patients with mild haemophilia A and a familial discrepancy between the one-stage and two-stage factor VIII:C methods. Br J Haematol. 1996;94:400–406. doi: 10.1046/j.1365-2141.1996.d01-1792.x. [DOI] [PubMed] [Google Scholar]
- 9.Pemberton S, Lindley P, Zaitsev V, Card G, Tuddenham EG, Kemball-Cook G. A molecular model for the triplicated A domains of human factor VIII based on the crystal structure of human ceruloplasmin. Blood. 1997;89:2413–2421. [PubMed] [Google Scholar]
- 10.Wakabayashi H, Varfaj F, Deangelis J, Fay PJ. Generation of enhanced stability factor VIII variants by replacement of charged residues at the A2 domain interface. Blood. 2008;112:2761–2769. doi: 10.1182/blood-2008-02-142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakabayashi H, Griffiths AE, Fay PJ. Combining mutations of charged residues at the A2 domain interface enhances factor VIII stability over single point mutations. J Thromb Haemost. 2009;7:438–444. doi: 10.1111/j.1538-7836.2008.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodeve AC, Reitsma PH, McVey JH Working Group on Nomenclature of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. Nomenclature of genetic variants in hemostasis. J Thromb Haemost. 2011;9:852–855. doi: 10.1111/j.1538-7836.2011.04191.x. [DOI] [PubMed] [Google Scholar]
- 13.Mimms LT, Zampighi G, Nozaki Y, Tanford C, Reynolds JA. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981;20:833–840. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- 14.Fay PJ, Smudzin TM. Characterization of the interaction between the A2 subunit and A1/A3-C1-C2 dimer in human factor VIIIa. J Biol Chem. 1992;267:13246–13250. [PubMed] [Google Scholar]
- 15.Doering C, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P. Expression and characterization of recombinant murine factor VIII. Thromb Haemost. 2002;88:450–458. [PubMed] [Google Scholar]
- 16.Wakabayashi H, Freas J, Zhou Q, Fay PJ. Residues 110-126 in the A1 domain of factor VIII contain a Ca2+ binding site required for cofactor activity. J Biol Chem. 2004;279:12677–12684. doi: 10.1074/jbc.M311042200. [DOI] [PubMed] [Google Scholar]
- 17.Fay PJ, Mastri M, Koszelak ME, Wakabayashi H. Cleavage of factor VIII heavy chain is required for the functional interaction of a2 subunit with factor IXA. J Biol Chem. 2001;276:12434–12439. doi: 10.1074/jbc.M009539200. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi H, Griffiths AE, Fay PJ. Increasing hydrophobicity or disulfide bridging at the factor VIII A1 and C2 domain interface enhances procofactor stability. J Biol Chem. 2011;286:25748–25755. doi: 10.1074/jbc.M111.241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Jagannathan I, Ichikawa HT, Kruger T, Fay PJ. Identification of residues in the 558-loop of factor VIIIa A2 subunit that interact with factor IXa. J Biol Chem. 2009;284:32248–32255. doi: 10.1074/jbc.M109.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansong C, Fay PJ. Factor VIII A3 domain residues 1954-1961 represent an A1 domain-interactive site. Biochemistry. 2005;44:8850–8857. doi: 10.1021/bi050145o. [DOI] [PubMed] [Google Scholar]
- 22.Pipe SW, Kaufman RJ. Characterization of a genetically engineered inactivation-resistant coagulation factor VIIIa. Proc Natl Acad Sci U S A. 1997;94:11851–11856. doi: 10.1073/pnas.94.22.11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene TK, Wang C, Hirsch JD, Zhai L, Gewirtz J, Thornton MA, et al. In vivo efficacy of platelet-delivered, high specific activity factor VIII variants. Blood. 2010;116:6114–6122. doi: 10.1182/blood-2010-06-293308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gale AJ, Pellequer JL. An engineered interdomain disulfide bond stabilizes human blood coagulation factor VIIIa. J Thromb Haemost. 2003;1:1966–1971. doi: 10.1046/j.1538-7836.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 25.Radtke KP, Griffin JH, Riceberg J, Gale AJ. Disulfide bond-stabilized factor VIII has prolonged factor VIIIa activity and improved potency in whole blood clotting assays. J Thromb Haemost. 2007;5:102–108. doi: 10.1111/j.1538-7836.2006.02283.x. [DOI] [PubMed] [Google Scholar]
- 26.Leong L, Thompson T, Tran K, Zhu D, Yegneswaran S, Patel C, Xu Y, Liu P, Mallari C, Sim D, Wakabayashi H, Fay PJ, Kretschmer P, Laux V, Murphy JE. Reduction of A2 Subunit Dissociation Increases Factor VIII Potency and Efficacy in HemA Mice. J Thromb Haemost. 2013;11:145. s2. [Google Scholar]


